Micromorphology of Barleria albostellata (Grey Barleria) Flower and Pollen Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Stereomicroscopy
2.3. Scanning Electron Microscopy (SEM)
3. Results and Discussion
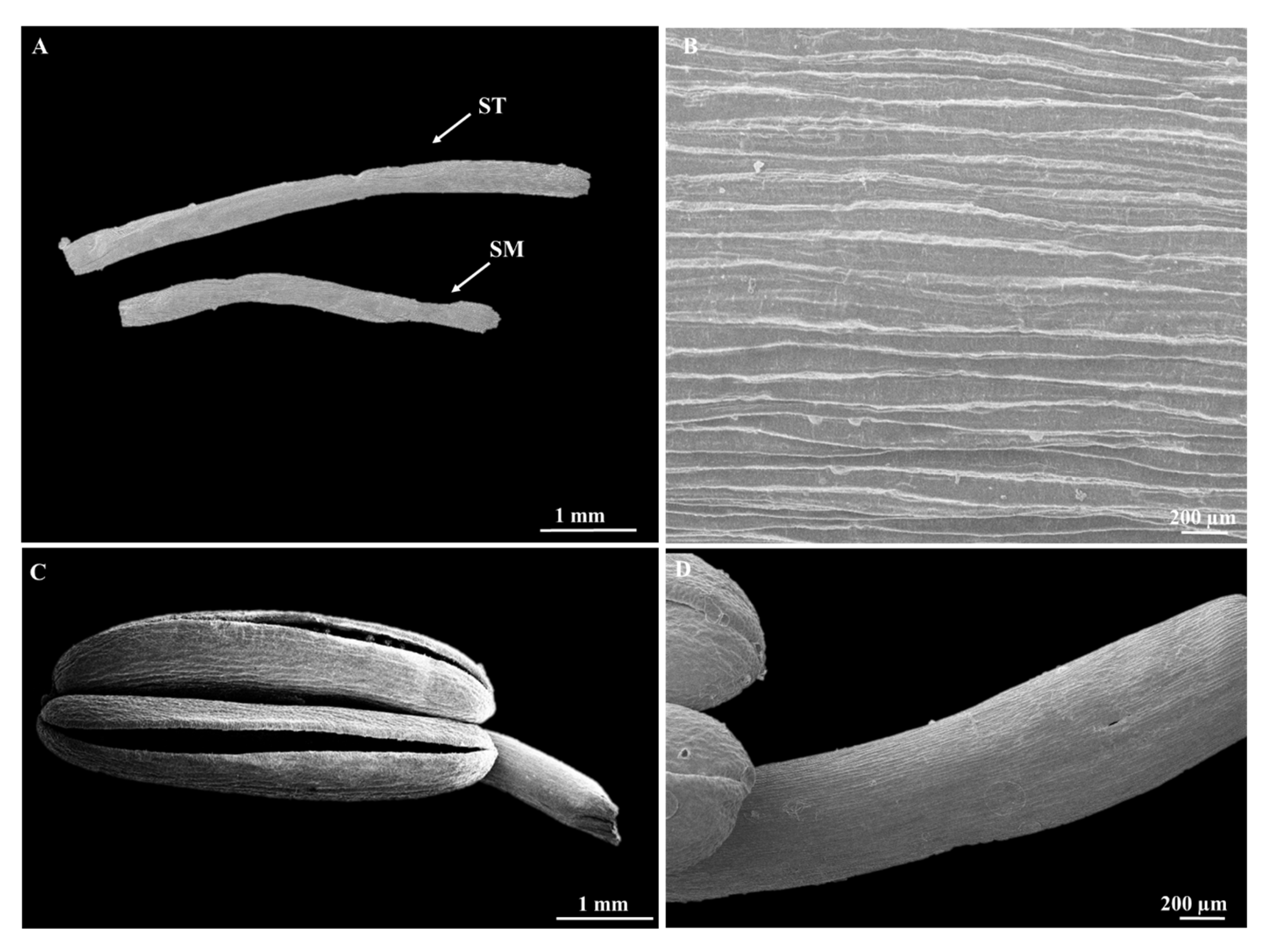
3.1. Analysis of Floral Structures via Stereomicroscopy
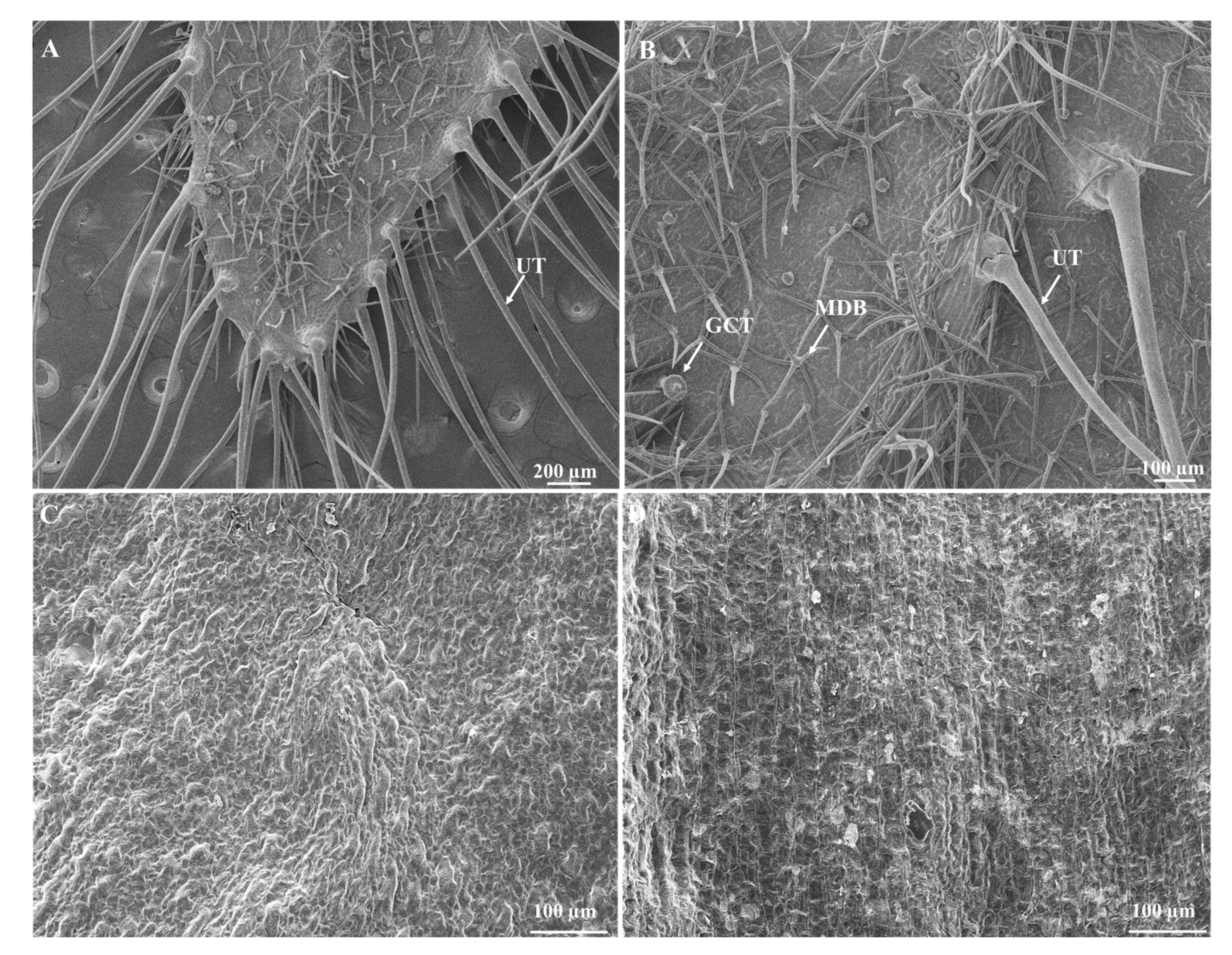
3.2. Floral Structures Observed via Scanning Electron Microscopy
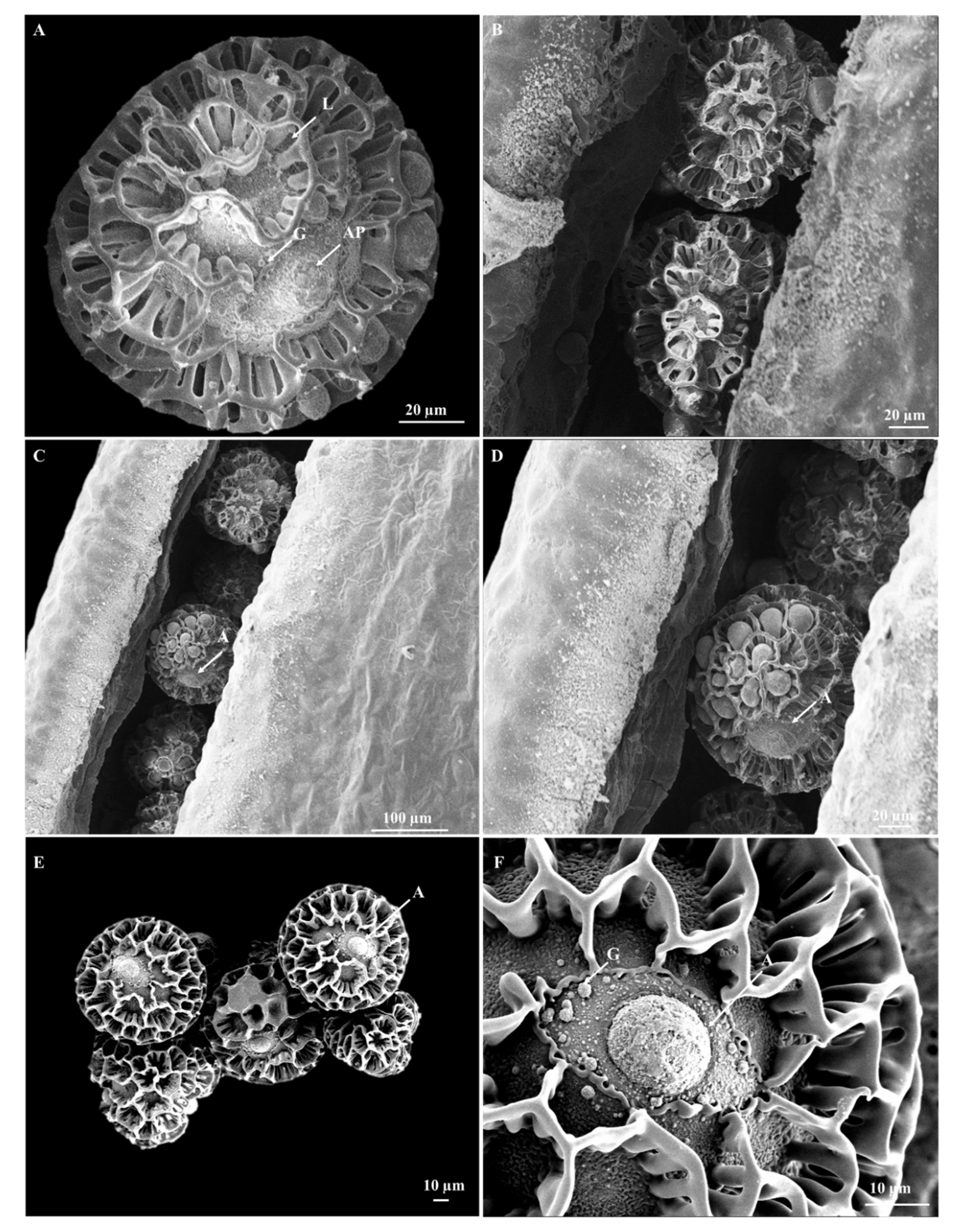
3.3. Pollen Morphology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Lin, M.; Chen, H.; Zhu, Q.; Chen, X. Floral biology and pistil receptivity of the drumstick tree (Moringa oleifera Lam.). Arch. Biol. Sci. 2018, 70, 299–305. [Google Scholar] [CrossRef]
- Liu, F.; Gao, C.; Chen, M.; Tang, G.; Sun, Y.; Li, K. The impacts of flowering phenology on the reproductive success of the narrow endemic Nouelia insignis Franch (Asteraceae). Ecol. Evol. 2021, 11, 9396–9409. [Google Scholar] [CrossRef] [PubMed]
- Harder, L.D.; Williams, N.M.; Jordan, C.Y.; Nelson, W.A. The effects of floral design and display on pollinator economics and pollen dispersal. In Cognitive Ecology of Pollination: Animal Behaviour and Floral Evolution; Chittka, L., Thomson, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 297–317. [Google Scholar]
- Budečević, S.; Hočevar, K.; Manitašević Jovanović, S.; Vuleta, A. Phenotypic Selection on Flower Traits in Food-Deceptive Plant Iris pumila L.: The Role of Pollinators. Symmetry 2023, 15, 1149. [Google Scholar] [CrossRef]
- McMullen, C.K.; Close, D.D. Wind pollination in the Galápagos Islands. Not. Galápagos 1993, 52, 12–17. [Google Scholar]
- Dafni, A.; Firmage, D. Pollen viability and longevity: Practical, ecological and evolutionary implications. Plant Syst. Evol. 2000, 222, 113–132. [Google Scholar] [CrossRef]
- Christopher, D.A.; Mitchell, R.J.; Karron, J.D. Pollination intensity and paternity in flowering plants. Ann. Bot. 2020, 125, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kay, K.M.; Sargent, R.D. The role of animal pollination in plant speciation: Integrating ecology, geography, and genetics. Annu. Rev. Ecol. Syst. 2009, 40, 637–656. [Google Scholar] [CrossRef]
- Sletvold, N.; Trunschke, J.; Smit, M.; Verbeek, J.; Ågren, J. Strong pollinator-mediated selection for increased flower brightness and contrast in a deceptive orchid. Evolution 2016, 70, 716–724. [Google Scholar] [CrossRef]
- Mabberley, J.B. The Plant—Book: A Portable Dictionary of the Vascular Plants, 2nd ed.; Cambridge University Press: Bath, UK, 1997. [Google Scholar]
- Darbyshire, I.; Vollesen, K.; Kelbessa, E. Acanthaceae, part 2. In Flora Zambesiaca; Timberlake, J.R., Martins, E.S., Eds.; Royal Botanic Gardens: London, UK, 2015; 8p. [Google Scholar]
- Kumari, R.; Kumar, S.; Kumar, A.; Goel, K.K.; Dubey, R.C. Antibacterial, antioxidant and immuno-modulatory properties in extracts of Barleria lupulina Lindl. BMC Complemen. Altern. Med. 2017, 17, 484. [Google Scholar] [CrossRef]
- Al-Hakimi, A.S.; Faridah, Q.; Abdulwahab, A.; Latiff, A. Pollen and seed morphology of Barleria L. (Barlerieae: Ruellioideae: Acanthaceae) of Yemen. S. Afr. J. Bot. 2018, 116, 185–191. [Google Scholar] [CrossRef]
- Balkwill, M.J.; Balkwill, K. A preliminary analysis of distribution patterns in a large, pantropical genus, Barleria L. (Acanthaceae). J. Biogeog. 1998, 25, 95–110. [Google Scholar] [CrossRef]
- Svoboda, K.P.; Svoboda, T.G.; Syred, A.D. Secretory Structures of Aromatic and Medicinal Plants: A Review and Atlas of Micrographs; Microscopix Publications: Knighton, UK, 2000; pp. 7–12. [Google Scholar]
- Schmid, N.B.; Giehl, R.F.; Döll, S.; Mock, H.P.; Strehmel, N.; Scheel, D.; Kong, X.; Hider, R.C.; Von Wirén, N. Feruloyl-CoA 6′-Hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol. 2014, 164, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Froneman, W.; Le Roux, L.N. Barleria albostellata. 2007. Available online: http://pza.sanbi.org/barleria-albostellata (accessed on 2 February 2019).
- Balkwill, M.J.; Balkwill, K. Delimitation and infra-generic classification of Barleria (Acanthaceae). Kew Bull. 1997, 52, 535–573. [Google Scholar] [CrossRef]
- Amoo, S.O.; Finnie, J.F.; Van Staden, J. In vitro pharmacological evaluation of three Barleria species. J. Ethnopharmacol. 2009, 121, 274–277. [Google Scholar] [CrossRef]
- Amoo, S.O.; Ndhlala, A.R.; Finnie, J.F.; Van Staden, J. Antifungal, acetylcholinesterase inhibition, antioxidant and phytochemical properties of three Barleria species. S. Afr. J. Bot. 2011, 77, 435–445. [Google Scholar] [CrossRef]
- Yosook, C.; Panpisutchai, Y.; Chaichana, S.; Santisuk, T.; Reutrakul, V. Evaluation of anti-HSV-2 activities of Barleria lupulina and Clinacanthus nutans. J. Ethnopharmacol. 1999, 67, 179–187. [Google Scholar] [CrossRef]
- Wang, B.U.; Wu, M.; Perchellet, E.M.; Mcilvain, C.J.; Sperfslage, B.J.; Huang, X.; Tamura, M.; Stephany, H.A.; Hua, D.H.; Perchellet, J.P. Asynthetic triptycene bisquinone which blocks nucleoside transport and induces DNA fragmentation, retains its cytotoxic efficacy in daunorubicin-resistant HL-60 cell lines. Int. J. Oncol. 2001, 19, 1169–1178. [Google Scholar]
- Jassim, S.A.A.; Naji, A.M. Novel antiviral agents: A medicinal plant perspective. J. App. Microbiol. 2003, 95, 412–427. [Google Scholar] [CrossRef]
- Suba, V.; Murugesan, T.; Arunachalam, G.; Mandal, S.C.; Saha, B.P. Anti-diabetic potential of Barleria lupulina extract in rats. Phytomedicine 2004, 11, 202–205. [Google Scholar] [CrossRef]
- Suba, V.; Murugesan, T.; Kumaravelrajan, R.; Mandal, S.C.; Saha, B.P. Antiinflammatory, analgesic and antiperoxidative efficacy of Barleria lupulina Lindl. extract. Phytother. Res. 2005, 19, 695–699. [Google Scholar] [CrossRef]
- Chomnawang, M.T.; Surassmo, S.; Nukoolkarn, V.S.; Gritsanapan, W. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J. Ethnopharma 2005, 101, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Raj, B. Pollen morphological studies in the Acanthaceae. Grana Palynol. 1961, 3, 3–108. [Google Scholar]
- Graham, V.A.W. Delimitation and infra-generic classification of Justicia (Acanthaceae). Kew Bull. 1988, 43, 551–624. [Google Scholar] [CrossRef]
- Daniel, T.F. Pollen morphology of Mexican Acanthaceae: Diversity and systematic significance. Proc. Calif. Acad. Sci. 1998, 508, 217–256. [Google Scholar]
- Bhatt, A.; Naidoo, Y.; Nicholas, A. The foliar trichomes of Hypoestes aristata (Vahl) Sol. ex Roem. & Schult var aristata (Acanthaceae) a widespread medicinal plant species in tropical sub-Saharan Africa: With comments on its possible phylogenetic significance. Biol. Res. 2010, 43, 403–409. [Google Scholar]
- Choopan, T.; Grote, P.J. Cystoliths in the leaves of the genus Pseuderanthemum (Acanthaceae) in Thailand. Int. J. Sci. 2015, 12, 13–20. [Google Scholar]
- House, A.; Balkwill, K. FIB-SEM enhances the potential taxonomic significance of internal pollen wall structure at the generic level. Flora 2017, 236, 44–57. [Google Scholar] [CrossRef]
- McDade, L.A.; Masta, S.E.; Moody, M.L.; Waters, E. Phylogenetic relationships among Acanthaceae: Evidence from two genomes. Syst. Bot. 2000, 25, 106–121. [Google Scholar] [CrossRef]
- Daniel, T.F.; McDade, L.A.; Manktelow, M.; Kiel, C.K. The “Tetramerium Lineage” (Acanthaceae: Acanthoideae: Justicieae): Delimitation and intra-lineage relationships based on cp and nrlTS sequence data. Syst. Bot. 2008, 33, 416–436. [Google Scholar] [CrossRef]
- McDade, L.A.; Daniel, T.F.; Kiel, C.A. The Tetramerium Lineage (Acanthaceae, Justicieae) Revisited: Phylogenetic relationships reveal polyphyly of many new world genera accompanied by rampant evolution of floral morphology. Syst. Bot. 2018, 43, 97–116. [Google Scholar] [CrossRef]
- McDade, L.A.; Daniel, T.F.; Kiel, C.A. Toward a comprehensive understanding of phylogenetic relationships among lineages of Acanthaceae s.l. (Lamiales). Am. J. Bot. 2008, 95, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Darbyshire, I.; Vollesen, K.; Kelbessa, E. Acanthaceae, part 2. In Flora of Tropical East Africa; Beentje, H., Ed.; Royal Botanic Gardens: London, UK, 2010. [Google Scholar]
- Scott, J. Dimorphism in Eranthemum. J. Bot. 1872, 10, 161–166. [Google Scholar]
- Raza, J.; Ahmad, M.; Zafar, M.; Athar, M.; Sultana, S.; Majeed, S.; Yaseen, G.; Imran, M.; Nazish, M.; Hussain, A. Comparative foliar anatomical and pollen morphological studies of Acanthaceae using light microscope and scanning electron microscope for effective microteaching in community. Microsc. Res. Techniq. 2020, 83, 103–1117. [Google Scholar] [CrossRef] [PubMed]
- Nilanthi, R.M.R.; Samarakoon, H.; Jayawardana, N.; Hathurusinghe, B.; Wijesundara, S.; Bandaranayake, P.C.G. Strobilanthes glandulata (Acanthaceae), a new species from Sri Lanka based on the morphological and molecular evidences. Phytotaxa 2022, 573, 1–14. [Google Scholar]
- Pooley, E. A Field Guide to Wild Flowers Kwazulu-Natal and the Eastern Region; Natal Flora Publications Trust: Durban, South Africa, 1998. [Google Scholar]
- Scott-Shaw, C.R.; Johnson, I.M.; Styles, D.; Makholela, T.; Von Staden, L. Barleria greenii (Balkwill, M., Balkwill, K.). National Assessment: Red List of South African Plants Version 2007, 1. Available online: http://redlist.sanbi.org/species.php?species=3909-26 (accessed on 10 October 2021).
- Froneman, W.; Plants of South Africa. South Africa: Lowveld National Botanical Garden. 2010. Available online: http://www.plantzafrica.com/plantab/barleriapriondel.htm (accessed on 2 February 2019).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.J. Epidermal hairs of Acanthaceae. Blumea-Biodivers. Evol. Biogeogr. Plants 1978, 24, 101–117. [Google Scholar]
- Werker, E. Trichome diversity and development. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar]
- Wagner, G.J.; Wang, E.; Shepherd, R.W. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. 2004, 93, 3–11. [Google Scholar] [CrossRef]
- Levin, D.A. The role of trichomes in plant defense. Q. Rev. Biol. 1973, 48, 3–15. [Google Scholar] [CrossRef]
- Baur, R.; Binder, S.; Benz, G. Non-glandular leaf trichomes as short-term inducible defense of the grey alder, Alnus incana (L.), against the chrysomelid beetle, Agelastica alni L. Oecologia 1991, 87, 219–226. [Google Scholar] [CrossRef]
- Szyndler, M.W.; Haynes, K.F.; Potter, M.F.; Corn, R.M.; Loudon, C. Entrapment of bed bugs by leaf trichomes inspires microfabrication of biomimetic surfaces. J. R. Soc. Interface 2013, 10, 20130174. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.; Pitman, N. Trees of Southern Africa; Balkema: Amsterdam, The Netherlands; Cape Town, South Africa, 1972. [Google Scholar]
- Schmidt, S.; Lotter, M.; McCleland, W. Trees and Shrubs of Mpumalanga and the Kruger National Park; Jacana: Johannesburg, South Africa, 2002. [Google Scholar]
- Fenner, M. The phenology of growth and reproduction in plants. Perspec. Plant Ecol. Evol. Syst. 1998, 1, 78–91. [Google Scholar] [CrossRef]
- Balkwill, M.J.; Balkwill, K.; Vincent, P.L.D. Systematic studies in the Acanthaceae: A new species of Barleria from Natal. S. Afr. J. Bot. 1990, 56, 571–576. [Google Scholar] [CrossRef]
- Burkhardt, D. Colour discrimination in insects. Adv. Insect Physiol. 1964, 3, 131–173. [Google Scholar]
- Faegri, K.; Van der Pijl, L. A short history of the study of pollination ecology. In Principles of Pollination Ecology; Elsevier: Amsterdam, The Netherlands, 1979; pp. 1–77. [Google Scholar]
- Caissard, J.C.; Joly, C.; Bergougnoux, V.; Hugueney, P.; Mauriat, M.; Baudino, S. Secretion mechanisms of volatile organic compounds in specialized cells of aromatic plants. Recent Res. Dev. Cell Biol. 2004, 2, 1–15. [Google Scholar]
- Oelschlägel, B.; Gorb, S.; Wanke, S.; Neinhuis, C. Structure and biomechanics of trapping flower trichomes and their role in the pollination biology of Aristolochia plants (Aristolochiaceae). New Phytol. 2009, 184, 988–1002. [Google Scholar] [CrossRef]
- Makholela, T.M.; Van der Bank, F.H.; Balkwill, K.; Manning, J.C. Allozyme variation in Barleria saxatilis (Acanthaceae) is lower than in two congeneric endemics. S. Afr. J. Bot. 2004, 70, 515–520. [Google Scholar] [CrossRef]
- Obermeijer, A.A. A revision of the South African species of Barleria. Ann. Transvaal Mus. 1933, 15, 123–180. [Google Scholar]
- Darbyshire, I. New species in Barleria sect. Stellatohirta (Acanthaceae) from Africa. Kew Bull. 2008, 63, 261–268. [Google Scholar] [CrossRef]
- Gosavi, K.V.C.; Nalawade, A.D.; Yadav, S.R. Taxonomic identity, rediscovery and epitypification of Barleria sepalosa (Acanthaceae) from northern Western Ghats, India. Rheedea 2013, 24, 23–26. [Google Scholar]
- Ravikumar, K.; Narasimhan, D.; Devanathan, K.; Gnanasekaran, G. Barleria durairajii (Acanthaceae): A new species from Tamil Nadu, India. Rheedea 2016, 26, 136–141. [Google Scholar]
- Scotland, R.W.; Vollesen, K. Classification of Acanthaceae. Kew Bull. 2000, 3, 513–589. [Google Scholar] [CrossRef]
- Tripathi, S.; Singh, S.; Roy, R.K. Pollen morphology of Bougainvillea (Nyctaginaceae): A popular ornamental plant of tropical and subtropical gardens of the world. Rev. Palaeobot. Palynol. 2017, 239, 31–46. [Google Scholar] [CrossRef]
- Darbyshire, I.; Tripp, E.A.; Dexter, K.G. A new species and a revised record in Namibian Barleria (Acanthaceae). Kew Bull. 2012, 67, 759–766. [Google Scholar] [CrossRef]
- Aronne, G.; Buonanno, M.; De Micco, V. Reproducing under a warming climate: Long winter flowering and extended flower longevity in the only Mediterranean and maritime Primula. Plant Biol. 2014, 17, 535–544. [Google Scholar] [CrossRef]
- Shendage, S.M.; Yadav, S.R. Pollen Morphology of Barleria L. (Acanthaceae) from India. Phytomorphology 2009, 59, 121–126. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangaram, S.; Naidoo, Y.; Dewir, Y.H.; Singh, M.; Magyar-Tábori, K. Micromorphology of Barleria albostellata (Grey Barleria) Flower and Pollen Grains. Horticulturae 2023, 9, 732. https://doi.org/10.3390/horticulturae9070732
Gangaram S, Naidoo Y, Dewir YH, Singh M, Magyar-Tábori K. Micromorphology of Barleria albostellata (Grey Barleria) Flower and Pollen Grains. Horticulturae. 2023; 9(7):732. https://doi.org/10.3390/horticulturae9070732
Chicago/Turabian StyleGangaram, Serisha, Yougasphree Naidoo, Yaser Hassan Dewir, Moganavelli Singh, and Katalin Magyar-Tábori. 2023. "Micromorphology of Barleria albostellata (Grey Barleria) Flower and Pollen Grains" Horticulturae 9, no. 7: 732. https://doi.org/10.3390/horticulturae9070732
APA StyleGangaram, S., Naidoo, Y., Dewir, Y. H., Singh, M., & Magyar-Tábori, K. (2023). Micromorphology of Barleria albostellata (Grey Barleria) Flower and Pollen Grains. Horticulturae, 9(7), 732. https://doi.org/10.3390/horticulturae9070732









