The Biotechnological “Provence” of the Future Provided by Antisense Oligoilators and Olinscides for Horticulturae
Abstract
1. Introduction
2. Kingdom of Scents in Kingdom of Plants
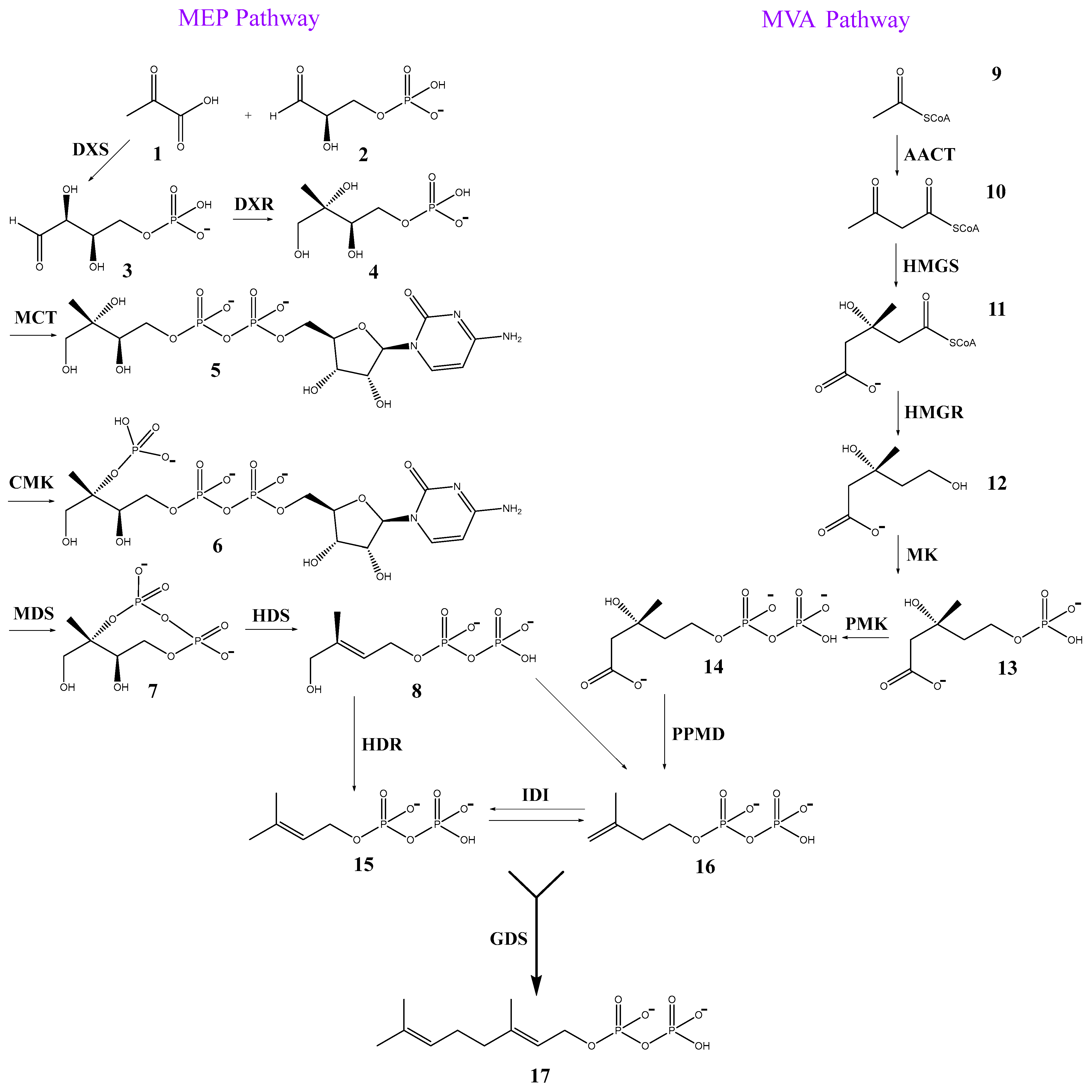
3. Biosynthesis of Essential Oils: Far, Far Away in Nucleotide Land
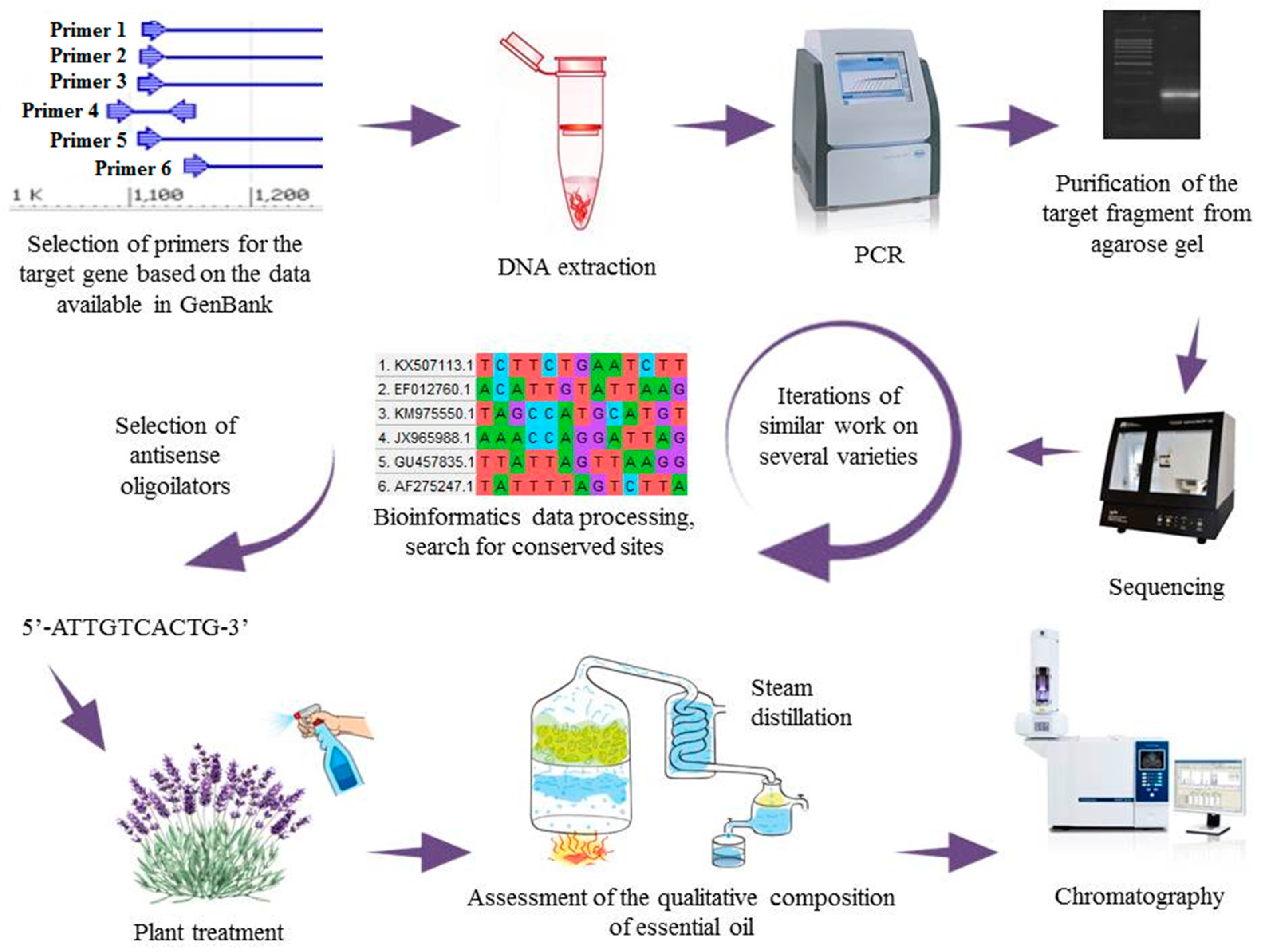
4. Field of Lavender Dreams: A Magic Wand Made of an Oligonucleotide Strand
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenaerts, B.; Collard, B.C.Y.; Demont, M. Review: Improving global food security through accelerated plant breeding. Plant Sci. 2019, 287, 110207. [Google Scholar] [CrossRef]
- Zimny, T.; Sowa, S.; Tyczewska, A.; Twardowski, T. Certain new plant breeding techniques and their marketability in the context of EU GMO legislation—Recent developments. New Biotechnol. 2019, 51, 49–56. [Google Scholar] [CrossRef]
- Butt, H.; Eid, A.; Momin, A.A.; Bazin, J.; Crespi, M.; Arold, S.T.; Mahfouz, M.M. CRISPR directed evolution of the spliceosome for resistance to splicing inhibitors. Gen. Biol. 2019, 20, 1–73. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Biradar, C.M.; Xiao, X.; Dong, J.; Zhou, Y.; Qin, Y.; Zhang, Y.; Liu, F.; Ding, M.; Thomas, R.J. Exacerbated grassland degradation and desertification in Central Asia during 2000–2014. Ecol. Appl. 2018, 28, 442–456. [Google Scholar] [CrossRef]
- Chang, T.-H.; Hsiech, F.L.; Ko, T.P.; Teng, K.-H.; Liang, P.-H.; Wang, A.H. Structure of a Heterotetrameric Geranyl Pyrophosphate Synthase from Mint (Mentha piperita) Reveals Intersubunit Regulation. Plant Cell. 2010, 22, 454–467. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2012, 21, 7382. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, H.; Ma, X.; Zhan, R.; Chen, W. Triterpenoid Saponin Biosynthetic Pathway Profiling and Candidate Gene Mining of the Ilex asprella Root Using RNA-Seq. Int. J. Mol. Sci. 2014, 15, 5970–5987. [Google Scholar] [CrossRef]
- Jia, Q.D.; Chen, F. Catalytic functions of the isoprenyl diphosphate synthase superfamily in plants: A growing repertoire. Mol. Plant 2016, 9, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.-L.; Chang, T.-H.; Ko, T.-P.; Wang, A.H.-J. Structure and Mechanism of an Arabidopsis Medium/Long-Chain-Length Prenyl Pyrophosphate Synthase. Plant Physiol. 2011, 155, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T.A. Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Huang, J. Anti-malarial drug: The emerging role of artemisinin and its derivatives in liver disease treatment. Chin. Med. 2021, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Caputi, L.; Aprea, E. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat. Food Nutr. Agric. 2011, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Madrid, R.; Carballo-Uicab, V.M.; Cárdenas-Conejo, Y.; Aguilar-Espinosa, M.; Siva, R. Overview of carotenoids and beneficial effects on human health. In Carotenoids: Properties, Processing and Applications; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, D.; Yang, J.; Wu, Z.; Luo, C.; Han, L.; Yang, F.; Lin, J.; Yang, M. Review of aromatherapy essential oils and their mechanism of action against migraines. J. Ethnopharmacol. 2020, 265, 113326. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St Rose, T.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Getahun, T.; Sharma, V.; Gupta, N. The genus Laggera (Asteraceae)—Ethnobotanical and Ethnopharmacological Information, Chemical Composition as well as Biological Activities of Its Essential Oils and Extracts: A Review. Chem. Biodivers. 2019, 16, e1900131. [Google Scholar] [CrossRef]
- Nahar, L.; El-Seedi, H.R.; Khalifa, S.A.; Mohammadhosseini, M.; Sarker, S.D. Ruta Essential Oils: Composition and Bioactivities. Molecules 2021, 26, 4766. [Google Scholar] [CrossRef]
- El-Shemy, H.A. Potential of Essential Oils; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Barbieri, C.; Borsotto, P. Essential oils: Market and legislation. In Potential of Essential Oils; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Torres Neto, L.; Monteiro, M.L.G.; Galvan, D.; Conte-Junior, C.A. An Evaluation of the Potential of Essential Oils against SARS-CoV-2 from In Silico Studies through the Systematic Review Using a Chemometric Approach. Pharmaceuticals 2021, 14, 1138. [Google Scholar] [CrossRef]
- Bochar, D.A.; Friesen, J.A.; Stauffacher, C.V.; Rodwell, V.W. Comprehensive Natural Product Chemistry: Isoprenoids Including Carotenoids and Isoprenoids; Cane, D.E., Ed.; Pergamon: Oxford, UK, 1999; Volume 2, pp. 15–44. [Google Scholar]
- Grochowski, L.L.; Xu, H.; White, R.H. Methenocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate. J. Bacteriol. 2006, 188, 3192–3198. [Google Scholar] [CrossRef]
- Hemmerlin, A.; Harwood, J.L.; Bach, T.J. A raison d’être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lipid Res. 2012, 51, 95–148. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, F.; Rahier, A.; Camara, B. Biogenesis, molecular regulation and function of plant isoprenoids. Prog. Lipid Res. 2005, 44, 357–429. [Google Scholar] [CrossRef] [PubMed]
- Adam, P.; Hecht, S.; Eisenreich, W.; Kaiser, J.; Grawert, T.; Arigoni, D.; Bacher, A.; Rohdich, F. Biosynthesis of terpenes: Studies on 1- hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase. Proc. Nat. Acad. Sci. USA 2002, 99, 12108–12113. [Google Scholar] [CrossRef] [PubMed]
- Dillies, M.-A.; Rau, A.; Aubert, J.; Antier, C.H.; Jeanmougin, M.; Servant, N. A comprehensive evaluation of normalization methods for Illumina high-throughput RNA sequencing data analysis. Brief. Bioinform. 2013, 14, 671–683. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, Y.; Zhong, Y.; Wu, Y.; Li, Z.; Xu, L.; Xu, M. Transcriptome analysis and identification of genes related to terpenoid biosynthesis in Cinnamomum camphora. BMC Genom. 2018, 19, 550. [Google Scholar] [CrossRef]
- Adal, A.M.; Sarker, L.S.; Malli, R.P.N.; Liang, P.; Mahmoud, S.S. RNA-Seq in the discovery of a sparsely expressed scent-determining monoterpene synthase in lavender (Lavandula). Plant 2019, 249, 271–290. [Google Scholar] [CrossRef]
- Malli, R.P.; Adal, A.M.; Sarker, L.S.; Liang, P.; Mahmoud, S.S. De novo sequencing of the Lavandula angustifolia genome reveals highly duplicated and optimized features for essential oil production. Planta 2019, 249, 251–256. [Google Scholar] [CrossRef]
- Oberemok, V.V.; Laikova, K.V.; Novikov, I.A.; Galchinsky, N.V.; Useinov, R.Z. Controlling the accumulation of secondary metabolites by plants using antisense oligonucleotides. In Plant Canada; The University of Guelph: Guelph, ON, Canada, 2019. [Google Scholar]
- Gal’chinsky, N.V.; Useinov, R.Z.; Yatskova, E.V.; Laikova, K.V.; Novikov, I.A.; Gorlov, M.V. A breakthrough in the efficiency of contact DNA insecticides: Rapid high mortality rates in the sap-sucking insects Dynaspidiotus britannicus Comstock and Unaspis euonymi Newstead. J. Plant Prot. Res. 2020, 60, 220–223. [Google Scholar] [CrossRef]
- Useinov, R.Z.; Gal’chinsky, N.V.; Yatskova, E.V.; Novikov, I.A.; Puzanova, Y.V.; Trikoz, N.N.; Sharmagiy, A.K.; Plugatar, Y.V.; Laikova, K.V.; Oberemok, V.V. To bee or not to bee: Creating DNA insecticides to replace non-selective organophosphate insecticides for use against the soft scale insect Ceroplastes japonicus Green. J. Plant Prot. Res. 2020, 60, 406–409. [Google Scholar] [CrossRef]
- Oberemok, V.V.; Laikova, K.V.; Useinov, R.Z.; Gal’chinsky, N.V.; Yurchenko, K.A.; Novikov, I.A. Successful Management of Secondary Metabolite Biosynthesis of Essential Oil Plants Using Unmodified Antisense Oligonucleotides in a Lavandula angustifolia Mill. In Vitro Cell. Dev. Biol.-Anim. 2020, 56, 42–65. [Google Scholar] [CrossRef]
- Kuo, Y.; Falk, B.W. RNA interference approaches for plant disease control. BioTechniques 2020, 69, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.D.; Kang, Y.; Kim, C. Application of Genomic Big Data in Plant Breeding: Past, Present, and Future. Plants 2020, 9, 1454. [Google Scholar] [CrossRef] [PubMed]
- Oberemok, V.; Laikova, K.; Useinov, R.; Gal’chinsky, N.; Novikov, I.; Gorlov, M. High Mortality of Sap-sucking Insects One Week After Topical Application of DNA Insecticides. Animal Posters. In Vitro Cell. Dev. Biol.-Anim. 2020, 56, 31–39. [Google Scholar] [CrossRef]
- Puzanova, Y.V.; Novikov, I.A.; Marochkin, N.A.; Eken, E.; Sharmagiy, A.K.; Oberemok, V.V. Another successful target in the suborder Sternorrhyncha (Hemiptera): Green oligonucleotide insecticides for aphid control. In Proceedings of the 2022 Meeting of the Society for In Vitro Biology, Sandiego, CA, USA, 4–7 June 2022; Volume P-3048, pp. 33–34. [Google Scholar]
- Wdowikowska, A.; Janicka, M. Antisense oligonucleotide technology as a research tool in plant biology. Funct. Plant Biol. 2021, 49, 1–12. [Google Scholar] [CrossRef]
- Liao, Y.W.K.; Sun, Z.H.; Zhou, Y.H.; Shi, K.; Li, X.; Zhang, G.Q. The role of hydrogen peroxide and nitric oxide in the induction of plant-encoded RNA-dependent RNA polymerase 1 in the basal defense against tobacco mosaic virus. PLoS ONE 2013, 8, e76090. [Google Scholar] [CrossRef]
- Dinç, E.; Tóth, S.Z.; Schansker, G.; Ayaydin, F.; Kovács, L.; Dudits, D. Synthetic antisense oligodeoxynucleotides to transiently suppress different nucleus-and chloroplast-encoded proteins of higher plant chloroplasts. Plant Physiol. 2011, 157, 1628–1641. [Google Scholar] [CrossRef]
- Wojtasik, W.; Kulma, A.; Boba, A.; Szopa, J. Oligonucleotide treatment causes flax β-glucanase up-regulation via changes in gene-body methylation. BMC Plant Biol. 2014, 14, 261. [Google Scholar] [CrossRef]
- Dzialo, M.; Szopa, J.; Czuj, T.; Zuk, M. Oligodeoxynucleotides Can Transiently Up- and Downregulate CHS Gene Expression in Flax by Changing DNA Methylation in a Sequence-Specific Manner. Front. Plant Sci. 2017, 8, 755. [Google Scholar] [CrossRef]
- Dzialo, M.; Szopa, J.; Hnitecka, A.; Zuk, M. Transgenerational perpetuation of CHS gene expression and dna methylation status induced by short oligodeoxynucleotides in flax (Linum usitatissimum). Int. J. Mol. Sci. 2019, 20, 3983. [Google Scholar] [CrossRef]
- Tsutsumi, N.; Kanayama, K.; Tano, S. Suppression of alpha-amylase gene expression by antisense oligodeoxynucleotide in barley cultured aleurone layers. Jpn. J. Genet. 1992, 67, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Estruch, J.J.; Kadwell, S.; Merlin, E.; Crossland, L. Cloning and characterization of a maize pollen-specific calcium-dependent calmodulin-independent protein kinase. Proc. Nat. Acad. Sci. USA 1994, 91, 8837–8841. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Höglund, A.-S.; Olsson, H.; Mangelsen, E.; Jansson, C. Antisense oligodeoxynucleotide inhibition as a potent strategy in plant biology: Identification of SUSIBA2 as a transcriptional activator in plant sugar signalling. Plant J. 2005, 44, 128–138. [Google Scholar] [CrossRef]
- Xie, Z.; Sundström, J.F.; Jin, Y.; Liu, C.; Jansson, C.; Sun, C.A. A selection strategy in plant transformation based on antisense oligodeoxynucleotide inhibition. Plant J. 2014, 77, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Chen, X.; Xu, F.; Ding, M.; Ye, W.; Kawai, Y.; Toda, Y.; Hayashi, Y.; Suzuki, T.; et al. Plasma membrane H+-ATPase overexpression increases rice yield via simultaneous enhancement of nutrient uptake and photosynthesis. Nat. Commun. 2021, 12, 735. [Google Scholar] [CrossRef]
- Hunter, W.B.; Cooper, W.R.; Sandoval-Mojica, A.F.; McCollum, G.; Aishwarya, V.; Pelz-Stelinski, K.S. Improving Suppression of Hemipteran Vectors and Bacterial Pathogens of Citrus and Solanaceous Plants: Advances in Antisense Oligonucleotides (FANA). Front. Agron. 2021, 3, 675247. [Google Scholar] [CrossRef]
- Wang, Z.; Siwkowski, A.; Lima, W.F.; Olsen, P.; Ravikumar, V.T. Antisense oligonucleotides: Efficient synthesis of 2′-O-methoxyethyl phosphorothioate oligonucleotides using 4,5-dicyanoimidazole. Are these oligonucleotides comparable to those synthesized using 1H-tetrazole as coupling activator? Bioorg. Med. Chem. 2006, 14, 5049–5060. [Google Scholar] [CrossRef]
- Prakash, T.P.; Brad Wan, W.; Low, A.; Yu, J.; Chappell, A.E.; Gaus, H.; Kinberger, G.A.; Østergaard, M.E.; Migawa, M.T.; Swayze, E.E.; et al. Solid-phase synthesis of 5′-triantennary N-acetylgalactosamine conjugated antisense oligonucleotides using phosphoramidite chemistry. Bioorg. Med. Chem. Lett. 2015, 25, 4127–4130. [Google Scholar] [CrossRef]
- Pon, R.T. Solid-Phase Supports for Oligonucleotide Synthesis. Curr. Protoc. Nucleic Acid Chem. 2000, 3.1.1–3.1.28. [Google Scholar] [CrossRef]
- Bonora, G.M.; Zaramella, S.; Veronese, F.M. Synthesis by high-efficiency liquid-phase (HELP) method of oligonucleotides conjugated with high-molecular weight polyethylene glycols (PEGs). Biol. Proced. Online 1998, 1, 59–69. [Google Scholar] [CrossRef]
- Lönnberg, H. Synthesis of oligonucleotides on a soluble support. Beilstein J. Org. Chem. 2017, 13, 1368–1387. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.G.; Sanghvi, Y.S. Liquid-phase oligonucleotide synthesis: Past, present, and future predictions. Curr. Protoc. Nucleic Acid Chem. 2019, 77, e82. [Google Scholar] [CrossRef] [PubMed]
- Nyadar, P.M.; Adeyemi, T.A. DNA insecticides: The lethal potency of LdMNPV IAP-2 gene antisense oligonucleotides in pre-infected gypsy moth (Lymantria dispar L.) larvae. Int. J. Pest Manag. 2018, 64, 173–177. [Google Scholar] [CrossRef]
- Laikova, K.V.; Oberemok, V.V.; Krasnodubets, A.; Gal’chinsky, N.V.; Useinov, R.Z.; Novikov, I.A. Advances in the Understanding of Skin Cancer: Ultraviolet Radiation, Mutations, and Antisense Oligonucleotides as Anticancer Drugs. Molecules 2019, 24, 1516. [Google Scholar] [CrossRef] [PubMed]
- Makalish, T.P.; Golovkin, I.O.; Oberemok, V.V.; Laikova, K.V.; Temirova, Z.Z.; Serdyukova, O.A.; Novikov, I.A.; Rosovskyi, R.A.; Gordienko, A.I.; Zyablitskaya, E.Y.; et al. Anti-Rheumatic Effect of Antisense Oligonucleotide Cytos-11 Targeting TNF-α Expression. Int. J. Mol. Sci. 2021, 22, 1022. [Google Scholar] [CrossRef]
- Gal’chinsky, N.V.; Yatskova, E.V.; Novikov, I.A.; Useinov, R.Z.; Kouakou, N.J.; Kouame, K.F.; Kra, K.D.; Sharmagiy, A.K.; Plugatar, Y.V.; Laikova, K.V.; et al. Icerya purchasi Maskell (Hemiptera: Monophlebidae) Control Using Low Carbon Footprint Oligonucleotide Insecticides. Int. J. Mol. Sci. 2023, 24, 11650. [Google Scholar] [CrossRef]
- PlaBi-PD Data Base. Available online: http://www.plabipd.de (accessed on 22 November 2022).
- Oberemok, V.V. Ecological Basis for Controlling the Number of Leaf-Eating Insects Using DNA Insecticides. Ph.D. Thesis, V.I. Vernadsky Crimean Federal University, Simferopol, Crimea, 2019. Available online: http://obr.nbgnsc.ru/wp-content/uploads/2019/07/Диccepтaция_Oбepeмoк-BB_-экoлoгия-и-зaщитa-pacтeний.pdf (accessed on 18 June 2023).
- Novikov, I.A. A Method for Increasing the Content of Linalyl Acetate in the Essential Oil of Narrow-Leaved Lavender (Lavandula angustifolia Mill.). Russian Patent RU 2.743.395.C1, 18 February 2021. [Google Scholar]
- Pokajewicz, K.; Czarniecka-Wiera, M.; Krajewska, A.; Maciejczyk, E.; Wieczorek, P.P. Lavandula × intermedia—A Bastard Lavender or a Plant of Many Values? Part I. Biology and Chemical Composition of Lavandin. Molecules 2023, 28, 2943. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops—An Identification and Information Guide. In The Natural History Museum; John Wiley and Sons, Ltd.: New York, NY, USA, 2020. [Google Scholar]
- Oberemok, V.V.; Laikova, K.V.; Gal’chinsky, N.V.; Useinov, R.Z.; Novikov, I.A.; Temirova, Z.Z.; Shumskykh, M.N.; Krasnodubets, A.M.; Repetskaya, A.I.; Dyadichev, V.V.; et al. DNA insecticide developed from the Lymantria dispar 5.8S ribosomal RNA gene provides a novel biotechnology for plant protection. Sci. Rep. 2019, 9, 6197. [Google Scholar] [CrossRef]
- Drobotova, E.N. Pests of Essential Oil Crops Grown at the Research Institute of Agriculture of Crimea. V International Scientific Conference ‘Current State, Problems and Prospects of the Development of Agrarian Science. Simferopol, Russia. 2020, pp. 47–49. Available online: https://www.semanticscholar.org/paper/Pests-of-essential-oil-crops-grown-at-the-Research-Drobotova/8f80b6e465bdcf18984f84a9db2a6efef611e879 (accessed on 18 June 2023).
- Nardi, G.; Vomero, V. (Eds.) Artropodi del parco nazionale del Vesuvio: Ricerche preliminari. In Conservazione Habitat Invertebrati; Centro Nazionale per lo Studio e la Conservazione della Biodiversità Forestale: Verona, Italy, 2007; Volume 4, pp. 377–454. [Google Scholar]


| Oil Component | Variant of Experiment | |
|---|---|---|
| Control | oligoMEP-11 | |
| menthol | 13.12 ± 1.64% | 6.47 ± 1.04% * |
| menthone | 53.50 ± 1.74% | 61.20 ± 1.31% * |
| Oil Component | Variant of Experiment | |
|---|---|---|
| Control | LAVAN-11 | |
| linalyl acetate | 21.91 ± 0.73% | 30.55 ± 2.18% * |
| linalool | 43.52 ± 0.50% | 37.39 ± 0.99% * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oberemok, V.V.; Puzanova, Y.V.; Novikov, I.A. The Biotechnological “Provence” of the Future Provided by Antisense Oligoilators and Olinscides for Horticulturae. Horticulturae 2023, 9, 896. https://doi.org/10.3390/horticulturae9080896
Oberemok VV, Puzanova YV, Novikov IA. The Biotechnological “Provence” of the Future Provided by Antisense Oligoilators and Olinscides for Horticulturae. Horticulturae. 2023; 9(8):896. https://doi.org/10.3390/horticulturae9080896
Chicago/Turabian StyleOberemok, Volodymyr V., Yelizaveta V. Puzanova, and Ilya A. Novikov. 2023. "The Biotechnological “Provence” of the Future Provided by Antisense Oligoilators and Olinscides for Horticulturae" Horticulturae 9, no. 8: 896. https://doi.org/10.3390/horticulturae9080896
APA StyleOberemok, V. V., Puzanova, Y. V., & Novikov, I. A. (2023). The Biotechnological “Provence” of the Future Provided by Antisense Oligoilators and Olinscides for Horticulturae. Horticulturae, 9(8), 896. https://doi.org/10.3390/horticulturae9080896







