Effect of Red Visible Lighting on Postharvest Ripening of Bananas via the Regulation of Energy Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
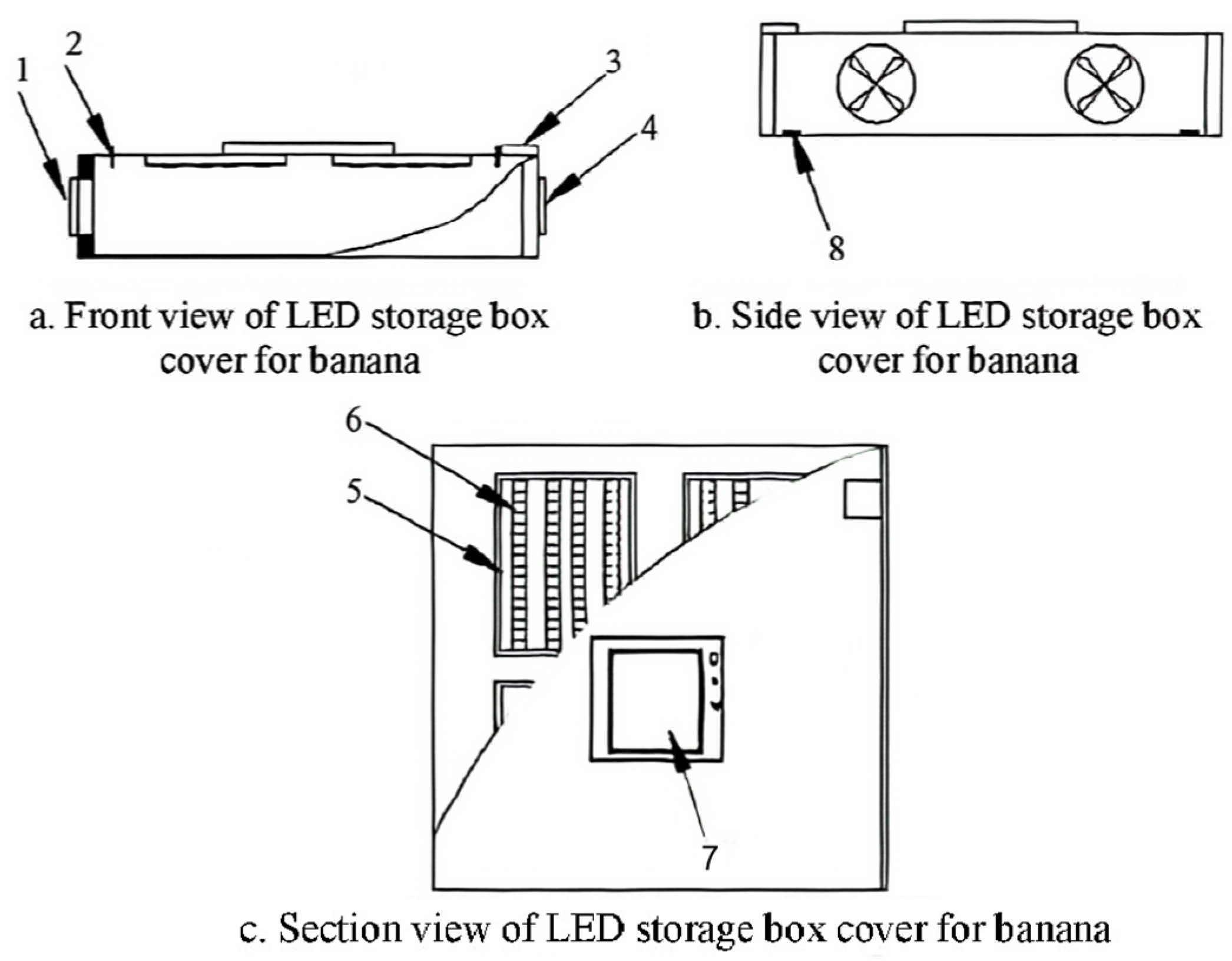
2.2.1. Experimental Grouping and Conditions
2.2.2. Determination of the Ripening Parameters
2.2.3. Determination of the Energy Metabolism Index
2.2.4. Determination of Pigment Content
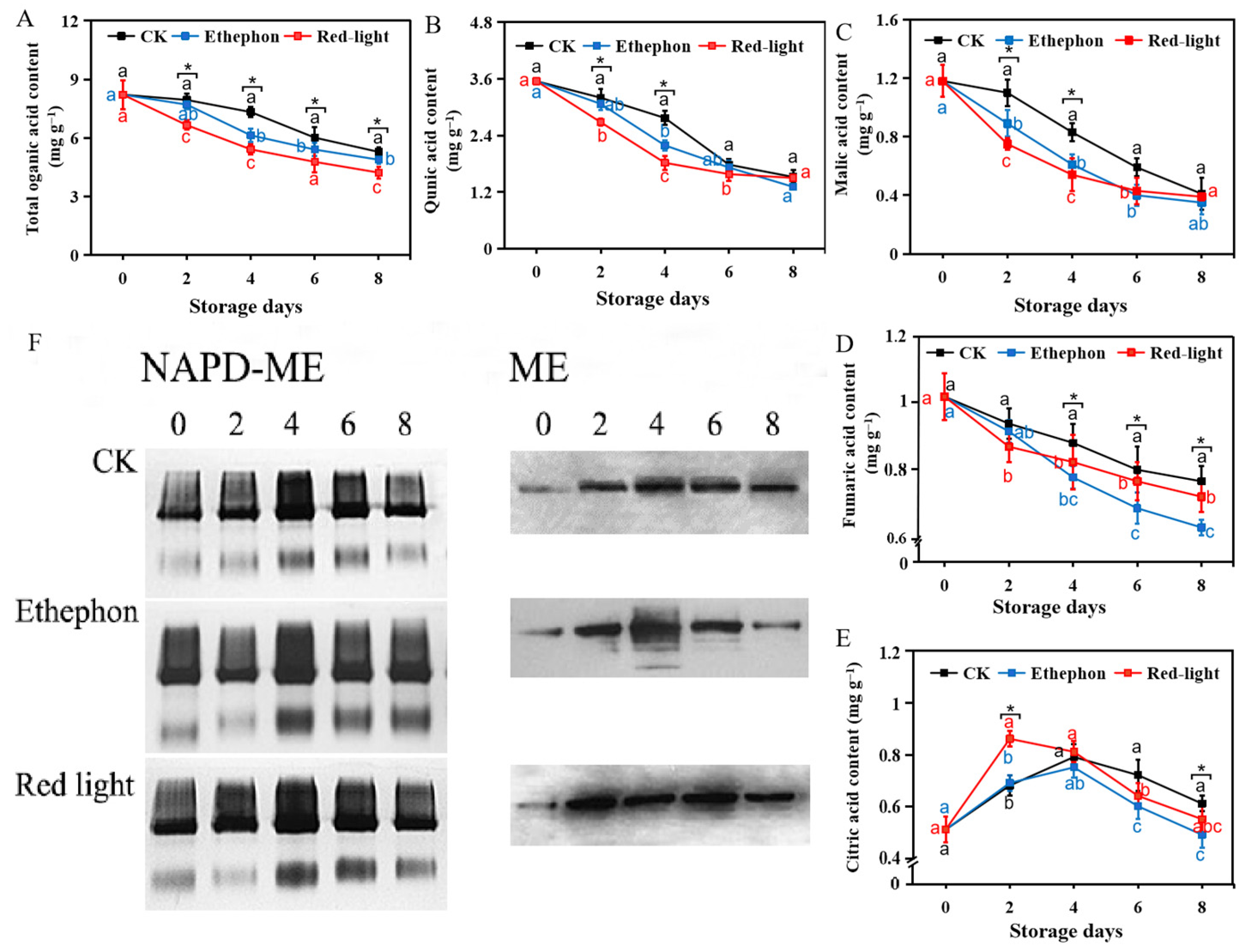
2.2.5. Detection of Organic Acid Contents
2.2.6. Determination of the NADP-Malic Enzyme Activity and Malic Enzyme Protein Content of Banana
2.3. Statistical Analysis
3. Results and Analysis
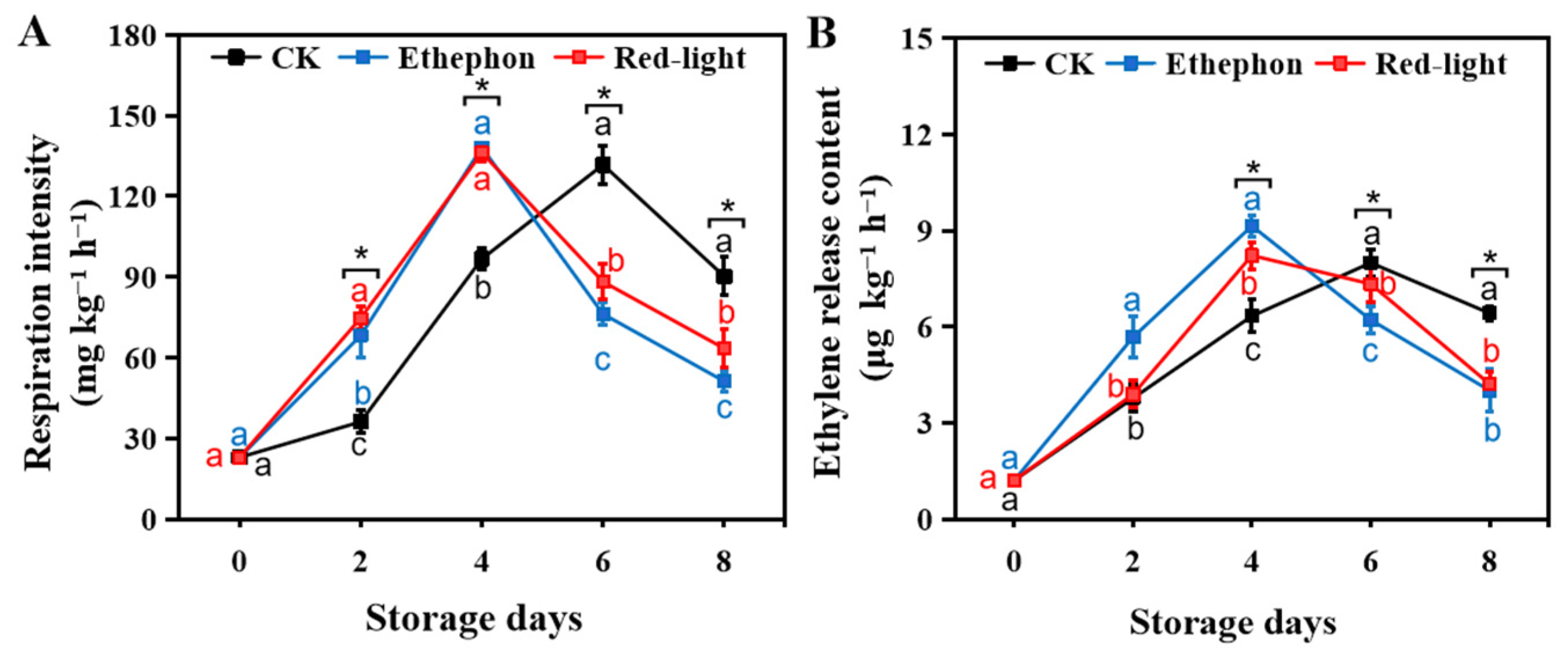
3.1. Effect of LED Red Light Irradiation on the Physiological Quality of the Banana Fruit
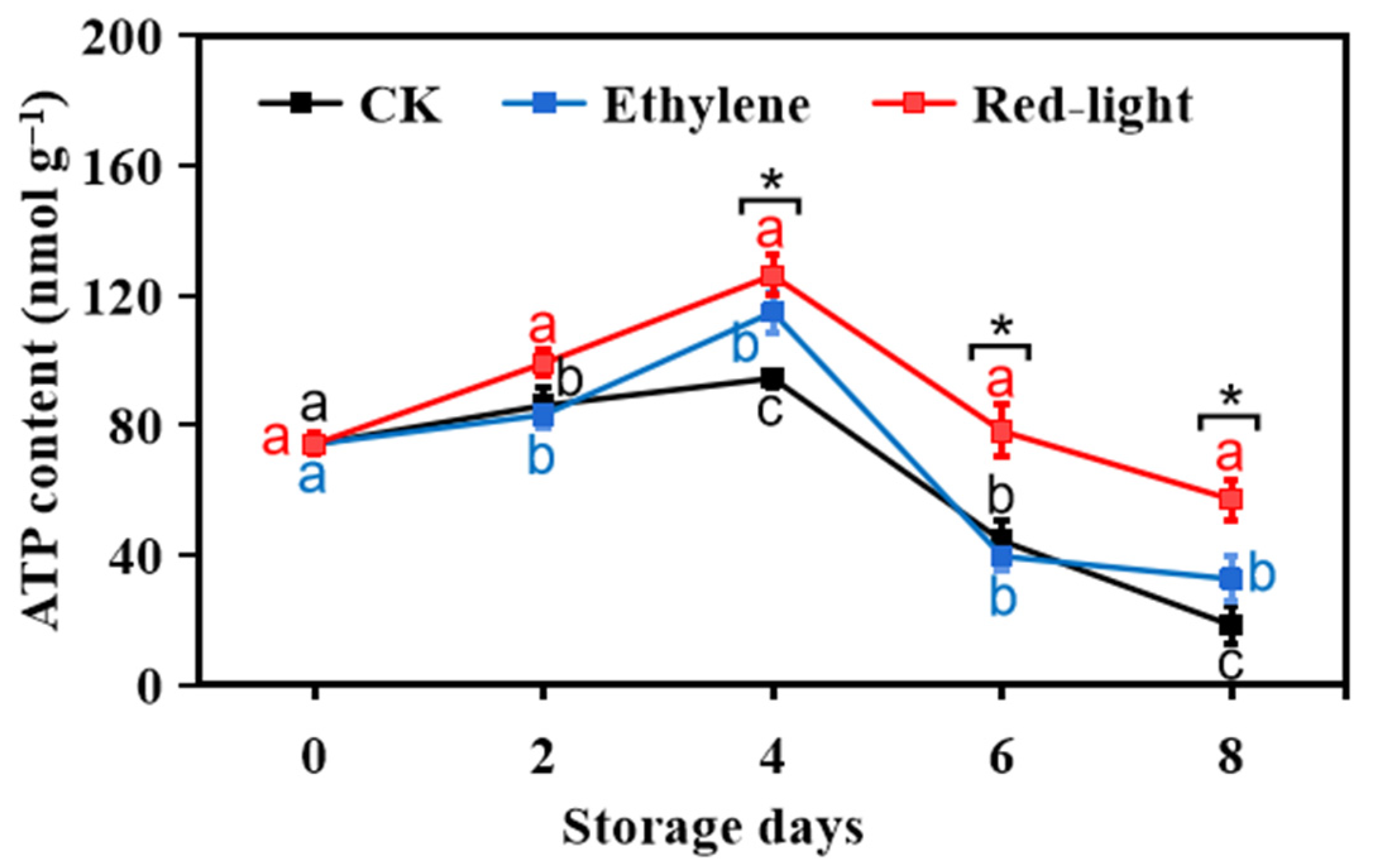
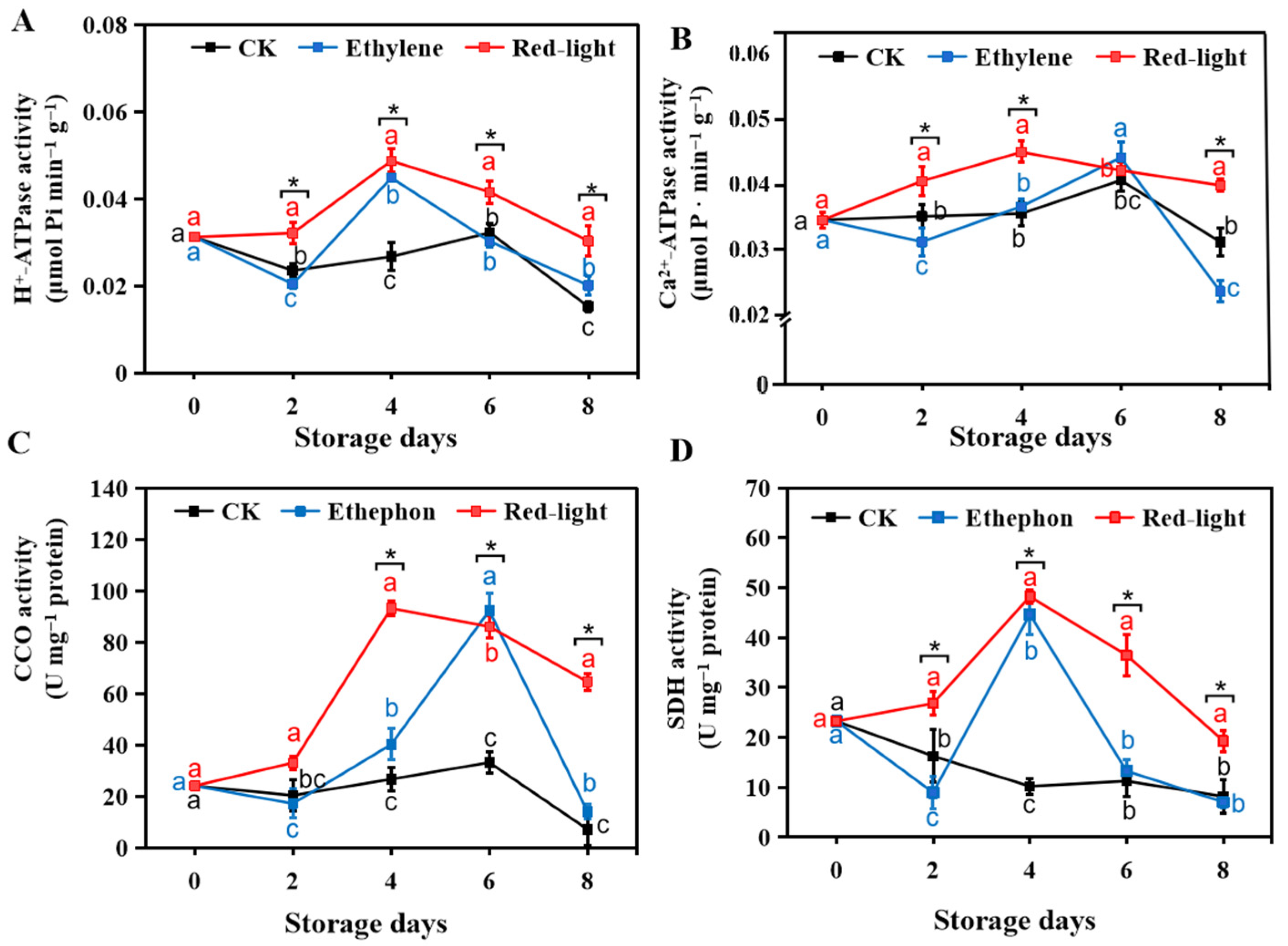
3.2. Effect of LED Red Light Irradiation on the Energy Metabolism of Banana Fruits
3.3. Effect of LED Red Light Irradiation on the Metabolism of Malic Acid in Bananas
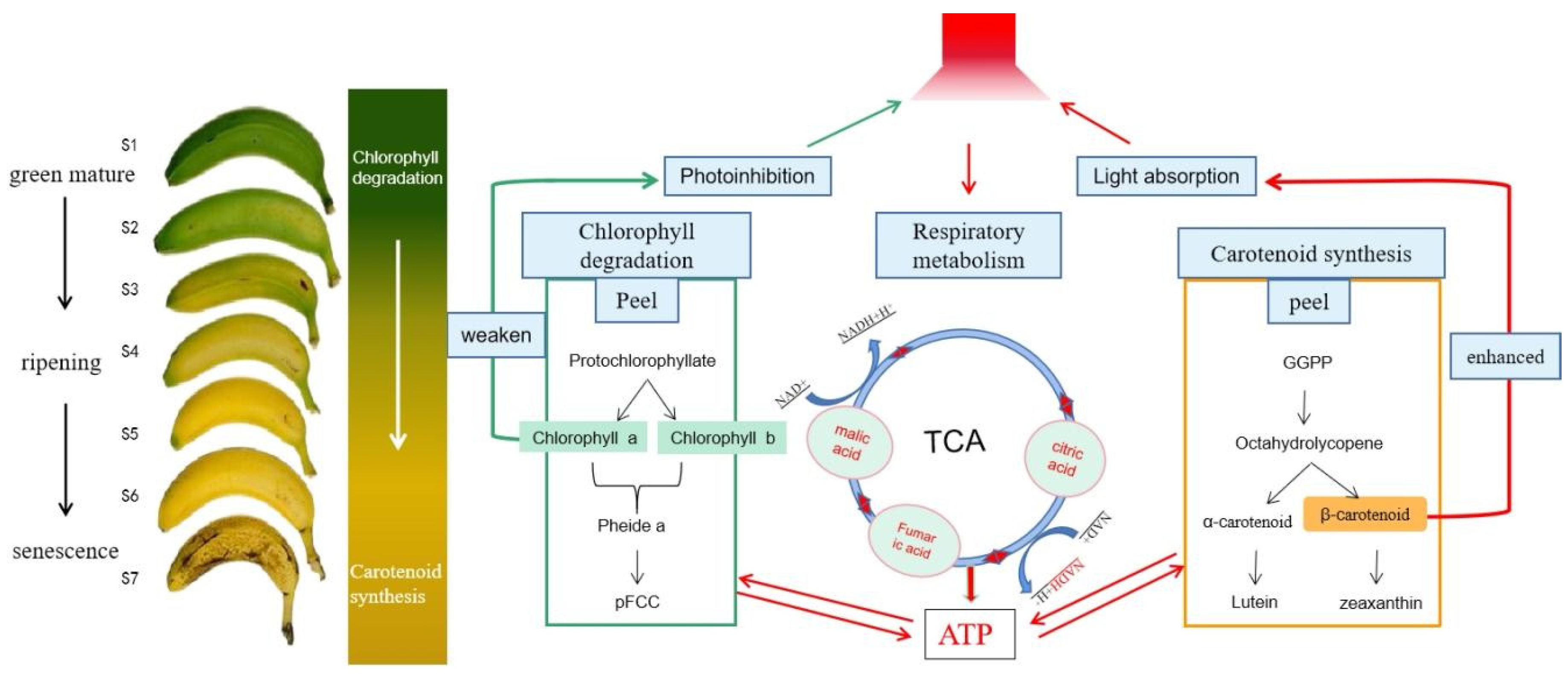
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohandas, S.; Ravishankar, K.V. Banana: Genomics and Transgenic Approaches for Genetic Improvement; Springer: Singapore, 2016; pp. 112–113. [Google Scholar] [CrossRef]
- Bantayehu, M.; Alemayehu, M. Efficacy of post-harvest technologies on ripening behavior and quality of banana varieties grown in Ethiopia. Int. J. Fruit Sci. 2020, 20, 59–75. [Google Scholar] [CrossRef]
- Gamrasni, D.; Feldmesser, E.; Ben-Arie, R.; Raz, A.; Tabatznik, A.A.; Glikman, M.; Goldway, M. Gene expression in 1-Methylcyclopropene (1-MCP) treated tomatoes during pre-climacteric ripening suggests shared regulation of methionine biosynthesis, ethylene production and respiration. Agronomy 2020, 10, 1669. [Google Scholar] [CrossRef]
- Parijadi, A.; Yamamoto, K.; Ikram, M.; Dwivany, F.; Wikantika, K.; Putri, S.; Fukusaki, E. Metabolome analysis of banana (Musa acuminata) treated with chitosan coating and low temperature reveals different mechanisms modulating delayed ripening. Front. Sustain. Food Syst. 2022, 6, 835978. [Google Scholar] [CrossRef]
- Lu, W.; Mao, L.; Chen, J.; Han, X.; Ren, X.; Ying, T.; Luo, Z. Interaction of abscisic acid and auxin on gene expression involved in banana ripening. Acta Physiol. Plant. 2018, 40, 46. [Google Scholar] [CrossRef]
- Ishra, R.; Khananm, R.; Soar, J. Influence of food safety concerns on safe food purchasing at rural and urban consumers in Bangladesh. Appetite 2022, 179, 106306. [Google Scholar] [CrossRef]
- Hossain, R. Safe Food in Bangladesh: Perception and Influences on Safe Food Purchasing; Griffith University: Brisbane, Australia, 2019. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and opportunities of light-emitting diode (Led) as key to modulate antioxidant compounds in plants. a review. Antioxidants 2020, 10, 42. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, Z.; Xie, J.; Yao, S.; Zeng, K. Sensitivity to ethephon degreening treatment is altered by blue LED light irradiation in mandarin fruit. J. Agric. Food Chem. 2017, 65, 6158–6168. [Google Scholar] [CrossRef]
- Shu, C.; Cao, J.; Jiang, W. Post-harvest vibration-induced apple quality deterioration is associated with the energy dissipation system. Food Chem. 2022, 386, 132767. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, L.; Wang, H.; Qu, H.; Ma, S.; Liu, Y.; Jiang, Y. Energy status of kiwifruit stored under different temperatures or exposed to long-term anaerobic conditions or pure oxygen. Post-Harvest Biol. Technol. 2014, 98, 56–64. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Ke, Z.; Sun, J.; Zhou, X.; Sun, J. Effects of LED light on the ripening regulation of green mature banana during storage and transportation. Trans. Chin. Soc. Agric. Eng. 2021, 37, 295–302. (in Chinese). [Google Scholar] [CrossRef]
- Liu, B.; Jiao, W.; Wang, B.; Shen, J.; Zhao, H.; Jiang, W. Near freezing point storage compared with conventional low temperature storage on apricot fruit flavor quality (volatile, sugar, organic acid) promotion during storage and related shelf life. Sci. Hortic. 2019, 249, 100–109. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, Y.; Xu, F.; Wang, H.; Chen, M.; Shao, X. Changes in the chlorophyll absorbance index (I AD) are related to peach fruit maturity. N. Z. J. Crop Hortic. Sci. 2020, 48, 34–46. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Li, Q.; Cai, T.; Jiang, W. Partial properties of an aspartic protease in bitter gourd (Momordica charantia L.) fruit and its activation by heating. Food Chem. 2008, 108, 496–502. [Google Scholar] [CrossRef]
- Mehrabani, L.V.; Hassanpouraghdam, M.B. Developmental variation of phenolic compounds in fruit tissue of two apple cultivars. Acta scientiarum polonorum. Technol. Aliment. 2012, 11, 259–264. Available online: http://www.food.actapol.net/volume11/issue3/5_3_2012 (accessed on 5 March 2023).
- Mishra, M.; Tiwari, S.; Gomes, A.V. Protein purification and analysis: Next generation Western blotting techniques. Expert Rev. Proteom. 2017, 14, 1037–1053. [Google Scholar] [CrossRef]
- Xie, C.; Tang, J.; Xiao, J.; Geng, X.; Guo, L. Purple light-emitting diode (LED) lights controls chlorophyll degradation and enhances nutraceutical quality of post-harvest broccoli florets. Sci. Hortic. 2022, 294, 110768. [Google Scholar] [CrossRef]
- Chen, L.S.; Li, P.; Cheng, L. Comparison of thermotolerance of sun-exposed peel and shaded peel of ‘Fuji’apple. Environ. Exp. Bot. 2009, 66, 110–116. [Google Scholar] [CrossRef]
- Moosavi-Nezhad, M.; Boshra, A.; Ahmad, E.; Nazim, S.; Sasan, A. Growth, Biomass Partitioning, and Photosynthetic Performance of Chrysanthemum Cuttings in Response to Different Light Spectra. Plants 2022, 11, 3337. [Google Scholar] [CrossRef]
- Muzzopappa, F.; Kirilovsky, D. Changing color for photoprotection: The orange carotenoid protein. Trends Plant Sci. 2020, 25, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, S.; Xiang, T.; Kitazawa, H.; Sun, H.; Guo, Y. Effects of light irradiation on the textural properties and energy metabolism of postharvest shiitake mushrooms (Lentinula edodes). J. Food Process. Preserv. 2021, 45, e16066. [Google Scholar] [CrossRef]
- Aghdam, M.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, H.; Wang, J.; Chen, J.; Wang, X.; Zheng, Y. Effect of methyl jasmonate on energy metabolism in peach fruit during chilling stress. J. Sci. Food Agric. 2013, 93, 1827–1832. [Google Scholar] [CrossRef]
- Osorio, S.; Scossa, F.; Fernie, A. Molecular regulation of fruit ripening. Front. Plant Sci. 2013, 4, 198. [Google Scholar] [CrossRef] [PubMed]
- González-Agüero, M.; Tejerina Pardo, L.; Zamudio, M.S.; Contreras, C.; Undurraga, P.; Defilippi, B.G. The unusual acid-accumulating behavior during ripening of cherimoya (Annona cherimola Mill.) is linked to changes in transcription and enzyme activity related to citric and malic acid metabolism. Molecules 2016, 21, 398. [Google Scholar] [CrossRef]
- Silva, G.; Silva, W.; Medeiros, D.; Salvador, A.; Cordeiro, M.; Silva, N.; Mizobutsi, G. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.; Medeiros, D.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, B.; Ding, H.; Zhang, J.; Li, S. The role of NADP-malic enzyme in plants under stress. Plant Sci. 2019, 281, 206–212. [Google Scholar] [CrossRef]
- Jiang, C.; Fang, Z.; Zhou, D.; Pan, S.; Ye, X. Changes in secondary metabolites, organic acids and soluble sugars during the development of plum fruit cv. ‘Furongli’ (Prunus salicina Lindl). J. Sci. Food Agric. 2019, 99, 1010–1019. [Google Scholar] [CrossRef]
- Onik, J.C.; Xie, Y.; Duan, Y.; Hu, X.; Wang, Z.; Lin, Q. UV-C treatment promotes quality of early ripening apple fruit by regulating malate metabolizing genes during postharvest storage. PLoS ONE 2019, 14, e0215472. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Cheng, J.; Sun, J.; Guo, S.; Guo, X.; Chen, Q.; Wang, X.; Zhu, X.; Liu, B. Effect of Red Visible Lighting on Postharvest Ripening of Bananas via the Regulation of Energy Metabolism. Horticulturae 2023, 9, 840. https://doi.org/10.3390/horticulturae9070840
Zhou X, Cheng J, Sun J, Guo S, Guo X, Chen Q, Wang X, Zhu X, Liu B. Effect of Red Visible Lighting on Postharvest Ripening of Bananas via the Regulation of Energy Metabolism. Horticulturae. 2023; 9(7):840. https://doi.org/10.3390/horticulturae9070840
Chicago/Turabian StyleZhou, Xinqun, Jianhu Cheng, Jing Sun, Shuzhen Guo, Xuexia Guo, Quan Chen, Xiaomei Wang, Xuan Zhu, and Bangdi Liu. 2023. "Effect of Red Visible Lighting on Postharvest Ripening of Bananas via the Regulation of Energy Metabolism" Horticulturae 9, no. 7: 840. https://doi.org/10.3390/horticulturae9070840
APA StyleZhou, X., Cheng, J., Sun, J., Guo, S., Guo, X., Chen, Q., Wang, X., Zhu, X., & Liu, B. (2023). Effect of Red Visible Lighting on Postharvest Ripening of Bananas via the Regulation of Energy Metabolism. Horticulturae, 9(7), 840. https://doi.org/10.3390/horticulturae9070840





