Essential Oil Quality of Lavender Grown Outside Its Native Distribution Range: A Study from Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil Samples
2.2. Physicochemical and Sensory Properties of Lavender Plants Essential Oils
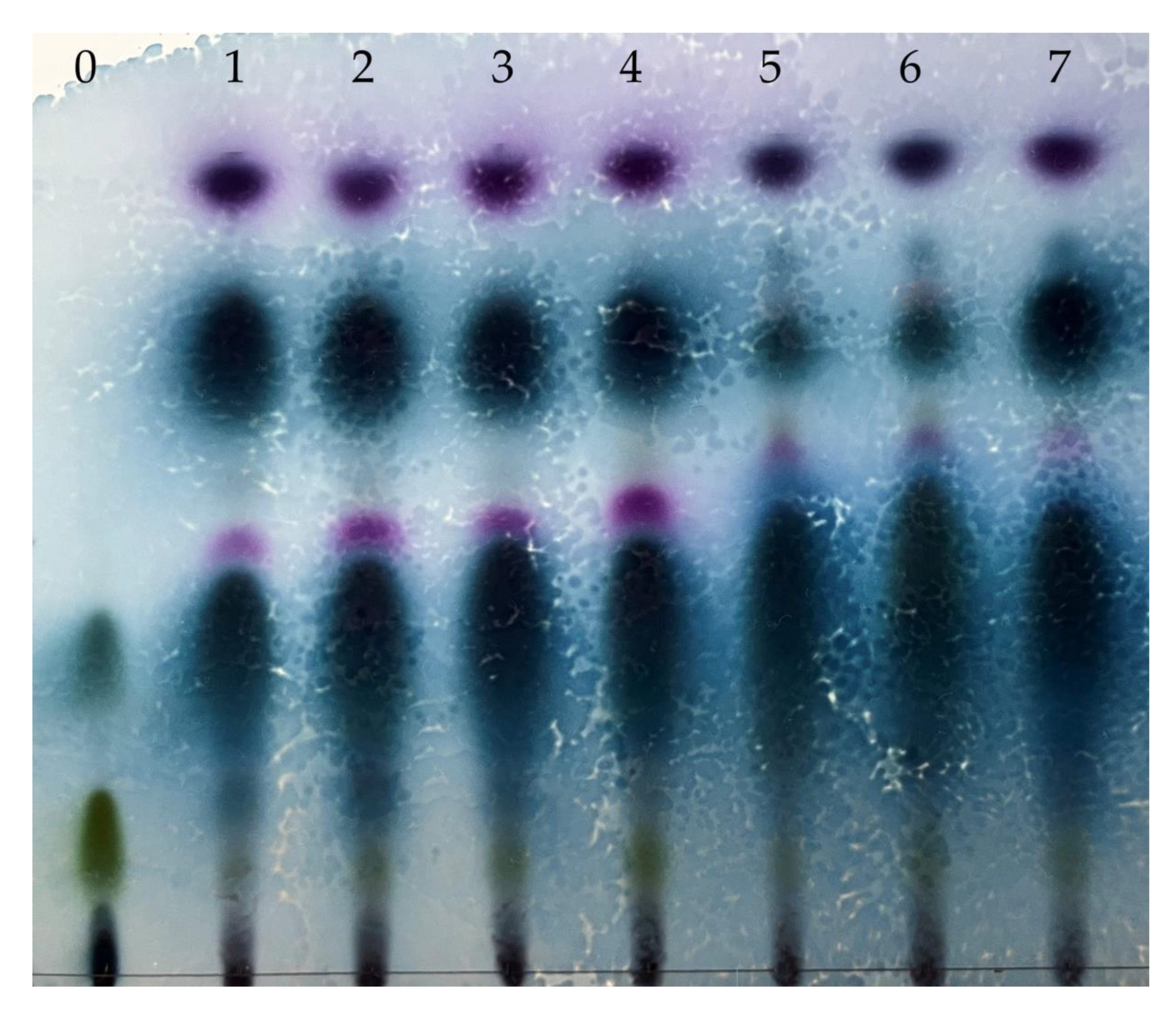
2.2.1. Refractive Indices and TLC Analysis of Lavender Essential Oils
2.2.2. GC-MS Analysis of Lavender Essential Oils
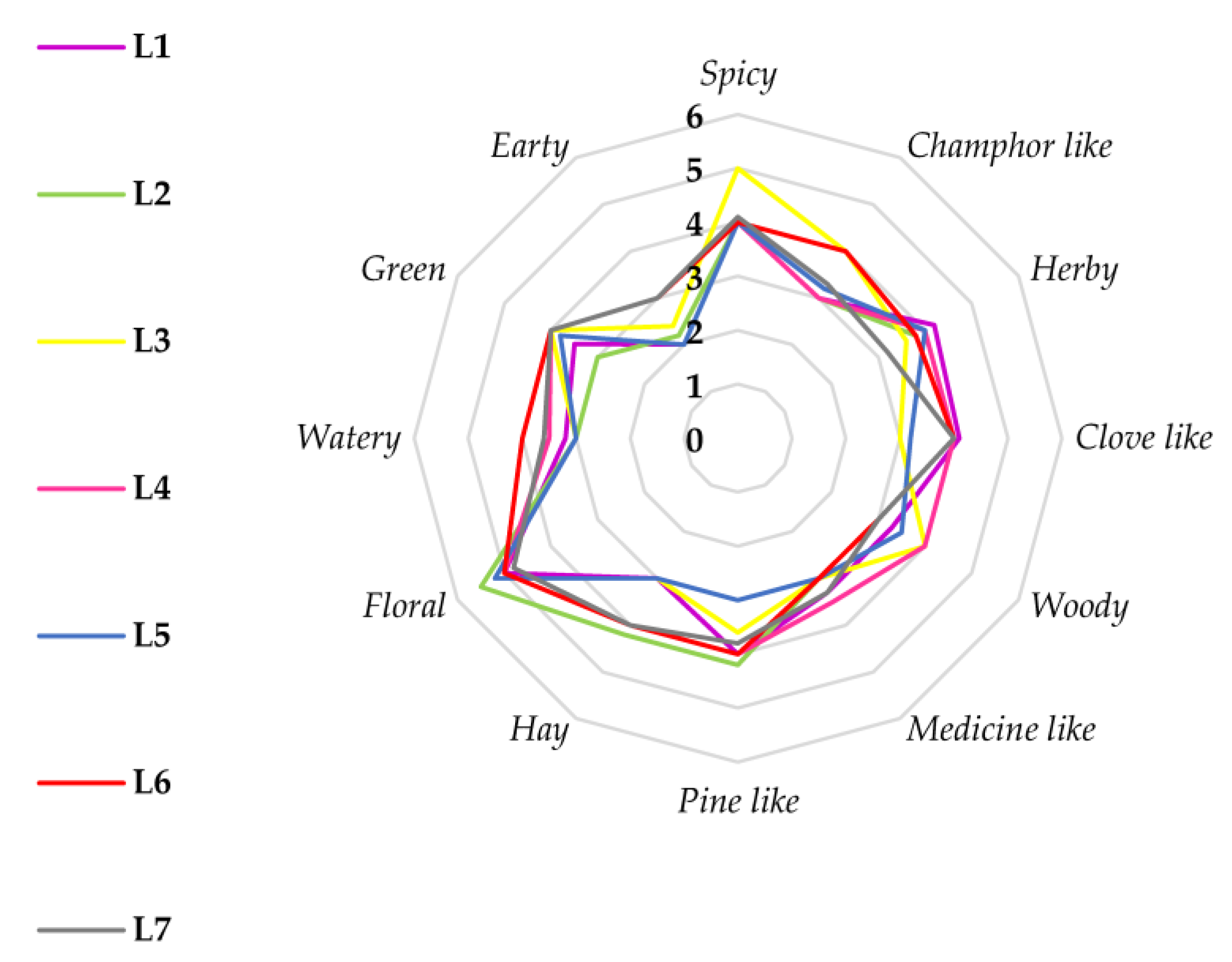
2.2.3. Sensory Odor Analysis
2.3. Bioactivity of Lavender Essential Oils
2.3.1. Antimicrobial Activity of Lavender Essential Oils
2.3.2. Antioxidant Activity of Lavender Essential Oils
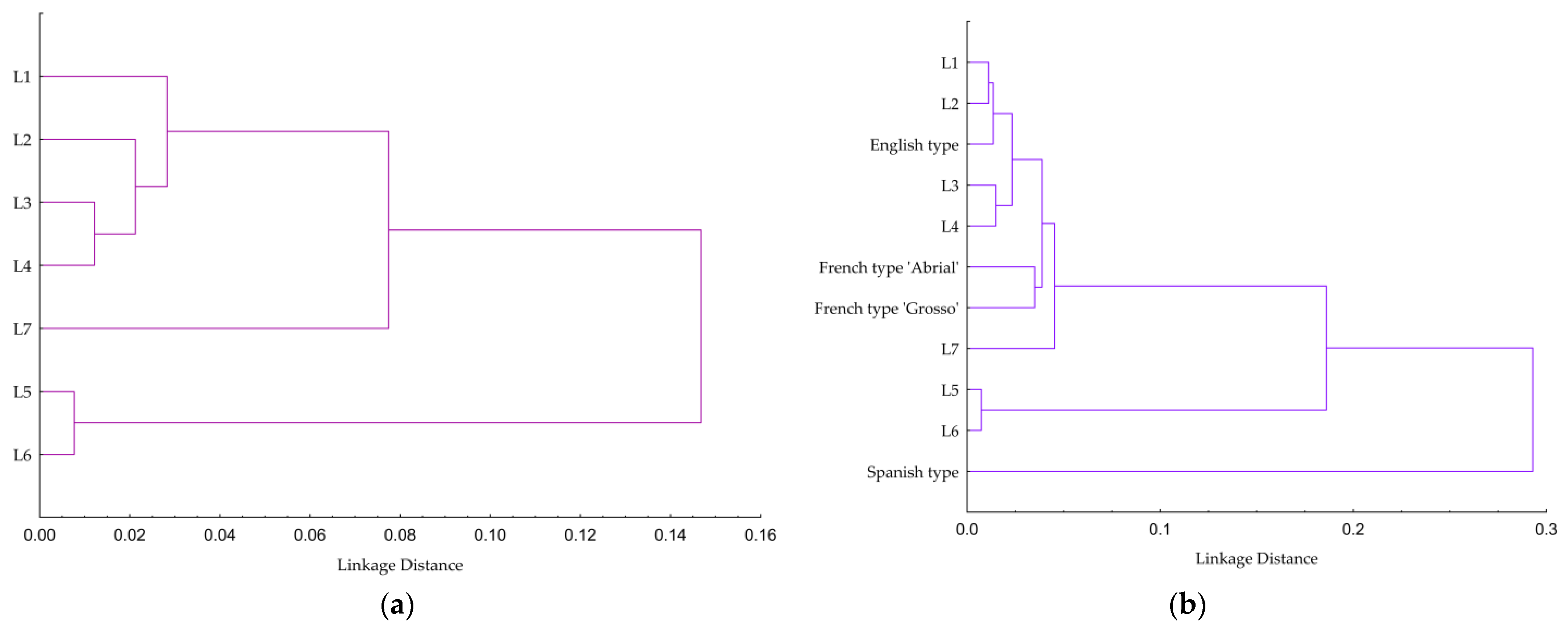
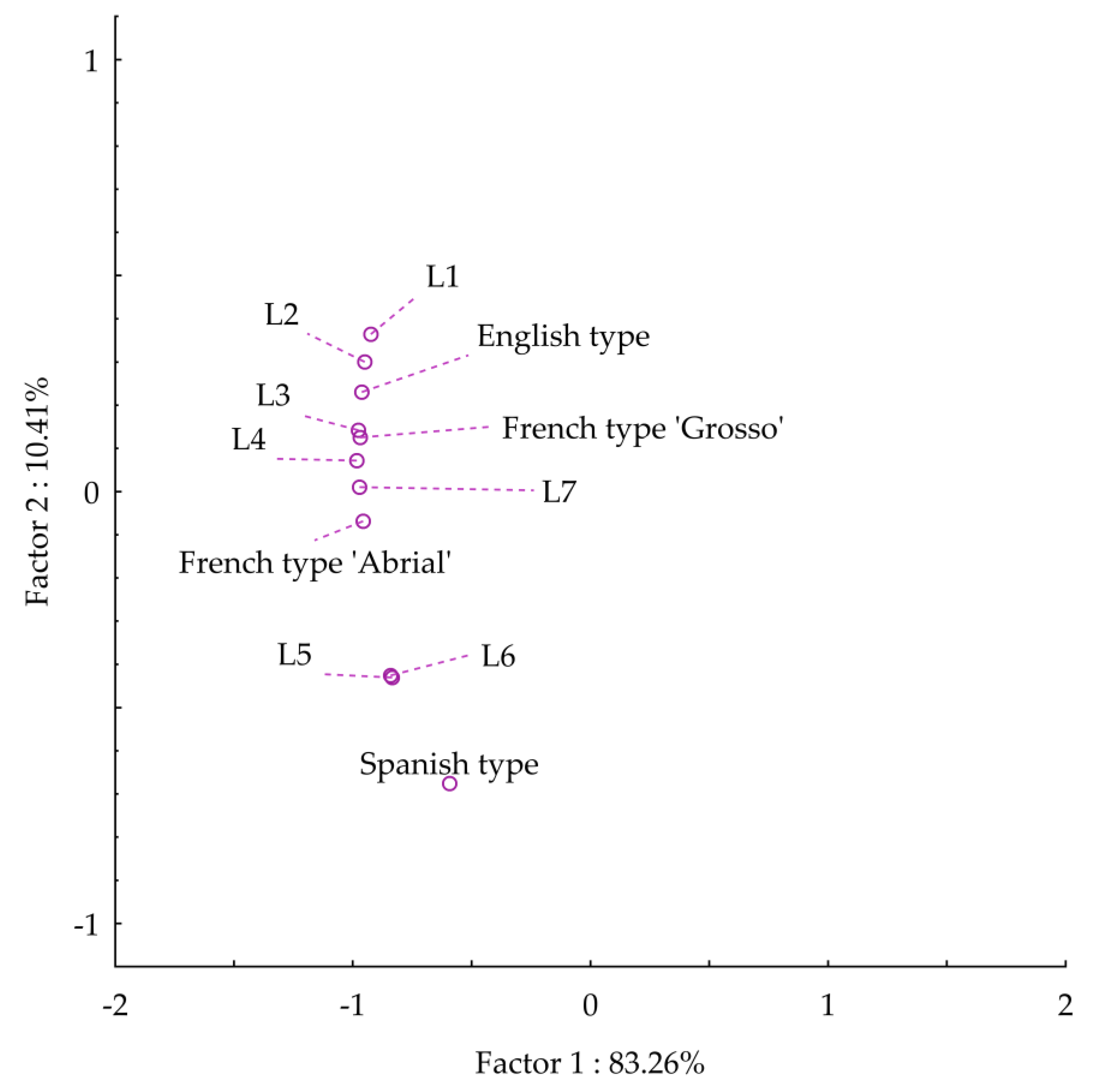
2.4. Statistical Analysis
3. Results
3.1. Physicochemical and Sensory Properties of Lavender Essential Oils
3.1.1. Refractive Indices and TLC Analysis of Lavender Essential Oils
3.1.2. GC-MS Analysis of Lavender Essential Oils
3.1.3. Sensory Analysis
3.2. Bioactivity of Lavender Essential Oils
3.2.1. Antimicrobial Activity of Lavender Essential Oils
3.2.2. Antioxidant Activity of Lavender Essential Oils
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Royal Botanic Gardens Kew. Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 12 December 2022).
- Upson, T. The taxonomy of the genus Lavandula L. In Lavender: The Genus Lavandula. Medicinal and Aromatic Plants—Industrial Profiles, 1st ed.; Lis-Balchin, M., Ed.; Taylor & Francis Inc.: London, UK; New York, NY, USA, 2002; pp. 10–14. [Google Scholar] [CrossRef]
- Tucker, A.O. Lavender, spike, and lavandin. Herbarist 1985, 51, 44–50. [Google Scholar]
- ISO 3515; Oil of Lavender (Lavandula angustifolia Mill), 3rd ed. International Standards: Geneva, Switzerland, 2002; pp. 2–5.
- ISO 4719; Essential Oil of Spike Lavender (Lavandula latifolia Medikus), Spanish Type, 3rd ed. International Standards: Geneva, Switzerland, 2012; pp. 2–5.
- ISO 8902; Oil of Lavandin Grosso (Lavandula angustifolia Mill. x Lavandula latifolia Medik.), French Type, 3rd ed. International Standards: Geneva, Switzerland, 2009; pp. 2–5.
- ISO 3054; Essential Oil of Lavandin Abrial (Lavandula angustifolia Mill. x Lavandula latifolia Medik.), French Type, 4th ed. International Standards: Geneva, Switzerland, 2017; pp. 2–5.
- Denys, J.C.; Renaud, E.N.C.; Simon, J.E. Comparative study of essential oil quantity and composition from ten cultivars of organically grown lavender and lavandin. In Lavender: The Genus Lavandula. Medicinal and Aromatic Plants—Industrial Profiles, 1st ed.; Lis-Balchin, M., Ed.; Taylor & Francis Inc.: London, UK; New York, NY, USA, 2002; pp. 232–242. [Google Scholar]
- Ph. Eur. VIII. European Pharmacopoeia, 8th ed.; European Pharmacopoeia Commission, and the European Directorate for the Quality of Medicines & HealthCare (EDQM): Strasbourg, France, 2013; Volume 1, pp. 1291–1292. [Google Scholar]
- Xiao, Z.; Li, Q.; Niu, Y.; Zhou, X.; Liu, J.; Xu, Y.; Xu, Z. Odor-active compounds of different lavender essential oils and their correlation with sensory attributes. Ind. Crops Prod. 2017, 108, 748–755. [Google Scholar] [CrossRef]
- The Good Scents Company. Available online: http://www.thegoodscentscompany.com/ (accessed on 3 January 2023).
- Guo, X.; Wang, P. Aroma characteristics of lavender extract and essential oil from Lavandula angustifolia Mill. Molecules 2020, 25, 5541. [Google Scholar] [CrossRef] [PubMed]
- Baydar, H.; Kineci, S. Scent composition of essential oil, concrete, absolute and hydrosol from lavandin (Lavandula × intermedia Emeric ex Loisel.). J. Essent. Oil Bear. Plants 2009, 12, 131–136. [Google Scholar] [CrossRef]
- Giray, F.H. An analysis of world lavender oil markets and lessons for Turkey. J. Essent. Oil Bear. Plants 2018, 21, 1612–1623. [Google Scholar] [CrossRef]
- Pokajewicz, K.; Białoń, M.; Svydenko, L.; Hudz, N.; Balwierz, R.; Marciniak, D.; Wieczorek, P.P. Comparative evaluation of the essential oil of the new Ukrainian Lavandula angustifolia and Lavandula x intermedia cultivars grown on the same plots. Molecules 2022, 27, 2152. [Google Scholar] [CrossRef] [PubMed]
- Hrnjak, I.; Lukić, T.; Gavrilov, M.B.; Marković, S.B.; Unkašević, M.; Tošić, I. Aridity in Vojvodina, Serbia. Theor. Appl. Climatol. 2014, 115, 323–332. [Google Scholar] [CrossRef]
- Kontic, L.; Stanojević, O.Z.; Vasić, M. Organic production of lavender in Serbia-economic and financial analysis. Econ. Agric. 2022, 69, 911–924. [Google Scholar] [CrossRef]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. Trends Anal. Chem. 2015, 66, 146–157. [Google Scholar] [CrossRef]
- Renaud, E.N.C.; Charles, D.J.; Simon, J.E. Essential oil quantity and composition from 10 cultivars of organically grown lavender and lavandin. J. Essent. Oil Res. 2001, 13, 269–273. [Google Scholar] [CrossRef]
- Imre, S.; Eşianu, S.; Miklos, A.; Tiuca, I.; Dicher, I.; Tero-Vescan, A.; Muntean, D.L.; Oprean, R. Qualitative assay of essential oils of lavender and peppermint in commercial products through spectral and chromatographic methods. J. Farm. 2016, 64, 857–862. [Google Scholar]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring pharmacological mechanisms of lavender (Lavandula angustifolia) essential oil on central nervous system targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef]
- Donelli, D.; Antonelli, M.; Bellinazzi, C.; Gensini, G.F.; Firenzuoli, F. Effects of lavender on anxiety: A systematic review and meta-analysis. Phytomedicine 2019, 65, 153099. [Google Scholar] [CrossRef]
- Caputo, L.; Souza, L.F.; Alloisio, S.; Cornara, L.; De Feo, V. Coriandrum sativum and Lavandula angustifolia essential oils: Chemical composition and activity on central nervous system. Int. J. Mol. Sci. 2016, 17, 1999. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V.R.; Grekov, D.F.; Kisyov, V.K.; Ivanov, K.I. Potential of lavender (Lavandula vera L.) for phytoremediation of soils contaminated with heavy metals. Int. J. Agric. Biosyst. Eng. 2015, 9, 522–529. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis. A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996; p. 181. [Google Scholar]
- Aćimović, M.; Zeremski, T.; Šovljanski, O.; Lončar, B.; Pezo, L.; Zheljazkov, V.; Pezo, M.; Šuput, D.; Kurunci, Z. Seasonal Variations in Essential Oil Composition of Immortelle Cultivated in Serbia. Horticulturae 2022, 8, 1183. [Google Scholar] [CrossRef]
- Aćimović, M.; Pezo, L.; Zeremski, T.; Lončar, B.; Marjanović Jeromela, A.; Stanković Jeremić, J.; Cvetković, M.; Sikora, V.; Ignjatov, M. Weather Conditions Influence on Hyssop Essential Oil Quality. Processes 2021, 9, 1152. [Google Scholar] [CrossRef]
- NIST. Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 16 March 2023).
- Aćimović, M.; Varga, A.; Cvetković, M.; Pezo, L.; Lončar, B.; Ignjatov, M.; Zeremski, T. Chemical composition of hyssop cv. “Domaći ljubičasti” essential oil and its antimicrobial activity. Ratar. Povrt. 2021, 58, 23–30. [Google Scholar] [CrossRef]
- Panda, S.K. Assay Guided Comparison for Enzymatic and Non-Enzymatic Antioxidant Activities with Special Reference to Medicinal Plants. In Biochemistry, Genetics and Molecular Biology; El-Missiry, M.A., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Astel, A.; Tsakovski, S.; Barbieri, P.; Simeonov, V. Comparison of self-organizing maps classification approach with cluster and principal components analysis for large environmental data sets. Water Res. 2007, 41, 4566–4578. [Google Scholar] [CrossRef]
- Ranitović, A.; Šovljanski, O.; Aćimović, M.; Pezo, L.; Tomić, A.; Travičić, V.; Saveljić, A.; Cvetković, D.; Ćetković, G.; Vulić, J.; et al. Biological Potential of Alternative Kombucha Beverages Fermented on Essential Oil Distillation By-Products. Fermentation 2022, 8, 625. [Google Scholar] [CrossRef]
- Kıvrak, Ş. Essential oil composition and antioxidant activities of eight cultivars of Lavender and Lavandin from western Anatolia. Ind. Crops Prod. 2018, 117, 88–96. [Google Scholar] [CrossRef]
- Boelens, M.H. Chemical and sensory evaluation of Lavandula oils. Perfum. Flavorist 1995, 20, 23. [Google Scholar]
- Naef, R.; Morris, A.F. Lavender-Lavadin—A comparison. Riv. Ital. EPPOS 1992, Numero Speciale, 364–377. Available online: https://scholar.google.com/scholar_lookup?journal=Rivista+Italiana+EPPOS&title=Lavender%E2%80%94Lavandin.+A+Comparison&author=R.+Naef&author=A.F.+Morris&volume=Special+edition&publication_year=1992&pages=364-377& (accessed on 3 January 2023).
- Jianu, C.; Pop, G.; Gruia, A.T.; Horhat, F.G. Chemical composition and antimicrobial activity of essential oils of lavender (Lavandula angustifolia) and lavandin (Lavandula x intermedia) grown in Western Romania. Int. J. Agric. Biol. 2013, 15, 772–776. [Google Scholar]
- Robu, S.; Chesaru, B.I.; Diaconu, C.; Dumitriu, B.O.; Tutunaru, D.; Stanescu, U.; Lisa, E.L. Lavandula hybrida: Microscopic characterization and the evaluation of the essential oil. Farmacia 2016, 64, 914–917. [Google Scholar]
- Blažeković, B.; Yang, W.; Wang, Y.; Li, C.; Kindl, M.; Pepeljnjak, S.; Vladimir-Knežević, S. Chemical composition, antimicrobial and antioxidant activities of essential oils of Lavandula x intermedia ‘Budrovka’ and L. angustifolia cultivated in Croatia. Ind. Crops Prod. 2018, 123, 173–182. [Google Scholar] [CrossRef]
- Oroian, C.; Odagiu, A.; Racz, C.P.; Oroian, I.; Mureșan, I.C.; Duda, M.; Ilea, M.; Brașovean, I.; Iederan, C.; Marchiș, Z. Composition of Lavandula angustifolia L. cultivated in Transylvania, Romania. Not. Bot. Hort. Agrobot. 2019, 47, 643–650. [Google Scholar] [CrossRef]
- Détár, E.; Zámboriné Németh, É.; Gosztola, B.; Demján, I.; Pluhár, Z. Effects of variety and growth year on the essential oil properties of lavender (Lavandula angustifolia Mill.) and lavandin (Lavandula x intermedia Emeric ex Loisel.). Biochem. Syst. Ecol. 2020, 90, 104020. [Google Scholar] [CrossRef]
- Garzoli, S.; Laghezza Masci, V.; Franceschi, S.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Headspace/GC–MS analysis and investigation of antibacterial, antioxidant and cytotoxic activity of essential oils and hydrolates from Rosmarinus officinalis L. and Lavandula angustifolia Miller. Foods 2021, 10, 1768. [Google Scholar] [CrossRef]
- Pokajewicz, K.; Białoń, M.; Svydenko, L.; Fedin, R.; Hudz, N. Chemical compositionof the essential oils of the new cultivars of Lavandula angustifolia Mill. Bred in Ukraina. Molecules 2021, 26, 5681. [Google Scholar] [CrossRef]
- Walasek-Janusz, M.; Grzegorczyk, A.; Zalewski, D.; Malm, A.; Gajcy, S.; Gruszecki, R. Variation in the Antimicrobial Activity of Essential Oils from Cultivars of Lavandula angustifolia and L. x intermedia. Agronomy 2022, 12, 2955. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. Dis. 2003, 9, ix–xv. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, Y.W.; Hou, C.Y. Antioxidant and antibacterial activity of seven predominant terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Blažeković, B.; Vladimir-Knežević, S.; Brantner, A.; Štefan, M.B. Evaluation of antioxidant potential of Lavandula x intermedia Emeric ex Loisel. ‘Budrovka’: A comparative study with L. angustifolia Mill. Molecules 2010, 15, 5971–5987. [Google Scholar] [CrossRef] [PubMed]
- Sariri, R.; Seifzadeh, S.; Sajedi, R.H. Anti-tyrosinase and antioxidant activity of Lavandula sp. extracts. Pharmacol. Online 2009, 2, 413–420. Available online: https://pharmacologyonline.silae.it/files/archives/2009/vol2/040.Sariri.pdf (accessed on 3 January 2023).
- Ferreira, A.; Proença, C.; Serralheiro, M.; Araújo, M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef]
- Tița, O.; Constantinescu, M.A.; Tița, M.A.; Opruța, T.I.; Dabija, A.; Georgescu, C. Valorization on the antioxidant potential of volatile oils of Lavandula angustifolia Mill., Mentha piperita L. and Foeniculum vulgare L. in the production of kefir. Appl. Sci. 2022, 12, 10287. [Google Scholar] [CrossRef]
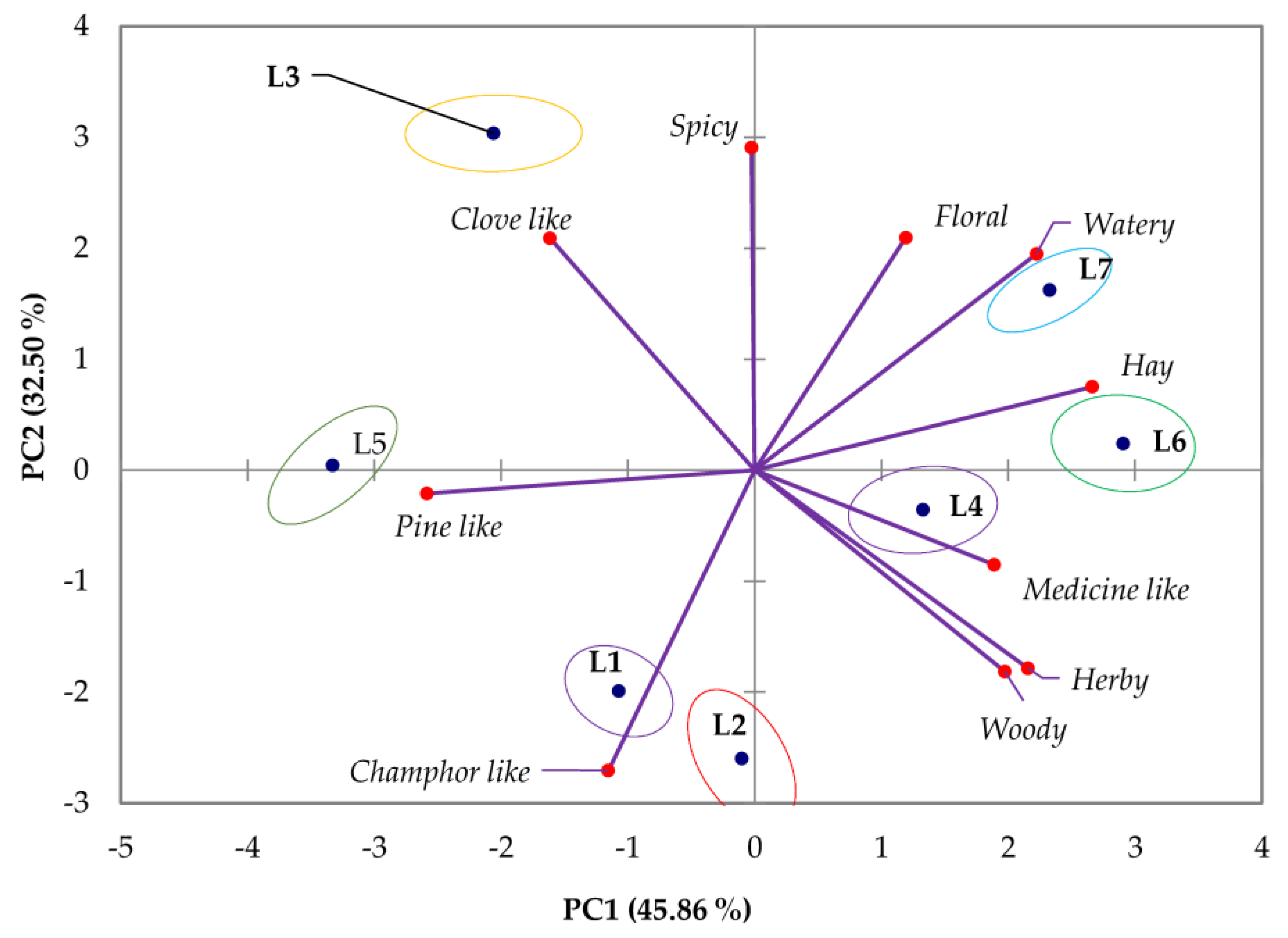
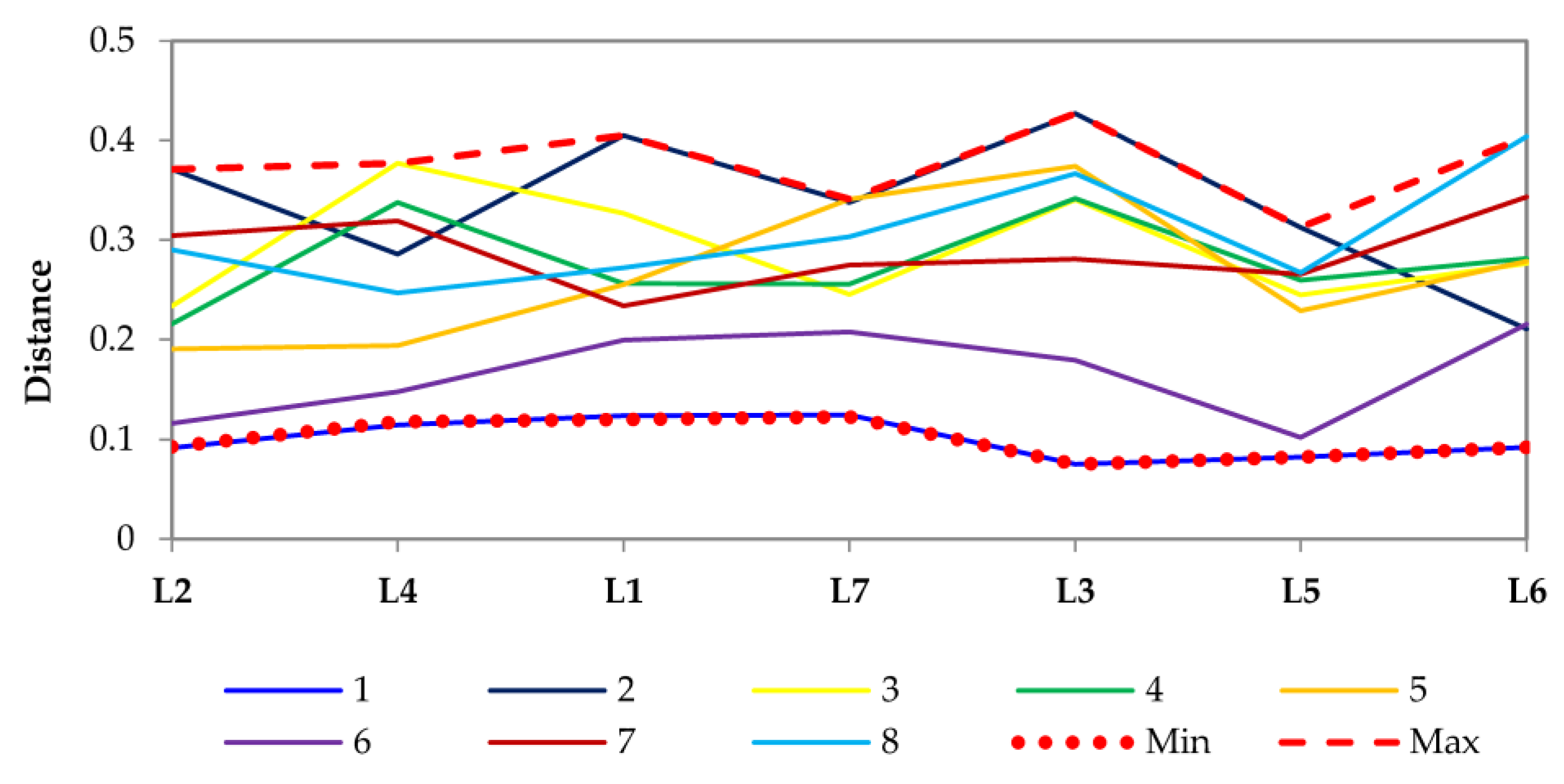
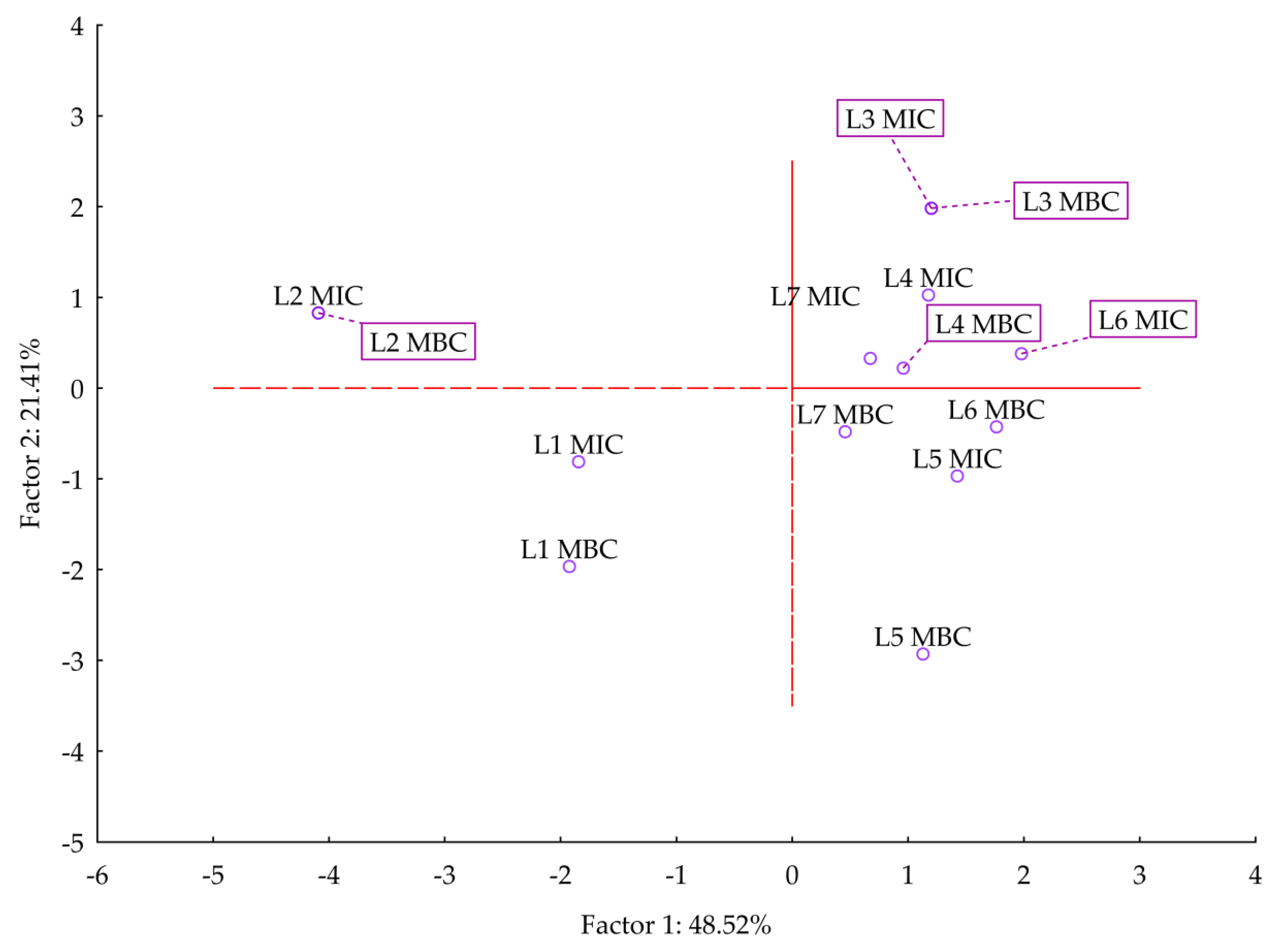
| Compound | English Type, True Lavender * | Spanish Type, Spike Lavender | French Type, Lavandin ‘Abrial’ | French Type, Lavandin ‘Grosso’ | Odor Type |
|---|---|---|---|---|---|
| myrcene | nd | nd | 0.4–0.9 | 0.3–1.0 | balsamic, spice [10] |
| β-phellandrene | tr-1.0 | nd | nd | nd | minty terpenic [11] |
| cis-β-ocimene or Z-β-ocimene | 1.0–10.0 | nd | 1.4–3.0 | 0.5–1.5 | sweet, floral, herbal [10] |
| trans-β-ocimene or E-β-ocimene | 1.0–6.0 | nd | 2.5–6.0 | nd-1.0 | sweet herbal [11] |
| limonene | 0.3–1.0 | 0.5–3.0 | 0.5–1.5 | 0.5–1.5 | terpene, pine, herbal, peppery [11] |
| 1,8-cineole | 0.5–3.0 | 16.0–39.0 | 6.0–12.5 | 4.0–8.0 | floral, minty, fruity 10] |
| linalool | 22.0–45.0 | 34.0–50.0 | 28.0–38.0 | 24.0–37.0 | floral [12] |
| camphor | tr-1.5 | 8.0–16.0 | 7.0–11.0 | 6.0–8.5 | camphor [10] |
| borneol | nd | nd | 1.5–3.5 | 1.5–3.5 | camphor [10] |
| lavandulol | nd | nd | 0.4–1.2 | 0.2–1.0 | herbal-rosy scent [12] |
| trepinen-4-ol | 1.2–8.0 | nd | 0.3–1.2 | 1.5–5.0 | peppery, woody [11] |
| 3-octanone | tr-5.0 | nd | nd | nd | herbal, mushroom [11] |
| α-terpineol | 0.5–2.0 | 0.2–2.0 | 0.3–1.2 | 0.3–1.3 | oil, anise, mint [10] |
| hexyl butyrate | nd | nd | 0.2–0.5 | 0.3–0.5 | sweet, fruit [10] |
| linalyl acetate | 25.0–47.0 | nd-1.6 | 19.0–29.0 | 25.0–38.0 | sweet, citrus, floral, woody [10,12] |
| lavandulyl acetate | 1.0–8.0 | nd | 1.0–2.0 | 1.5–3.5 | herbal-rosy scent [12] |
| β-caryophyllene | nd | nd | 1.5–2.5 | nd | sweet, woody, spicy [11] |
| trans-α-bisabolene | nd | 0.4–2.5 | nd | nd | fruity, citrus, woody [11] |
| Odor type [11] | lavender, floral, herbal, woody | camphor, eucalyptus, fresh, herbal, rosemary, woody, floral | herbal, spicy, camphoreous, soapy, floral, balsamic, woody | camphoraceous, lavender, herbal, floral, fruity |
| EO Sample | RI ± Se |
|---|---|
| L. angustifolia Mill. | 1.465 ± 0.33 |
| L. agustifolia ‘Hidcote blue’ | 1.466 ± 0.00 |
| L. angustifolia ‘Munstead’ | 1.466 ± 0.00 |
| L. angustifolia ‘Primorska’ | 1.466 ± 0.33 |
| ‘Budrovka’ lavandin | 1.468 ± 0.33 |
| Lavandula sp. | 1.467 ± 0.33 |
| ‘Grosso’ lavandin | 1.464 ± 0.00 |
| Compound | LRIref | LRIexp | L1 | L2 | L3 | L4 | L5 | L6 | L7 |
|---|---|---|---|---|---|---|---|---|---|
| α-Thujene | 930 | 927 | 0.13 | 0.23 | 0.25 | 0.12 | 0.15 | 0.21 | 0.25 |
| α-Pinene | 937 | 934 | 0.32 | 0.47 | 0.49 | 0.28 | 0.97 | 1.13 | 1.07 |
| Camphene | 952 | 949 | 0.22 | 0.36 | 0.37 | 0.30 | 0.45 | 0.52 | 0.61 |
| Sabinene | 975 | 973 | nd | 0.18 | 0.10 | 0.06 | 0.30 | 0.40 | 0.27 |
| β-Pinene | 979 | 978 | 0.19 | 0.48 | 0.44 | 0.54 | 1.48 | 1.91 | 1.25 |
| 3-Octanone | 986 | 985 | 0.35 | 0.36 | 0.87 | 0.49 | 0.08 | 0.04 | 0.05 |
| Myrcene | 991 | 989 | 0.68 | 1.07 | 0.81 | 0.52 | 0.59 | 0.65 | 0.96 |
| α-Phellandrene | 1004 | 1005 | 0.04 | 0.12 | 0.08 | 0.03 | 0.07 | 0.08 | 0.06 |
| δ-3-Carene | 1011 | 1010 | 0.15 | 1.16 | 0.39 | 0.16 | 0.38 | 0.27 | 0.05 |
| Hexyl acetate | 1011 | 1011 | 0.40 | 0.53 | 0.28 | 0.31 | 0.13 | 0.26 | 0.17 |
| δ-2-Carene | 1015 | 1016 | 0.05 | 0.05 | 0.08 | 0.06 | 0.09 | 0.11 | 0.12 |
| o-Cymene | 1020 | 0.07 | 0.18 | 0.11 | 0.23 | 0.11 | 0.05 | nd | |
| p-Cymene | 1025 | 1023 | 0.27 | 0.49 | 0.40 | 0.71 | 0.53 | 0.39 | 0.27 |
| Limonene | 1030 | 1027 | 0.42 | 2.05 | 0.82 | 0.85 | nd | nd | nd |
| 1,8-Cineole | 1032 | 1029 | 0.74 | 1.83 | 1.29 | 1.91 | nd | nd | nd |
| Limonene + 1,8-Cineole | 6.87 | Nd | nd | nd | 15.32 | 14.61 | 10.25 | ||
| β-Z-Ocimene | 1038 | 1036 | 2.91 | 4.20 | 6.11 | 2.80 | 2.93 | 3.99 | 0.46 |
| β-E-Ocimene | 1049 | 1046 | 0.16 | 2.92 | 2.94 | 0.94 | 0.35 | 0.39 | 0.38 |
| γ-Terpinene | 1060 | 1056 | 0.06 | 0.16 | 0.28 | 0.17 | 0.27 | 0.30 | 0.32 |
| cis-Sabinene-hydrate | 1068 | 1064 | 0.17 | 0.05 | 0.07 | 0.05 | 0.06 | 0.23 | 0.11 |
| cis-Linalool oxide | 1074 | 1070 | 0.04 | 0.18 | 0.26 | 0.43 | 0.18 | 0.23 | 0.25 |
| Terpinolene | 1088 | 1087 | 0.23 | 0.30 | 0.36 | 0.46 | 0.40 | 0.45 | 0.56 |
| Linalool | 1099 | 1105 | 23.90 | 25.15 | 29.31 | 30.22 | 31.94 | 36.89 | 28.86 |
| 1-Octen-3-yl-acetate | 1111 | 1112 | 1.03 | 0.34 | 0.57 | 0.82 | nd | 0.04 | 0.34 |
| Camphor | 1144 | 1142 | 0.30 | 0.34 | 0.36 | 1.04 | 5.07 | 2.25 | 8.25 |
| Borneol | 1166 | 1164 | 0.86 | 2.02 | 1.36 | 3.55 | nd | nd | 3.59 |
| Lavandulol | 1168 | 1166 | 0.79 | 0.72 | 0.98 | 1.55 | 11.53 | 10.57 | nd |
| Terpinen-4-ol | 1177 | 1176 | 5.62 | 3.46 | 6.32 | 4.19 | 5.95 | 6.71 | 5.75 |
| p-Cymen-8-ol | 1183 | 1181 | 0.10 | 0.13 | 0.09 | 0.23 | 0.11 | 0.04 | nd |
| Cryptone | 1184 | 1184 | 0.24 | 0.66 | 0.30 | 0.91 | 0.27 | 0.20 | 0.07 |
| α-Terpineol | 1189 | 1190 | 1.78 | 1.94 | 1.29 | 1.02 | 1.38 | 0.94 | 1.66 |
| Hexyl butanoate | 1196 | 1192 | nd | 0.64 | 0.46 | 0.45 | 0.67 | 0.73 | 0.46 |
| Eucarvone | 1206 | nd | 0.09 | 0.05 | 0.21 | 0.10 | 0.06 | 0.06 | |
| Nerol | 1228 | 1228 | 0.27 | 0.33 | 0.19 | 0.17 | 0.48 | 0.26 | 0.19 |
| Cuminaldehyde | 1239 | 1237 | 0.11 | 0.31 | 0.16 | 0.50 | 0.40 | 0.39 | 0.18 |
| Linalyl acetate | 1257 | 1259 | 32.22 | 28.90 | 24.07 | 22.18 | 7.16 | 6.90 | 20.68 |
| Z-Isocitral | 1272 | 0.05 | 0.09 | nd | 0.13 | 0.05 | nd | nd | |
| Bornyl acetate | 1285 | 1284 | 0.34 | 0.17 | 0.21 | 0.18 | 0.12 | 0.05 | 0.04 |
| Lavandulyl acetate | 1289 | 1292 | 4.84 | 5.52 | 5.05 | 6.53 | 0.77 | 0.93 | 3.43 |
| Neryl acetate | 1364 | 1364 | 0.57 | 0.70 | 0.44 | 0.41 | 0.18 | 0.07 | 0.40 |
| α-Copaene | 1373 | 0.07 | Nd | nd | nd | nd | nd | nd | |
| Geranyl acetate | 1382 | 1383 | 1.07 | 1.30 | 0.89 | 0.86 | 0.35 | 0.22 | 0.87 |
| Sesquithujene | 0.11 | 0.05 | 0.07 | 0.08 | 0.16 | 0.15 | 0.11 | ||
| E-Carryophyllene | 1405 | 1417 | 5.18 | 4.11 | 5.48 | 5.53 | 1.23 | 0.76 | 1.51 |
| α-cis-Bergamotene | 1415 | 1433 | 0.23 | 0.34 | 0.17 | 0.26 | 0.13 | 0.12 | 0.16 |
| α-Humulene | 1454 | 1450 | 0.14 | 0.10 | 0.16 | 0.14 | 0.04 | nd | 0.05 |
| β-E-Farnesene | 1457 | 1456 | 3.75 | 1.22 | 2.00 | 2.04 | 3.14 | 3.00 | 1.23 |
| Germacrene D | 1480 | 1477 | 0.42 | 0.36 | 0.27 | 0.54 | 0.53 | 0.40 | 0.50 |
| Sesquisabinene | 1482 | 0.10 | 0.13 | 0.06 | 0.12 | 0.14 | nd | 0.04 | |
| γ-Cadinene | 1513 | 1510 | 0.14 | 0.24 | 0.53 | 0.27 | 0.46 | 0.37 | 0.65 |
| Caryophyllene oxide | 1581 | 1578 | 0.32 | 0.73 | 0.49 | 1.33 | 0.21 | 0.13 | 0.17 |
| epi-α-Cadinol | 1640 | 1637 | nd | Nd | 0.35 | 0.14 | 0.04 | nd | 0.24 |
| Total identified | 98.76 | 96.81 | 98.15 | 96.13 | 97.22 | 98.20 | 96.90 | ||
| Total monoterpenes | 88.15 | 89.03 | 88.14 | 85.82 | 90.59 | 92.48 | 91.67 | ||
| Total sesquiterpenes | 10.45 | 7.27 | 9.57 | 10.46 | 6.09 | 4.93 | 4.67 | ||
| Total esters | 0.40 | 1.17 | 0.74 | 0.76 | 0.80 | 0.98 | 0.63 |
| Gram-Positive Bacteria | Gram-Negative Bacteria | Yeast | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. cereus | E. faecalis | L. monocytogenes | S. aureus | E. coli | P. aeruginosa | S. enteritidis | S. Typhimurium | C. albicans | ||
| L1 | MIC | 3.5 | 227.2 | 14.2 | 227.2 | 14.2 | 227.2 | 454.5 | 227.2 | 14.2 |
| MBC | 3.5 | 227.2 | 14.2 | 227.2 | 14.2 | 227.2 | 454.5 | 454.5 | 14.2 | |
| L2 | MIC | 14.2 | 454.5 | 28.4 | 227.2 | 14.2 | 227.2 | 454.5 | 113.6 | 14.2 |
| MBC | 14.2 | 454.5 | 28.4 | 227.2 | 14.2 | 227.2 | 454.5 | 113.6 | 14.2 | |
| L3 | MIC | 7.1 | 227.2 | 7.1 | 113.6 | 3.5 | 113.6 | 113.6 | 56.8 | 7.1 |
| MBC | 7.1 | 227.2 | 7.1 | 113.6 | 3.5 | 113.6 | 113.6 | 56.8 | 7.1 | |
| L4 | MIC | 3.5 | 227.5 | 7.1 | 227.2 | 7.1 | 227.5 | 113.6 | 113.6 | 3.5 |
| MBC | 3.5 | 227.5 | 14.2 | 227.2 | 7.1 | 454.5 | 113.6 | 113.6 | 3.5 | |
| L5 | MIC | 7.1 | 28.4 | 7.1 | 56.81 | 14.2 | 227.2 | 56.81 | 227.2 | 7.1 |
| MBC | 7.1 | 28.4 | 14.2 | 56.81 | 14.2 | 454.5 | 56.81 | 454.5 | 7.1 | |
| L6 | MIC | 7.1 | 28.4 | 7.1 | 56.81 | 7.1 | 227.2 | 56.81 | 113.6 | 7.1 |
| MBC | 7.1 | 28.4 | 14.2 | 56.81 | 7.1 | 454.5 | 56.81 | 113.6 | 7.1 | |
| L7 | MIC | 7.1 | 227.2 | 7.1 | 113.6 | 7.1 | 227.2 | 227.2 | 227.2 | 7.1 |
| MBC | 7.1 | 227.2 | 14.2 | 113.6 | 7.1 | 454.5 | 227.2 | 227.2 | 7.1 | |
| EO Sample | ± Se | Duncan’s Test |
|---|---|---|
| L. angustifolia Mill. | 0.237 ± 0.003 | a |
| L. angustifolia ‘Hidcote blue’ | 0.280 ± 0.012 | ab |
| L. angustifolia ‘Munstead’ | 0.240 ± 0.006 | a |
| L. angustifolia ‘Primorska’ | 0.423 ± 0.032 | cd |
| ‘Budrovka’ lavandin | 0.567 ± 0.015 | e |
| Lavandula sp. | 0.497 ± 0.064 | de |
| ‘Grosso’ lavandin | 0.593 ± 0.052 | e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiprovski, B.; Zeremski, T.; Varga, A.; Čabarkapa, I.; Filipović, J.; Lončar, B.; Aćimović, M. Essential Oil Quality of Lavender Grown Outside Its Native Distribution Range: A Study from Serbia. Horticulturae 2023, 9, 816. https://doi.org/10.3390/horticulturae9070816
Kiprovski B, Zeremski T, Varga A, Čabarkapa I, Filipović J, Lončar B, Aćimović M. Essential Oil Quality of Lavender Grown Outside Its Native Distribution Range: A Study from Serbia. Horticulturae. 2023; 9(7):816. https://doi.org/10.3390/horticulturae9070816
Chicago/Turabian StyleKiprovski, Biljana, Tijana Zeremski, Ana Varga, Ivana Čabarkapa, Jelena Filipović, Biljana Lončar, and Milica Aćimović. 2023. "Essential Oil Quality of Lavender Grown Outside Its Native Distribution Range: A Study from Serbia" Horticulturae 9, no. 7: 816. https://doi.org/10.3390/horticulturae9070816
APA StyleKiprovski, B., Zeremski, T., Varga, A., Čabarkapa, I., Filipović, J., Lončar, B., & Aćimović, M. (2023). Essential Oil Quality of Lavender Grown Outside Its Native Distribution Range: A Study from Serbia. Horticulturae, 9(7), 816. https://doi.org/10.3390/horticulturae9070816









