Alternations in Physiological and Phytochemical Parameters of German Chamomile (Matricaria chamomilla L.) Varieties in Response to Amino Acid Fertilizer and Plasma Activated-Water Treatments
Abstract
1. Introduction
2. Material and Methods
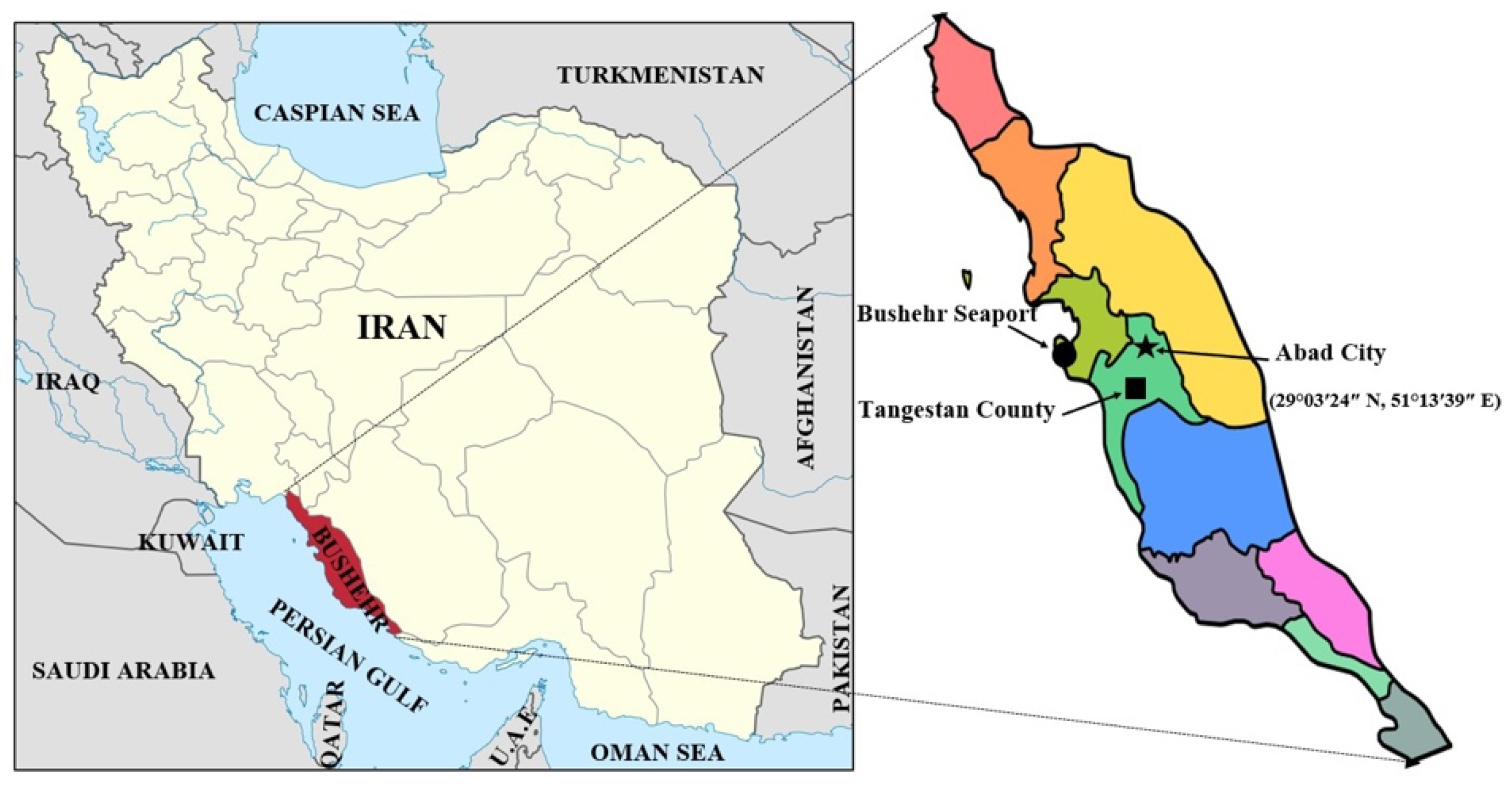
2.1. Field Experiment Description
2.2. Experimental Design
2.3. Field Management
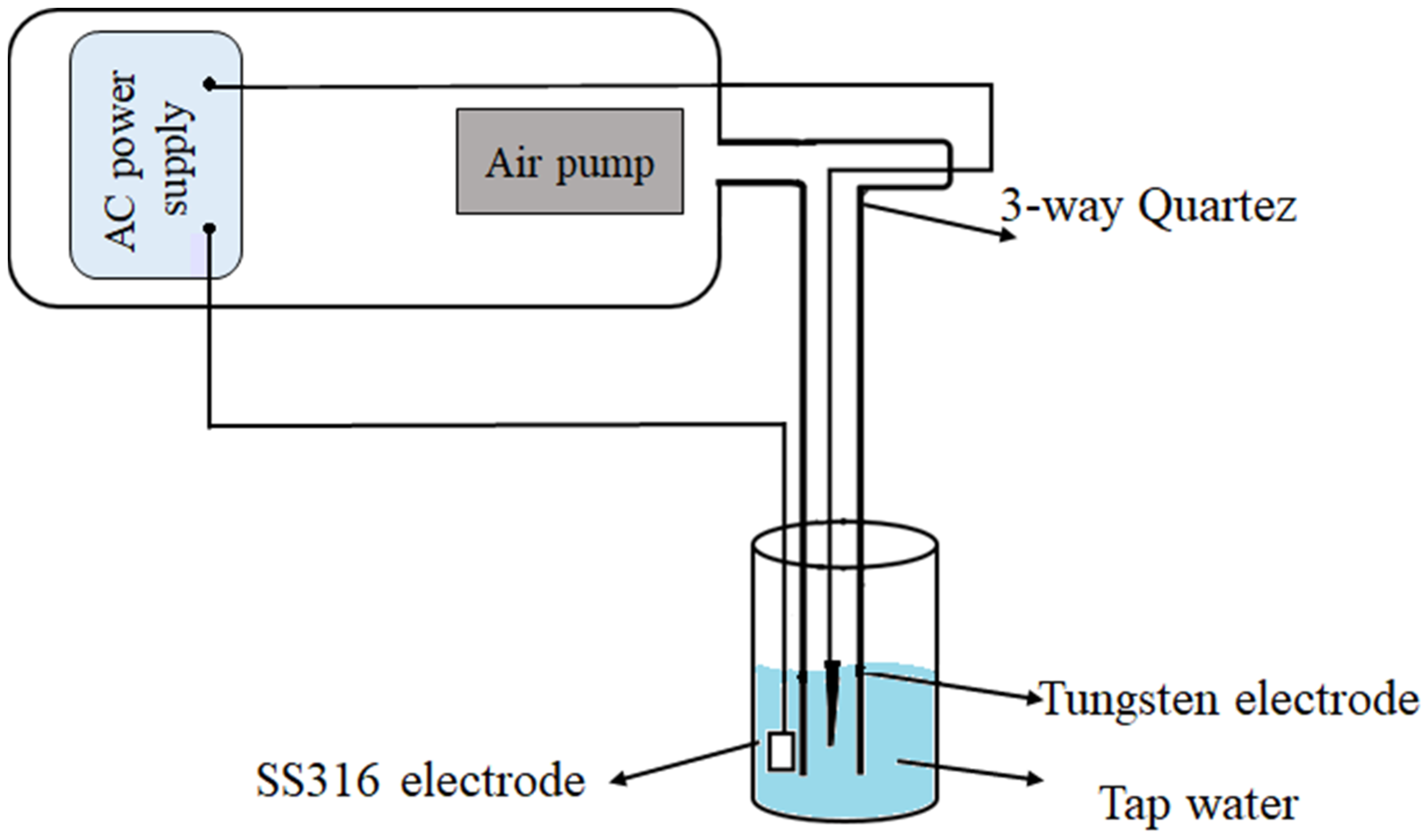
2.4. Preparation of Plasma Activated Water (PAW)
2.5. Application of Amino Acid Fertilizer and Plasma Activated Water Treatments
2.6. Physiological Characteristics
2.7. Leaf Chlorophyll a, b, and Carotenoids Contents
2.8. Elemental Analysis and Carbon-to-Nitrogen Ratio
2.9. Amino Acids Content Measurement
2.10. Flower Harvesting and Oil Extraction
2.11. Chamomile Oil Analysis
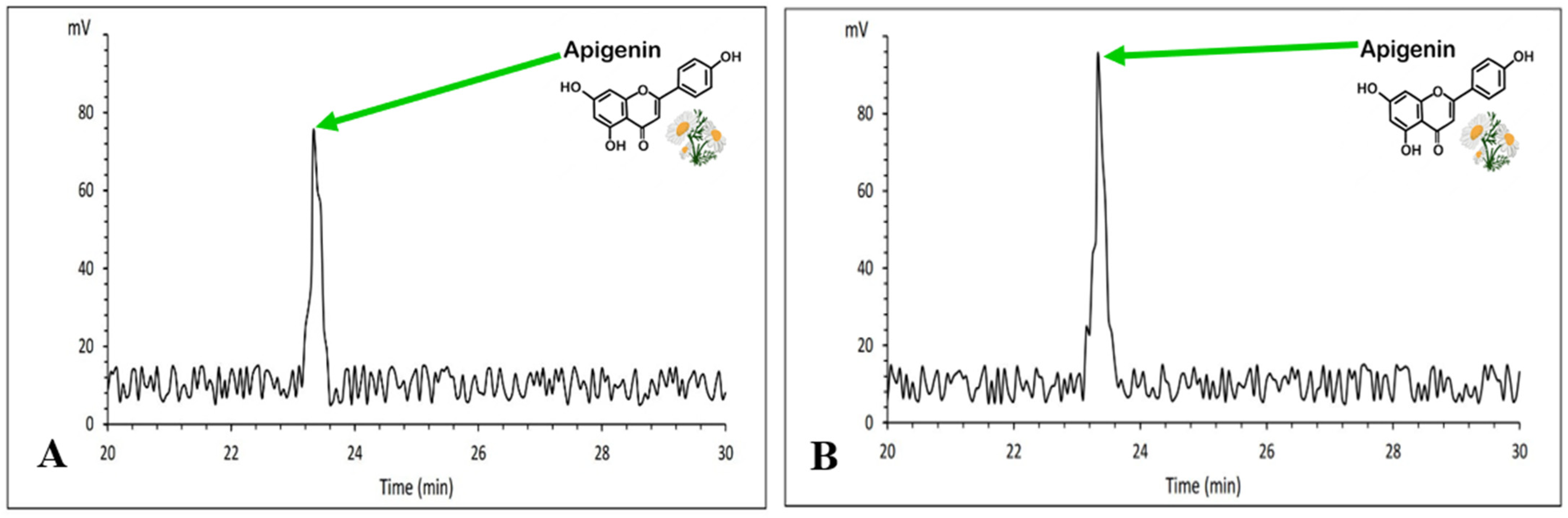
2.12. Determination of Total Apigenin Content
2.13. Statistical Analysis
3. Results
3.1. Physiological Characteristics
3.2. Chlorophyll a, b, and Carotenoids Content
3.3. Carbon, Hydrogen, and Nitrogen Percentage
3.4. C: N Ratio and Total Protein Content
3.5. Essential Oil Yield
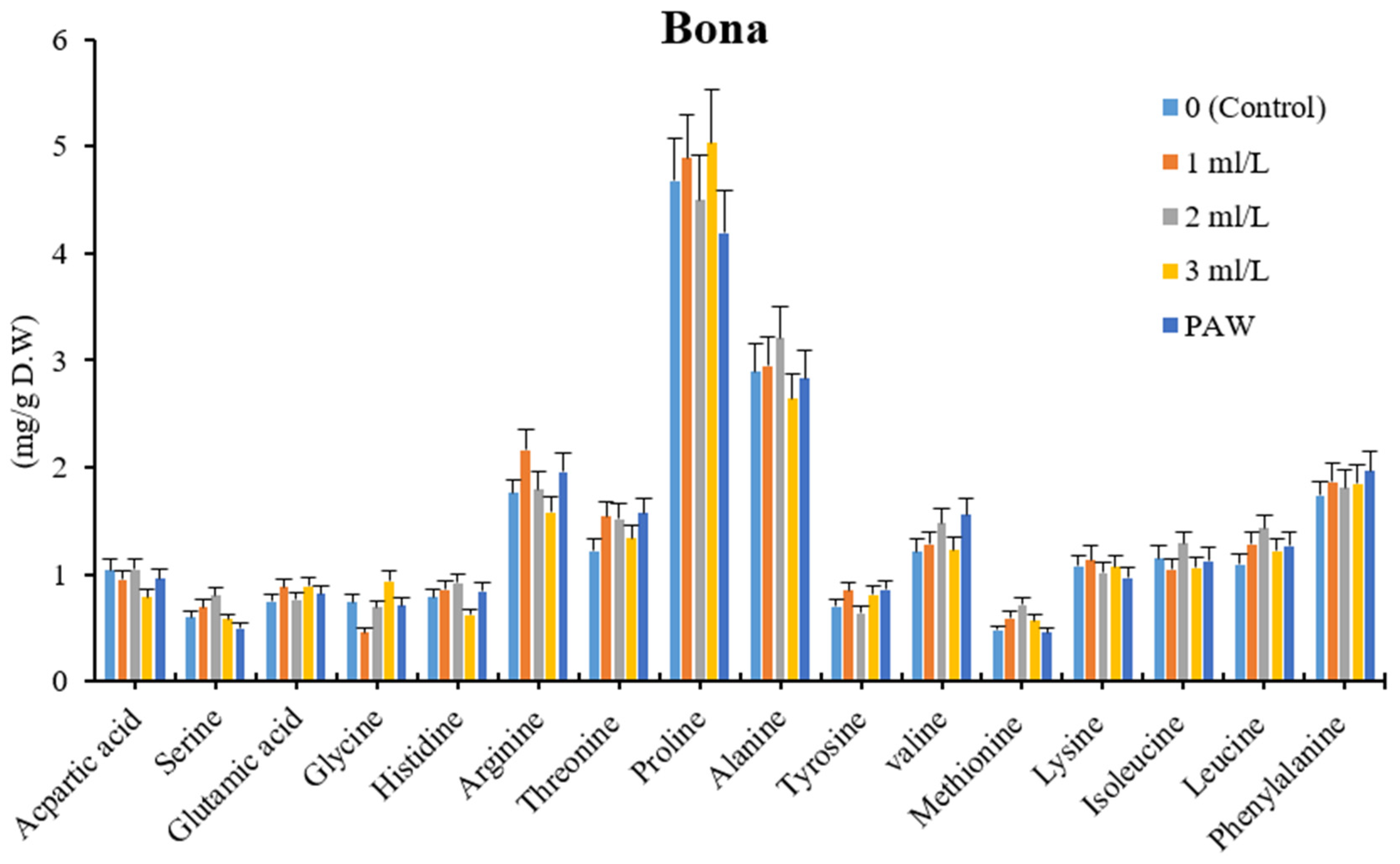
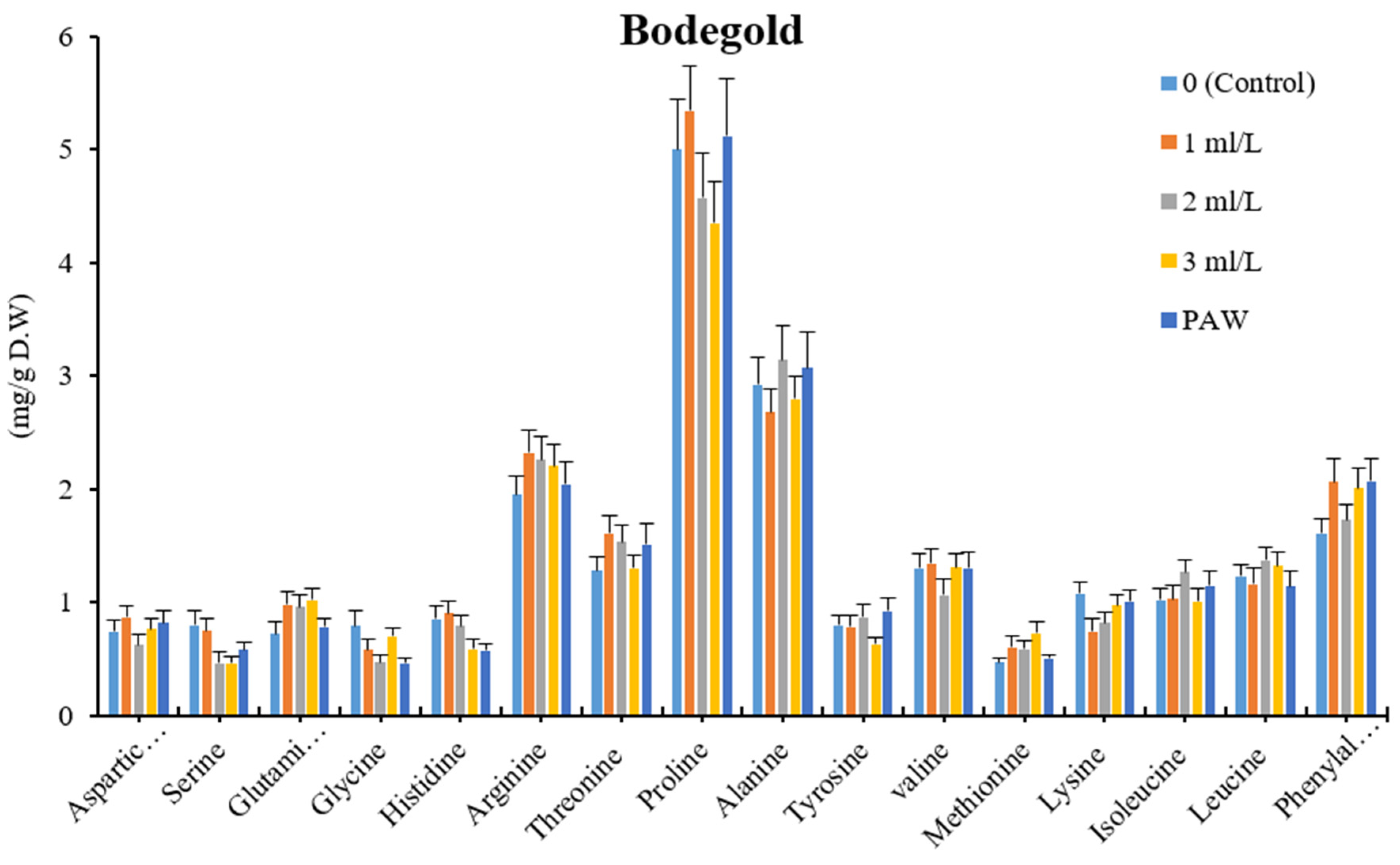
3.6. Amino Acids Profile Analysis
3.7. Secondary Metabolite Profile Analysis
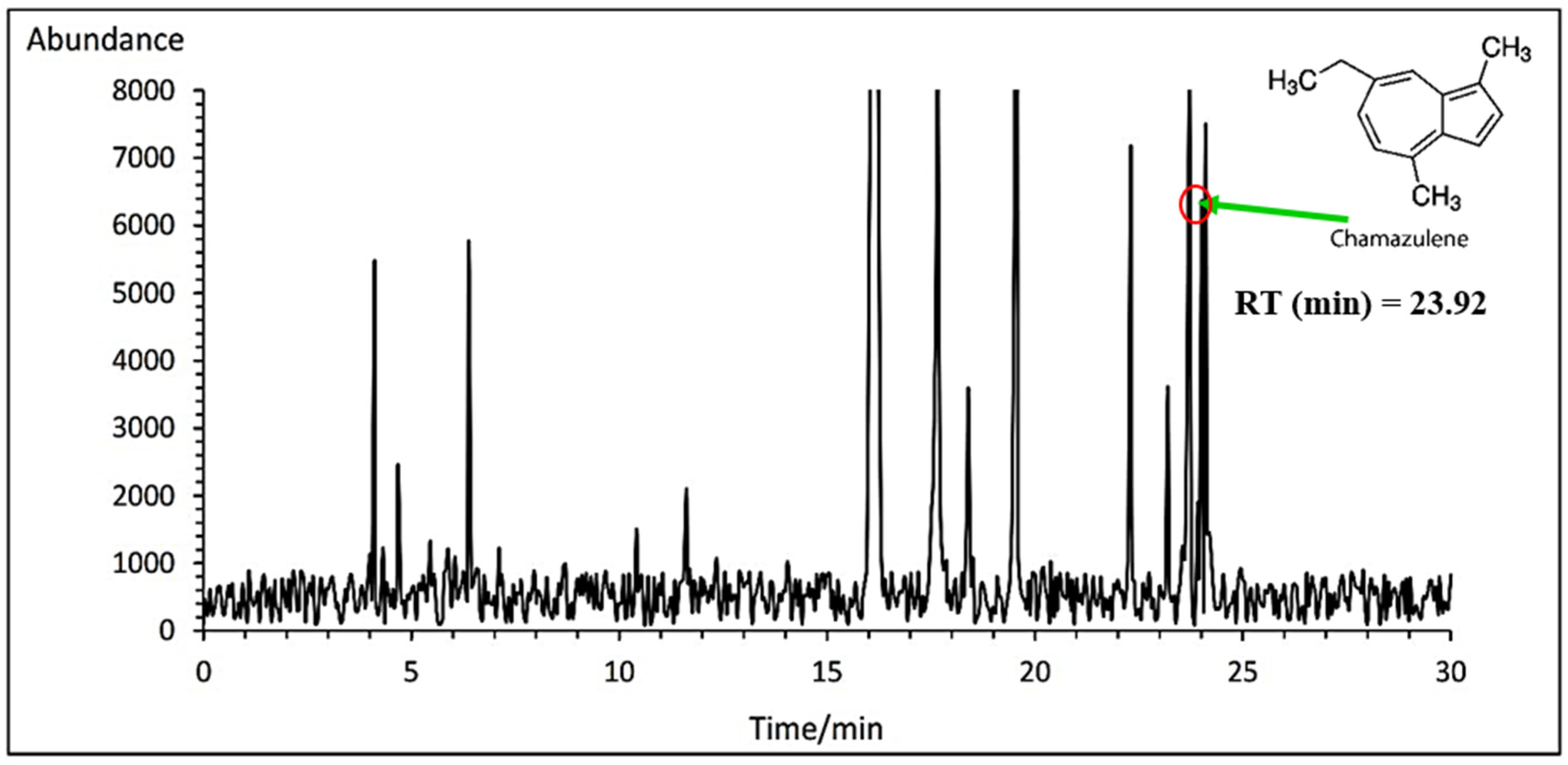
3.7.1. Chamazulene
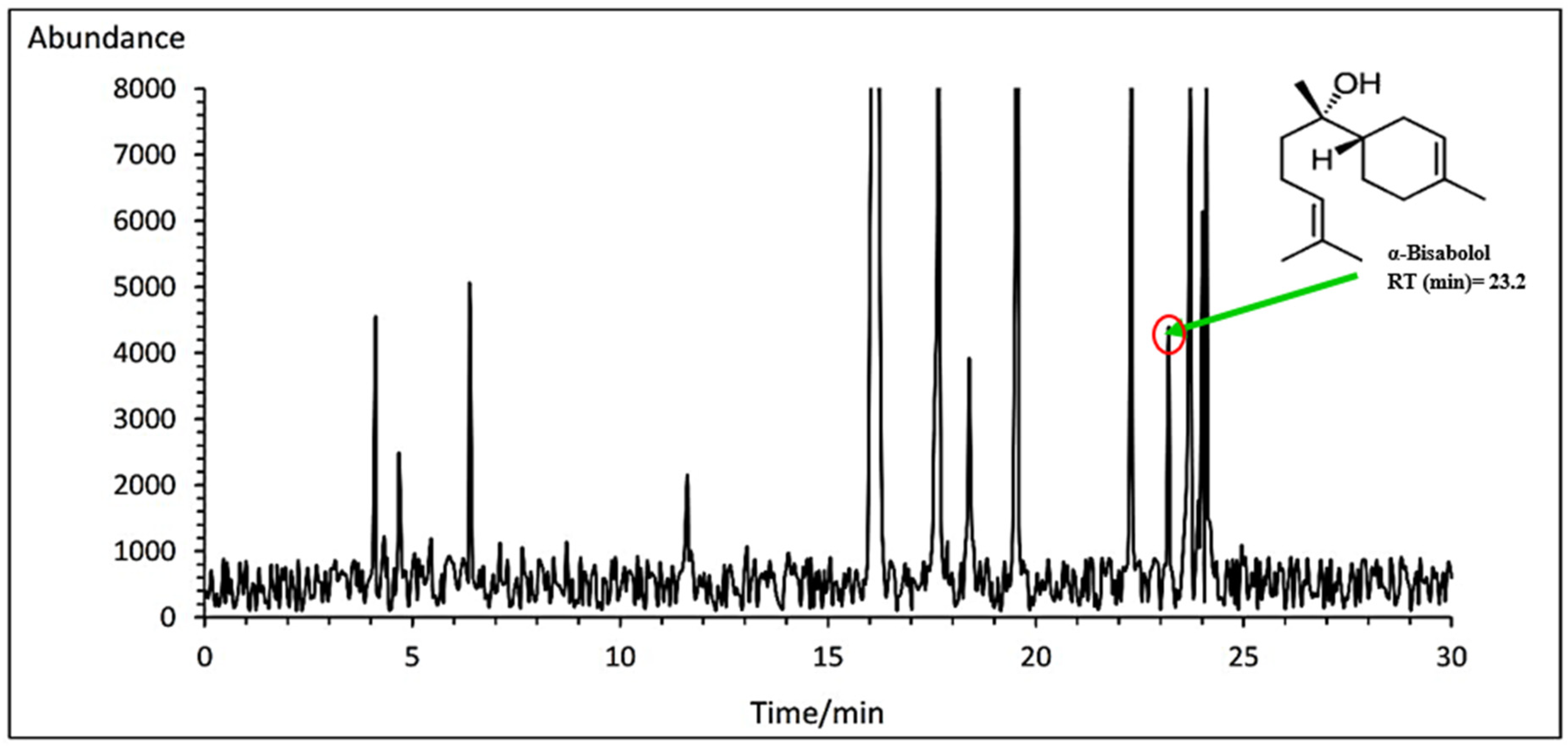
3.7.2. α- Bisabolol
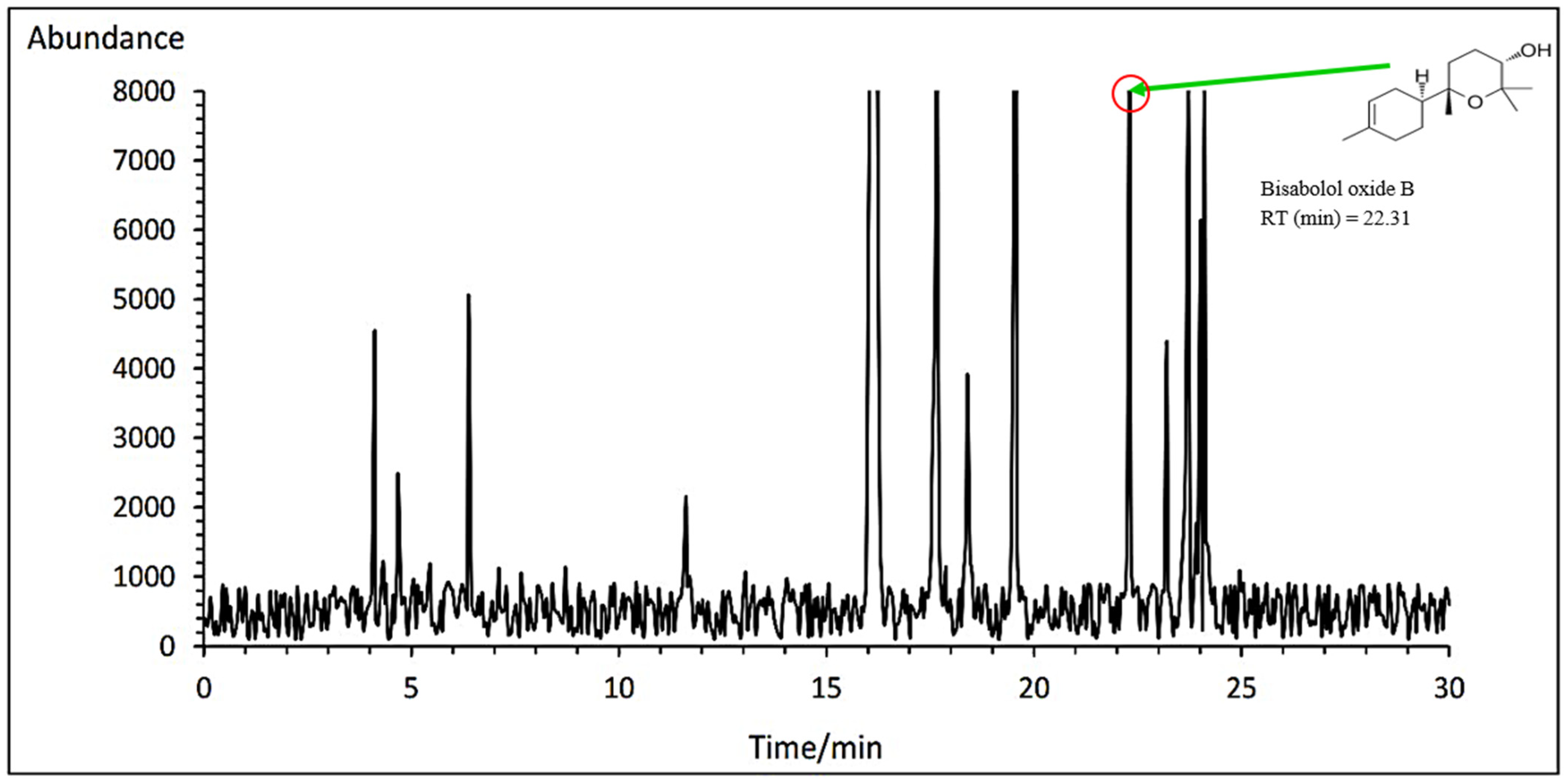
3.7.3. Bisabolol Oxide A and B
3.7.4. Bisabolone Oxide A
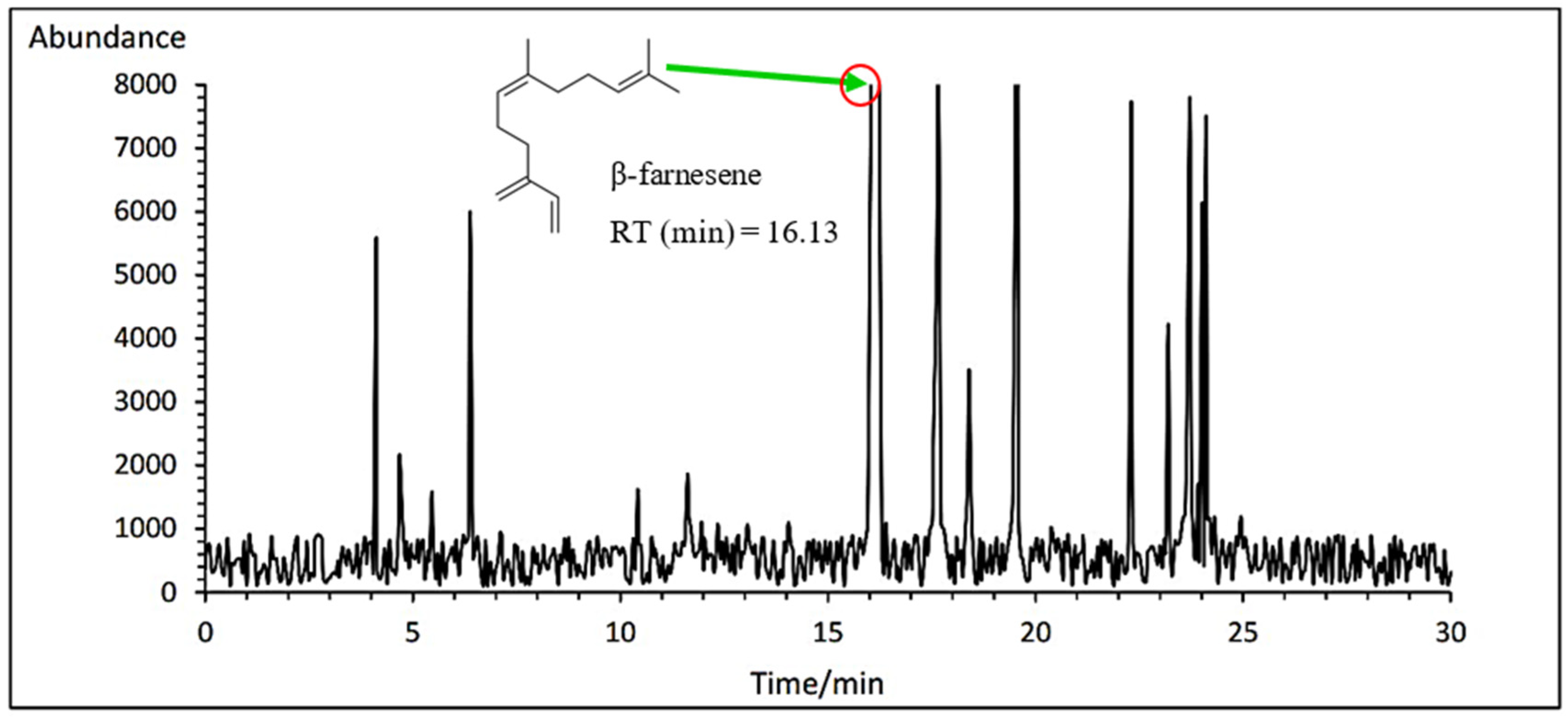
3.7.5. α- and β- Farnesene
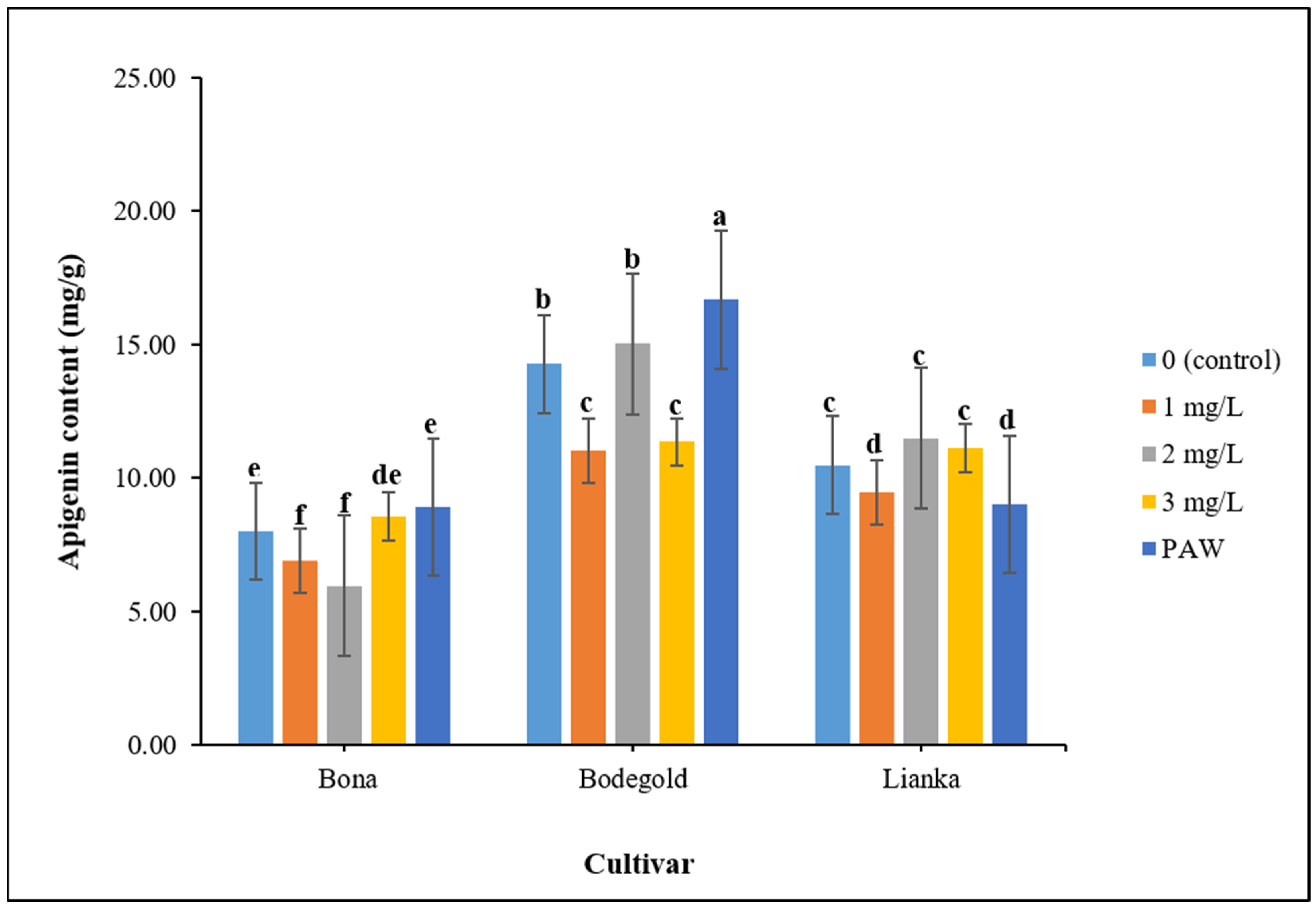
3.8. Apigenin Content Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghasemi, M.; Modarresi, M.; Babaeian Jelodar, N.; Bagheri, N.; Jamali, A. The Evaluation of Exogenous Application of Salicylic Acid on Physiological Characteristics, Proline and Essential Oil Content of Chamomile (Matricaria chamomila L.) under Normal and Heat Stress Conditions. Agriculture 2016, 6, 31. [Google Scholar] [CrossRef]
- Salamon, I.; Ibraliu, A.; Kryvtsova, M. Essential Oil Content and Composition of the Chamomile Inflorescences (Matricaria recutita L.) Belonging to Central Albania. Horticulturae 2023, 9, 47. [Google Scholar] [CrossRef]
- Ghasemi, M.; Jelodar, N.B.; Modarresi, M.; Bagheri, N.; Jamali, A. Increase of chamazulene and α-bisabolol contents of the essential oil of german chamomile (Matricaria chamomilla L.) using salicylic acid treatments under normal and heat stress conditions. Foods 2016, 5, 56. [Google Scholar] [CrossRef]
- Shams-ardakani, M.; Ghannadi, A.; Rahimzadeh, A.; Asteraceae, L.; Garden, B.; Matricaria, L. Volatile Constituents of Matricaria chamomilla L. from Isfahan, Iran traditionally have been used for the treatment macological effects of chamomile are mainly connected with its essential oil for its antispasmodic, antimicrobial and disinfective and a. Analysis 2006, 2, 57–60. [Google Scholar]
- Zhuri, N.; Gixhari, B.; Ibraliu, A. Variability of chamomile (Matricaria chamomilla) populations as a valuable medicinal plant in Albania evaluated by morphological traits Documentation of Plant Genetic Resources and quality of characterization and evaluation data recorded in genebank datab. Albanian J. Agric. Sci. 2019, 4, 83–89. Available online: https://www.researchgate.net/publication/333995481 (accessed on 5 June 2023).
- Šalamon, I. Effect of the internal and external factors on yield and qualitative quantitative characteristics of chamomile essential oil. Acta Hortic. 2007, 749, 45–64. [Google Scholar] [CrossRef]
- Rafieiolhossaini, M.; Sodaeizadeh, H.; Adams, A.; De Kimpe, N.; Van Damme, P. Effects of planting date and seedling age on agro-morphological characteristics, essential oil content and composition of German chamomile (Matricaria chamomilla L.) grown in Belgium. Ind. Crops Prod. 2010, 31, 145–152. [Google Scholar]
- Baghalian, K.; Abdoshah, S.; Khalighi-Sigaroodi, F.; Paknejad, F. Physiological and phytochemical response to drought stress of German chamomile (Matricaria recutita L.). Plant Physiol. Biochem. PPB 2011, 49, 201–207. [Google Scholar] [CrossRef]
- Baghalian, K.; Haghiry, A.; Naghavi, M.R.; Mohammadi, A. Effect of saline irrigation water on agronomical and phytochemical characters of chamomile (Matricaria recutita L.). Sci. Hortic. 2008, 116, 437–441. [Google Scholar] [CrossRef]
- Sharafzadeh, S.; Alizadeh, O. German and roman chamomile. J. Appl. Pharm. Sci. 2011, 1, 1–5. [Google Scholar]
- Salamon, I. Chamomile Biodiversity of the Essential Oil Qualitative-Quantitative Characteristics; Springer Press: Berlin/Heidelberg, Germany, 2009; pp. 83–90. [Google Scholar]
- Sashidhara, K.V.; Verma, S.R.; Ram, P. Essential oil composition of Matricaria recotita L. from the lower region of the Himalayas. Flavor Fragr. J. 2006, 21, 274–276. [Google Scholar] [CrossRef]
- Omer, E.A.; Ahl, H.A.H.S.-A.; El Gendy, A.E.G.; Hussein, M.S. Effect of Amino Acids Application on Production, Volatile Oil and Chemical. J. Appl. Sci. Res. 2013, 9, 3006–3021. [Google Scholar]
- Nahed, G.; Abdel Aziz, A.; Mazher, A.M.; Farahat, M.M. Response of Vegetative Growth and Chemical Constituents of Thuja orientalis L. Plant to Foliar Application of Different Amino Acids at Nubaria. J. Am. Sci. 2010, 6, 295–301. [Google Scholar]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef]
- Velička, A.; Tarasevičienė, Ž.; Hallmann, E.; Kieltyka-Dadasiewicz, A. Impact of Foliar Application of Amino Acids on Essential Oil Content, Odor Profile, and Flavonoid Content of Different Mint Varieties in Field Conditions. Plants 2022, 11, 2938. [Google Scholar] [CrossRef]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2018, 15, 1700174. [Google Scholar] [CrossRef]
- Takashima, K.; Nor, A.S.B.A.; Ando, S.; Takahashi, H.; Kaneko, T. Evaluation of plant stress due to plasma-generated reactive oxygen and nitrogen species using electrolyte leakage. Jpn. J. Appl. Phys. 2021, 60, 010504. [Google Scholar] [CrossRef]
- Stoleru, V.; Burlica, R.; Mihalache, G.; Dirlau, D.; Padureanu, S.; Teliban, G.-C.; Astanei, D.; Cojocaru, A.; Beniuga, O.; Patras, A. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci. Rep. 2020, 10, 20920. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Annor, G.A. Potential of Cold Plasma Technology in Ensuring the Safety of Foods and Agricultural Produce: A Review. Foods 2020, 9, 1435. [Google Scholar] [CrossRef]
- Sajib, S.A.; Billah, M.; Mahmud, S.; Miah, M.; Hossain, F.; Omar, F.B.; Roy, N.C.; Hoque, K.M.F.; Talukder, M.R.; Kabir, A.H.; et al. Plasma activated water: The next generation eco-friendly stimulant for enhancing plant seed germination, vigor and increased enzyme activity, a study on black gram (Vigna mungo L.). Plasma Chem. Plasma Process. 2020, 40, 119–143. [Google Scholar] [CrossRef]
- Feng, X.; Ma, X.; Liu, H.; Xie, J.; He, C.; Fan, R. Argon plasma effects on maize: Pesticide degradation and quality changes. J. Sci. Food Agric. 2019, 99, 5491–5498. [Google Scholar] [CrossRef] [PubMed]
- Sayed, W.A.A.; Hassan, R.S.; Sileem, T.M.; Rumpold, B.A. Impact of plasma irradiation on Tribolium castaneum. J. Pest Sci. 2021, 94, 1405–1414. [Google Scholar] [CrossRef]
- Li, H. Measurements of Electron Energy Distribution Function and Neutral Gas Temperature in an Inductively Coupled Plasma. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2006. [Google Scholar]
- Chen, T.; Liang, J.; Su, T. Plasma-activated water: Antibacterial activity and artifacts? Environ. Sci. Pollut. Res. Int. 2018, 25, 26699–26706. [Google Scholar] [CrossRef] [PubMed]
- Škarpa, P.; Klofáč, D.; Krčma, F.; Šimečková, J.; Kozáková, Z. Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea mays L.). Water 2020, 12, 3545. [Google Scholar] [CrossRef]
- Mandici, A.; Cretu, D.E.; Burlica, R.; Astanei, D.; Beniuga, O.; Rosu, C.; Topa, D.C.; Aostacioaei, T.G.; Aprotosoaie, A.C.; Miron, A. Preliminary Study on the Impact of Non-Thermal Plasma Activated Water on the Quality of Triticum aestivum L. cv. Glosa Sprouts. Horticulturae 2022, 8, 1158. [Google Scholar] [CrossRef]
- Yan D, Lin L, Zvansky M, Kohanzadeh L, Taban S, Chriqui S, et al. Improving Seed Germination by Cold Atmospheric Plasma. Plasma. 2022, 5, 98–110.
- Soni, A.; Choi, J.; Brightwell, G. Plasma-activated water (PAW) as a disinfection technology for bacterial inactivation with a focus on fruit and vegetables. Foods 2021, 10, 166. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Punith, N.; Harsha, R.; Lakshminarayana, R.; Hemanth, M.; Anand, M.S.; Dasappa, S. Plasma Activated Water Generation and its Application in Agriculture. Adv. Mater. Lett. 2019, 10, 700–704. [Google Scholar] [CrossRef]
- Tsoukou, E.; Bourke, P.; Boehm, D. Temperature stability and effectiveness of plasma-activated liquids over an 18 months period. Water 2020, 12, 3021. [Google Scholar] [CrossRef]
- Lin, C.M.; Chu, Y.C.; Hsiao, C.P.; Wu, J.S.; Hsieh, C.W.; Hou, C.Y. The optimization of plasma-activated water treatments to inactivate Salmonella enteritidis (ATCC 13076) on shell eggs. Foods 2019, 8, 520. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzym. 1987, 148, 350–382. [Google Scholar]
- Gill, S.S.; Willette, S.; Dungan, B.; Jarvis, J.M.; Schaub, T.; Van Leeuwen, D.M.; St Hilaire, R.; Omar Holguin, F. Suboptimal Temperature Acclimation Affects Kennedy Pathway Gene Expression, Lipidome and Metabolite Profile of Nannochloropsis salina during PUFA Enriched TAG Synthesis. Mar. Drugs 2018, 16, 425. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, D.; Li, X.; Meng, L. Chromatographic method for determination of the free amino acid content of chamomile flowers. Pharmacogn. Mag. 2015, 11, 176–179. [Google Scholar] [CrossRef]
- Šalamon, I. The Slovak gene pool of German chamomile (Matricaria recutita L.) and comparison in its parameters. Hortic. Sci. 2004, 31, 70–75. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, Ľ.; Hensel, K. Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process. Polym. 2019, 16, 1800131. [Google Scholar] [CrossRef]
- D’Andrea, L. Yield and Essential Oil Components in Common Chamomile (Chamomilla recutita (L.) Rauschert) Cultivars Grown in Southern Italy. J. Herbs Spices. Med. Plants 2002, 9, 359–365. [Google Scholar] [CrossRef]
- Circella, G.; De Mastro, G.; Nano, G.M.; D’Andrea, L. Comparison of Chamomile Biotypes (chamomilla recutal. Rauschert). In Proceedings of the WOCMAP I-Medicinal and Aromatic Plants Conference, Maastricht, The Netherlands, 1 April 1993; ISHS Acta Horticulturae: Leuven, Belgium, 1993. [Google Scholar]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Fait, A.; Nesi, A.N.; Angelovici, R.; Lehmann, M.; Pham, P.A.; Song, L.; Haslam, R.P.; Napier, J.A.; Galili, G.; Fernie, A.R. Targeted enhancement of glutamate-to-γ-aminobutyrate conversion in Arabidopsis seeds affects carbon-nitrogen balance and storage reserves in a development-dependent manner. Plant Physiol. 2011, 157, 1026–1042. [Google Scholar] [CrossRef]
- Hu, W.; Coomer, T.D.; Loka, D.A.; Oosterhuis, D.M.; Zhou, Z. Potassium deficiency affects the carbon-nitrogen balance in cotton leaves. Plant Physiol. Biochem. 2017, 115, 408–417. [Google Scholar] [CrossRef]
- Lo Porto, C.; Ziuzina, D.; Los, A.; Boehm, D.; Palumbo, F.; Favia, P.; Tiwari, B.; Bourke, P.; Cullen, P.J. Plasma activated water and airborne ultrasound treatments for enhanced germination and growth of soybean. Innov. Food Sci. Emerg. Technol. 2018, 49, 13–19. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, J.H.; Sun, D.W. Enhancement of Wheat Seed Germination, Seedling Growth and Nutritional Properties of Wheat Plantlet Juice by Plasma Activated Water. J. Plant Growth Regul. 2022, 42, 2006–2022. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Chapman, C.; Yuan, B.; Huang, B. Improved heat tolerance in creeping bentgrass by γ-aminobutyric acid, proline, and inorganic nitrogen associated with differential regulation of amino acid metabolism. Plant Growth Regul. 2021, 93, 231–242. [Google Scholar] [CrossRef]
- Rossi, S.; Chapman, C.; Huang, B. Suppression of heat-induced leaf senescence by γ-aminobutyric acid, proline, and ammonium nitrate through regulation of chlorophyll degradation in creeping bentgrass. Environ. Exp. Bot. 2020, 177, 104116. Available online: https://www.sciencedirect.com/science/article/pii/S0098847220301428 (accessed on 25 January 2020). [CrossRef]
- Abd El-Gawad, H.G.; Mukherjee, S.; Farag, R.; Abd Elbar, O.H.; Hikal, M.; Abou El-Yazied, A.; Abd Elhady, S.A.; Helal, N.; ElKelish, A.; El Nahhas, N.; et al. Exogenous γ-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L. Plant Signal. Behav. 2021, 16, 1853384. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Rowshan, V.; Khoi, M.K.; Javidnia, K. Effects of Salicylic Acid on Quality and Quantity of Essential oil Components in Salvia macrosiphon. J. Biol. Environ. Sci. 2010, 4, 77–82. [Google Scholar]
- Honcariv, R.; Repcak, M. Chemotypes of Matricaria chamomilla L. Herba Pol. 1979, 25, 261–267. [Google Scholar]
- Mann, C.; Staba, E. The Chemistry, Pharmacology, Commercial Formulation of Chamomile. Herbs Spices Med. Plants 1975, 1, 235–279. [Google Scholar]
- Sadeghia, F.; Hadian, J.; Hadavi, M.; Mohamadi, A.; Ghorbanpour, M.; Ghafarzadegan, R. Effects of Exogenous Salicylic Acid Application on Growth, Metabolic Activities and Essential Oil Composition of Satureja khuzistanica Jamzad. J. Med. Plants 2013, 12, 70–82. [Google Scholar]
- Galambosi, B.; Repcok, M. Varition The Yield and essential oil of four chamomile varieties grown in Finland in 1985–1988. J. Agric. 1991, 63, 403–410. [Google Scholar]
- Letchamo, W. A Comparative Study of Chamomile yield Essential oil Flavonoides Content under two sowing season and nitrogen levels. Herbs Spices Med. Plants 1992, 2, 342–352. [Google Scholar]
- Kwiatkowski, C.A. Yield and quality of chamomile (Chamomilla recutita (L.) Rausch.) raw material depending on selected foliar sprays and plant spacing. Acta Sci. Pol. Hortorum Cultus 2015, 14, 143–156. [Google Scholar]
- Dixon, R.; Paiva, N. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Nikolova, M.T.; Ivancheva, S.V. Quantitative flavonoid variations of Artemisia vulgaris L. and Veronica chamaedrys L. in relation to altitude and polluted environment. Acta Biol. Szeged. 2005, 49, 29–32. [Google Scholar]



| Evaporation (mm) | Average of Sunny Hours (h) | Precipitation (mm) | Average of Relative Humidity (%) | Average of Temperature (°C) | Month and Year | ||
|---|---|---|---|---|---|---|---|
| Max | Min | Max | Min | ||||
| 5.4 | 7.5 | 39.3 | 62 | 26 | 30.8 | 17.9 | November 2020 |
| 2.8 | 7.9 | 62.4 | 83 | 46 | 21.7 | 10.6 | December 2020 |
| 2.9 | 6.8 | 32.2 | 77 | 44 | 22.4 | 10.8 | January 2021 |
| 3.5 | 5.6 | 33.9 | 76 | 38 | 21.6 | 10.5 | February 2021 |
| 5.3 | 6.9 | 22.2 | 68 | 28 | 24.1 | 11.3 | March 2021 |
| 8.9 | 6.7 | 10.5 | 65 | 22 | 32.2 | 19.1 | April 2021 |
| Mn (ppm) | Cu (ppm) | Zn (ppm) | Fe (ppm) | K (ppm) | P (ppm) | N (%) | O.C (%) | T.N.V (%) | pH | SP (%) | EC (ds·m−1) | Texture | Soil Depth (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.3 | 0.74 | 2 | 3.14 | 124 | 0 | 0.02 | 0.23 | 52.5 | 7.5 | 51 | 10.68 | Sandy loam | 0–30 |
| SAR | TDS | TH | Alkalinity | Na+ | Ca2+ + Mg2+ | SO4− | Cl− | CO3 | HCO3− | pH | EC (ds m−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| meq L−1 | |||||||||||
| 2.22 | 4467.2 | 2850 | 240 | 11.9 | 57 | 40.6 | 24 | 0 | 4 | 6.78 | 6.98 |
| Water Sample | pH | Nitrate mg/kg | Nitrite μg/kg |
|---|---|---|---|
| Untreated water | 7.58 | 6.16 ± 0.31 | 10.12 ± 0.21 |
| 30 min treated water | 3.16 | 45.92 ± 1.33 | 52.32 ± 1.3 |
| Compositions | Content (%) |
|---|---|
| Total Nitrogen (water-soluble organic nitrogen) | 4.50 |
| A phosphate (P2O5) | 0.50 |
| Soluble Potash (K2O) | 2.90 |
| Chelated Iron (Fe) | 0.10 |
| Total Amino Acid | 22.70 |
| Total Carbon | 11.70 |
| Times of Spraying | Growth Stage | Date of Spraying |
|---|---|---|
| 1 | 30 days after planting | 15 December 2020 |
| 2 | Vegetative phase | 15 January 2020 |
| 3 | Flowering phase | 15 February 2020 |
| Car (μg/g dw) | Chl b (mg/g dw) | Chl a (mg/g dw) | Dried Flower Weight (g m−2) | Fresh Flower Weight (g m−2) | Plant Height (cm) | Fertilizer | Cultivar |
|---|---|---|---|---|---|---|---|
| 701.08 g,h | 2.22 b | 5.06 a,b | 31.1 e,f | 124.00 g | 40.70 b,c,d | 0 | Bona |
| 598.89 g,h | 2.15 b | 4.96 b | 36.51 c | 146.00 c | 42.50 b,c | 1 | |
| 479.52 h | 2.19 b | 4.99 a,b | 33.15 d | 132.00 e,f | 54.67 a | 2 | |
| 810.64 f,g | 1.03 d | 5.18 a | 38.68 b | 154.70 b | 43.50 b | 3 | |
| 1010.18 e,f | 0.99 d | 4.99 a,b | 33.56 d | 134.00 e | 54.50 a | PAW | |
| 3832.02 b | 1.01 d | 3.82 d | 23.57 i | 99.00 i | 35.33 e,f | 0 | Bodegold |
| 3699.03 b | 2.55 a | 3.99 c,d | 31.74 e | 133.30 e | 37.80 d,e | 1 | |
| 3388.64 c | 2.57 a | 4.00 c,d | 21.3 j | 90.00 j | 31.27 g | 2 | |
| 3352.85 c | 2.54 a | 4.12 c | 27.1 h | 114.60 h | 31.00 g | 3 | |
| 4656.06 a | 1.14 c | 2.61 f | 30.7 f | 130.00 f | 32.60 f,g | PAW | |
| 1187.35 e | 1.15 c | 3.57 e | 36.24 c | 154.00 b | 43.00 b | 0 | Lianka |
| 1770.47 d | 1.14 c | 3.59 e | 41.91 a | 177.67 a | 41.80 b,c | 1 | |
| 1893.44 d | 1.03 d | 3.43 e | 33.42 d | 142.00 d | 32.70 f,g | 2 | |
| 1940.15 d | 1.02 d | 3.41 e | 29.65 g | 126.00 g | 38.67 c,d,e | 3 | |
| 1121.58 e | 1.03 d | 3.83 d | 41.92 a | 178.00 a | 40.07 b,c,d | PAW | |
| EO Yield (%w/w) | Total Protein (%) | C:N Ratio | N (%) | H (%) | C (%) | Fertilizer | Cultivar |
| 0.93 c,d | 46.08 f | 7.62 h | 9.65 d | 5.75 c,d | 73.44 a | 0 | Bona |
| 0.69 g | 39.87 g | 8.22 g | 8.34 e,f | 6.04 b,c | 68.58 c,d | 1 | |
| 0.80 e | 32.03 j | 9.01 e,f | 6.63 h | 4.61 f,g | 60.35 i | 2 | |
| 0.73 f | 38.34 h | 8.65 f,g | 8.02 e,f,g | 6.12 b,c | 69.4 c | 3 | |
| 0.74 f | 39.91 g | 7.15 h | 8.35 e | 5.95 b,c | 59.72 i | PAW | |
| 0.96 c | 63.38 a | 5.14 j,k | 13.26 a | 5.24 d,e | 68.14 d | 0 | Bodegold |
| 1.27 a | 53.01 c | 5.9 i | 11.09 b | 4.88 e,f,g | 65.44 e | 1 | |
| 1.11 b | 61.28 b | 5.01 k | 12.82 a | 5.04 e,f | 64.28 f | 2 | |
| 0.90 d | 48.66 e | 6.29 i | 10.18 c,d | 4.41 g | 64.03 f | 3 | |
| 1.09 b | 50.62 d | 5.72 i,j | 10.59 b,c | 4.80 e,f,g | 60.56 i | PAW | |
| 0.34 h | 27.1 l | 11.11 c | 5.70 i | 6.36 a,b | 62.98 g | 0 | Lianka |
| 0.95 c,d | 28.68 k | 10.27 d | 6.00 h,i | 6.86 a | 61.63 h | 1 | |
| 0.81 e | 16.6 m | 16.87 b | 3.36 j | 6.29 a,b,c | 56.68 k | 2 | |
| 0.96 c | 15.77 m | 17.56 a | 3.30 j | 6.43 a,b | 57.94 j | 3 | |
| 0.73 f,g | 36.57 i | 9.29 e | 7.65 e,g | 6.38 a,b | 71.09 b | PAW |
| α-Fa (%) | β-Fa (%) | α-BnA (%) | α-BoB (%) | α-BoA (%) | α-Bo (%) | Ch (%) | Fertilizer (mL/L) | Cultivar |
|---|---|---|---|---|---|---|---|---|
| 10.60 b | 34.52 b,c,d,e | 4.80 f | 5.89 e,f | 4.28 c,d,e,f | 2.81 a,b | 0.73 g | 0 | Bona |
| 9.67 e | 33.55 d,e | 5.00 e | 6.03 c,d,e | 4.12 d,e,f | 2.43 e,f | 1.19 a,b,c | 1 | |
| 10.37 c | 35.04 b,c,d | 4.94 e,f | 5.92 d,e,f | 4.48 a,b,c,d,e | 2.26 f | 0.69 g | 2 | |
| 8.23 h | 33.95 c,d,e | 5.37 b,c,d | 6.06 c,d,e | 4.53 a,b,c,d | 2.48 e,f | 0.61 g | 3 | |
| 10.04 d | 35.40 b,c | 5.31 c,d | 5.67 g | 4.05 e,f | 2.82 a,b | 0.85 f | PAW | |
| 10.59 b | 29.64 f | 5.43 b,c | 5.81 f,g | 4.84 a | 2.62 b,c,d,e | 1.11 b,c | 0 | Bodegold |
| 11.15 a | 30.88 f | 5.79 a | 5.90 e,f | 4.77 a,b | 2.78 a,b,c,d | 1.14 a,b,c | 1 | |
| 10.38 c | 33.94 c,d,e | 5.40 b.c | 6.40 b | 4.36 b,c,d,e,f | 2.79 a,b,c | 1.11 b,c | 2 | |
| 8.60 f | 36.01 b | 4.95 e,f | 6.23 b,c | 4.10 d,e,f | 2.31 f | 0.90 e,f | 3 | |
| 9.51 e | 33.01 e | 5.46 b | 6.80 a | 4.18 d,e,f | 2.99 a | 1.21 a,b | PAW | |
| 10.09 d | 35.67 b,c | 5.04 e | 5.44 h | 3.98 f | 2.56 d,e | 0.99 d,e | 0 | Lianka |
| 8.39 g,h | 37.64 a | 5.26 d | 4.64 j | 4.12 d,e,f | 2.34 f | 1.23 a | 1 | |
| 8.53 f,g | 35.18 b,c,d | 5.42 b,c | 6.12 c,d | 4.69 a,b,c | 2.62 b,c,d,e | 0.72 g | 2 | |
| 7.88 i | 37.81 a | 5.04 e | 6.42 b | 3.97 f | 2.57 c,d,e | 0.65 g | 3 | |
| 8.47 f,g | 38.96 a | 4.99 e | 4.95 i | 3.93 f | 2.70 b,c,d | 1.09 c,d | PAW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omrani, M.; Ghasemi, M.; Modarresi, M.; Salamon, I. Alternations in Physiological and Phytochemical Parameters of German Chamomile (Matricaria chamomilla L.) Varieties in Response to Amino Acid Fertilizer and Plasma Activated-Water Treatments. Horticulturae 2023, 9, 857. https://doi.org/10.3390/horticulturae9080857
Omrani M, Ghasemi M, Modarresi M, Salamon I. Alternations in Physiological and Phytochemical Parameters of German Chamomile (Matricaria chamomilla L.) Varieties in Response to Amino Acid Fertilizer and Plasma Activated-Water Treatments. Horticulturae. 2023; 9(8):857. https://doi.org/10.3390/horticulturae9080857
Chicago/Turabian StyleOmrani, Malihe, Mojtaba Ghasemi, Mohammad Modarresi, and Ivan Salamon. 2023. "Alternations in Physiological and Phytochemical Parameters of German Chamomile (Matricaria chamomilla L.) Varieties in Response to Amino Acid Fertilizer and Plasma Activated-Water Treatments" Horticulturae 9, no. 8: 857. https://doi.org/10.3390/horticulturae9080857
APA StyleOmrani, M., Ghasemi, M., Modarresi, M., & Salamon, I. (2023). Alternations in Physiological and Phytochemical Parameters of German Chamomile (Matricaria chamomilla L.) Varieties in Response to Amino Acid Fertilizer and Plasma Activated-Water Treatments. Horticulturae, 9(8), 857. https://doi.org/10.3390/horticulturae9080857






