Optimizing Different Medium Component Concentration and Temperature Stress Pretreatment for Gynogenesis Induction in Unpollinated Ovule Culture of Sugar Beet (Beta vulgaris L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing Conditions for Donor Plants
2.2. Sterilization of Explants
2.3. In Vitro Culture of Unpollinated Ovules
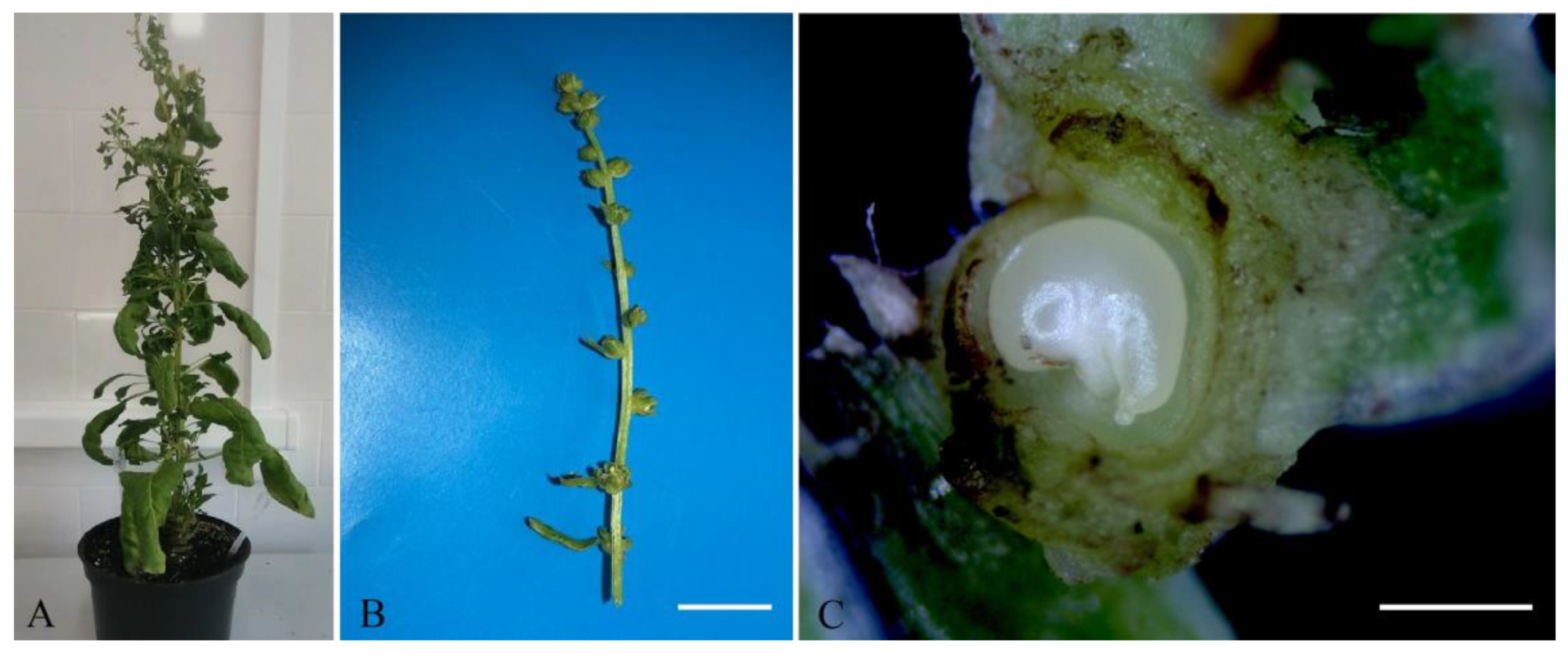
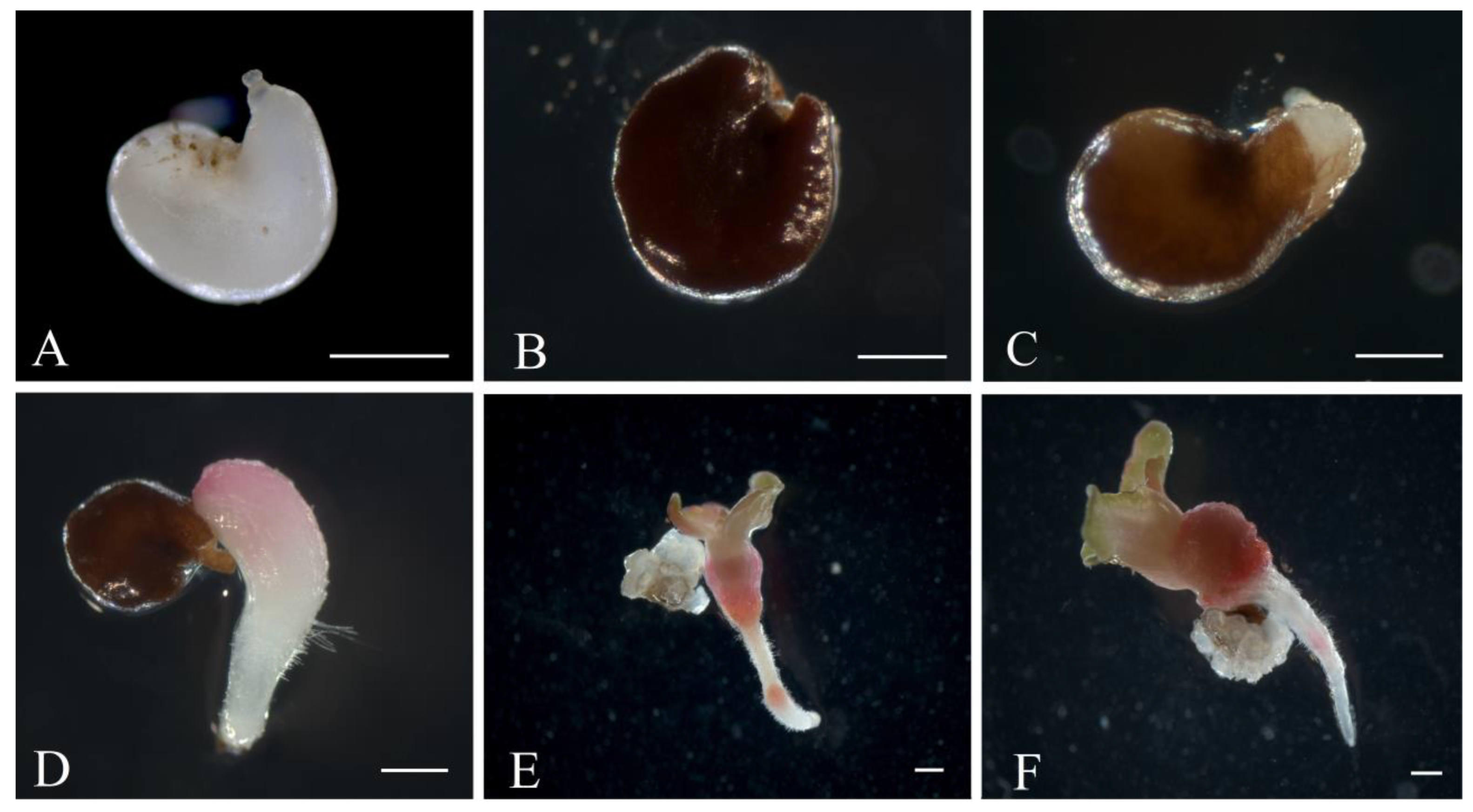
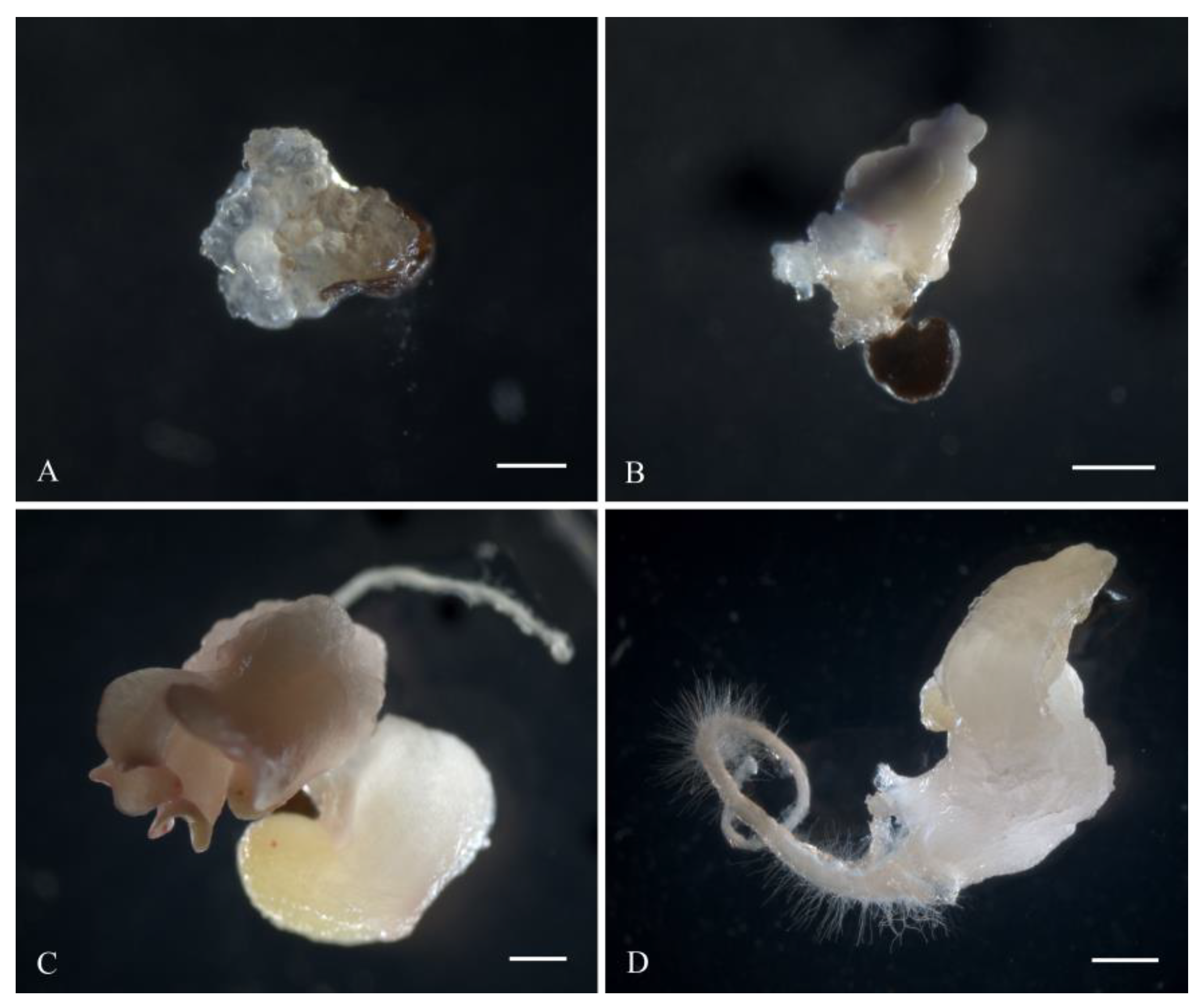
2.4. Obtaining Regenerant Plants in Unpollinated Ovule Culture
2.5. Cultivation of Regenerated Plants
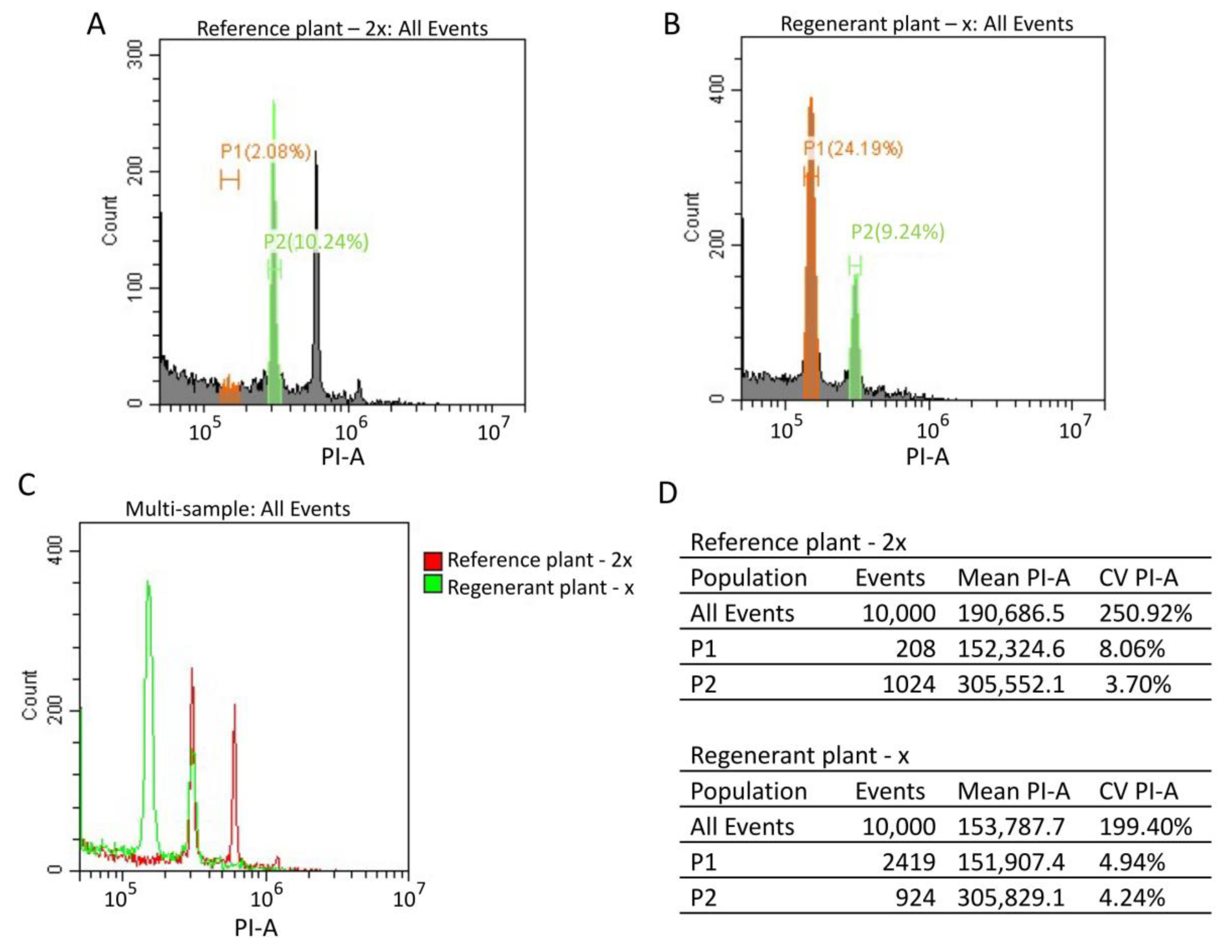
2.6. Determination of Ploidy Level by Flow Cytometry
2.7. Determination of Ploidy Level by Chromosome Counting
2.8. Regenerant Plant Ploidy Determination by Leaf Stomata Guard Cell Chloroplast Counting
2.9. Statistical Analysis
3. Results
3.1. Testing Effects of Different Medium Component Concentration and Temperature Stress Pretreatment on Gynogenesis in Different Genotypes
3.2. The Formation of Regenerating Plants
3.3. Ploidy-Level Analysis of Sugar Beet Regenerant Plants Obtained in an Unpollinated Ovule Culture In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Letschert, J.P.W.; Lange, W.; Frese, L.; Van Der Berg, D. Taxonomy of the section Beta. J. Sugar Beet Res. 1993, 31, 69–85. [Google Scholar] [CrossRef]
- Lange, W.; Brandenburg, W.A.; De Bock, T.S.M. Taxonomy and cultonomy of beet (Beta vulgaris L.). Bot. J. Linnean Soc. 1999, 130, 81–96. [Google Scholar] [CrossRef]
- Hassani, M.; Heidari, B.; Dadkhodaie, A.; Stevanato, P. Genotype by environment interaction components underlying variations in root, sugar and white sugar yield in sugar beet (Beta vulgaris L.). Euphytica. 2018, 79, 214. [Google Scholar] [CrossRef]
- Valli, V.; Gomez-Caravaca, A.M.; Di Nunzio, M.; Danesi, F.; Caboni, M.F.; Bordoni, A. Sugar cane and sugar beet molasses, antioxidant-rich alternatives to refined sugar. J. Agric. Food Chem. 2012, 60, 12508–12515. [Google Scholar] [CrossRef] [PubMed]
- Koga, N. An energy balance under a conven tional crop rotation system in northern Japan: Perspectives on fuel ethanol production from sugar beet. Agric. Ecosyst. Environm. 2008, 125, 101–110. [Google Scholar] [CrossRef]
- Gerbens-Leenesa, W.; Hoekstraa, A.Y.; Van der Meerb, T.H. The water footprint of bioenergy. Proc. Nat. Acad. Sci. USA 2009, 106, 10219–10223. [Google Scholar] [CrossRef]
- Asadi, M. Beet Sugar Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 435–450. [Google Scholar]
- OECD. Section 8—Sugar Beet (Beta vulgaris L.). In Safety Assessment of Transgenic Organisms; OECD Consensus Documents; OECD Publishing: Paris, France, 2006; Volume 1, pp. 174–196. [Google Scholar] [CrossRef]
- Ford-Lloyd, B.V. Sources of genetic variation, Genus Beta. In Genetics and Breeding of Sugar Beet; Biancardi, E., Campbell, L.G., Skaracis, G.N., De Biaggi, M., Eds.; Science Publishers Inc.: Enfield, NH, USA, 2005; pp. 25–33. [Google Scholar]
- De Bock, T.S.M. The genus Beta: Domestication, taxonomy and interspecific hybridization for plant breeding. Acta Hortic. 1986, 182, 335–343. [Google Scholar] [CrossRef]
- Knapp, E. Beta Rüben. In Handbuch der Pflanzenzüchtung; Roemer, T., Rudorf, W., Eds.; Paul Parey: Berlin, Germany, 1958; Volume 3, pp. 196–284. [Google Scholar]
- Fischer, H.E. Origin of the “Weisse schlesische Rübe” (White Silesian beet) and resyntesis of sugar beet. Euphytica 1989, 41, 75–80. [Google Scholar] [CrossRef]
- Shilov, I.A.; Aniskina, Y.V.; Shalaeva, T.V.; Kolobova, O.S.; Velishaeva, N.S.; Mischenko, V.N.; Logvinov, A.V. Creation of modern sugar beet hybrids using microsatellite analysis. Sugar 2020, 8, 27–31. [Google Scholar] [CrossRef]
- Biancardi, E.; McGrath, J.M.; Panella, L.W.; Lewellen, R.T.; Stevanato, P. Sugar Beet. In Root and Tuber Crops, Handbook of Plant Breeding; Bradshaw, J.E., Ed.; Springer: New York, NY, USA, 2010; Volume 7, pp. 173–219. [Google Scholar]
- Smith, G.A. Sugar beet. In Principles of Cultivar Development; Fehr, W.R., Ed.; Macmillan: New York, NY, USA, 1987; Volume 2, pp. 577–625. [Google Scholar]
- Oltmann, W. Verfahren und Erfolge der Zuckerrübenzüchtung seit Mitte des 19. Jahrhunderts bis zur Gegenwart. In Geschichte der Zuckerrübe: 200 Jahre Anbau und Züchtung; Bartens, A., Ed.; Verlag Dr.: Berlin, Germany, 1984; pp. 48–66. [Google Scholar]
- Elliott, M.C.; Weston, G.D. Biology and physiology of the sugar-beet plant. In The Sugar Beet Crop; World Crop Series; Cooke, D.A., Scott, R.K., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 37–66. [Google Scholar] [CrossRef]
- Finkenstadt, V.L. A Review on the complete utilization of the sugarbeet. Sugar Technol. 2013, 4, 339–346. [Google Scholar] [CrossRef]
- Mordenti, A.L.; Giaretta, E.; Campidonico, L.; Parazza, P.; Formigoni, A. A Review Regarding the Use of Molasses in Animal Nutrition. Animals 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Burenin, V.I.; Pivovarov, V.F. Beet; VNIISSOK: St. Petersburg, Russia, 1998; p. 215. [Google Scholar]
- Heikkila, H.O.; Melaja, J.A.; Millner, D.E.D.; Virtanen, J.J. Betaine Recovery Process. U.S. Patent 4,359,430, 16 November 1982. [Google Scholar]
- Kotsiopoulou, N.G.; Liakos, T.I.; Lazaridis, N.K. Melanoidin chromophores and betaine osmoprotectant separation from aqueous solutions. J. Mol. Liq. 2016, 216, 496–502. [Google Scholar] [CrossRef]
- Escudero, I.; Ruiz, M.O. Extraction of betaine from beet molasses using membrane contactors. J. Membr. Sci. 2011, 372, 258–268. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/ru (accessed on 25 November 2022).
- Lima, I.M.; White, P.M.J. Sugarcane bagasse and leaf residue biochars as soil amendment for increased sugar and cane yields. Int. Sugar J. 2017, 119, 1421. [Google Scholar]
- Patel, H. Environmental valorisation of bagasse fly ash: A review. R. Soc. Chem. Adv. 2020, 10, 31611–31621. [Google Scholar] [CrossRef]
- Giacobello, S.; Storti, G.; Tola, G. Design of a simulated moving bed for sucrose-betaine separations. J. Chromatogr. 2000, 872, 23–35. [Google Scholar] [CrossRef]
- McFarlane, J.S. Variety development. In Advances in Sugar Beet Production; Jonhson, R.T., Alexander, J.T., Bush, G.E., Hawkes, G.R., Eds.; Iowa State University Press: Ames, IA, USA, 1971; pp. 402–435. [Google Scholar]
- Vasilchenko, E.N.; Zhuzhalova, T.P.; Kolesnikova, E.O. Accelerated production of new homozygous sugar beet lines (B. vulgaris L.). Sugar 2020, 2, 30–32. [Google Scholar]
- Abbasi, Z.; Arzani, A.; Majidi, M.M. Evaluation of genetic diversity of sugar beet (Beta vulgaris L.) crossing parents using agromorphological traits and molecular markers. J. Agric. Sci. Technol. 2014, 16, 1397–1411. [Google Scholar]
- Larsen, K. Self incompatibility in Beta vulgaris L. Four gametophytic complementary S-loci in sugar beet. Hereditas 1977, 85, 227–248. [Google Scholar] [CrossRef]
- Svirshchevskaya, A.; Dolezel, J. Production and Performance of Gynogenetic Sugar beet Lines. J. Sugar Beet Res. 2000, 37, 117–133. [Google Scholar] [CrossRef][Green Version]
- De La Fuente, G.N.; Frei, U.K.; Lübberstedt, T. Accelerating plant breeding. Trends Plant Sci. 2013, 18, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Chodacka, M.; Baranski, R. Comparison of haploid and doubled haploid sugar beet clones in their ability to micropropagate and regenerate. Electron. J. Biotechnol. 2013, 16, fulltext-3. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Malik, A.; Mbira, K.G. In vitro haploid and dihaploid production via unfertilized ovule culture. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 104, 311–319. [Google Scholar] [CrossRef]
- Vjurtts, T.S.; Shmykova, N.A.; Fedorova, M.I.; Zayachkovskaya, T.V.; Domblides, E.A. Creation of doubled haploid lines of carrot (Daucus carota L.) using biotechnological methods. Plant Prot. Bull. 2016, 3, 43–44. [Google Scholar]
- Mezei, S.; Kovacev, L.; Nagl, N. Sugar beet micropropagation. Biotechnol. Biotechnol. Equip. 2006, 20, 9–14. [Google Scholar] [CrossRef][Green Version]
- Van Geyt, J.; D’Halluin, K.; Jacobs, M. Induction of nuclear and cell divisions in microspores of sugar beet (Beta vulgaris L.). Z. Pflanzenzuecht. 1985, 95, 325–335. [Google Scholar]
- Speckmann, G.J.; Van Geyt, J.P.C.; Jacobs, M. The induction of haploids of sugar beet (Beta vulgaris L.) using anther and free pollen culture or ovule and ovary culture. In Genetic Manipulation in Plant Breeding; Horn, W., Jensen, C.J., Odenbach, W., Schieder, O., Eds.; De Gruyter: Berlin, Germany, 1986; pp. 351–353. [Google Scholar]
- Rogozinska, J.H.; Goska, M.; Kuzdowicz, A. Induction of plants from anthers of Beta vulgaris cultured in vitro. Acta Soc. Bot. Pol. 1977, 46, 471–479. [Google Scholar] [CrossRef]
- Pazuki, A.; Aflaki, F.; Gürel, E.; Ergül, A.; Gürel, S. Gynogenesis Induction in Sugar Beet (Beta vulgaris) Improved by 6- Benzylaminopurine (BAP) and Synergized with Cold Pretreatment. Sugar Tech. 2017, 20, 69–77. [Google Scholar] [CrossRef]
- Gurel, S.; Gurel, E.; Kaya, Z. Doubled haploid plant production from unpollinated ovules of sugar beet (Beta vulgaris L.). Plant Cell Rep. 2000, 19, 1155–1159. [Google Scholar] [CrossRef]
- Nagl, N.; Mezei, S.; Kovacev, L.; Vasic, D.; Cacic, N. Induction and micropropagation potential of sugar beet haploids. Genetika 2004, 36, 187–194. [Google Scholar] [CrossRef]
- Aflaki, F.; Pazuki, A.; Gurel, S.; Stevanato, S.; Biancardi, E.; Gurel, E. Doubled haploid sugar beet: An integrated view of factors influencing the processes of gynogenesis and chromosome doubling. Int. Sugar J. 2017, 119, 884–895. [Google Scholar]
- Hosemans, D.; Bossoutrot, D. Induction of haploid plant from in vitro culture of unpollinated beet ovules (Beta vulgaris L.). J. Plant Breed. 1983, 91, 74–77. [Google Scholar]
- D’Halluin, K.; Keimer, B. Production of haploid sugar beets (Beta vulgaris L.) by ovule culture. In Genetic Manipulation in Plant Breeding; Horn, W., Jensen, C.J., Odenbach, W., Schieder, O., Eds.; De Gruyter: Berlin, Germany, 1986; pp. 307–309. [Google Scholar]
- Van Geyt, J.; Speckmann, G.J.; D’Halluin, K.; Jacobs, M. In vitro induction of haploid plants from unpollinated ovules and ovaries of the sugarbeet (Beta vulgaris L.). Theor. Appl. Genet. 1987, 73, 920–925. [Google Scholar] [CrossRef]
- Doctrinal, M.; Sangwan, R.S.; Sangwan-Norreel, B.S. In vitro gynogenesis in Beta vulgaris L.: Effects of plant growth regulators, temperature, genotypes and season. Plant Cell Tissue Organ Cult. 1989, 17, 1–12. [Google Scholar] [CrossRef]
- Lux, H.; Herrmann, L.; Wetzel, C. Production on haploid sugar beet (Beta vulgaris L.) by culturing unpollinated ovules. Plant Breed. 1990, 104, 177–183. [Google Scholar] [CrossRef]
- Goska, M. Monographs and Scientific Dissertations. In Haploids and Sub-Haploids of Sugar Beet (Beta vulgaris L.) and Possibilities of Their Use in Breeding; Institute of Plant Breeding and Acclimatization: Radzików, Poland, 1997. [Google Scholar]
- Weich, E.W.; Levall, M.W. Doubled haploid production of sugar beet (Beta vulgaris L.): Published protocols for other crop plant species. In Doubled Haploid Production in Crop Plants: A Manual; Maluszynski, M., Kasha, K.J., Forster, B.P., Szareiko, J., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 255–263. [Google Scholar] [CrossRef]
- Pazuki, A.; Aflaki, F.; Gürel, S.; Ergül, A.; Gürel, E. Production of doubled haploids in sugar beet (Beta vulgaris): An efficient method by a multivariate experiment. Plant Cell Tissue Organ Cult. 2018, 132, 85–97. [Google Scholar] [CrossRef]
- Tomaszewska-Sowa, M. Effect of growth regulators and other components of culture medium on morphogenesis of sugar beet (Beta vulgaris L.) in unfertilised ovule in vitro cultures. Acta Agrobot. 2012, 65, 91–100. [Google Scholar] [CrossRef]
- Tomaszewska-Sowa, M.; Keutgen, A.J. Plant Regeneration from Unpollinated Ovules of Sugar Beet (Beta vulgaris L.) on Growing Media with Different Carbohydrates. Sugar Tech. 2022, 24, 542–550. [Google Scholar] [CrossRef]
- Galatowitsch, M.W.; Smith, G.A. Regeneration from unfertilized ovule callus of sugarbeet (Beta vulgaris L.). Can. J. Plant Sci. 1990, 70, 83–89. [Google Scholar] [CrossRef]
- Svirshchevskaya, A.M.; Kozyrevich, T.P.; Bormotov, V.E. Haploids in the culture of unfertilized ovules in beet. Dokl. Akad. Nauk Belar. 1993, 37, 74–76. [Google Scholar]
- Hansen, A.L.; Gertz, A.; Joersbo, M.; Andersen, S.B. Short-duration colchicine treatment for in vitro chromosome doubling during ovule culture of Beta vulgaris L. Plant Breed. 1995, 114, 515–519. [Google Scholar] [CrossRef]
- Sohrabi, S.; Abdollahi, M.R.; Mirzaie-Asl, A.; Koulaei, H.E.; Aghaeezadeh, M.; Seguí-Simarro, J.M. A refined method for ovule culture in sugar beet (Beta vulgaris L.). Plant Cell Tissue Organ Cult. 2021, 146, 259–267. [Google Scholar] [CrossRef]
- Gurel, S.; Pazuki, A.; Aflaki, F.; Gurel, E. Production of Doubled Haploid Sugar Beet (Beta vulgaris L.) Plants through Gynogenesis. In Doubled Haploid Technology: Emerging Tools, Cucurbits, Trees, Other Species; Methods in Molecular Biology; Segui-Simarro, J.M., Ed.; Humana: New York, NY, USA, 2021; Volume 3, pp. 313–323. [Google Scholar] [CrossRef]
- Pedersen, H.C.; Keimer, B. Haploidy in sugar beet (Beta vulgaris L.). In In Vitro Haploid Production in Higher Plants; Important Selected Plants; Jain, S.M., Sopory, S.K., Veilleux, R.E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; Volume 3, pp. 17–36. [Google Scholar] [CrossRef]
- Gémes-Juhász, A.; Balogh, P.; Ferenczy, A.; Kristóf, Z. Effect of optimal stage of female gametophyte and heat treatment on in vitro gynogenesis induction in cucumber (Cucumis sativus L.). Plant Cell Rep. 2002, 21, 105–111. [Google Scholar]
- Diao, W.-P.; Jia, Y.-Y.; Song, H.; Zhang, X.-Q.; Lou, Q.-F.; Chen, J.-F. Efficient embryo induction in cucumber ovary culture and homozygous identification of the regenetants using SSR markers. Sci. Hortic. 2009, 3, 246–251. [Google Scholar] [CrossRef]
- Zhang, C.L.; Chen, D.F.; Elliott, M.C.; Slater, A. Thidiazuron-induced organogenesis and somatic embryogenesis in sugar beet (Beta vulgaris L.). In Vitro Cell. Dev. Biol.-Plant 2001, 37, 305–310. [Google Scholar] [CrossRef]
- Zayachkovskaya, T.; Domblides, E.; Zayachkovsky, V.; Kan, L.; Domblides, A.; Soldatenko, A. Production of Gynogenic Plants of Red Beet (Beta vulgaris L.) in Unpollinated Ovule Culture In Vitro. Plants 2021, 10, 2703. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Skaptsov, M.V.; Smirnov, S.V.; Kutsev, M.G.; Shmakov, A.I. Problems of a standardization in plant flow cytometry. Turczaninowia 2016, 19, 120–122. [Google Scholar]
- Domblides, E.; Ermolaev, A.; Belov, S.; Kan, L.; Skaptsov, M.; Domblides, A. Efficient Methods for Evaluation on Ploidy Level of Cucurbita pepo L. Regenerant Plants Obtained in Unpollinated Ovule Culture In Vitro. Horticulturae 2022, 8, 1083. [Google Scholar] [CrossRef]
- Yudanova, S.S.; Maletskaya, E.I.; Maletsky, S.I. Variability in the number of chloroplasts in populations of stomata guard cells in sugar beet (Beta vulgaris L.). Genetics 2002, 38, 72–78. [Google Scholar]
- Subrahmanyeswari, T.; Gantait, S. Advancements and prospectives of sugar beet (Beta vulgaris L.) biotechnology. Appl. Microbiol. Biotechnol. 2022, 106, 7417–7430. [Google Scholar] [CrossRef] [PubMed]
- Levan, A. A haploid sugar beet after colchicine treatment. Hereditas 1945, 31, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, E.N.; Zhuzhzhalova, T.P. Biotechnology methods in the genus Beta. In Collection of Articles of the Thirty-Fifth International Scientific Practical Conference, Russian, Belgorod, 1 February 2021; Science and Education and Foreign Experience GK LLC, EDN FNHHTW: Belgorod, Russia, 2021; pp. 65–69. [Google Scholar]
- Pazuki, A.; Aflaki, F.; Gurel, S.; Ergul, A.; Gurel, E. The effects of proline on in vitro proliferation and propagation of doubled haploid sugar beet (Beta vulgaris). Turk. J. Bot. 2018, 42, 280–288. [Google Scholar] [CrossRef]
- Ferrant, V.; Bouharmont, J. Origin of gynogenetic embryos of Beta vulgaris L. Sex. Plant Reprod. 1994, 7, 12–16. [Google Scholar] [CrossRef]
- Dubois, F.; Lenee, P.; Sangwan, R.S.; Sangwan-Norreel, B.S. Do developmental stages of ovules influence in vitro induction of gynogenetic embryos in sugar beet. In Seeds: Genesis of Natural and Artificial Forms; Le Biopole Vegetal: Amiens, France, 1990; Volume 249. [Google Scholar]
- Bossoutrot, D.; Hosemans, D. Gynogenesis in Beta vulgaris L.: From in vitro culture of unpollinated ovules to the production of doubled haploid plants in soil. Plant Cell Rep. 1985, 4, 300–303. [Google Scholar] [CrossRef]
- Mukhambetzhanov, S.K. Culture of nonfertilized female gametophytes in vitro. Plant Cell Tissue Organ Cult. 1997, 48, 111–119. [Google Scholar] [CrossRef]
- Tomaszewska Sowa, M.; Olszewska, D. Evaluation of genetic stability of sugar beet (Beta vulgaris L.) plants obtained from unfertilized ovules using RAPD markers. J. Cent. Eur. Agric. 2019, 20, 928–937. [Google Scholar] [CrossRef]
- Chu, C.C.; Wang, C.C.; Sun, C.S.; Hsu, C.; Yin, K.C.; Chu, C.Y.; Bi, F.Y. Anther culture of cereal. Sci. Sin. 1975, 18, 659–668. [Google Scholar]
- Baranski, R. In vitro gynogenesis in red beet (Beta vulgaris L.): Effects of ovule culture conditions. Acta Soc. Bot. Pol. Tow. Bot. 1996, 65, 57–60. [Google Scholar] [CrossRef]
- Kiszczak, W.; Burian, M.; Kowalska, U.; Gorecka, K.; Podwyszynska, M. Production of Homozygous Red Beet (Beta vulgaris L. subsp. vulgaris) Plants by Ovule Culture. In Doubled Haploid Technology: Emerging Tools, Cucurbits, Trees, Other Species, Methods in Molecular Biology; Segui-Simarro, J.M., Ed.; Humana: New York, NY, USA, 2021; Volume 3, pp. 301–312. [Google Scholar] [CrossRef]
- Arndt, F.; Rusxh, R.; Stilfried, H.V.S.N. 49537 A new cotton defoliant. Plant Physiol. 1976, 57, 99. [Google Scholar]
- Mok, M.C.; Mok, D.W.S.; Armstrong, D.J. Cytokinin activity of N-phenyl-N’-l,2,3-thiadiazol-5-yl urea (Thidiazuron). Phytochemistry 1982, 21, 1509–1511. [Google Scholar] [CrossRef]
- Malik, K.A.; Saxena, P.K. Regeneration in Phaseolus vulgaris L.: High-frequency induction of direct shoot formation in intact seedlings by Nt-benzylaminopurine and thidiazuron. Planta 1992, 186, 384–389. [Google Scholar] [CrossRef]
- Huetteman, C.; Preece, J. Thidiazuron: A potent cytokinin for woody plant tissue culture. Plant Cell Tissue. Organ Cult. 1993, 33, 105–119. [Google Scholar] [CrossRef]
- Li, Z.; Jarret, R.L.; Pittman, R.N.; Demski, J.W. Shoot organogenesis from cultured seed explants of peanut (Arachis hypogaea L.) using thidiazuron. In Vitro Cell. Dev. Biol.–Plant 1994, 30, 187–191. [Google Scholar] [CrossRef]
- Capelle, S.C.; Mok, D.W.S.; Kirchner, S.C.; Mok, M.C. Effects of thidiazuron on cytokinin autonomy and the metabolism of Nt-(/x 2-isopentenyl) [8-14C]adenosine in callus tissues of Phaseolus lunatus L. Plant Physiol. 1983, 73, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.C.; Katterman, F.R. Cytokinin activity induced by thidiazuron. Plant Physiol. 1986, 81, 681–683. [Google Scholar] [CrossRef]
- Kaminek, M.; Armstrong, D.J. Genotypic variation in cytokinin oxidase from Phaseolus callus cultures. Plant Physiol. 1990, 93, 1530–1538. [Google Scholar] [CrossRef]
- Domblides, E.; Belov, S.; Soldatenko, A.; Pivovarov, V. Obtaining doubled haploids of cucumber (Cucumis sativus L.). Veg. Crop Russ. 2019, 5, 3–14. [Google Scholar]
- Kiełkowska, A.; Adamus, A.; Baranski, R. An improved protocol for carrot haploid and doubled haploid plant production using induced parthenogenesis and ovule excision in vitro. In Vitro Cell. Dev. Biol.-Plant. 2014, 50, 376–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Review: Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Ciereszko, I. Sucrose metabolism control in plants as response to changes of environmental conditions. Kosmos 2006, 55, 229–241. Available online: http://kosmos.icm.edu.pl/PDF/2006/229.pdf (accessed on 4 August 2023).
- Grigolava, T.R.; Vishnyakova, A.V.; Sinitsyna, A.A.; Voronina, A.V.; Zubko, O.N.; Zudova, O.V.; Monakhos, S.G. Methodological approaches for producing doubled haploids in sugar beet and red beet (Beta vulgaris L.). Vavilovskii Zhurnal Genet Selektsii 2021, 25, 276–283. [Google Scholar] [CrossRef] [PubMed]
- San Noeum, L.H. Haploides d’Hordeum vulgare L. par culture in vitro non fe´conde´s. Annales de l’ame´lioration des plantes 1976, 26, 751–754. [Google Scholar]
- Gos´ka, M.; Jassem, B. Histological observations of sugar—Beet ovules in in vitro cultures. Bull. Pol. Acad. Sci. Biol. 1988, 36, 171–175. [Google Scholar]
- Yu, M.H. Growth and reproduction performance of ovule—Induced sugar beet plants. Sabrao J. Breed. Genet. 1992, 24, 47–55. [Google Scholar]





| Variety (Factor A) | Average Number of Induced Ovules,% | Two-Way ANOVA/Proportion of Influence of Factors A and B,% | |
|---|---|---|---|
| TDZ Concentration, mg/L (Factor B) | |||
| 0.4 | 0.8 | ||
| b.a. 37130 | 16.7 1 a 3/A 4 | 8.6 a/B | Factor A 2 */36.1% Factor B */31.5% Factor A × Factor B ns |
| b.a. 37131 | 8.0 b/A | 0 b/B | |
| b.a. 36764 | 11.3 ab/A | 7.3 a/A | |
| Genotype (Factor A) | Average Number of Induced Ovules,% | Two-Way ANOVA/Proportion of Influence of Factors A and B,% | ||
|---|---|---|---|---|
| Sucrose Concentration,% (Factor B) | ||||
| 2% | 5% | 8% | ||
| b.a. 37130 | 2.0 1 a 3/B 4 | 12.7 a/A | 0 a/B | Factor A 2 */10.8% Factor B */63.1% Factor A × Factor B */12.3% |
| b.a. 37131 | 0 b/B | 4.7 b/A | 0 a/B | |
| b.a. 36764 | 0 b/B | 6.0 b/A | 1.3 a/B | |
| Genotype (Factor A) | Average Number of Induced Ovules,% | Two-Way ANOVA/Fraction of Influence of Factors A and B,% | |||
|---|---|---|---|---|---|
| Pretreatment Duration, Days (Factor B) | |||||
| 0 | 2 | 4 | 6 | ||
| b.a. 37130 | 16.0 1 a 3/A 4 | 0 a/C | 6.7 a/B | 0 a/C | Factor A 2 */18.6% Factor B * 47.2% Factor A × Factor B */22.4% |
| b.a. 37131 | 3.3 b/A | 0 a/B | 0 b/B | 0 a/B | |
| b.a. 36764 | 5.3 b/A | 0 a/B | 1.3 b/B | 0 a/B | |
| Genotype | Total Induced Ovules, pcs. | Average Number of Microshoots Per Ovule, pcs. | Total Microrosettes Received, pcs. | Total Microshoots, Remaining in In Vitro Culture, pcs. | Total Plants Regenerated under Ex Vitro Conditions, pcs. | Total Plants Adapted to Ex Vitro Conditions, pcs. |
|---|---|---|---|---|---|---|
| b.a. 37130 | 126 | 7.2 1 a 2 | 231 | 106 | 31 | 22 |
| b.a. 37131 | 33 | 2.0 b | 18 | 11 | 0 | 0 |
| b.a. 36764 | 67 | 3.2 c | 56 | 14 | 5 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayachkovskaya, T.; Alyokhina, K.; Mineykina, A.; Romanova, O.; Vjurtts, T.; Tukuser, Y.; Zayachkovsky, V.; Ermolaev, A.; Kan, L.; Fomicheva, M.; et al. Optimizing Different Medium Component Concentration and Temperature Stress Pretreatment for Gynogenesis Induction in Unpollinated Ovule Culture of Sugar Beet (Beta vulgaris L.). Horticulturae 2023, 9, 900. https://doi.org/10.3390/horticulturae9080900
Zayachkovskaya T, Alyokhina K, Mineykina A, Romanova O, Vjurtts T, Tukuser Y, Zayachkovsky V, Ermolaev A, Kan L, Fomicheva M, et al. Optimizing Different Medium Component Concentration and Temperature Stress Pretreatment for Gynogenesis Induction in Unpollinated Ovule Culture of Sugar Beet (Beta vulgaris L.). Horticulturae. 2023; 9(8):900. https://doi.org/10.3390/horticulturae9080900
Chicago/Turabian StyleZayachkovskaya, Tatyina, Ksenia Alyokhina, Anna Mineykina, Olga Romanova, Tatiana Vjurtts, Yana Tukuser, Vladimir Zayachkovsky, Alexey Ermolaev, Lyudmila Kan, Maria Fomicheva, and et al. 2023. "Optimizing Different Medium Component Concentration and Temperature Stress Pretreatment for Gynogenesis Induction in Unpollinated Ovule Culture of Sugar Beet (Beta vulgaris L.)" Horticulturae 9, no. 8: 900. https://doi.org/10.3390/horticulturae9080900
APA StyleZayachkovskaya, T., Alyokhina, K., Mineykina, A., Romanova, O., Vjurtts, T., Tukuser, Y., Zayachkovsky, V., Ermolaev, A., Kan, L., Fomicheva, M., & Domblides, E. (2023). Optimizing Different Medium Component Concentration and Temperature Stress Pretreatment for Gynogenesis Induction in Unpollinated Ovule Culture of Sugar Beet (Beta vulgaris L.). Horticulturae, 9(8), 900. https://doi.org/10.3390/horticulturae9080900








