Physicochemical Response of External Plant Growth Regulator in the Cutting Process of Mulberry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Test Method
2.2.1. Cutting Method
2.2.2. Dynamic Observation and Index Observation of Rooting
2.2.3. Determination of Physiological and Biochemical Indexes
2.2.4. Data Processing and Statistical Analysis
3. Results and Analysis
3.1. Growth and Development of Adventitious Roots
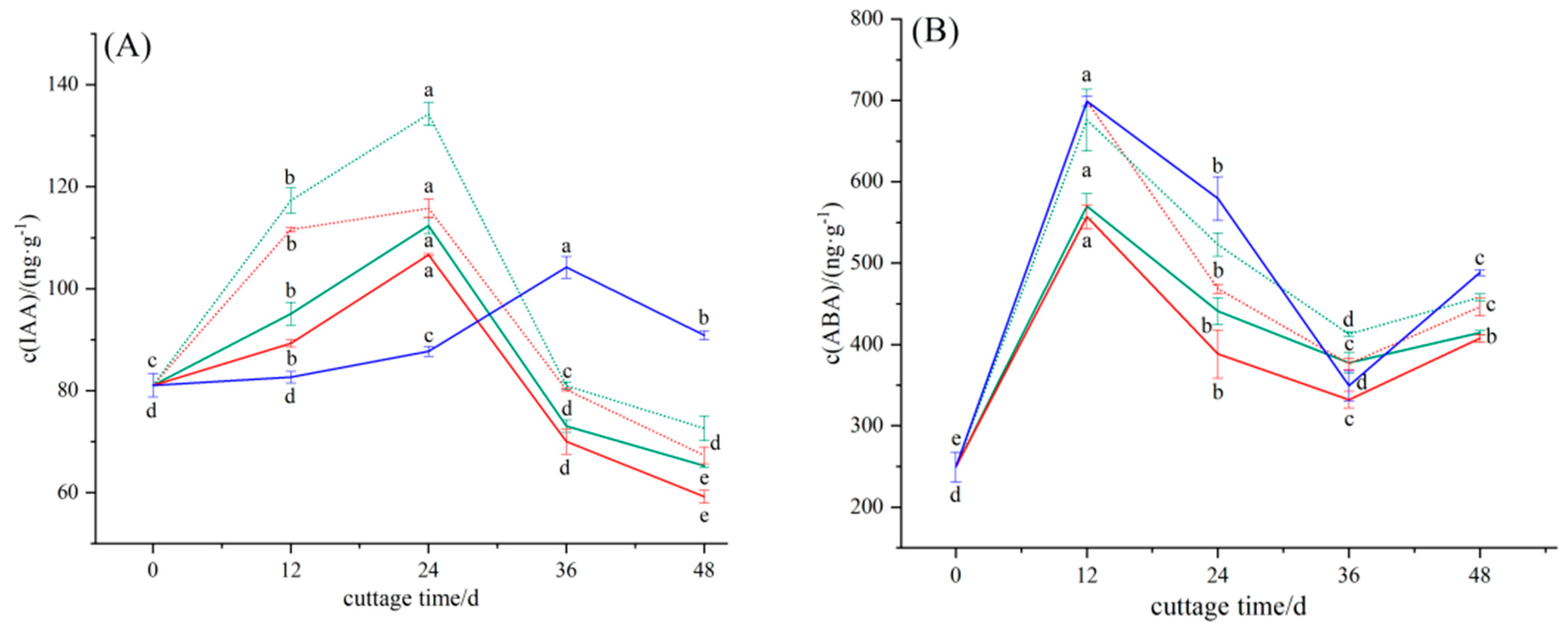
3.2. Endogenous Hormone Content during Adventitious Root Formation
3.2.1. Dynamic Changes in Endogenous Hormone IAA in the Rooting Process of Cuttings
3.2.2. Dynamic Changes in Endogenous Hormone ABA in the Rooting Process of Cuttings
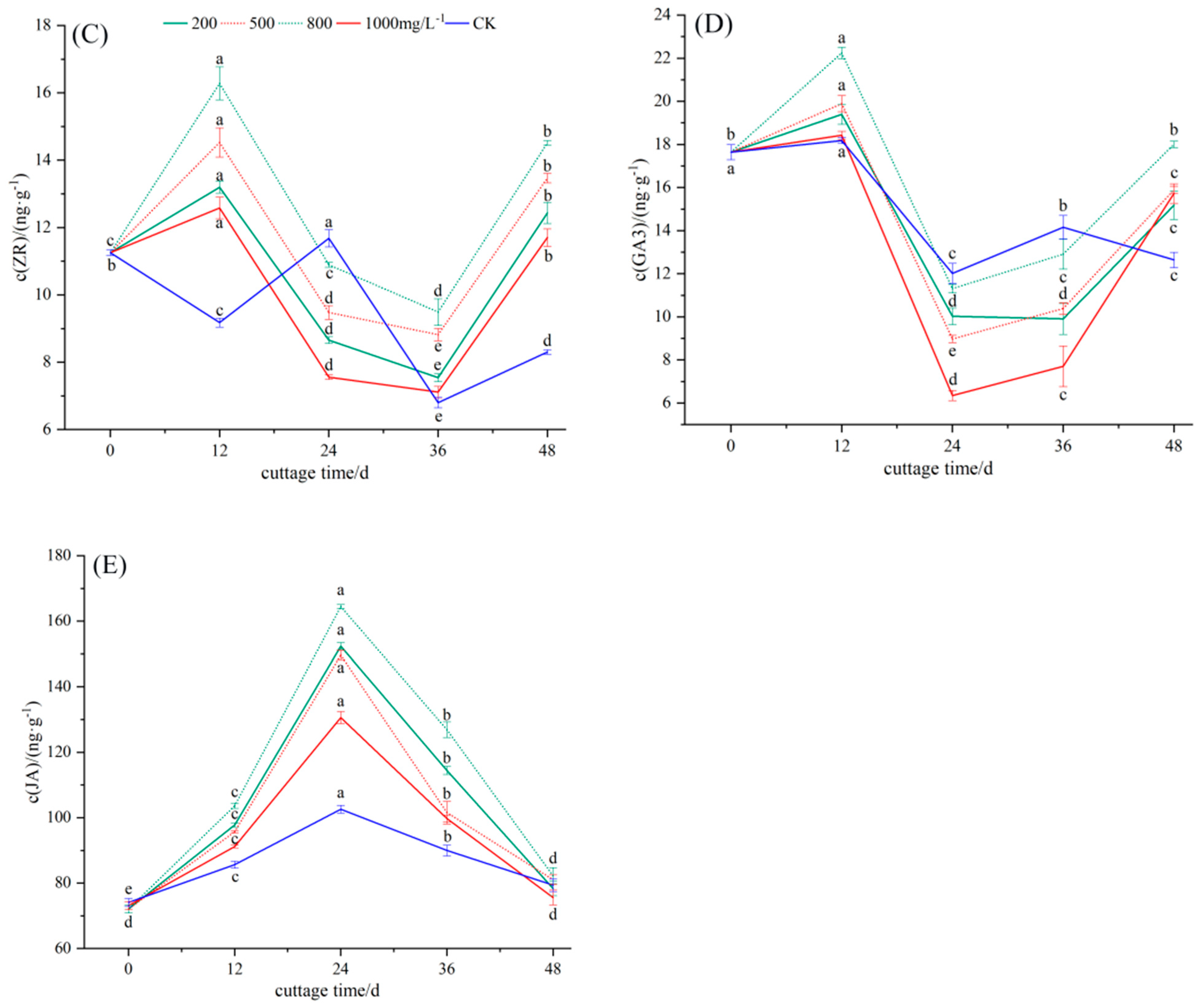
3.2.3. Dynamic Changes in Endogenous Hormone ZR in the Rooting Process of Cuttings
3.2.4. Dynamic Changes in Endogenous Hormone GA3 in the Rooting Process of Cuttings
3.2.5. Dynamic Changes in Endogenous Hormone JA in the Rooting Process of Cuttings
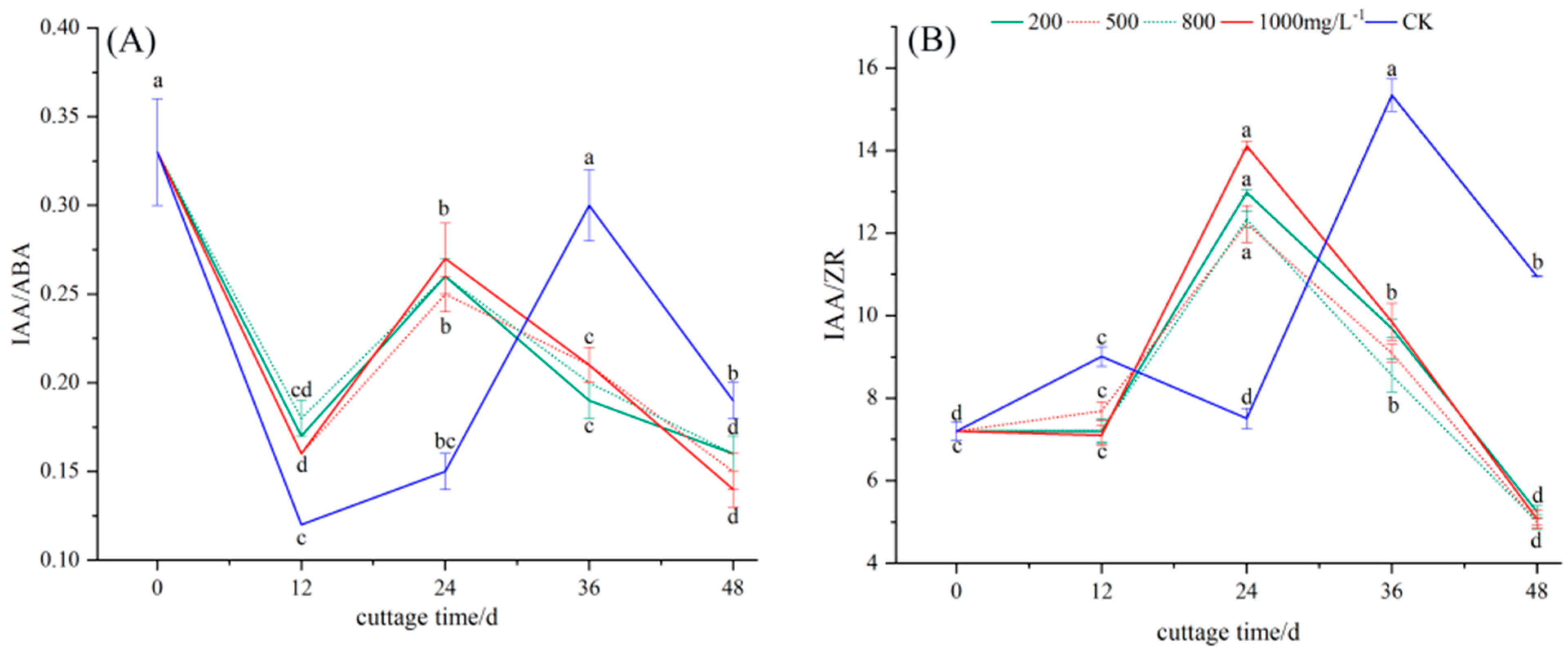
3.2.6. Changes in Endogenous Hormone Ratio in Cuttings Treated with Different Concentrations of ABT1
3.3. Changes in Oxidase Activity during Rooting of Cuttings
4. Discussion
4.1. The Relationship between Exogenous Hormone Treatment and Rooting of Cuttings
4.2. The Relationship between Adventitious Root Formation and Endogenous Hormones in Twig Cuttings of Mulberry
4.3. Relationship between Adventitious Root Formation and Related Enzyme Activity in Twig Cutting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gundogdu, M.; Muradoğlu, F.; Sensoy, R.I.; Yılmaz, H. Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci. Hortic. 2011, 132, 37–41. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Chen, L.; Hu, W.J.; Yu, C.L.; Shi, Y.B.; Chen, X.L.; Wang, J.S.; Hu, H.Q.; Shen, G.X. The expression changes of ABI4 and PIN1 genes and their correlation with rooting performance in mulberry green branch cuttings. Seric. Sci. 2015, 41, 204–210. [Google Scholar]
- Sourati, R.; Sharifi, P.; Poorghasemi, M.; Alves Vieira, E.; Seidavi, A.; Anjum, N.A.; Sehar, Z.; Sofo, A. Effects of Naphthaleneacetic Acid, Indole-3-Butyric Acid and Zinc Sulfate on the Rooting and Growth of Mulberry Cuttings. Int. J. Plant Biol. 2022, 13, 245–256. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Luo, X. Research Progress of fruit tree Cutting Propagation (Literature Review). J. Hubei Agric. Col. 1992, 12, 57–62. [Google Scholar]
- Fang, C.G. Exploration and application of mulberry cuttage raising technology. China Agric. Inform. 2014, 3, 221–222. [Google Scholar]
- Ahmad, I.; Kamran, M.; Ali, S.; Cai, T.; Bilegjargal, B.; Liu, T.L.; Han, Q.F. Seed filling in maize and hormones crosstalk regulated by exogenous application of uniconazole in semiarid regions. Environ. Sci. Pollut. Res. 2018, 25, 33225–33239. [Google Scholar] [CrossRef]
- Hedden, P. Gibberellin metabolism and its regulation. Plant Growth Regul. 2001, 20, 317–318. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. The regulatory signaling of gibberellin metabolism and its crosstalk with phytohormones in response to plant abiotic stresses. In Plant Signaling Molecules; Woodhead Publishers: Cambridge, UK, 2019; pp. 333–339. [Google Scholar]
- Zhang, B.L.; Wang, W.; Wu, X.L. Application of ABT root powder on mulberry. Anhui Agric. Sci. 1993, 21, 367–370. [Google Scholar]
- Reed, R.C.; Brady, S.R.; Muday, G.K. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998, 118, 1369–1378. [Google Scholar] [CrossRef]
- Chen, H.; Lei, Y.; Sun, J.; Ma, M.; Deng, P.; Quan, J.; Bi, H. Effects of Different Growth Hormones on Rooting and Endogenous Hormone Content of Two Morus alba L. Cuttings. Horticulturae 2023, 9, 552. [Google Scholar] [CrossRef]
- Shang, C.Q. Comparative Study on Physiological and Transcriptional Regulation Mechanism of Different Rooting Types in Mulberry Hard Branch Cuttings; Jiangsu University of Science and Technology: Jiangsu, China, 2020. [Google Scholar]
- Du, W. Study on Rooting Mechanism of Mulberry Green Branch Cuttings by Artificial Induction; Jiangsu University of Science and Technology: Zhenjiang, China, 2010. [Google Scholar]
- Wen, S.; Miao, D.; Cui, H.; Li, S.; Gu, Y.; Jia, R.; Leng, Y. Physiology and transcriptomic analysis of endogenous hormones regulating in vitro adventitious root formation in tree peony. Sci. Hortic. 2023, 318, 112–122. [Google Scholar] [CrossRef]
- Quan, J.E.; Ni, R.Y.; Wang, Y.G.; Sun, J.J.; Ma, M.Y.; Bi, H.T. Effects of Different Growth Regulators on the Rooting of Catalpa bignonioides Softwood Cuttings. Life 2022, 12, 1231. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; He, X.; Ma, Y.; Fei, Y. Effects of ABT on the morphogenesis and inclusions of Taxus chinensis (Pilger) Rehd f. baokangsis cutting rooting. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12200. [Google Scholar] [CrossRef]
- Pan, J. Research on Rooting Mechanism of Cuttings of Three Species of Suzuki; Nanjing Forestry University: Nanjing, China, 2007. [Google Scholar]
- Zhuo, M.; Sakuraba, Y.; Yanagisawa, S. A jasmonate-activated myc2-dof2.1-myc2 transcriptional loop promotes leaf senescence in Arabidopsis. Plant Cell 2019, 32, 242–262. [Google Scholar] [CrossRef] [PubMed]
- Intapruk, C.; Yamamoto, K.; Sekine, M.; Takano, M.; Shinmyo, A. Regulatory sequences involved in the peroxidase gene expression in Arabidopsis thaliana. Plant Cell Rep. 1994, 13, 123–129. [Google Scholar] [CrossRef]
- Graham, M.Y.; Graham, T.L. Rapid Accumulation of Anionic Peroxidases and Phenolic Polymers in Soybean Cotyledon Tissues following Treatment with Phytophthora megasperma f. sp. Glycinea Wall Glucan. Plant Physiol. 1991, 97, 1445–1455. [Google Scholar] [CrossRef]
- Song, L.H.; Cao, B.H. Study on the changes of indole-acetic acid oxidase, polyphenol oxidase and peroxidase activities of cutting roots of Guangyoussonetia Broussonetia. J. Plant Sci. 2005, 23, 347–350. [Google Scholar]
- Ma, F.Q.; Yang, Q.; Guo, Q.S.; Zhang, G.J.; Qin, A.L.; Wu, H.; Cai, S.Y.; Wang, L. Effects of picking position, substrate and growth regulator on cuttings of Cypress cypress. J. For. Eng. 2003, 164, 901–909. [Google Scholar]
- Zhang, Z.W.; Wang, Z.H. Effects of different concentrations of ABT root powder on cuttage rooting of 3 tea cultivars. Mol. Plant Breed. 2021, 19, 6574–6580. [Google Scholar]
- Yan, T.W.; Chen, G.H.; Li, G. Effects of ABT1 on rooting, nutrient content and enzyme activity in cuttings of Quercus mongolicus. J. Inn. Mong. Agric. Univ. (Soc. Sci. Ed.) 2022, 43, 34–39. [Google Scholar]
- Sun, J.J.; Li, H.Y.; Chen, H.L.; Wang, T.T.; Quan, J.E.; Bi, H.T. The Effect of Hormone Types, Concentrations, and Treatment Times on the Rooting Traits of Morus ‘Yueshenda 10’ Softwood Cuttings. Life 2023, 13, 1032. [Google Scholar] [CrossRef] [PubMed]
- Owusu Adjei, M.; Xiang, Y.; He, Y.; Zhou, X.; Mao, M.; Liu, J.; Hu, H.; Luo, J.; Zhang, H.; Feng, L.; et al. Adventitious root primordia formation and development in the stem of Ananas comosus var. bracteatus slip. Plant Signal Behav. 2021, 16, 1949147. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ji, K.-S.; Zhai, J.-R. Relationship between rooting ability and endogenous phytohormone changes in successive continuous generation cuttings of Buxus sinica var. parvifolia, an endangered woody species in China. For. Stud. China 2007, 9, 189–197. [Google Scholar] [CrossRef]
- Kumar, P.; Patel, P.K.; Sonkar, M.K. Propagation through juvenile shoot cuttings in difficult-to-root Dalbergia latifolia—examining role of endogenous IAA in adventitious rooting. Plant Physiol. Rep. 2022, 27, 242–249. [Google Scholar] [CrossRef]
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M.M. Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of arabidopsis. Ann. Bot. 2013, 112, 1395–1407. [Google Scholar] [CrossRef]
- Tahir, M.M.; Chen, S.; Ma, X.; Li, S.; Zhang, X.; Shao, Y.; Shalmani, A.; Zhao, C.; Bao, L.; Zhang, D. Transcriptome analysis reveals the promotive effect of potassium by hormones and sugar signaling pathways during adventitious roots formation in the apple rootstock. Plant Physiol. Biochem. 2021, 165, 123–136. [Google Scholar] [CrossRef]
- Wang, Q.M.; Peng, W.X.; Zhang, J.P.; Pei, D. Study on the morphological structure and hormone regulation of root of young stem of walnut in vitro. J. Hortic. 2006, 33, 5. [Google Scholar]
- Gao, J.; Zeng, X.F.; Liu, X.H.; Yang, S.X. Cutting propagation of Periploca forrestii and dynamic analyses of physiological and biochemical characteristitics related to adventitious roots formation. J. Chin. Med. Mater. 2011, 34, 841–845. [Google Scholar]
- Pfaff, W.; Schopfer, P. Hormones are no causal links in phytochrome-mediated adventitious root formation in mustard seedlings (Sinapis alba L.). Planta 1980, 150, 321–329. [Google Scholar] [CrossRef]
- Brian, P.W.; Hemming, H.G.; Lowe, D. Inhibition of rooting of cuttings by gibberellic acid. Ann. Bot. 1960, 24, 407–419. [Google Scholar] [CrossRef]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. 2014, 78, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Zheng, Y.; Chen, L.G. Study on root culture and changes of endogenous hormone content of tissue culture seedlings of Cuckoo. J. Fujian For. Col. 2021, 31, 5. [Google Scholar]
- Zhang, F.; Wang, H. Research progress on rooting mechanism of peach hardwood cuttings. J. Plant Physiol. 2019, 55, 1595–1606. [Google Scholar]
- Sipes, D.L.; Einset, J.W. Cytokinin stimulation of abscission in lemon pistil explants. J. Plant Growth Regul. 1983, 2, 73–80. [Google Scholar] [CrossRef]
- Lup, S.D.; Tian, X.; Xu, J.; Pérez-Pérez, J.M. Wound signaling of regenerative cell reprogramming. Plant Sci. Int. J. Exp. Plant Biol. 2016, 250, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Song, P.F.; Chen, H.J.; Jiang, Y.Q.; Wei, J.G.; Li, J.H. Effect of IBA on Rooting and Endogenous Hormone Changes of Rabbit Eyed Blue Berry Twig Cutting. Chin. Agri. Sci. Bull. 2014, 30, 117–122. [Google Scholar]
- Guo, S.J.; Ling, H.Q.; Li, F.L. A Study on the Physiological and Biochemical Basis of Rooting of Pinus bungeana cuttings. J. Beijing Fore. Univ. 2004, 26, 43–47. [Google Scholar]
- Wang, Y.; Yao, R.L. Increased endogenous indole-3-acetic acid:abscisic acid ratio is a reliable marker of Pinus massoniana rejuvenation. Biotech. Histochem. 2019, 94, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, H.; Taşkin, T.; Otludil, B. Polyphenol Oxidase Activity during Rooting in Cuttings of Grape (Vitis vinifera L.) Varieties. Turk. J. Bot. 2003, 27, 495–498. [Google Scholar]
- Bassuk, N.L.; Hunter, L.D.; Howard, B.H. The apparent involvement of polyphenol oxidase and phloridzin in the production of apple rooting cofactors. J. Hortic. Sci. 1981, 56, 313–322. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, K.; Zhang, A.; Zhu, W.; Zhang, H.; Tan, F.; Huang, Q.; Wu, X.; Zha, D. Metabolomic Analysis, Combined with Enzymatic and Transcriptome Assays, to Reveal the Browning Resistance Mechanism of Fresh-Cut Eggplant. Foods 2022, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R. Effect of Auxins on Adventitious Root Development from Single Node Cuttings of Camellia sinensis (L.) Kuntze and Associated Biochemical Changes. Plant Growth Regul. 2006, 48, 111–117. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Hausman, J.; Berthon, J.; Ripetti, V. Practical uses of peroxidase activity as a predictive marker of rooting performance of micropropagated shoots. Agronomie 1992, 12, 757–765. [Google Scholar] [CrossRef]
- Su, C.F.; Duan, G.Z.; Fan, G.H. Physiological and biochemical analysis of Lycium barbarum cutting rooting. North. Hortic. 2023, 520, 90–97. [Google Scholar]
- Nag, S.; Saha, K.; Choudhuri, M.A. Role of Auxin and Polyamines in Adventitious Root Formation in Relation to Changes in Compounds Involved in Rooting. J. Plant Growth Regul. 2001, 20, 182–194. [Google Scholar] [CrossRef]


| Index | ABT1 Concentration (mg/L−1) | ||||
|---|---|---|---|---|---|
| 200 | 500 | 800 | 1000 | 0 (CK) | |
| Rooting rate (%) | 46.67 ± 0.58 b | 45.77 ± 0.51 b | 64.63 ± 0.35 a | 20.73 ± 1.41 c | 18.20 ± 0.60 c |
| Average number of roots (Strip/Plant) | 5.22 ± 0.17 b | 5.56 ± 0.20 b | 8.36 ± 0.18 a | 2.89 ± 1.11 c | 2.30 ± 0.05 c |
| Average root length (cm) | 4.73 ± 0.06 bc | 4.77 ± 0.05 b | 5.86 ± 0.10 a | 4.62 ± 0.06 c | 1.07 ± 0.07 d |
| Longest root length (cm) | 6.37 ± 0.23 c | 8.53 ± 0.23 b | 9.17 ± 0.13 a | 3.50 ± 0.13 d | 3.25 ± 0.15 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Dou, H.; Chen, H.; Wang, Y.; Wang, T.; Quan, J.; Bi, H. Physicochemical Response of External Plant Growth Regulator in the Cutting Process of Mulberry. Horticulturae 2023, 9, 1006. https://doi.org/10.3390/horticulturae9091006
Sun J, Dou H, Chen H, Wang Y, Wang T, Quan J, Bi H. Physicochemical Response of External Plant Growth Regulator in the Cutting Process of Mulberry. Horticulturae. 2023; 9(9):1006. https://doi.org/10.3390/horticulturae9091006
Chicago/Turabian StyleSun, Jiajia, Hao Dou, Hanlei Chen, Yilin Wang, Tiantian Wang, Jin’e Quan, and Huitao Bi. 2023. "Physicochemical Response of External Plant Growth Regulator in the Cutting Process of Mulberry" Horticulturae 9, no. 9: 1006. https://doi.org/10.3390/horticulturae9091006
APA StyleSun, J., Dou, H., Chen, H., Wang, Y., Wang, T., Quan, J., & Bi, H. (2023). Physicochemical Response of External Plant Growth Regulator in the Cutting Process of Mulberry. Horticulturae, 9(9), 1006. https://doi.org/10.3390/horticulturae9091006





