Morphological and Biochemical Response of Potatoes to Exogenous Application of ZnO and SiO2 Nanoparticles in a Water Deficit Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles [ZnO and SiO2] Characterization
2.2. Experimental Materials, Setup and Design
2.3. Data Recorded
2.3.1. Morphological and Growth Traits
2.3.2. Mineral Concentrations in Potato Leaves
Nitrogen
P, K1+, Zn2+, Mn2+, and Fe2+
2.4. Enzymatic Component
2.4.1. Enzyme Extraction
2.4.2. Enzyme Assay
2.5. Non-Enzymatic Component
2.5.1. Total Flavonoid Content (TFC)
2.5.2. Total Phenolic Content (TPC)
2.6. Oxidative Stress Biomarkers
2.6.1. Lipid Peroxidation (Malondialdehyde (C3H4O2))
2.6.2. Hydrogen Peroxide (H2O2)
2.7. Statistical Analysis
3. Results and Discussion
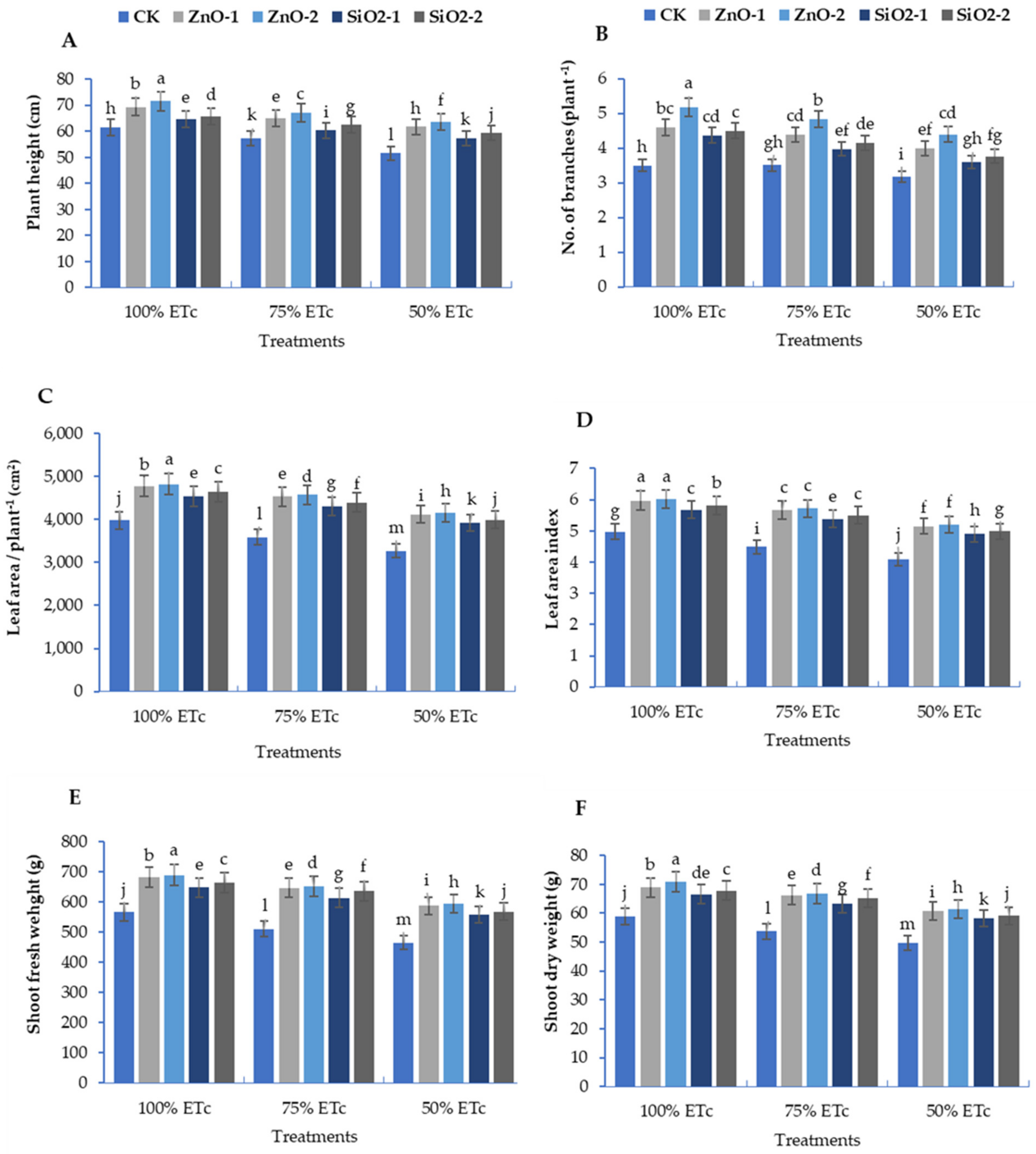
3.1. Morphological and Growth Traits
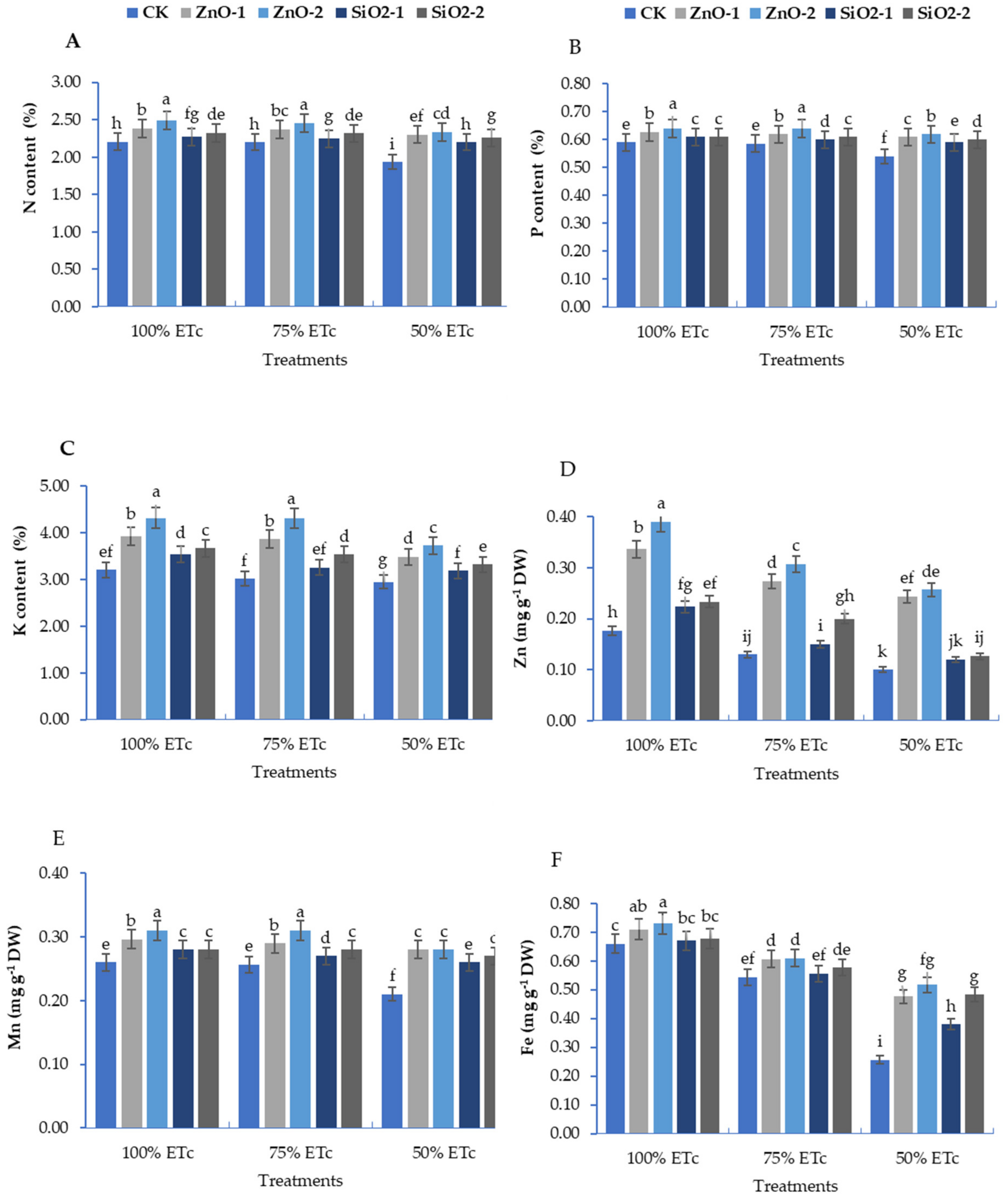
3.2. Mineral Contents
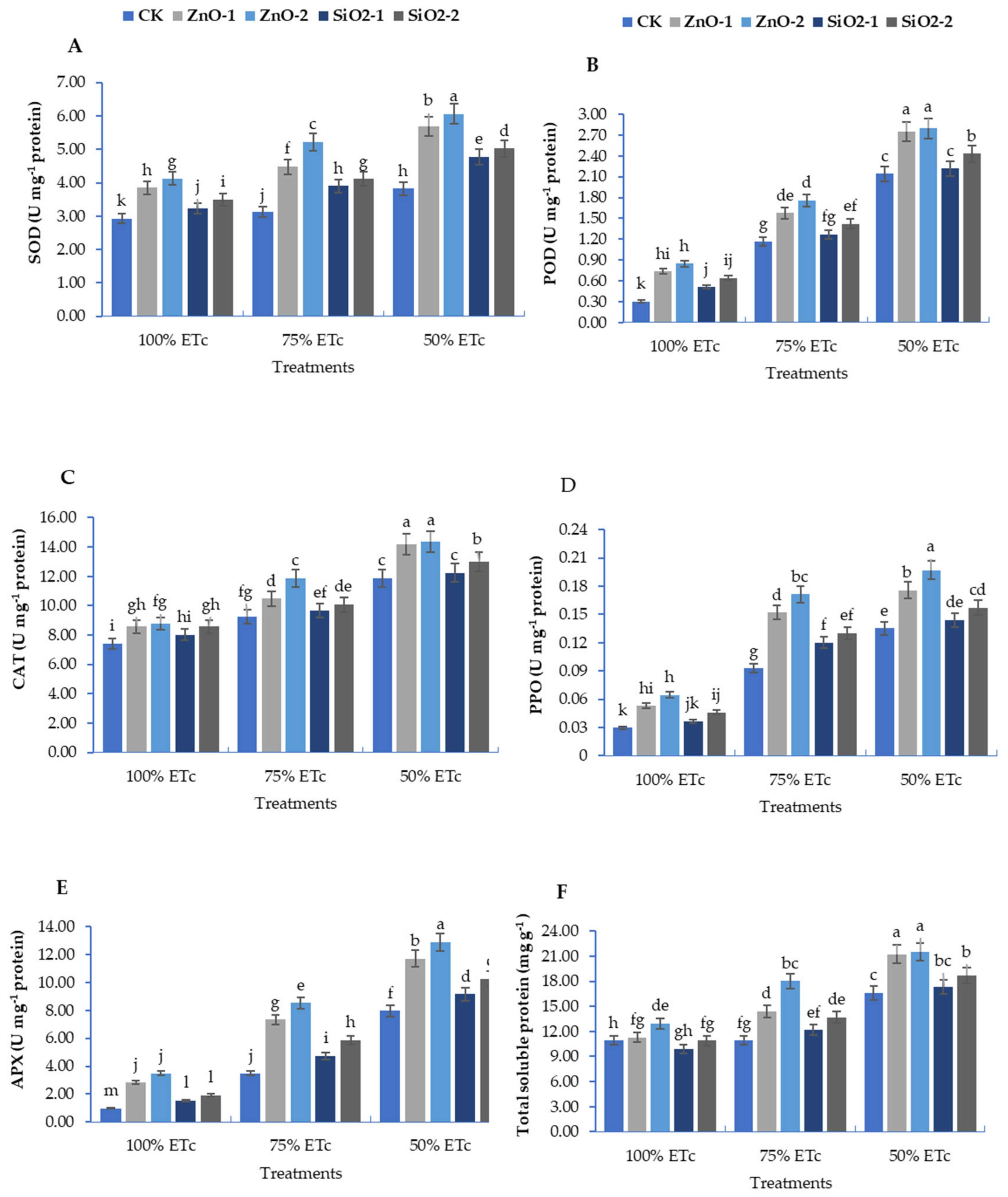
3.3. Antioxidants Enzymatic Activity
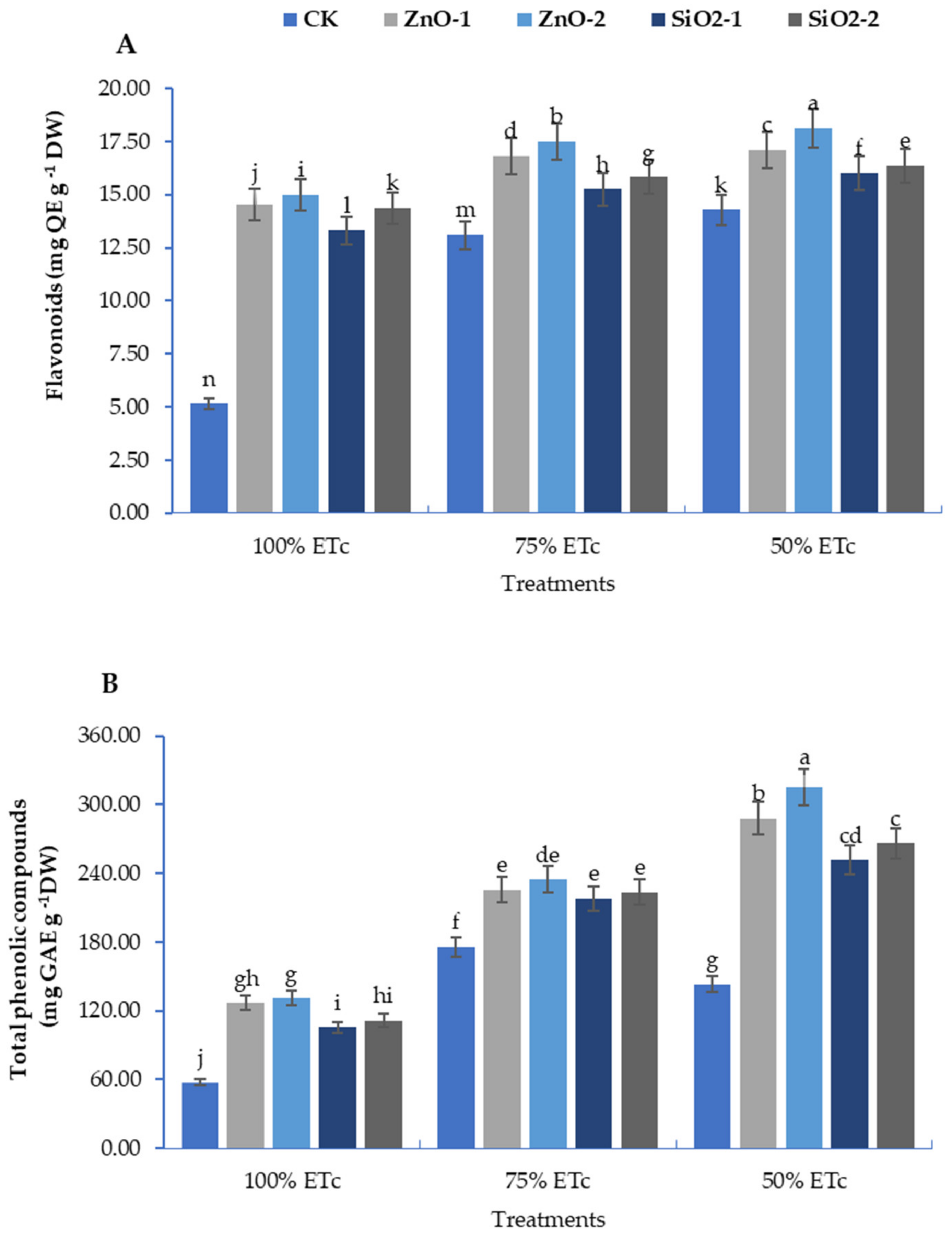
3.4. Non-Enzymatic Antioxidant Compounds: Total Flavonoids and Phenolic Contents
3.5. Oxidative Stress Biomarkers (MDA) and (H2O2)
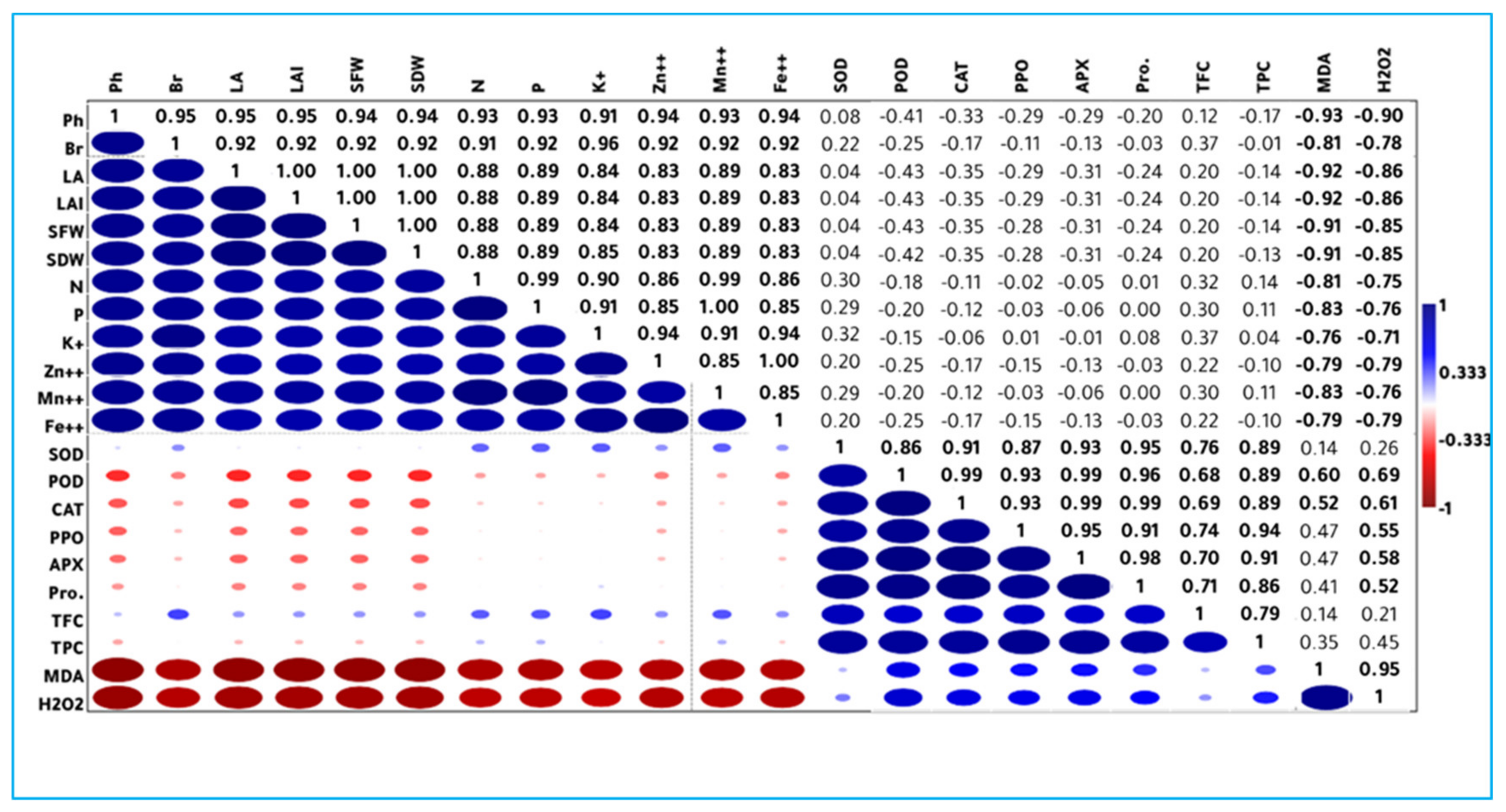
3.6. Correlation Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Winter, J.; Lopez, J.; Ruane, A.; Young, C.; Scanlon, B.; Rosenzweig, C. Representing water scarcity in future agricultural assessments. Anthropocene 2017, 18, 15–26. [Google Scholar] [CrossRef]
- Eid, M.A.M.; Abdel-Salam, A.A.; Salem, H.M.; Mahrous, S.E.; Seleiman, M.F.; Alsadon, A.A.; Solieman, T.H.I.; Ibrahim, A.A. Interaction effects of nitrogen source and irrigation regime on tuber quality, yield, and water use efficiency of Solanum tuberosum L. Plants 2020, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Kheir, A.M.S.; Al-Dhumri, S.; Alghamdi, A.G.; Omar, E.-S.H.; Aboelsoud, H.M.; Abdella, K.A.; Abou El Hassan, W.H. Exploring optimal tillage improved soil characteristics and productivity of wheat irrigated with different water qualities. Agronomy 2019, 9, 233. [Google Scholar] [CrossRef]
- Kheir, A.M.S.; Alkharabsheh, H.M.; Seleiman, M.F.; Al-Saif, A.M.; Ammar, K.A.; Attia, A.; Zoghdan, M.G.; Shabana, M.M.A.; Aboelsoud, H.; Schillaci, C. Calibration and validation of AQUACROP and APSIM models to optimize wheat yield and water saving in arid regions. Land 2021, 10, 1375. [Google Scholar] [CrossRef]
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Control of drought stress in wheat using plant-growth-promoting bacteria. J. Plant Growth Regul. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Refay, Y.; Al-Suhaibani, N.; Al-Ashkar, I.; El-Hendawy, S.; Hafez, E.M. Integrative effects of rice-straw biochar and silicon on oil and seed quality, yield and physiological traits of Helianthus annuus L. grown under water deficit stress. Agronomy 2019, 9, 637. [Google Scholar] [CrossRef]
- Ding, Z.; Ali, E.F.; Elmahdy, A.M.; Ragab, K.E.; Seleiman, M.F.; Kheir, A.M.S. Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agric. Water Manag. 2021, 244, 106626. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; Hafez, Y.M.; El-Afry, M.M.; Tantawy, D.S.; Alshaal, T. Effect of some osmoregulators on photosynthesis, lipid peroxidation, antioxidative capacity, and productivity of barley (Hordeum vulgare L.) under water deficit stress. Environ. Sci. Pollut. Res. 2018, 25, 30199–30211. [Google Scholar] [CrossRef]
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; Rashed, S.H.; Hossain, A.; Sabagh, A.E. Yield and quality of two sugar beet (Beta vulgaris L. ssp. vulgaris var. altissima Döll) cultivars are influenced by foliar application of salicylic acid, irrigation timing, and planting density. Acta Agric. Slov. 2020, 115, 273–282. [Google Scholar] [CrossRef]
- AlKahtani, M.D.; Hafez, Y.M.; Attia, K.; Rashwan, E.; Husnain, L.A.; AlGwaiz, H.I.; Abdelaal, K.A. Evaluation of silicon and proline application on the oxidative machinery in drought-stressed sugar beet. Antioxidants 2021, 10, 398. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Semida, W.M.; Rady, M.M.; Mohamed, G.F.; Hemida, K.A.; Alhammad, B.A.; Hassan, M.M.; Shami, A. Sequential Application of antioxidants rectifies ion imbalance and strengthens antioxidant systems in salt-stressed cucumber. Plants 2020, 9, 1783. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Ma, Q.; Yue, L.-J.; Zhang, J.-L.; Wu, G.-Q.; Bao, A.-K.; Wang, S.-M. Sodium chloride improves photosynthesis and water status in the succulent xerophyte Zygophyllum xanthoxylum. Tree Physiol. 2012, 32, 4–13. [Google Scholar] [CrossRef]
- Sattar, F.A.; Hamooh, B.T.; Wellman, G.; Ali, M.A.; Shah, S.H.; Anwar, Y.; Mousa, M.A.A. Growth and biochemical responses of potato cultivars under In Vitro lithium chloride and mannitol simulated salinity and drought stress. Plants 2021, 10, 924. [Google Scholar] [CrossRef]
- Lee, B.-R.; Zhang, Q.; Kim, T.-H. Lignification in Relation to the Influence of Water-deficit Stress in Brassica napus. J. Korean Soc. Grassl. Forage Sci. 2014, 34, 15–20. [Google Scholar] [CrossRef]
- Ghani, M.I.; Saleem, S.; Rather, S.A.; Rehmani, M.S.; Alamri, S.; Rajput, V.D.; Kalaji, H.M.; Saleem, N.; Sial, T.A.; Liu, M. Foliar application of zinc oxide nanoparticles: An effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 2022, 289, 133202. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Battaglia, M.L.; Bilal, H.M.; Alhammad, B.A.; Khan, K. Zinc oxide nanoparticles: A unique saline stress mitigator with the potential to increase future crop production. S. Afr. J. Bot. 2023, 159, 208–218. [Google Scholar] [CrossRef]
- Alhammad, B.A.; Abdel-Aziz, H.M.M.; Seleiman, M.F.; Tourky, S.M.N. How Can Biological and Chemical Silver Nanoparticles Positively Impact Physio-Chemical and Chloroplast Ultrastructural Characteristics of Vicia faba Seedlings? Plants 2023, 12, 2509. [Google Scholar] [CrossRef]
- Kumar, R.; Nehra, M.; Kumar, D.; Saharan, B.S.; Chawla, P.; Sadh, P.K.; Manuja, A.; Duhan, J.S. Evaluation of cytotoxicity, release behavior and phytopathogens control by Mancozeb-Loaded Guar Gum nanoemulsions for sustainable agriculture. J. Xenobiot. 2023, 13, 270–283. [Google Scholar] [CrossRef]
- Kumar, R.; Duhan, J.S.; Manuja, A.; Kaur, P.; Kumar, B.; Sadh, P.K. Toxicity Assessment and Control of Early Blight and Stem Rot of Solanum tuberosum L. by Mancozeb-Loaded Chitosan–Gum Acacia Nanocomposites. J. Xenobiot. 2022, 12, 74–90. [Google Scholar] [CrossRef]
- Talebi, S.M.; Ghorbanpour, M. Chapter 28—Nanoparticles treatment ameliorate the side effects of stresses in plants. In Plant Stress Mitigators; Ghorbanpour, M., Adnan Shahid, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 469–478. [Google Scholar]
- Garza-Alonso, C.A.; Juárez-Maldonado, A.; González-Morales, S.; Cabrera-De la Fuente, M.; Cadenas-Pliego, G.; Morales-Díaz, A.B.; Trejo-Téllez, L.I.; Tortella, G.; Benavides-Mendoza, A. ZnO nanoparticles as potential fertilizer and biostimulant for lettuce. Heliyon 2023, 9, e12787. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Alshahrani, T.S. Integrative Effects of Zinc Nanoparticle and PGRs to Mitigate Salt Stress in Maize. Agronomy 2023, 13, 1655. [Google Scholar] [CrossRef]
- Alhammad, B.A.; Ahmad, A.; Seleiman, M.F. Nano-Hydroxyapatite and ZnO-NPs Mitigate Pb Stress in Maize. Agronomy 2023, 13, 1174. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.; Hussain, I.; Singh, H.; Yadav, V.; Singh, S. Green synthesis of nano zinc oxide and evaluation of its impact on germination and metabolic activity of Solanum lycopersicum. J. Biotechnol. 2016, 233, 84–94. [Google Scholar] [CrossRef]
- Sun, L.; Song, F.; Guo, J.; Zhu, X.; Liu, S.; Liu, F.; Li, X. Nano-ZnO-induced drought tolerance is associated with melatonin synthesis and metabolism in maize. Int. J. Mol. Sci. 2020, 21, 782. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Qin, H.; Ding, H.; Li, Y.; Guo, T. Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. J. Plant Growth Regul. 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Irfan, M.; Maqsood, M.A.; Rehman, H.u.; Mahboob, W.; Sarwar, N.; Hafeez, O.B.A.; Hussain, S.; Ercisli, S.; Akhtar, M.; Aziz, T. Silicon Nutrition in Plants under Water-Deficit Conditions: Overview and Prospects. Water 2023, 15, 739. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Ashraf, M. Influence of foliar application of silicon on chlorophyll fluorescence, photosynthetic pigments, and growth in water-stressed wheat cultivars differing in drought tolerance. Turk. J. Bot. 2015, 39, 625–634. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 28 January 2023).
- Morales-Fernández, S.D.; Mora-Aguilar, R.; Salinas-Moreno, Y.; Rodríguez-Pérez, J.E.; Colinas-León, M.T.; Lozoya-Saldaña, H. Growth and sugar content of potato tubers in four maturity stages under greenhouse conditions. Rev. Chapingo Ser. Hortic. 2018, 24, 53–67. [Google Scholar]
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Potato Production: A Review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Djaman, K.; Irmak, S.; Koudahe, K.; Allen, S. Irrigation management in potato (Solanum tuberosum L.) production: A review. Sustainability 2021, 13, 1504. [Google Scholar] [CrossRef]
- Al-Selwey, W.A.; Alsadon, A.A.; Ibrahim, A.A.; Labis, J.P.; Seleiman, M.F. Effects of Zinc Oxide and Silicon Dioxide Nanoparticles on Physiological, Yield, and Water Use Efficiency Traits of Potato Grown under Water Deficit. Plants 2023, 12, 218. [Google Scholar] [CrossRef]
- Allen, R.G. Crop Evapotranspiration-Guideline for computing crop water requirements. Irrig. Drain 1998, 56, 300. [Google Scholar]
- Maynard, D.N.; Hochmuth, G.J. Knott’s Handbook for Vegetable Growers; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Watson, D.J. Comparative physiological studies on the growth of field crops: I. Variation in net assimilation rate and leaf area between species and varieties, and within and between years. Ann. Bot. 1947, 11, 41–76. [Google Scholar] [CrossRef]
- Azab, E.; Soror, A.-f.S. Physiological Behavior of the Aquatic Plant Azolla sp. in Response to Organic and Inorganic Fertilizers. Plants 2020, 9, 924. [Google Scholar] [CrossRef]
- Azab, E.M.A. Performance of Catharanthus roseus plants in response to gamma irradiation. Soc. Adv. Sci. 2016, 33, 130–140. [Google Scholar]
- Jackson, M. Soil Chemical Analysis Prentice; Hall of India Private Limited: New Delhi, India, 1967; Volume 498. [Google Scholar]
- Fuentes, H.R.; Absi, J.R. Métodos de Análisis de Suelos y Plantas: Criterios de Interpretación; Editorial Trillas: Mexico City, Mexico, 2015. [Google Scholar]
- Osman, H.S. Enhancing antioxidant–yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar] [CrossRef]
- Osman, H.S.; Salim, B.B. Enhancing antioxidants defense system of snap bean under NaCl salinity using foliar application of salicylic acid, spermidine and glycine betaine. Am. Eurasian J. Agric. Environ. Sci 2016, 16, 1200–1210. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, R.; Nuckles, E.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Kong, F.; Hu, W.; Chao, S.; Sang, W.; Wang, L. Physiological responses of the lichen Xanthoparmelia mexicana to oxidative stress of SO2. Environ. Exp. Bot. 1999, 42, 201–209. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Malick, C.; Singh, M. Plant Enzymology and Histo Enzymology; Kalyari Publishers: New Delhi, India, 1980; p. 286. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Ordonez, A.; Gomez, J.; Vattuone, M. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Rao, K.M.; Sresty, T. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Shao, H.-B.; Chu, L.-Y.; Jaleel, C.A.; Manivannan, P.; Panneerselvam, R.; Shao, M.-A. Understanding water deficit stress-induced changes in the basic metabolism of higher plants–biotechnologically and sustainably improving agriculture and the ecoenvironment in arid regions of the globe. Crit. Rev. Biotechnol. 2009, 29, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.C.; Jin, Y.I.; Nam, J.H.; Cheon, C.G.; Cho, J.H.; Kim, S.J.; Yu, H.-S. Early drought effect on canopy development and tuber growth of potato cultivars with different maturities. Field Crops Res. 2018, 215, 156–162. [Google Scholar] [CrossRef]
- Queiroz, M.S.; Oliveira, C.E.; Steiner, F.; Zuffo, A.M.; Zoz, T.; Vendruscolo, E.P.; Silva, M.V.; Mello, B.; Cabra, R.; Menis, F.T. Drought stresses on seed germination and early growth of maize and sorghum. J. Agric. Sci. 2019, 11, 310–318. [Google Scholar] [CrossRef]
- Patil, S.V.; Patil, C.D.; Mohite, B.V. Isolation and Screening of ACC Deaminase-Producing Microbes for Drought Stress Management in Crops. In Practical Handbook on Agricultural Microbiology; Humana: New York, NY, USA, 2022; pp. 361–367. [Google Scholar]
- Kesiime, V.; Tusiime, G.; Kashaija, I.; Edema, R.; Gibson, P.; Namugga, P.; Kakuhenzire, R. Characterization and evaluation of potato genotypes (Solanum tuberosum L.) for tolerance to drought in Uganda. Am. J. Potato Res. 2016, 93, 543–551. [Google Scholar] [CrossRef]
- Michel, A.; Teixeira, E.; Brown, H.; Dellow, S.; Maley, S.; Gillespie, R.; Richards, K. Water stress responses of three potato cultivars. Agron. N. Z. 2019, 49, 25–37. [Google Scholar]
- Kataria, S.; Jain, M.; Rastogi, A.; Živčák, M.; Brestic, M.; Liu, S.; Tripathi, D.K. Role of nanoparticles on photosynthesis: Avenues and applications. In Nanomaterials in Plants, Algae and Microorganisms; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–127. [Google Scholar]
- Zoufan, P.; Baroonian, M.; Zargar, B. ZnO nanoparticles-induced oxidative stress in Chenopodium murale L., Zn uptake, and accumulation under hydroponic culture. Environ. Sci. Pollut. Res. 2020, 27, 11066–11078. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Slimani, Y.; Tombuloglu, G.; Almessiere, M.; Baykal, A. Uptake and translocation of magnetite (Fe3O4) nanoparticles and its impact on photosynthetic genes in barley (Hordeum vulgare L.). Chemosphere 2019, 226, 110–122. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Slimani, Y.; Tombuloglu, G.; Alshammari, T.; Almessiere, M.; Korkmaz, A.D.; Baykal, A.; Samia, A.C.S. Engineered magnetic nanoparticles enhance chlorophyll content and growth of barley through the induction of photosystem genes. Environ. Sci. Pollut. Res. 2020, 27, 34311–34321. [Google Scholar] [CrossRef]
- El-Zohri, M.; Al-Wadaani, N.A.; Bafeel, S.O. Foliar sprayed green zinc oxide nanoparticles mitigate drought-induced oxidative stress in tomato. Plants 2021, 10, 2400. [Google Scholar] [CrossRef]
- Ahmed, R.; Uddin, M.; Quddus, M.; Samad, M.Y.A.; Hossain, M.; Haque, A.N.A. Impact of Foliar Application of Zinc and Zinc Oxide Nanoparticles on Growth, Yield, Nutrient Uptake and Quality of Tomato. Horticulturae 2023, 9, 162. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef]
- A El-Kereti, M.; A El-feky, S.; S Khater, M.; A Osman, Y.; A El-sherbini, E.-s. ZnO nanofertilizer and He Ne laser irradiation for promoting growth and yield of sweet basil plant. Recent Pat. Food Nutr. Agric. 2013, 5, 169–181. [Google Scholar] [CrossRef]
- Elshayb, O.M.; Nada, A.M.; Ibrahim, H.M.; Amin, H.E.; Atta, A.M. Application of silica nanoparticles for improving growth, yield, and enzymatic antioxidant for the hybrid rice ehr1 growing under water regime conditions. Materials 2021, 14, 1150. [Google Scholar] [CrossRef]
- Kashyap, D.; Siddiqui, Z.A. Effect of silicon dioxide nanoparticles and Rhizobium leguminosarum alone and in combination on the growth and bacterial blight disease complex of pea caused by Meloidogyne incognita and Pseudomonas syringae pv. pisi. Arch. Phytopathol. Plant Prot. 2021, 54, 499–515. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Bbiolog. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Bhaskara, G.B.; Yang, T.-H.; Verslues, P.E. Dynamic proline metabolism: Importance and regulation in water limited environments. Front. Plant Sci. 2015, 6, 484. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Andfood Chem. 2017, 65, 8552–8559. [Google Scholar] [CrossRef]
- Dawa, K.; Al-Gazar, T.; Abdel-Fatah, A. Response of Tomato Plants to Water Irrigation Levels and some Foliar Applications under Drip Irrigation System: 1-Vegetative Growth and Chemical Constituents of L. J. Plant Prod. 2019, 10, 265–273. [Google Scholar] [CrossRef]
- Ragab, M.E.; Arafa, Y.E.; Sawan, O.M.; Fawzy, Z.F.; El-Sawy, S.M. Effect of irrigation systems on vegetative growth, fruit yield, quality and irrigation water use efficiency of tomato plants (Solanum lycopersicum L.) grown under water stress conditions. Acta Sci. Agric. 2019, 3, 172–183. [Google Scholar]
- Elsakhawy, T.; Omara, A.E.-D.; Alshaal, T.; El-Ramady, H. Nanomaterials and plant abiotic stress in agroecosystems. Environ. Biodivers. Soil Secur. 2018, 2, 73–94. [Google Scholar] [CrossRef]
- Ghoto, K.; Simon, M.; Shen, Z.-J.; Gao, G.-F.; Li, P.-F.; Li, H.; Zheng, H.-L. Physiological and root exudation response of maize seedlings to TiO2 and SiO2 nanoparticles exposure. BioNanoScience 2020, 10, 473–485. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, A.H.; Mir, S.H.; Rehman, I.U.; Tahir, I.; Ahmad, P.; Rashid, I. Elucidating the role of silicon in drought stress tolerance in plants. Plant Physiol. Biochem. 2021, 165, 187–195. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef]
- Alam, P.; Arshad, M.; Al-Kheraif, A.A.; Azzam, M.A.; Al Balawi, T. Silicon Nanoparticle-Induced Regulation of Carbohydrate Metabolism, Photosynthesis, and ROS Homeostasis in Solanum lycopersicum Subjected to Salinity Stress. ACS Omega 2022, 7, 31834–31844. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Martínez-Villegas, N.; Meza-Figueroa, D.; Rivera-Cruz, M.d.C.; Cadenas-Pliego, G.; Juárez-Maldonado, A. SiO2 Nanoparticles improve nutrient uptake in tomato plants developed in the presence of arsenic. Rev. Bio Cienc. 2021, 8. [Google Scholar] [CrossRef]
- Zhang, H.; Du, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; White, J.C.; Keller, A.; Guo, H.; Ji, R.; Zhao, L. Metabolomics reveals how cucumber (Cucumis sativus) reprograms metabolites to cope with silver ions and silver nanoparticle-induced oxidative stress. Environ. Sci. Technol. 2018, 52, 8016–8026. [Google Scholar] [CrossRef]
- Naz, S.; Mushtaq, A.; Ali, S.; Muhammad, H.M.D.; Saddiq, B.; Ahmad, R.; Zulfiqar, F.; Hayat, F.; Tiwari, R.K.; Lal, M.K. Foliar application of ascorbic acid enhances growth and yield of lettuce (Lactuca sativa) under saline conditions by improving antioxidant defence mechanism. Funct. Plant Biol. 2022. [Google Scholar] [CrossRef]
- Nandy, S.; Mandal, S.; Gupta, S.K.; Anand, U.; Ghorai, M.; Mundhra, A.; Rahman, M.H.; Ray, P.; Mitra, S.; Ray, D. Role of Polyamines in Molecular Regulation and Cross-Talks Against Drought Tolerance in Plants. J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Kumar, R.; Lal, M.K.; Kumar, A.; Altaf, M.A.; Devi, R.; Mangal, V.; Naz, S.; Altaf, M.M.; Dey, A. Melatonin-Polyamine Interplay in the Regulation of Stress Responses in Plants. J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Noohpisheh, Z.; Amiri, H.; Mohammadi, A.; Farhadi, S. Effect of the foliar application of zinc oxide nanoparticles on some biochemical and physiological parameters of Trigonella foenum-graecum under salinity stress. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2021, 155, 267–280. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Zhao, J.; Xu, L.; Xu, L.; Yu, X.; Wei, Y.; Xing, B. Interaction of CuO nanoparticles with plant cells: Internalization, oxidative stress, electron transport chain disruption, and toxicogenomic responses. Environ. Sci. Nano 2018, 5, 2269–2281. [Google Scholar] [CrossRef]
- Srivastav, A.; Ganjewala, D.; Singhal, R.K.; Rajput, V.D.; Minkina, T.; Voloshina, M.; Srivastava, S.; Shrivastava, M. Effect of ZnO nanoparticles on growth and biochemical responses of wheat and maize. Plants 2021, 10, 2556. [Google Scholar] [CrossRef]
- Taran, N.; Storozhenko, V.; Svietlova, N.; Batsmanova, L.; Shvartau, V.; Kovalenko, M. Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res. Lett. 2017, 12, 1–6. [Google Scholar] [CrossRef]
- Foroutan, L.; Solouki, M.; Abdossi, V.; Fakheri, B.A. The effects of zinc oxide nanoparticles on enzymatic and osmoprotectant alternations in different Moringa peregrina populations under drought stress. Int. J. Basic Sci. Med. 2018, 3, 178–187. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Asif, M.; Ozturk, L. Supra-optimal growth temperature exacerbates adverse effects of low Zn supply in wheat. J. Plant Nutr. Soil Sci. 2019, 182, 656–666. [Google Scholar] [CrossRef]
- Cherif, J.; Mediouni, C.; Ammar, W.B.; Jemal, F. Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solarium lycopersicum). J. Environ. Sci. 2011, 23, 837–844. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Tan, Q.; Li, L.; Shi, K.; Zheng, Y.; Sun, X. Drought stress tolerance mediated by zinc-induced antioxidative defense and osmotic adjustment in cotton (Gossypium hirsutum). Acta Physiol. Plant. 2015, 37, 167. [Google Scholar] [CrossRef]
- Liu, W.; Worms, I.; Slaveykova, V.I. Interaction of silver nanoparticles with antioxidant enzymes. Environ. Sci. Nano 2020, 7, 1507–1517. [Google Scholar] [CrossRef]
- Chen, J.; Dou, R.; Yang, Z.; You, T.; Gao, X.; Wang, L. Phytotoxicity and bioaccumulation of zinc oxide nanoparticles in rice (Oryza sativa L.). Plant Physiol. Biochem. 2018, 130, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Azarin, K.; Usatov, A.; Minkina, T.; Plotnikov, A.; Kasyanova, A.; Fedorenko, A.; Duplii, N.; Vechkanov, E.; Rajput, V.D.; Mandzhieva, S. Effects of ZnO nanoparticles and its bulk form on growth, antioxidant defense system and expression of oxidative stress related genes in Hordeum vulgare L. Chemosphere 2022, 287, 132167. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Petrik, P.; Mushtaq, Z.; Khan, M.M.A.; Gulfishan, M.; Lux, A. Silicon nanoparticles in higher plants: Uptake, action, stress tolerance, and crosstalk with phytohormones, antioxidants, and other signalling molecules. Environ. Pollut. 2022, 310, 119855. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-Khaishany, M.Y. Role of Nanoparticles in Plants. In Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Siddiqui, M.H., Al-Whaibi, M.H., Mohammad, F., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 19–35. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.F.; Legay, S. Silicon and the Plant Extracellular Matrix. Front Plant Sci 2016, 7, 463. [Google Scholar] [CrossRef]
- Qados, A.M.A. Mechanism of nanosilicon-mediated alleviation of salinity stress in faba bean (Vicia faba L.) plants. Am. J. Exp. Agric. 2015, 7, 78–95. [Google Scholar] [CrossRef]
- Sutulienė, R.; Ragelienė, L.; Samuolienė, G.; Brazaitytė, A.; Urbutis, M.; Miliauskienė, J. The response of antioxidant system of drought-stressed green pea (Pisum sativum L.) affected by watering and foliar spray with silica nanoparticles. Horticulturae 2022, 8, 35. [Google Scholar] [CrossRef]
- Gowayed, M.; Al-Zahrani, H.S.; Metwali, E.M. Improving the salinity tolerance in potato (Solanum tuberosum) by exogenous application of silicon dioxide nanoparticles. Int. J. Agric. Biol. 2017, 19, 183–192. [Google Scholar]
- Kefeli, V.I.; Kalevitch, M.V.; Borsari, B. Phenolic cycle in plants and environment. J. Cell Mol. Biol 2003, 2, 13–18. [Google Scholar]
- Siracusa, L.; Gresta, F.; Sperlinga, E.; Ruberto, G. Effect of sowing time and soil water content on grain yield and phenolic profile of four buckwheat (Fagopyrum esculentum Moench.) varieties in a Mediterranean environment. J. Food Compos. Anal. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Hura, T.; Grzesiak, S.; Hura, K.; Thiemt, E.; Tokarz, K.; Wędzony, M. Physiological and biochemical tools useful in drought-tolerance detection in genotypes of winter triticale: Accumulation of ferulic acid correlates with drought tolerance. Ann. Bot. 2007, 100, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Roy, B.K. Population authentication of the traditional medicinal plant Cassia tora L. based on ISSR markers and FTIR analysis. Sci. Rep. 2018, 8, 10714. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.C. Phenolics as antioxidants. In Antioxidants and Reactive Oxygen Species in Plants; Wiley-Blackwell: New Delhi, India, 2005; pp. 141–168. [Google Scholar]
- Bors, W.; Michel, C.; Saran, M. Flavonoid antioxidants: Rate constants for reactions with oxygen radicals. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1994; Volume 234, pp. 420–429. [Google Scholar]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Guerrero, Z.H.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; González-Morales, S.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Form of silica improves yield, fruit quality and antioxidant defense system of tomato plants under salt stress. Agriculture 2020, 10, 367. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef]
- El-Yazied, A.A.; Ibrahim, M.F.; Ibrahim, M.A.; Nasef, I.N.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; Alaklabi, A.; Dessoky, E.S.; Alabdallah, N.M. Melatonin mitigates drought induced oxidative stress in potato plants through modulation of osmolytes, sugar metabolism, ABA homeostasis and antioxidant enzymes. Plants 2022, 11, 1151. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.-O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 2020, 10, 208. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Desoky, E.-S.M.; Babalghith, A.O.; El-Tahan, A.M.; Ibrahim, O.M.; Ebrahim, A.A.; Abd El-Mageed, T.A. Role of Nanoparticles in Enhancing Crop Tolerance to Abiotic Stress: A Comprehensive Review. Front. Plant Sci. 2022, 13, 946717. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, T.A.; Bayoumi, Y.; Eid, Y.; Elbasiouny, H.; Elbehiry, F.; Prokisch, J.; El-Ramady, H.; Ling, W. Can nanofertilizers mitigate multiple environmental stresses for higher crop productivity? Sustainability 2022, 14, 3480. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Selwey, W.A.; Ibrahim, A.A.; Shady, M.; Alsadon, A.A. Foliar applications of ZnO and SiO2 nanoparticles mitigate water deficit and enhance potato yield and quality traits. Agronomy 2023, 13, 466. [Google Scholar] [CrossRef]

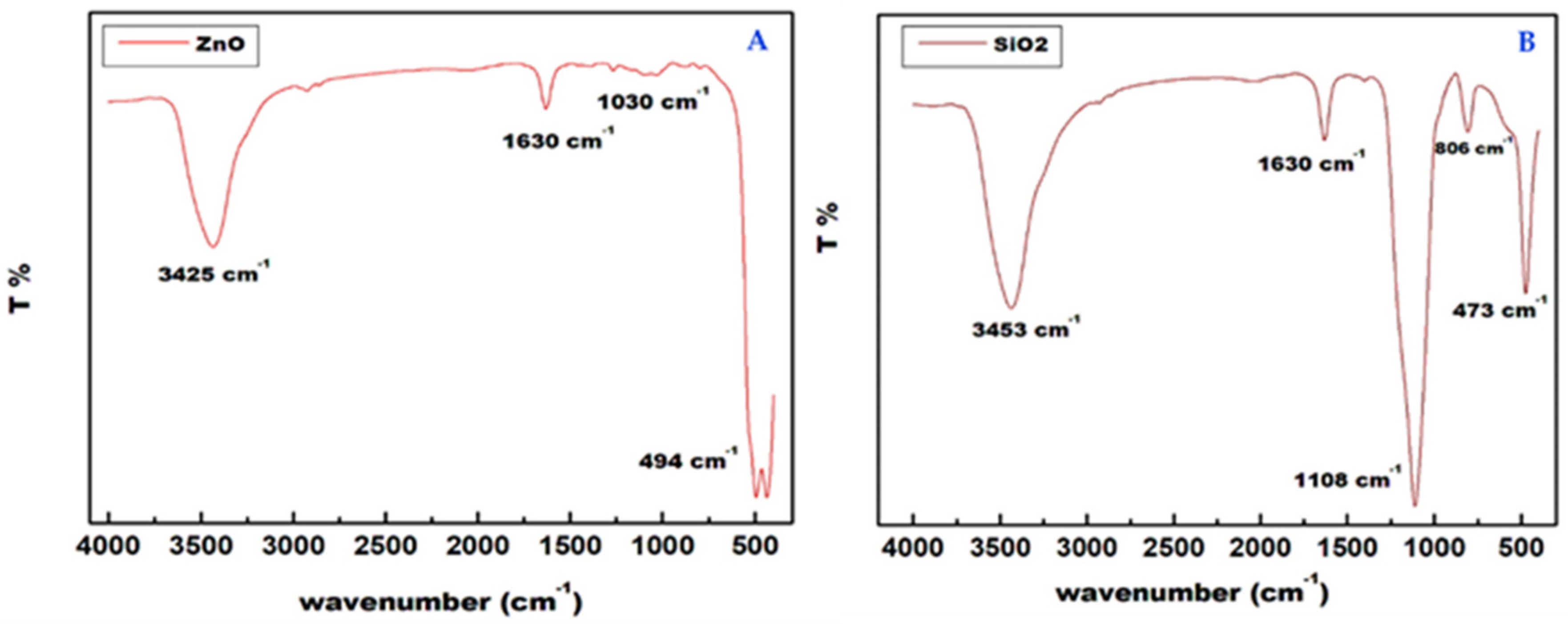



| Particle Size Distribution (%) | Texture Class | FC (%) | WP (%) | OM (%) | Ks (mm/h) | ρb (g cm−3) | pH | EC (dS/m) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand% | Silt% | Clay% | ||||||||
| 83.72 | 7.83 | 8.45 | Sandy Loam | 14.9 | 6.7 | 0.83 | 24.8 | 1.6 | 7.8 | 1.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Selwey, W.A.; Alsadon, A.A.; Alenazi, M.M.; Tarroum, M.; Ibrahim, A.A.; Ahmad, A.; Osman, M.; Seleiman, M.F. Morphological and Biochemical Response of Potatoes to Exogenous Application of ZnO and SiO2 Nanoparticles in a Water Deficit Environment. Horticulturae 2023, 9, 883. https://doi.org/10.3390/horticulturae9080883
Al-Selwey WA, Alsadon AA, Alenazi MM, Tarroum M, Ibrahim AA, Ahmad A, Osman M, Seleiman MF. Morphological and Biochemical Response of Potatoes to Exogenous Application of ZnO and SiO2 Nanoparticles in a Water Deficit Environment. Horticulturae. 2023; 9(8):883. https://doi.org/10.3390/horticulturae9080883
Chicago/Turabian StyleAl-Selwey, Wadei A., Abdullah A. Alsadon, Mekhled M. Alenazi, Mohamed Tarroum, Abdullah A. Ibrahim, Awais Ahmad, Mohamed Osman, and Mahmoud F. Seleiman. 2023. "Morphological and Biochemical Response of Potatoes to Exogenous Application of ZnO and SiO2 Nanoparticles in a Water Deficit Environment" Horticulturae 9, no. 8: 883. https://doi.org/10.3390/horticulturae9080883
APA StyleAl-Selwey, W. A., Alsadon, A. A., Alenazi, M. M., Tarroum, M., Ibrahim, A. A., Ahmad, A., Osman, M., & Seleiman, M. F. (2023). Morphological and Biochemical Response of Potatoes to Exogenous Application of ZnO and SiO2 Nanoparticles in a Water Deficit Environment. Horticulturae, 9(8), 883. https://doi.org/10.3390/horticulturae9080883






