Effect of Bed Preparation on Native Wildflower Establishment, Weed Control, and Arthropod Presence
Abstract
1. Introduction
2. Materials and Methods
2.1. Time of Study and Field Location
2.2. Experimental Design, Plot Layout, and Treatments
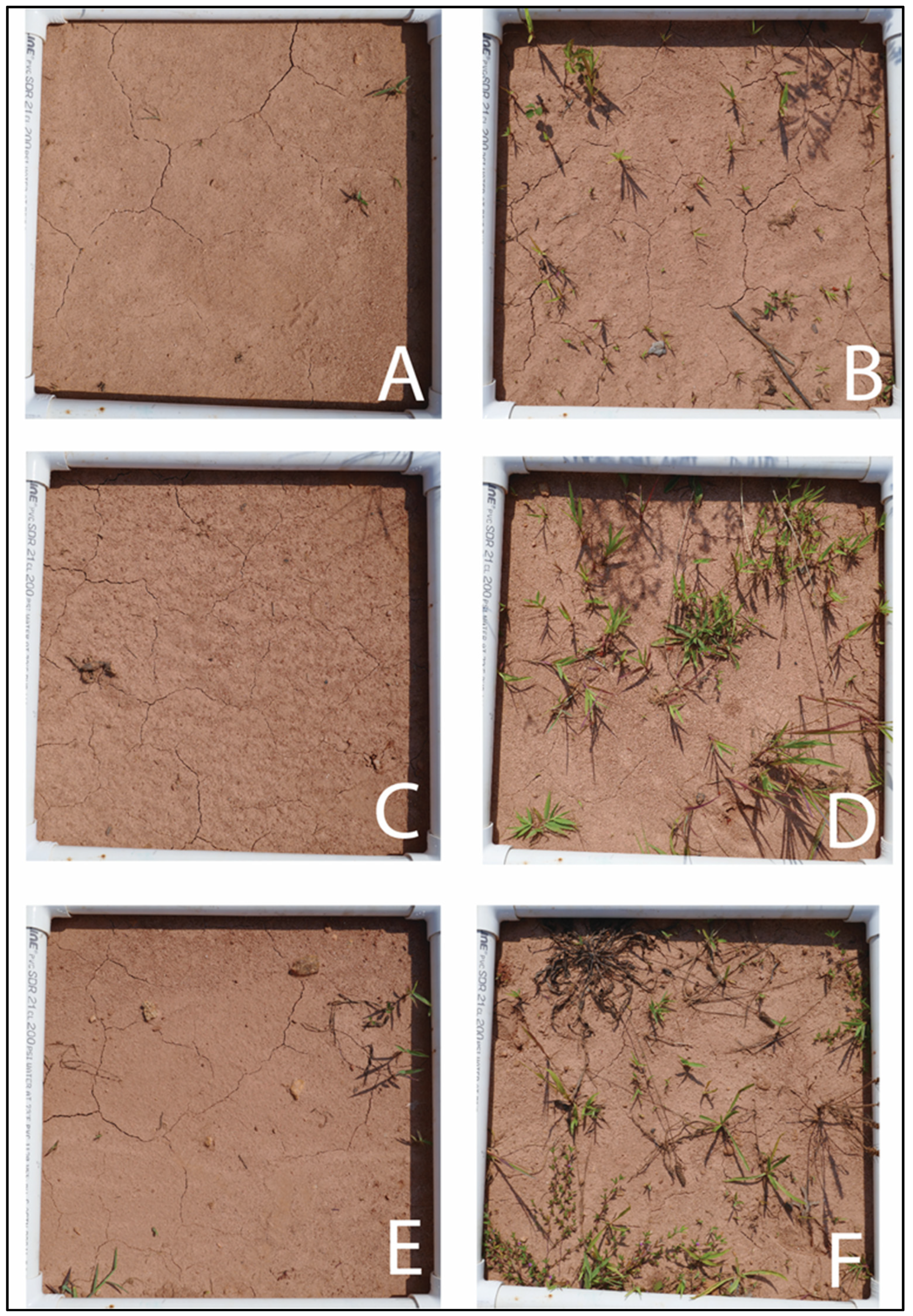
2.3. Bed Preparation
2.4. Plant Material
2.5. Insect-Trapping Methods
2.6. Data Measurements
2.6.1. Plants
2.6.2. Spontaneous Weeds
2.7. Statistical Analysis
3. Results
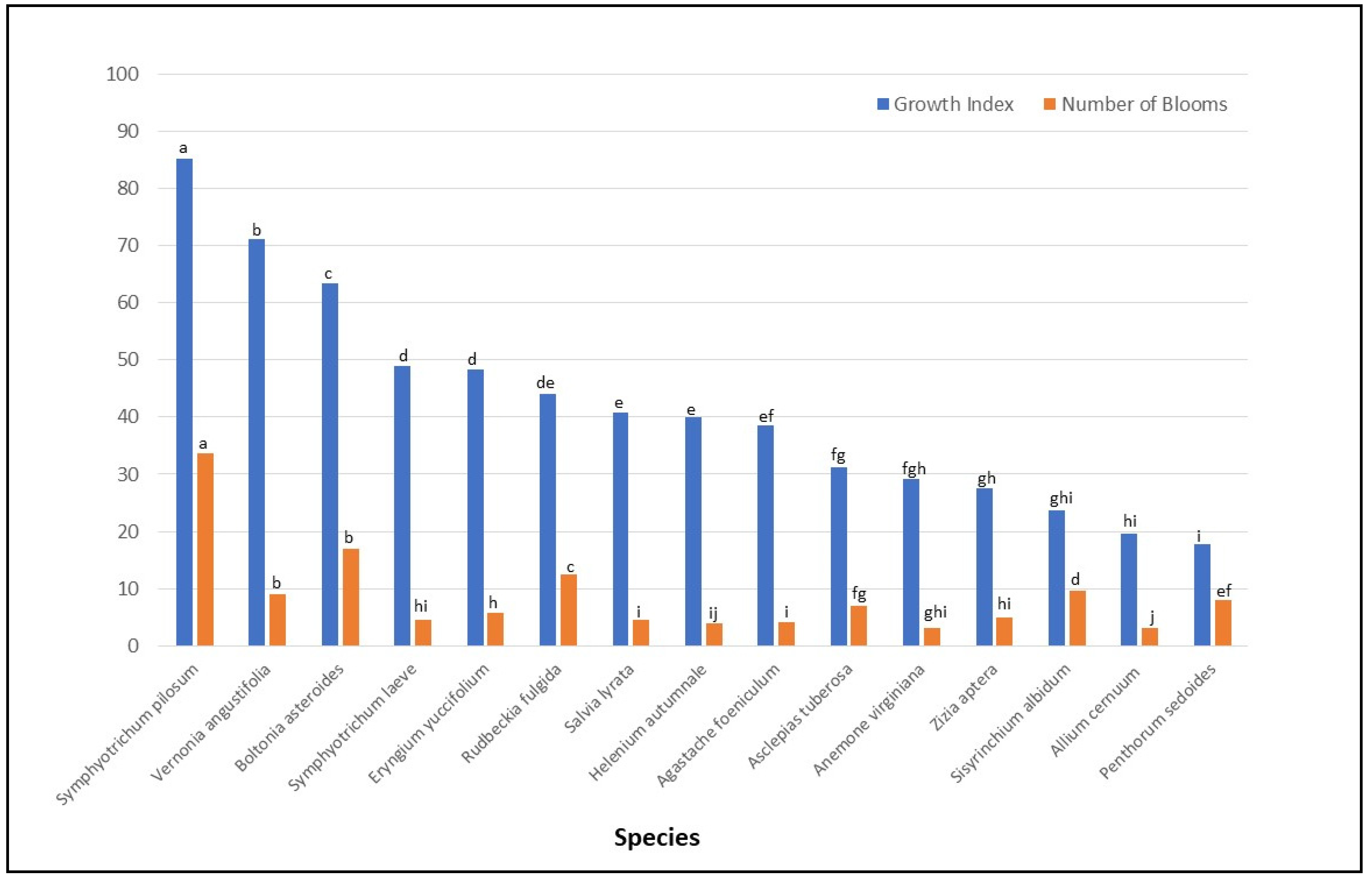
3.1. Plant Performance
3.2. Weed Control
3.3. Arthropod Presence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habel, J.C.; Samways, M.J.; Schmitt, T. Mitigating the precipitous decline of terrestrial European insects: Requirements for a new strategy. Biodivers. Conserv. 2019, 28, 1343–1360. [Google Scholar] [CrossRef]
- Brereton, T.M.; Warren, M.S.; Roy, D.B.; Stewart, K. The changing status of the Chalkhill Blue butterfly Polyommatus coridon in the UK: The impacts of conservation policies and environmental factors. J. Insect Conserv. 2008, 12, 629–638. [Google Scholar] [CrossRef]
- Modiba, R.V.; Joseph, G.S.; Seymour, C.L.; Fouché, P.; Foord, S.H. Restoration of riparian systems through clearing of invasive plant species improves functional diversity of Odonate assemblages. Biol. Conserv. 2017, 214, 46–54. [Google Scholar] [CrossRef]
- Carrié, R.; Ekroos, J.; Smith, H.G. Organic farming supports spatiotemporal stability in species richness of bumblebees and butterflies. Biol. Conserv. 2018, 227, 48–55. [Google Scholar] [CrossRef]
- Hopwood, J.; Black, S.H.; Lee-Mäder, E.; Charlap, A.; Preston, R.; Mozumder, K.; Fleury, S. Literature Review: Pollinator Habitat Enhancement and Best Management Practices in Highway Rights-of-Way; The Xerces Society for Invertebrate Conservation and ICF International, Federal Highway Administration: Washington, DC, USA, 2015. [Google Scholar]
- Perry, L. Successful Wildflower Meadows; University of Vermont Extension: Burlington, VT, USA, 2005; OH 84. [Google Scholar]
- Easton, V. Wildflower mixes: The good, the bad, the ugly. The Seattle Times, 25 February 2012. [Google Scholar]
- Haaland, C.; Nesbitt, R.E.; Bersier, O.F. Sown wildflower strips for insect conservation: A review. Insect Conserv. Divers. 2011, 4, 60–80. [Google Scholar] [CrossRef]
- Stokstad, E. Pesticides Under Fire for Risks to Pollinators. Science 2013, 340, 674–676. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Christmann, S. Pollinator protection strategies must be feasible for all nations. Nat. Ecol. Evol. 2020, 4, 896–897. [Google Scholar] [CrossRef]
- Bretzel, F.; Pezzarossa, B.; Carrai, C.; Malorgio, F. Wildflower plantings to reduce the management costs of urban gardens and roadsides. Acta Hortic. 2009, 813, 263–270. [Google Scholar] [CrossRef]
- Campbell, J.W.; Kimmel, C.B.; Grodsky, S.M.; Smithers, C.; Daniels, J.C.; Ellis, J.D. Wildflower plantings harbor increased arthropod richness and abundance within agricultural areas in Florida (USA). Ecosphere 2019, 10, e02890. [Google Scholar] [CrossRef]
- Ahern, J.; Barker, A. Roadside wildflower meadows: Summary of benefits and guidelines to successful establishment and management. Transp. Res. Rec. 1992, 1334, 46–53. [Google Scholar]
- Derr, J.F.; Appleton, B.L. Weed control with landscape fabrics. J. Environ. Hortic. 1989, 7, 129–133. [Google Scholar] [CrossRef]
- Corley, W.; Murphy, T.; Reynolds, K. Weed management options for wildflower meadows and beauty spots. SNA Res. Conf. Proc. 1993, 38, 135. [Google Scholar]
- Aldrich, J.H. Factors and Benefits in the Establishment of Modest-Sized Wildflower Plantings: A Review. Nativ. Plants J. 2002, 3, 67–86. [Google Scholar] [CrossRef]
- Skousen, J.; Venable, C. Establishing native plants on newly-constructed and older-reclaimed sites along West Virginia highways. Land Degrad. Dev. 2008, 19, 388–396. [Google Scholar] [CrossRef]
- Angelella, G.M.; Stange, L.; Scoggins, H.L.; O’rourke, M.E. Pollinator refuge establishment and conservation value: Impacts of seedbed preparations, seed mixtures, and herbicides. Hortscience 2019, 54, 445–451. [Google Scholar] [CrossRef]
- Johnston, C.R.; McCullough, P.E.; Shilling, D.G. Native plant establishment on georgia roadsides. Agron. J. 2015, 107, 990–996. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef]
- Quinn, J.P.; Peden, J.M.M.; Dick, R.E. Glyphosate tolerance and utilization by the microflora of soils treated with the herbicide. Appl. Microbiol. Biotechnol. 1988, 29, 511–516. [Google Scholar] [CrossRef]
- Gilreath, J.P.; Santos, B.M. Efficacy of methyl bromide alternatives on purple nutsedge (Cyperus rotundus) control in tomato and pepper. Weed Technol. 2004, 18, 341–345. [Google Scholar] [CrossRef]
- Fennimore, S.A.; Haar, M.J.; Goodhue, R.E.; Winterbottom, C.Q. Weed Control in Strawberry Runner Plant Nurseries with Methyl Bromide Alternative Fumigants. Hortscience 2008, 45, 1495–1500. [Google Scholar] [CrossRef]
- Czarnota, M.A. Georgia Pest Management Handbook; Commercial Edition; University of Georgia: Athens, GA, USA, 2016. [Google Scholar]
- Environmental Protection Agency. Climate Change Indicators: U.S. and Global Precipitation; United States Environmental Protection Agency: Washington, DC, USA, 2021. [Google Scholar]
- Norcini, J.G.; Aldrich, J.H. Establishment of Native Wildflower Plantings by Seed; ENH1046; University of Florida: Gainesville, FL, USA, 2004. [Google Scholar]
- Milstein, G.P. The uses and potential of wildflower seed in landscaping. In Flower Seeds: Biology and Technology; CABI Publishing: Columbus, OH, USA, 2005; pp. 39–51. [Google Scholar]
- Norcini, J.G.; Aldrich, J.H.; Halsey, L.A.; Lilly, J.G. Seed source affects performance of six wildflower species. Proc. Fla. State Hort. Soc. 1998, 111, 4–9. [Google Scholar] [CrossRef]
- Harris, B.A.; Braman, S.K.; Pennisi, S.V. Influence of plant taxa on pollinator, butterfly, and beneficial insect visitation. Hortscience 2016, 51, 1016–1019. [Google Scholar] [CrossRef]
- Dantas, B.F.; Moura, M.S.B.; Pelacani, C.R.; Angelotti, F.; Taura, T.A.; Oliveira, G.M.; Bispo, J.S.; Matias, J.R.; Silva, F.F.S.; Pritchard, H.W.; et al. Rainfall, not soil temperature, will limit the seed germination of dry forest species with climate change. Oecologia 2020, 192, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Whittinghill, L.J.; Rowe, D.B.; Ngouajio, M.; Cregg, B.M. Evaluation of nutrient management and mulching strategies for vegetable production on an extensive green roof. Agroecol. Sustain. Food Syst. 2016, 40, 297–318. [Google Scholar] [CrossRef]
- Gibson, D.R.; Rowe, L.; Isaacs, R.; A Landis, D. Screening Drought-Tolerant Native Plants for Attractiveness to Arthropod Natural Enemies in the U.S. Great Lakes Region. Environ. Èntomol. 2019, 48, 1469–1480. [Google Scholar] [CrossRef]
- Campbell, J.W.; Smithers, C.; Irvin, A.; Kimmel, C.B.; Stanley-Stahr, C.; Daniels, J.C.; Ellis, J.D. Trap nesting wasps and bees in agriculture: A comparison of sown wildflower and fallow plots in florida. Insects 2017, 8, 107. [Google Scholar] [CrossRef]
- Harris, B.A.; Poole, E.; Pennisi, S.V. Impact of biostimulant and cultural factors on whorled mountain mint Pycnanthemum verticillatum (Michx.) Pers. var. pilosum (Nutt.) Cooperr.: Growth performance and assessment of arthropod visitation. Nat. Plants J. 2022, 23, 97–114. [Google Scholar] [CrossRef]
- Braman, S.K.; Pennisi, S.V.; Fair, C.G.; Quick, J.C. Pollinator cultivar choice: An assessment of season-long pollinator visitation among coreopsis, aster, and salvia cultivars. Front. Sustain. Cities 2022, 4, 988966. [Google Scholar] [CrossRef]
- Kalaman, H.; Wilson, S.B.; Mallinger, R.E.; Knox, G.W.; Kim, T.; Begcy, K.; van Santen, E. Evaluation of native and nonnative ornamentals as pollinator plants in Florida: II. Floral resource value. HortScience 2021, 57, 137–143. [Google Scholar] [CrossRef]
- Palmersheim, M.C.; Schürch, R.; O’rourke, M.E.; Slezak, J.; Couvillon, M.J. If you grow it, they will come: Ornamental plants impact the abundance and diversity of pollinators and other flower-visiting insects in gardens. Horticulturae 2022, 8, 1068. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Larger wildflower plantings increase natural enemy density, diversity, and biological control of sentinel prey, without increasing herbivore density. Ecol. Èntomol. 2012, 37, 386–394. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G. Formation, characteristics and eco-environmental implications of urban soils—A review. Soil Sci. Plant Nutr. 2015, 61 (Suppl. S1), 30–46. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, G. Differences in shallow soil temperatures at urban and rural areas. J. Eng. Geol. 2012, 20, 58–65. [Google Scholar]
- Schellenberg, M.P.; Biligetu, B.; Lamb, E.G.; Mischkolz, J.M. World interest in diverse native plant stands. In PXXII International Grassland Congress Proceedings; University of Kentucky: Lexington, KY, USA, 2022. [Google Scholar]
- Bang, C.; Faeth, S.H. Variation in arthropod communities in response to urbanization: Seven years of arthropod monitoring in a desert city. Landsc. Urban Plan. 2011, 103, 383–399. [Google Scholar] [CrossRef]
- Sutter, L.; Albrecht, M.; Jeanneret, P. Landscape greening and local creation of wildflower strips and hedgerows promote multiple ecosystem services. J. Appl. Ecol. 2017, 55, 612–620. [Google Scholar] [CrossRef]
- Mody, K.; Lerch, D.; Müller, A.-K.; Simons, N.K.; Blüthgen, N.; Harnisch, M. Flower power in the city: Replacing roadside shrubs by wildflower meadows increases insect numbers and reduces maintenance costs. PLoS ONE 2020, 15, e0234327. [Google Scholar] [CrossRef]
- Ullmann, K.S.; Meisner, M.H.; Williams, N.M. Impact of tillage on the crop pollinating, ground-nesting bee, Peponapis pruinosa in California. Agric. Ecosyst. Environ. 2016, 232, 240–246. [Google Scholar] [CrossRef]
- Wilson, J.; Carril, O. The Bees in Your Backyard; Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar]
- Angelella, G.M.; O’rourke, M.E. Pollinator habitat establishment after organic and no-till seedbed preparation methods. Hortscience 2017, 52, 1349–1355. [Google Scholar] [CrossRef]
- Jordan, S.F.C.; Jessa, K.; Gill, K.; Hopwood, J.; Fowler, J.; Lee-Mader, E.; Vaughan, M. Wildflower Establishment, Organic Site Preparation Methods; Xerces Society for Invertebrate Conservation: Portland, OR, USA, 2016. [Google Scholar]
- Parys, K.A.; Esquivel, I.L.; Wright, K.W.; Griswold, T.; Brewer, M.J. Native Pollinators (Hymenoptera: Anthophila) in Cotton Grown in the Gulf South, United States. Agronomy 2020, 10, 698. [Google Scholar] [CrossRef]
- Harris, B.; Braman, S.; Pennisi, S. Pan trap designs for monitoring pollinators and other beneficial insects in conservation gardens. J. Èntomol. Sci. 2017, 52, 9–14. [Google Scholar] [CrossRef]
- Triplehorn, C.A.; Johnson, N.F. Chapter 28: Order Hymenoptera. In Borror and DeLong’s Introduction to the Study of Insects, 7th ed.; Brooks/Cole, Cengage Learning: Boston, MA, USA, 2019. [Google Scholar]
- Irmak, S.; Haman, D.Z.; Irmak, A.; Jones, J.W.; Campbell, K.L.; Crisman, T.L. Measurement and Analyses of Growth and Stress Parameters of Viburnum odoratissimum (Ker-gawl) Grown in a Multi-pot Box System. Hortscience 2004, 39, 1445–1455. [Google Scholar] [CrossRef]
- Shaner, D.C. Herbicide Handbook, 10th ed.; Weed Science Society of America: Westminster, CO, USA, 2014. [Google Scholar]
- Lyons, M.T. Composts, Biofumigation, Dazomet, and Integrated Treatments Influence Southern Blight, Yield and Microbial Community Composition in Plasticulture Tomato Production; The University of Tennessee: Knoxville, TN, USA, 2003. [Google Scholar]
- Nunes, M.R.; Karlen, D.L.; Moorman, T.B. Tillage intensity effects on soil structure indicators—A US meta-analysis. Sustainability 2020, 12, 2071. [Google Scholar] [CrossRef]
- Gardiner, M.M. Chapter 8: Predatory Flies. In Good Garden Bugs: Everything You Need to Know About Beneficial Predatory Insects; Quarry Books: Beverly, MA, USA, 2015. [Google Scholar]
- Eliyahu, D.; McCall, A.C.; Lauck, M.; Trakhtenbrot, A.; Bronstein, J.L. Minute pollinators: The role of thrips (Thysanoptera) as pollinators of pointleaf manzanita, Arctostaphylos pungens (Ericaceae). J. Pollinat. Ecol. 2015, 16, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Poythress, J.C.; Affolter, J.M. Ecological Value of Native Plant Cultivars versus Wild-Type Native Plants for Promoting Hemipteran Diversity in Suburban Areas. Environ. Èntomol. 2018, 47, 890–901. [Google Scholar] [CrossRef]
- Shelton, J. Does Bed Preparation Impact Native Wildflower Establishment? Cost Analysis and Implications for Biodiversity; University of Georgia: Athens, GA, USA, 2021. [Google Scholar]
- Huber, G. Understanding Perceptions to Improve the Success and Acceptance of Insect Pollinator Habitat in Public Spaces; University of Georgia: Athens, GA, USA, 2020. [Google Scholar]
- European Union Commission. Available online: https://food.ec.europa.eu/plants/pesticides/approval-active-substances/renewal-approval/glyphosate_en#:~:text=The%20Appeal%20Committee%20also%20did,glyphosate%20until%2015%20December%202023 (accessed on 1 May 2021).

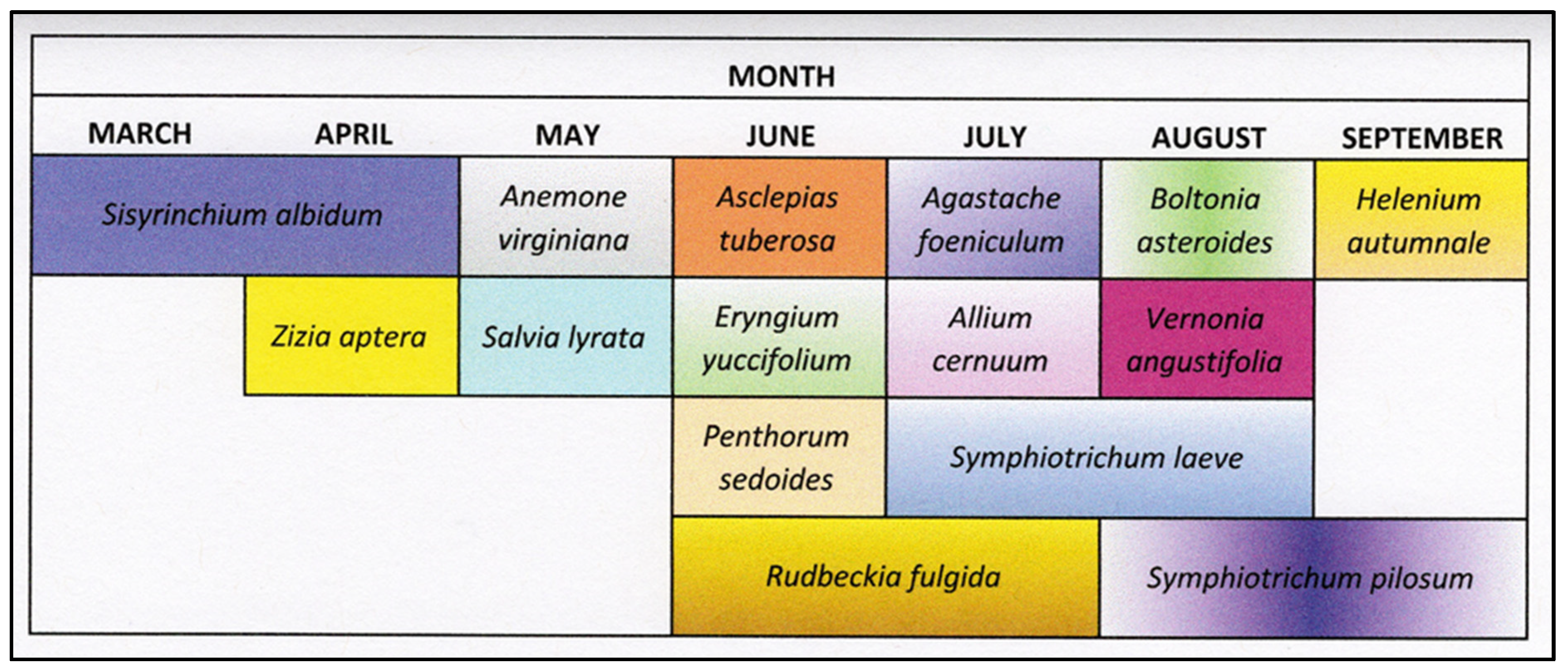
| Plant Species | Common Name | Native Range | Blooming Period |
|---|---|---|---|
| Agastache foeniculum ((Nutt.) Britton) (Lamiales; Lamiaceae) | anise hyssop | Northeastern United States | June to September |
| Allium cernuum Roth. (Liliales; Liliaceae) | wild-nodding onion | Northeastern United States | June to August |
| Anemone virginiana L. (Ranunculales; Ranunculaceae) | thimbleweed | United States | April to May |
| Asclepias tuberosa L. (Gentianales: Asclepiadaceae) | butterfly weed | Eastern North America | June to August |
| Boltonia asteroides L. (Asterales; Asteraceae) | white boltonia | Eastern North America | August to September |
| Eryngium yuccifolium Michx. (Apiales: Apiaceae) | rattlesnake master | United States | June to September |
| Helenium autumnale L. (Asterales; Asteraceae) | common sneezeweed | Eastern North America | August to October |
| Penthroum sedoides L. (Saxifragales; Penthoraceae) | ditch stonecrop | North America | July to September |
| Rudbeckia fulgida Aiton. (Asterales: Asteraceae) | orange coneflower | Southeastern United States | June to October |
| Salvia lyrata L. (Lamiales; Lamiaceae) | lyre-leaf sage | United States | April to June |
| Sisyrinchium albidum Raf. (Asparagales; Iridaceae) | blue-eyed grass | North America | May to July |
| Symphyotrichum laeve L. (Asterales; Asteraceae) | smooth blue aster | North America | September to October |
| Symphyotrichum pilosum L. (Asterales; Asteraceae) | hairy aster | North America | August to October |
| Vernonia angustifolia Michx. (Asterales; Asteraceae) | narrow-leaf ironweed | Eastern United States | June to September |
| Zizia aptera (A. Gray) Fernald (Apiales; Apiaceae) | meadow zizia | North America | May |
| Effect | Plant Morphometrics | |||
|---|---|---|---|---|
| Growth Index | Number of Blooms | |||
| F-Value | Pr > F | F-Value | Pr > F | |
| Bed Preparation | 182.4 | <0.0001 | 577.6 | <0.0001 |
| Plant Species | 136.6 | <0.0001 | 591.3 | <0.0001 |
| Bed Preparation × Plant Species | 10.4 | <0.0001 | 20.2 | <0.0001 |
| Species | Treatment | |||
|---|---|---|---|---|
| Tillage + Dazomet | Non-Tillage + Glyphosate | |||
| Growth Index | No. of Blooms | Growth Index | No. of Blooms | |
| Symphyotrichum pilosum | 92.7 (3.1) a | 50.0 (3.5) a | 77.8 (3.1) a | 22.7 (1.7) a |
| Vernonia angustifolia | 84.3 (2.3) ab | 16.1 (1.2) d | 58.1 (2.3) b | 5.1 (0.4) d |
| Boltonia asteroides | 75.3 (1.9) b | 28.0 (2.0) b | 51.6 (1.9) bc | 10.2 (0.8) b |
| Eryngium yuccifolium | 53.8 (1.9) c | 7.7 (0.6) g | 42.9 (1.0) cd | 4.2 (0.3) de |
| Agastache foeniculum | 52.8 (2.2) c | 6.0 (0.5) ghi | 24.3 (2.1) gh | 2.9 (0.4) ef |
| Symphyotrichum laeve | 51.0 (2.2) c | 6.8 (0.6) gh | 46.9 (2.4) bcd | 3.0 (0.4) ef |
| Helenium autumnale | 50.3 (2.4) c | 4.0 (0.5) ij | 29.6 (2.4) fgh | 4.0 (0.5) def |
| Rudbeckia fulgida | 46.6 (2.3) cd | 19.3 (1.4) c | 41.3 (2.3) cde | 7.9 (0.6) c |
| Salvia lyrata | 44.1 (2.3) cde | 6.0 (0.5) hi | 37.3 (2.3) def | 3.4 (0.3) ef |
| Anemone virginiana | 32.6 (2.9) defg | 5.5 (0.7) ghij | 25.7 (3.2) efgh | 4.2 (0.7) cdef |
| Aclepias tuberosa | 32.6 (2.3) ef | 9.5 (0.7) f | 29.9 (2.4) efg | 5.1 (0.4) d |
| Zizia aptera | 28.3 (2.4) fg | 5.9 (0.5) hi | 26.6 (2.4) fgh | 4.2 (0.3) de |
| Sisyrinchium albidum | 25.4 (2.3) fg | 12.4 (0.9) e | 21.8 (2.1) gh | 7.5 (0.6) c |
| Allium cernuum | 22.9 (2.3) fg | 3.7 (0.3) ij | 16.5 (2.3) h | 2.5 (0.3) f |
| Penthorum sedoides | 18.9 (2.4) g | 8.2 (0.9) fg | 16.9 (2.4) h | 7.7 (0.7) f |
| Bed Preparation | Number of Weeds | Weed Coverage |
|---|---|---|
| Tillage + dazomet | 8.1 (0.9) b | 462.5 (65.7) |
| Non-tillage + glyphosate | 33.8 (3.0) a | 1025.7 (145.7) |
| Arthropod Order/Group | Number | Mean |
|---|---|---|
| Diptera | 819 | 5.69 (1.22) a |
| Thysanoptera | 812 | 5.64 (0.07) a |
| Hymenoptera: bees | 177 | 1.23 (0.17) bc |
| Aranea | 158 | 1.10 (0.23) b |
| Parasitica | 126 | 0.87 (0.12) bcd |
| Hemiptera | 105 | 0.73 (0.12) bcd |
| Psocoptera | 95 | 0.66 (0.10) ced |
| Hemiptera: aphids | 76 | 0.52 (0.07) fed |
| Coleoptera | 66 | 0.46 (0.07) fed |
| Hymenoptera: wasps | 44 | 0.30 (0.05) feg |
| Collembola | 32 | 0.22 (0.04) fhg |
| Lepidoptera | 14 | 0.10 (0.02) hg |
| Orthoptera | 5 | 0.03 (0.01) h |
| Total number | 2529 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelton, J.S.; Pennisi, S.V.; Czarnota, M. Effect of Bed Preparation on Native Wildflower Establishment, Weed Control, and Arthropod Presence. Horticulturae 2023, 9, 854. https://doi.org/10.3390/horticulturae9080854
Shelton JS, Pennisi SV, Czarnota M. Effect of Bed Preparation on Native Wildflower Establishment, Weed Control, and Arthropod Presence. Horticulturae. 2023; 9(8):854. https://doi.org/10.3390/horticulturae9080854
Chicago/Turabian StyleShelton, Joseph S., Svoboda V. Pennisi, and Mark Czarnota. 2023. "Effect of Bed Preparation on Native Wildflower Establishment, Weed Control, and Arthropod Presence" Horticulturae 9, no. 8: 854. https://doi.org/10.3390/horticulturae9080854
APA StyleShelton, J. S., Pennisi, S. V., & Czarnota, M. (2023). Effect of Bed Preparation on Native Wildflower Establishment, Weed Control, and Arthropod Presence. Horticulturae, 9(8), 854. https://doi.org/10.3390/horticulturae9080854






