The Composition and the Content of ∆-5 Sterols, Fatty Acids, and the Activity of Acyl-Lipid Desaturases in the Shoots of Ephedra monosperma, Introduced in the Botanical Garden of the Cryolithozone of Yakutia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Field Experiment
2.3. Lipid Extraction
2.4. Neutral Lipid Composition Analysis by HPTLC-UV/Vis/FLD Method
2.5. Separation of Free and Bound Sterols
2.6. Silylation
2.7. GC-MS Analysis of Sterols and Their Ethers
2.8. Determination of FAs Content
2.9. Identification and Quantitative Analysis
2.10. Activity of Sterol Methyltransferase and Acyl-Lipid Desaturase Enzymes
2.11. Statistical Processing
3. Results
3.1. Composition of Neutral Lipids in E. monosperma Shoots by HPTLC−UV/Vis/FLD Profiling Method
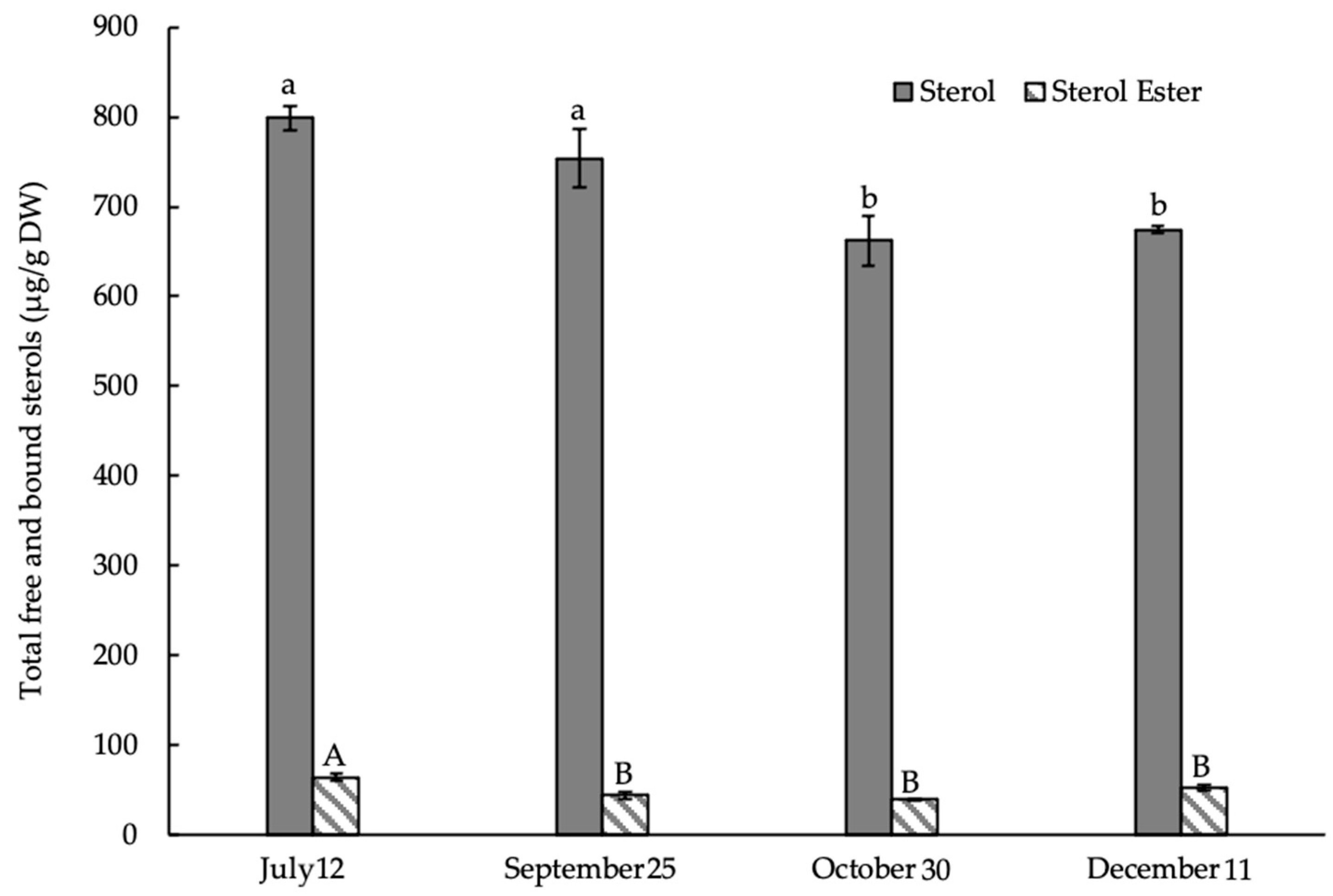
3.2. Composition and Content of Membrane Sterols in the Shrub E. monosperma Shoots
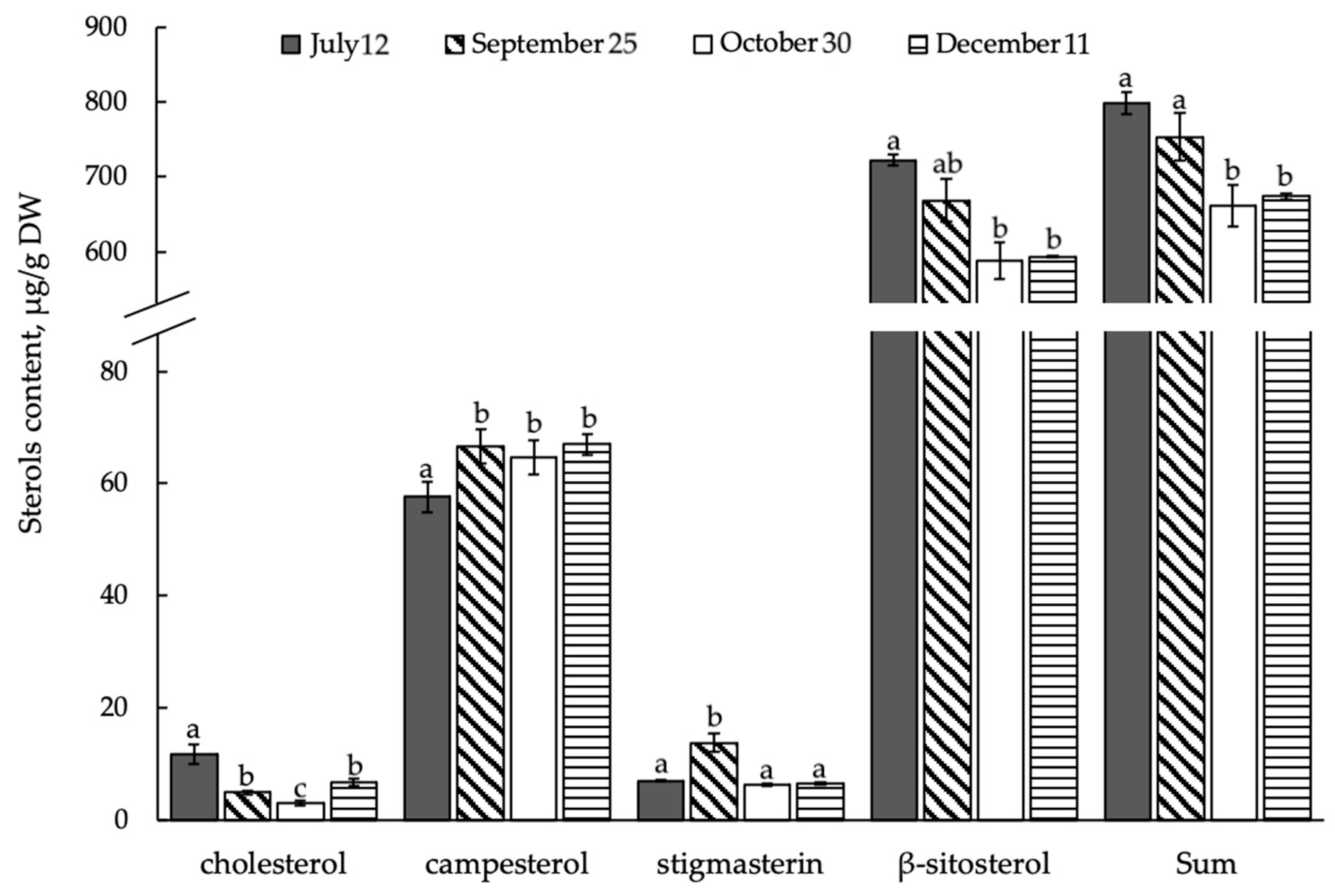
3.3. Composition and Content of Free ∆5-Sterols in Shoots of E. monosperma
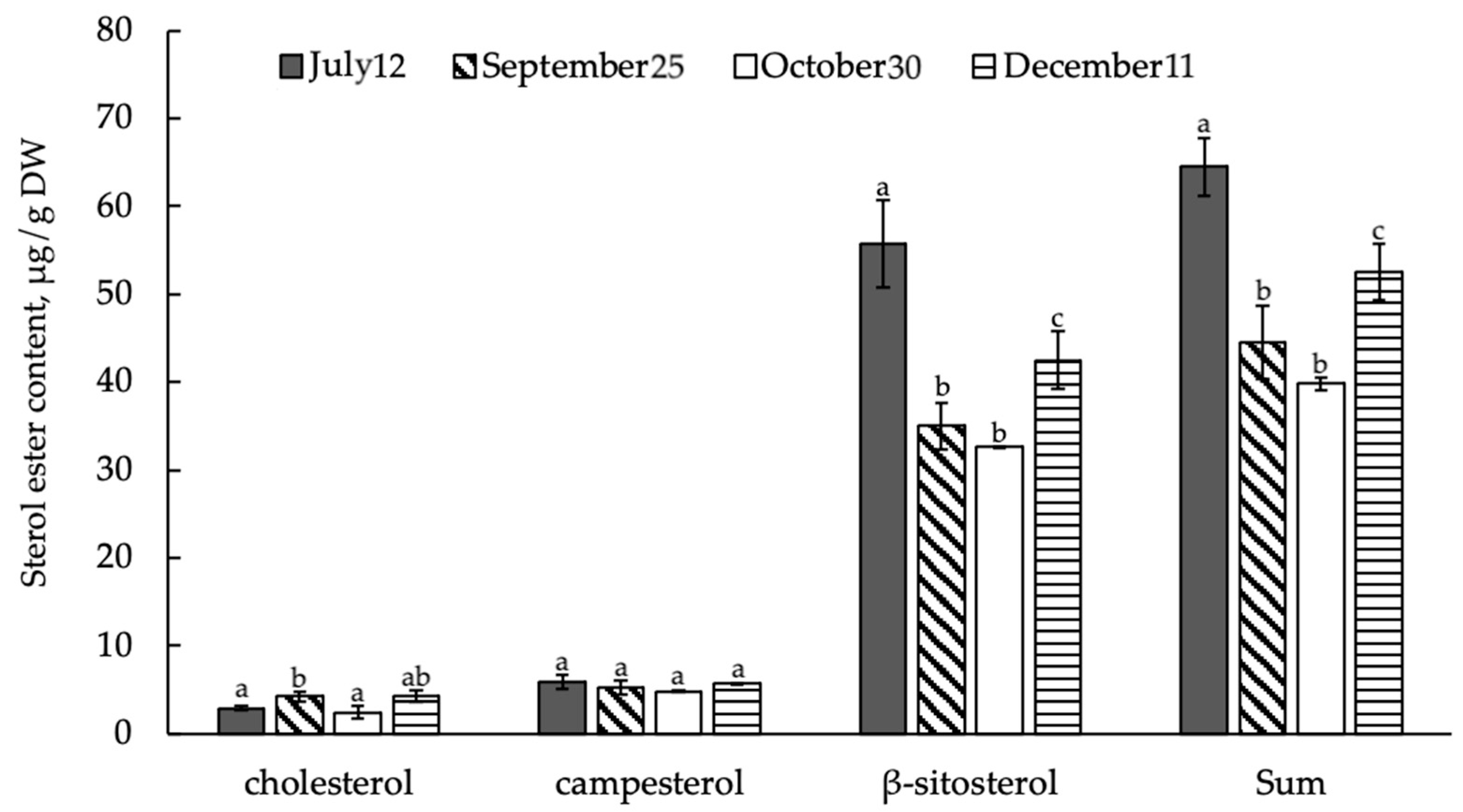
3.4. Composition and Content of Bound STs (Sterol Esters) in Shoots of E. monosperma
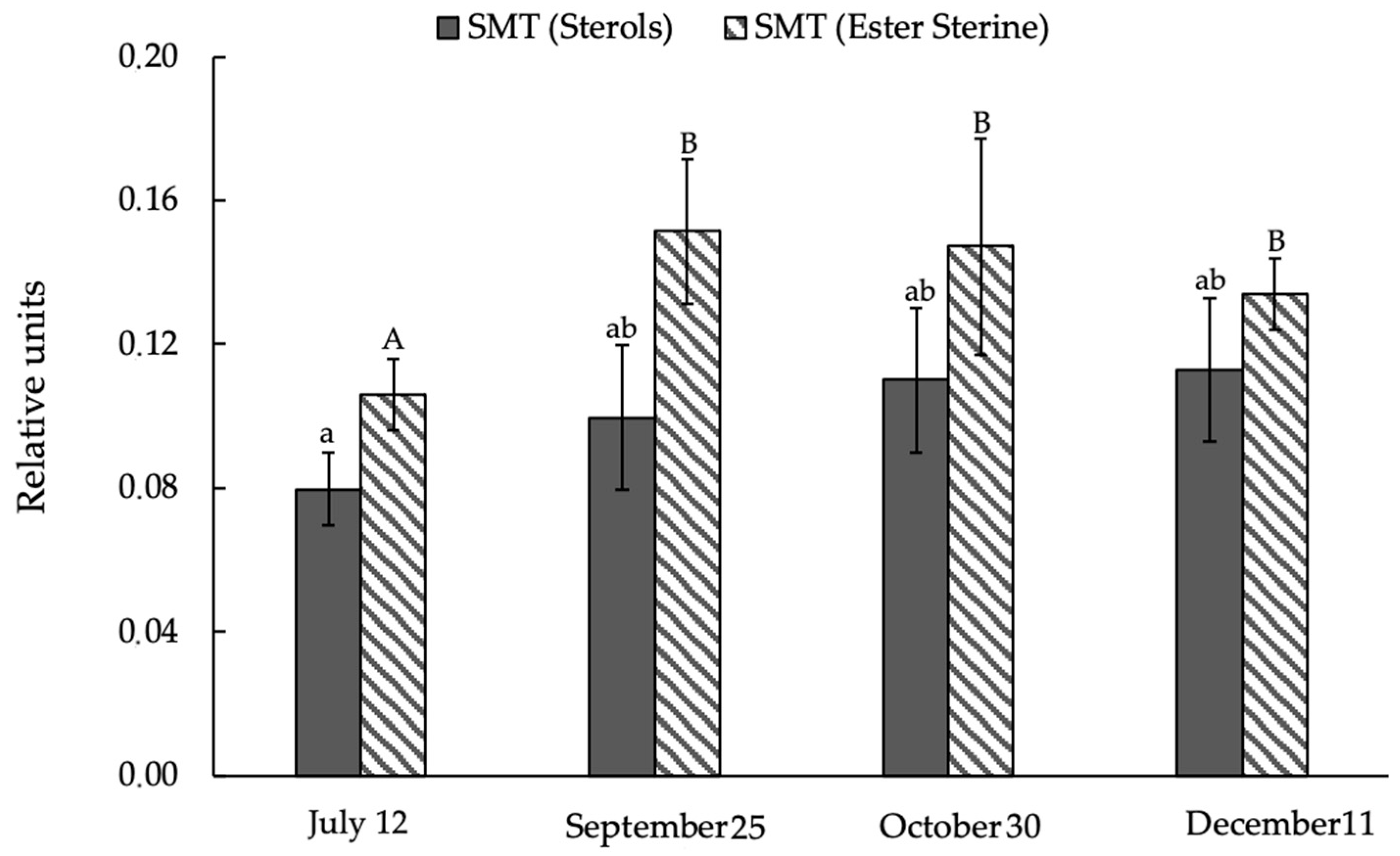
3.5. Activity of Sterol Methyltransferase (SMT) Enzymes in Ephedra Shoots during Quenching to Low Cryolithozone Temperatures
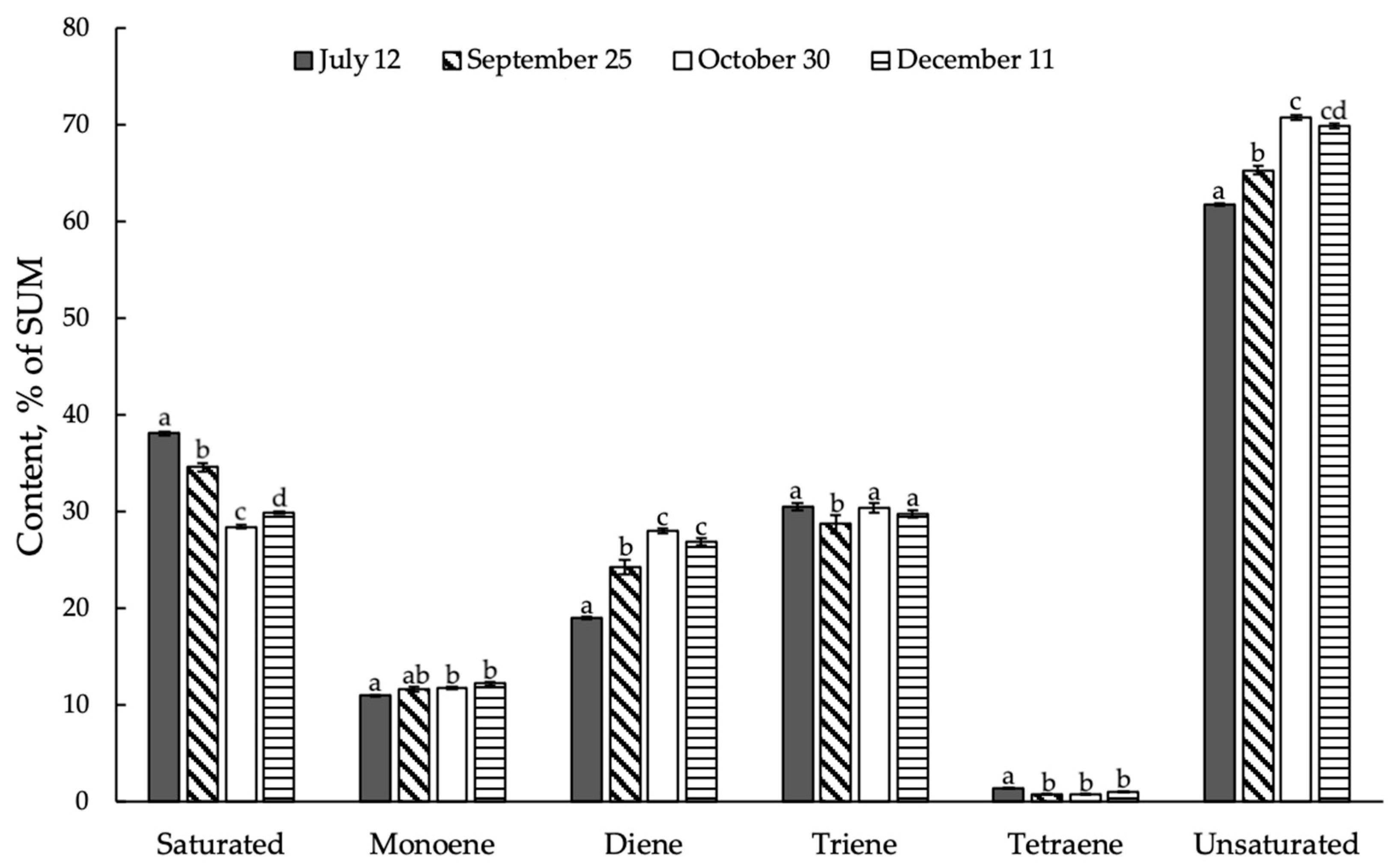
3.6. Seasonal Dynamics of Saturated and Unsaturated Fatty Acids in Ephedra Shoots
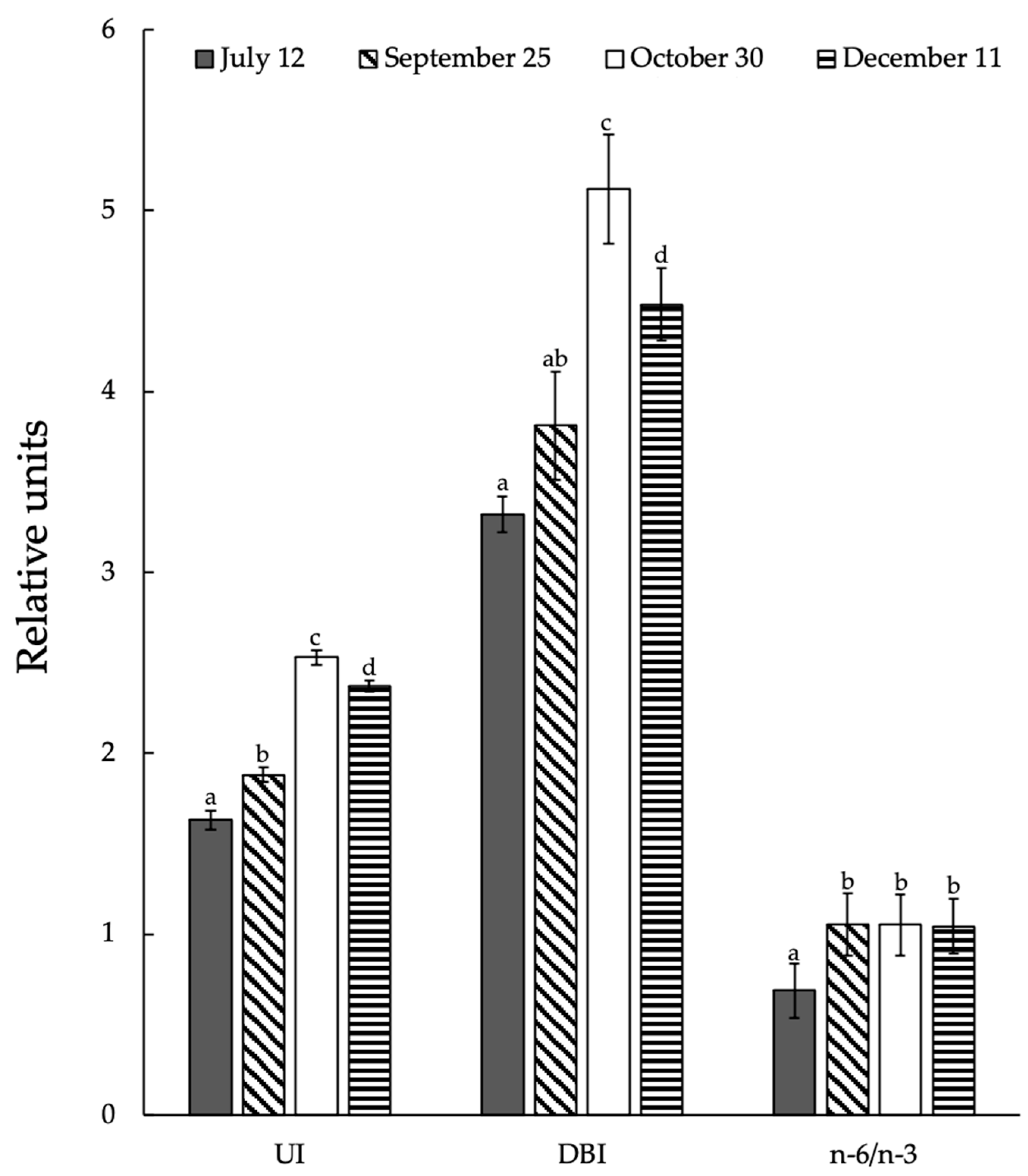
3.7. Seasonal Changes in Integral Indexes of Lipid Unsaturated Levels and Activity of Acyl-Lipid Desaturases in Ephedra Shoots
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eslamdoust, J. Bark content variations and the production of Populus deltoides as a source of bioenergy under temperate climate conditions. J. For. Res. 2022, 33, 1807–1815. [Google Scholar] [CrossRef]
- González-Juárez, D.E.; Escobedo-Moratilla, A.; Flores, J.; Hidalgo-Figueroa, S.; Martínez-Tagüeña, N.; Morales-Jiménez, J.; Muñiz-Ramírez, A.; Pastor-Palacios, G.; Pérez-Miranda, S.; Ramírez-Hernández, A.; et al. A Review of the Ephedra genus: Distribution, Ecology, Ethnobotany, Phytochemistry and Pharmacological Properties. Molecules 2020, 25, 3283. [Google Scholar] [CrossRef] [PubMed]
- Abourashed, E.A.; El-Alfy, A.T.; Khan, I.A.; Walker, L. Ephedra in perspective—A current review. Phytother. Res. 2003, 17, 703–712. [Google Scholar] [CrossRef] [PubMed]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources). Scientific opinion on safety evaluation of Ephedra species in food. EFSA J. 2013, 11, 3467–3546. [Google Scholar] [CrossRef]
- Ibragic, S.; Sofic, E. Chemical composition of various Ephedra species. Bosn. J. Basic Med. Sci. 2015, 15, 21–27. [Google Scholar] [CrossRef]
- Ephedra // Plantarium. Plants and Lichens of Russia and Neighboring Countries: Open Online Galleries and Plant Identification Guide. Available online: https://www.plantarium.ru/lang/en/page/view/item/44768.html (accessed on 11 June 2023).
- Dudareva, L.V.; Semenova, N.V.; Nochsorov, V.V.; Rudikovskaya, E.G.; Petrov, K.A. The component composition of the phytosterols of the aerial part of the horsetail variegated Equisétum variegatum Schleich. ex. Web. growing in North-East Yakutia. Khimiya Rastit. Syr’ya 2020, 2, 133–139. [Google Scholar] [CrossRef]
- Valitova, J.; Renkova, A.; Mukhitova, F.; Dmitrieva, S.; Beckett, R.P.; Minibayeva, F.V. Membrane sterols and genes of sterol biosynthesis are involved in the response of Triticum aestivum seedlings to cold stress. Plant Physiol. Biochem. 2019, 142, 452. [Google Scholar] [CrossRef]
- Xie, D.; Ye, J.; Lu, M.; Wang, S.; You, C.; Li, Y. Comparsion of Activities of Fatty Acyl Desaturases and Elongases among Six Teleosts with Different Feeding and Ecological Habits. Front. Mar. Sci. 2020, 7, 117. [Google Scholar] [CrossRef]
- Rozentsvet, O.A.; Kotlova, E.R.; Bogdanova, E.S.; Nesterov, V.N.; Senik, S.V.; Shavarda, A.L. Balance of Δ5-and Δ7-sterols and stanols in halophytes in connection with salinity tolerance. Phytochemistry 2022, 198, 113156. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant sterols: Diversity, biosynthesis, and physiological functions. Biochem. Mosc. 2016, 81, 819–834. [Google Scholar] [CrossRef]
- Senthil-Kumar, M.; Wang, K.; Mysore, K.S. AtCYP710A1 gene-mediated stigmasterol production plays a role in imparting temperature stress tolerance in Arabidopsis thaliana. Plant Signal. Behav. 2013, 8, e23142. [Google Scholar] [CrossRef]
- Zauber, H.; Burgos, A.; Garapati, P.; Schulze, W.X. Plasma membrane lipid-protein interactions affect signaling processes in sterol-biosynthesis mutants in Arabidopsis thaliana. Front. Plant Sci. 2014, 5, 78. [Google Scholar] [CrossRef]
- Ozolina, N.V.; Kapustina, I.S.; Gurina, V.V.; Nurminsky, V.N. Role of tonoplast microdomains in plant cell protection against osmotic stress. Planta 2022, 255, 65. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.-M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Vezza, T.; Canet, F.; de Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants 2020, 9, 1266. [Google Scholar] [CrossRef]
- Nokhsorov, V.V.; Senik, S.V.; Sofronova, V.E.; Kotlova, E.R.; Misharev, A.D.; Chirikova, N.K.; Dudareva, L.V. Role of Lipids of the Evergreen Shrub Ephedra monosperma in Adaptation to Low Temperature in the Cryolithozone. Plants 2023, 12, 15. [Google Scholar] [CrossRef]
- Alqarawi, A.A.; Hashem, A.; Abd Allah, E.F.; Alshahrani, T.S.; Huqail, A.A. Effect of salinity on moisture content, pigment system, and lipid composition in Ephedra alata Decne. Acta Biol. Hung. 2014, 65, 61–71. [Google Scholar] [CrossRef]
- Bondarenko, S.A.; Ianutsevich, E.A.; Danilova, O.A.; Grum-Grzhimaylo, A.A.; Kotlova, E.R.; Kamzolkina, O.V.; Bilanenko, E.N.; Tereshina, V.M. Membrane lipids and soluble sugars dynamics of the alkaliphilic fungus Sodiomyces tronii in response to ambient pH. Extremophiles 2017, 21, 743–754. [Google Scholar] [CrossRef]
- Wolff, R.L.; Christie, W.W.; Pedrono, F.; Marpeau, A.M.; Tsevegsüren, N.; Aitzetmüller, K.; Gunstone, F.D. Δ5-olefinic acids in the seed lipids from four Ephedra species and their distribution between the α and β positions of triacylglycerols. Characteristics common to coniferophytes and cycadophytes. Lipids 1999, 58, 101–115. [Google Scholar] [CrossRef]
- Nokhsorov, V.V.; Dudareva, L.V.; Petrov, K.A. Content and Composition of Lipids and Their Fatty Acids in Needles of Pinus sylvestris L. and Picea obovata Ledeb. upon Cold Hardening in the Cryolithozone of Yakutia. Russ. J. Plant Physiol. 2019, 66, 548–555. [Google Scholar] [CrossRef]
- Nokhsorov, V.V.; Dudareva, L.V.; Senik, S.V.; Chirikova, N.K.; Petrov, K.A. Influence of Extremely Low Temperatures of the Pole of Cold on the Lipid and Fatty-Acid Composition of Aerial Parts of the Horsetail Family (Equisetaceae). Plants 2021, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W.; Han, X. Lipid Analysis: Isolation, Separation, Identification and Lipidomic Analysis, 4th ed.; Woodhead Publishing: Bridgwater, UK, 2010; p. 448. [Google Scholar] [CrossRef]
- Christie, W.W. The AOCS Lipid Library: Methyl Esters of Fatty Acids—Archive of Mass Spectra. Available online: https://www.lipidmaps.org/resources/lipidweb/index.php?page=ms/methesters/me-arch/index.htm (accessed on 31 March 2023).
- Nes, W.D. Sterol methyltransferase: Enzymology and inhibition. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2000, 1529, 63–88. [Google Scholar] [CrossRef]
- Lyons, J.M.; Wheaton, T.A.; Pratt, H.K. Relationship between the Physical Nature of Mitochondrial Membranes and Chilling Sensitivity in Plants. Plant Physiol. 1964, 39, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Wismer, W.V.; Worthing, W.M.; Yada, R.Y.; Marangoni, A.G. Membrane lipid dynamics and lipid peroxidation in the early stages of low-temperature sweetening in tubers of Solanum tuberosum. Physiol. Plant. 1998, 102, 396–410. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahn, S.J.; Im, Y.J.; Cho, K.; Chung, G.C.; Cho, B.H.; Han, O. Differential impact of low temperature on fatty acid unsaturation and lipoxygenase activity in figleaf gourd and cucumber roots. Biochem. Biophys. Res. Commun. 2005, 330, 1194–1198. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V. Fatty Acids Composition of the Epiphytic Ferns, Platycerium bifurcatum and Asplenium nidus, and the Terrestrial Fern, Asplenium trichomanes. Am. Fern J. 2021, 111, 117–128. [Google Scholar] [CrossRef]
- O’Hare, T.J.; Trieu, H.H.; Topp, B.; Russell, D.; Pun, S.; Torrisi, C.; Liu, D. Assessing Fatty Acid Profiles of Macadamia Nuts. HortScience 2019, 54, 633–637. [Google Scholar] [CrossRef]
- Almeyda, M.D.; Scodelaro Bilbao, P.G.; Sanchez-Puerta, M.V.; Constenla, D.; Leonardi, P.I. Pavlova gyrans as a potential source of essential fatty acids, sterols and pigments: Culture under low temperature. J. Appl. Phycol. 2023, 35, 1073–1089. [Google Scholar] [CrossRef]
- Schaller, H. The role of sterols in plant growth and development. Prog. Lipid Res. 2003, 42, 163–175. [Google Scholar] [CrossRef]
- Ozolina, N.V.; Gurina, V.V.; Kapustina, I.S.; Spiridonova, E.V.; Nurminsky, V.N. Comparison of Changes in the Content of Plasma Membrane and Tonoplast Sterols under Oxidative and Osmotic Stress. Biochem. Mosc. Suppl. Ser. A 2023, 17, 180–182. [Google Scholar] [CrossRef]
- Ozolina, N.; Kapustina, I.; Gurina, V.; Spiridonova, E.; Nurminsky, V. The microdomains (rafts) of plasmalemma in the protection of the plant cell under oxidative stress. Protoplasma 2023, 1–10. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.; Luo, N.; Qu, R.; Guo, Z.; Lu, S. Cholesterol accumulation by suppression of SMT1 leads to dwarfism and improved drought tolerance in herbaceous plants. Plant Cell Environ. 2018, 41, 1417–1426. [Google Scholar] [CrossRef]
- Renkova, A.G.; Khabibrakhmanova, V.R.; Chasov, A.V.; Valitova, J.N.; Galeeva, E.I.; Minibayeva, F.V. Changes in Composition and Content of Lipophilic Compounds in the Seedlings of Triticum aestivum L. Treated with Stress Phytohormones. Russ. J. Plant Physiol. 2023, 70, 6. [Google Scholar] [CrossRef]
- Renkova, A.G.; Khabibrakhmanova, V.R.; Valitova, J.N.; Mukhitova, F.K.; Minibayeva, F.V. Effects of Stress Phytohormones on Sterol Metabolism of Triticum aestivum L. Russ. J. Plant Physiol. 2021, 68, 474–482. [Google Scholar] [CrossRef]
- Weng, X.C.; Wang, W. Antioxidant activity of compounds isolated from Salvia plebeian. Food Chem. 2000, 71, 489–493. [Google Scholar] [CrossRef]
- Wang, T.; Hicks, K.B.; Moreau, R. Antioxidant activity of phytosterols, oryzanol, and other phytosterol conjugates. J. Am. Oil Chem. Soc. 2002, 79, 1201–1206. [Google Scholar] [CrossRef]
- Vivancos, M.; Moreno, J.J. Beta-sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic. Biol. Med. 2005, 39, 91–97. [Google Scholar] [CrossRef]
- Pose, D.; Castanedo, I.; Borsani, O.; Nieto, B.; Rosado, A.; Taconnat, L.; Ferrer, A.; Dolan, L.; Valpuesta, V.; Botella, M.A. Identification of the Arabidopsis dry2/sqe1-5 mutant reveals a central role for sterols in drought tolerance and regulation of reactive oxygen species. Plant J. 2009, 59, 63–76. [Google Scholar] [CrossRef]
- Kendall, E.J.; McKersie, B.D. Free radical and freezing injury to cell membranes of winter wheat. Physiol. Plant. 1989, 76, 86–94. [Google Scholar] [CrossRef]
- Wegener, A.; Gimbel, W.; Werner, T.; Hani, J.; Ernst, D.; Sandermann, H., Jr. Molecular cloning of ozone-inducible protein from Pinus sylvestris L. with high sequence similarity to vertebrate 3-hydroxy-3-methylglutaryl-CoA-synthase. Biochim. Biophys. Acta Gene Struct. Expr. 1997, 1350, 247–252. [Google Scholar] [CrossRef]
- Dufourc, E.J. The role of phytosterols in plant adaptation to temperature. Plant Signal. Behav. 2008, 3, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Boutte, Y.; Grebe, M. Cellular processes relying on sterol function in plants. Curr. Opin. Plant Biol. 2009, 12, 705–713. [Google Scholar] [CrossRef]
- Deng, S.; Wei, T.; Tan, K.; Hu, M.; Li, F.; Zhai, Y.; Ye, S.; Xiao, Y.; Hou, L.; Pei, Y.; et al. Phytosterol content and the campesterol: Sitosterol ratio influence cotton fiber development: Role of phytosterols in cell elongation. Sci. China Life Sci. 2016, 59, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.; Han, B.; Forestier, E.; Hu, Z.; Gao, N.; Lu, W.; Shaller, H.; Li, J.; Hou, S. Sterols are required for cell fate commitment and maintenance of the stomatal lineage in Arabidopsis. Plant J. 2013, 74, 1029–1044. [Google Scholar] [CrossRef] [PubMed]
- Carland, F.M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Nelson, T. The identification of CVP1 reveals a role for sterols in vascular patterning. Plant Cell 2002, 14, 2045–2058. [Google Scholar] [CrossRef]
- Cunha, A.; Ferreira, M.F. Differences in free sterols content and composition associated with somatic embryogenesis, shoot organogenesis and calli growth of flax. Plant Sci. 1997, 124, 97–105. [Google Scholar] [CrossRef]
- Semenova, N.V.; Shmakov, V.N.; Konstantinov, Y.M.; Dudareva, L.V. Comparative Analysis of Sterol Composition of Embryogenic and Nonembryogenic Cell Lines of Larix sibirica Ledeb. Russ. J. Plant Physiol. 2023, 70, 181–191. [Google Scholar] [CrossRef]
- Cruz, R.P.D.; Golombieski, J.I.; Bazana, M.T.; Cabreira, C.; Silveira, T.F.; Silva, L.P.D. Alterations in fatty acid composition due to cold exposure at the vegetative stage in rice. Braz. J. Plant Physiol. 2010, 22, 199–207. [Google Scholar] [CrossRef]
- Voronkov, A.; Ivanova, T. Significance of Lipid Fatty Acid Composition for Resistance to Winter Conditions in Asplenium scolopendrium. Biology 2022, 11, 507. [Google Scholar] [CrossRef]
- Nejadsadeghi, L.; Maali-Amiri, R.; Zeinali, H.; Ramezanpour, S.; Sadeghzade, B. Membrane fatty acid compositions and cold-induced responses in tetraploid and hexaploid wheats. Mol. Biol. Rep. 2015, 42, 363–372. [Google Scholar] [CrossRef]
- Starikov, A.Y.; Sidorov, R.A.; Goriainov, S.V.; Los, D.A. Acyl-Lipid Δ6-Desaturase May Act as a First FAD in Cyanobacteria. Biomolecules 2022, 12, 1795. [Google Scholar] [CrossRef]
- Sato, N.; Wada, H. Lipid Biosynthesis and Its Regulation in Cyanobacteria. Lipids in Photosynthesis: Essential and Regulatory Functions; Wada, H., Murata, N., Eds.; Springer Science + Business Media B.V.: Amsterdam, The Netherlands, 2009; Volume 30, pp. 157–177. [Google Scholar] [CrossRef]

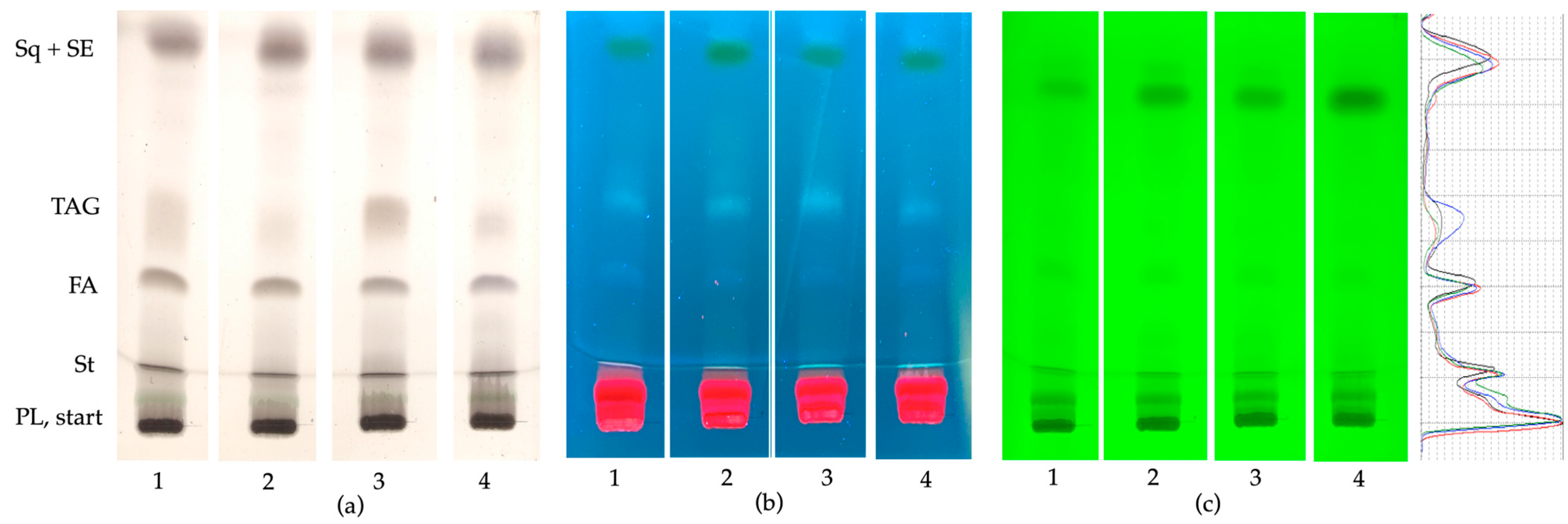
| Parameters | 12 July | 25 September | 30 October | 11 December |
|---|---|---|---|---|
| PDR | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.02 ± 0.00 a | 0.03 ± 0.01 a |
| SDR | 0.60 ± 0.18 a | 0.50 ± 0.16 a | 0.60 ± 0.13 a | 0.60 ± 0.13 a |
| ODR | 0.90 ± 0.09 a | 0.90 ± 0.13 a | 0.90 ± 0.13 a | 0.90 ± 0.07 a |
| LDR | 0.60 ± 0.04 a | 0.50 ± 0.03 b | 0.50 ± 0.03 b | 0.50 ± 0.04 b |
| C16 desaturation | 0.01 ± 0.00 a | 0.01 ± 0.00 a | *,a | 0.01 ± 0.00 a |
| C18 desaturation | 3.81 ± 0.24 a | 3.69 ± 0.18 a | 5.57 ± 0.31 b | 5.20 ± 0.22 b |
| C16 elongation | 2.90 ± 0.12 a | 3.40 ± 0.26 b | 3.99 ± 0.17 b | 3.78 ± 0.19 b |
| Sterol C-22 desaturase | 103.19 ± 10.3 a | 48.43 ± 5.8 b | 93.32 ± 7.6 a | 89.84 ± 8.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nokhsorov, V.V.; Dudareva, L.V.; Semenova, N.V.; Sofronova, V.E. The Composition and the Content of ∆-5 Sterols, Fatty Acids, and the Activity of Acyl-Lipid Desaturases in the Shoots of Ephedra monosperma, Introduced in the Botanical Garden of the Cryolithozone of Yakutia. Horticulturae 2023, 9, 858. https://doi.org/10.3390/horticulturae9080858
Nokhsorov VV, Dudareva LV, Semenova NV, Sofronova VE. The Composition and the Content of ∆-5 Sterols, Fatty Acids, and the Activity of Acyl-Lipid Desaturases in the Shoots of Ephedra monosperma, Introduced in the Botanical Garden of the Cryolithozone of Yakutia. Horticulturae. 2023; 9(8):858. https://doi.org/10.3390/horticulturae9080858
Chicago/Turabian StyleNokhsorov, Vasiliy V., Luybov V. Dudareva, Natalia V. Semenova, and Valentina E. Sofronova. 2023. "The Composition and the Content of ∆-5 Sterols, Fatty Acids, and the Activity of Acyl-Lipid Desaturases in the Shoots of Ephedra monosperma, Introduced in the Botanical Garden of the Cryolithozone of Yakutia" Horticulturae 9, no. 8: 858. https://doi.org/10.3390/horticulturae9080858
APA StyleNokhsorov, V. V., Dudareva, L. V., Semenova, N. V., & Sofronova, V. E. (2023). The Composition and the Content of ∆-5 Sterols, Fatty Acids, and the Activity of Acyl-Lipid Desaturases in the Shoots of Ephedra monosperma, Introduced in the Botanical Garden of the Cryolithozone of Yakutia. Horticulturae, 9(8), 858. https://doi.org/10.3390/horticulturae9080858






