Hop Tropicalization: Chemical Compositions of Varieties Grown under Organic and Conventional Systems in Subtropical Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area
2.2. Treatments and Experimental Design
2.2.1. Description of Varieties
2.2.2. Conventional Cultivation
2.2.3. Organic Farming
2.3. Evaluations
2.3.1. Chemical Composition of the Essential Oil
2.3.2. Quantification of Alpha Acids, Beta Acids and Xanthohumol
2.4. Statistical Analysis
3. Results
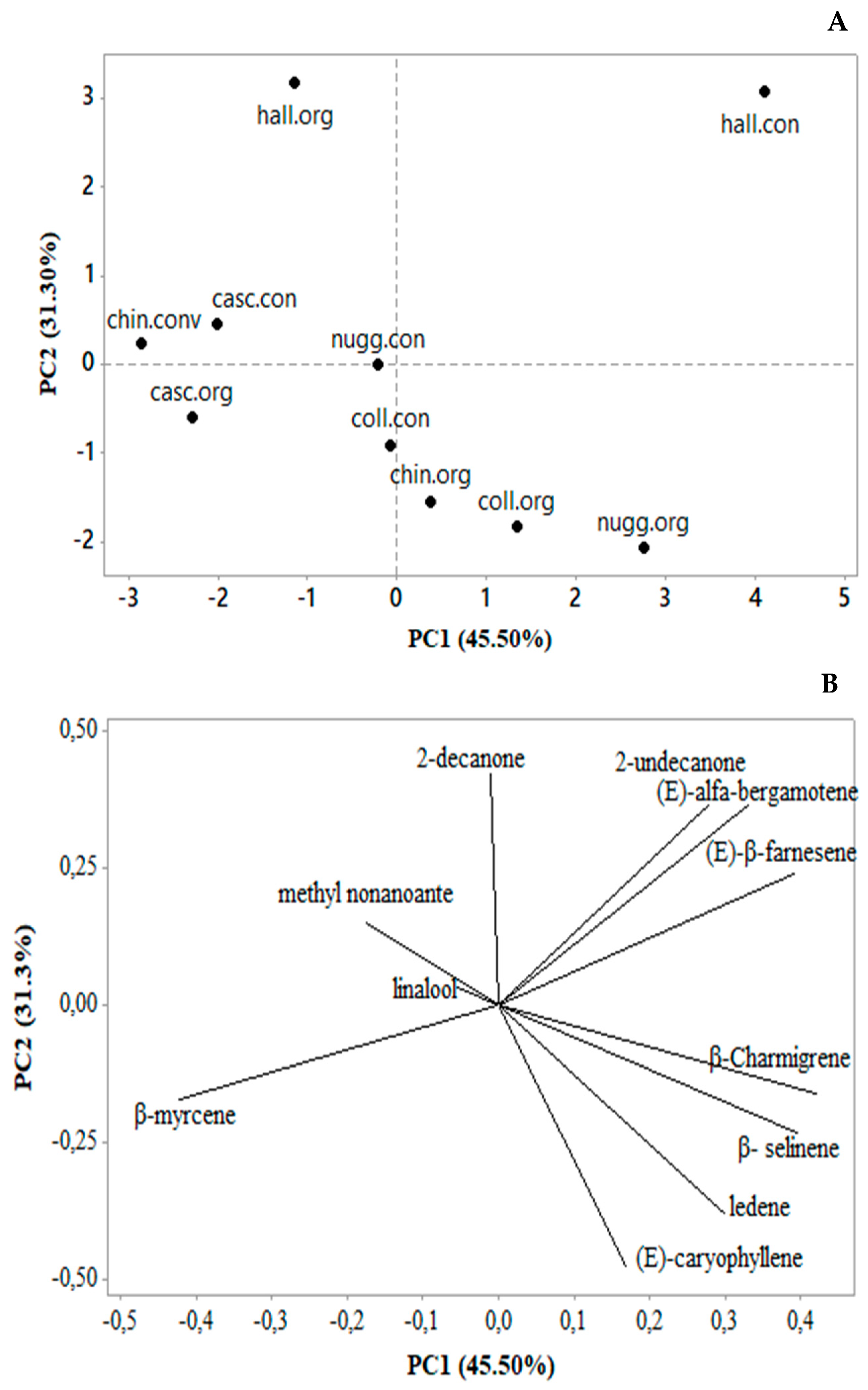
3.1. Chemical Composition of the Essential Oils
3.2. Alpha acids and Beta Acids Contents
4. Discussion
4.1. Chemical Composition of the Essential Oils
4.2. Alpha Acid and Beta Acid Contents
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| pH | OM | P | K | Ca | Mg | CEC | V% | S | B | Cu | Fe | Mn | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaCl2 | G·dm−3 | mg·dm−3 | mmolc·dm−3 | % | mg·dm−3 | ||||||||||
| Date | Sys./Depth (cm) | ||||||||||||||
| Nov. 2018 | Org 0–20 | 5.4 | 29 | 49 | 13.5 | 32 | 15 | 84 | 72 | 124 | 0.44 | 4.7 | 34 | 6.9 | 3.0 |
| Org 20–40 | 5.0 | 22 | 35 | 7.5 | 25 | 12 | 84 | 53 | 77 | 0.63 | 4.9 | 34 | 5.3 | 1.6 | |
| Conv 0–20 | 5.6 | 19 | 29 | 9.6 | 26 | 9 | 65 | 68 | 139 | 0.38 | 5.4 | 27 | 4.7 | 0.6 | |
| Conv 20–40 | 5.3 | 18 | 12 | 2.6 | 14 | 5 | 51 | 42 | 52 | 0.31 | 5.5 | 39 | 4.6 | 0.4 | |
| Apr. 2019 | Org 0–20 | 5.3 | 17 | 27 | 1.9 | 25 | 11 | 63 | 60 | 4 | 0.43 | 2.0 | 34 | 6.8 | 1.3 |
| Org 20–40 | 5.0 | 17 | 23 | 2.9 | 14 | 11 | 67 | 42 | 25 | 0.55 | 1.2 | 34 | 5.7 | 1.1 | |
| Conv 0–20 | 5.4 | 22 | 34 | 1.4 | 29 | 10 | 70 | 58 | 27 | 0.48 | 1.9 | 35 | 4.5 | 0.5 | |
| Conv 20–40 | 4.5 | 16 | 10 | 1.2 | 12 | 7 | 63 | 33 | 43 | 0.44 | 0.5 | 36 | 2.1 | 0.4 | |
| Aug. 2019 | Org 0–20 | 5.7 | 21 | 14 | 1.6 | 33 | 12 | 64 | 72 | 17 | 0.34 | 3.6 | 18 | 2.1 | 1.2 |
| Org 20–40 | 4.4 | 15 | 3 | 0.5 | 11 | 5 | 64 | 26 | 67 | 0.33 | 4.5 | 18 | 0.8 | 0.1 | |
| Conv 0–20 | 5.0 | 19 | 17 | 0.3 | 19 | 7 | 58 | 45 | 34 | 0.30 | 4.1 | 21 | 2.4 | 0.2 | |
| Conv 20–40 | 4.3 | 15 | 4 | 0.4 | 11 | 4 | 70 | 21 | 48 | 0.35 | 4.8 | 17 | 1.7 | 0.2 | |
| Nov. 2019 | Org 0–20 | 6.0 | 24 | 39 | 4.3 | 47 | 13 | 84 | 76 | 101 | 0.39 | 4.2 | 24 | 3.7 | 3.9 |
| Org 20–40 | 5.6 | 22 | 40 | 2.8 | 40 | 17 | 83 | 71 | 80 | 0.63 | 3.8 | 24 | 3.4 | 3.4 | |
| Conv 0–20 | 5.8 | 25 | 44 | 4.0 | 80 | 10 | 119 | 79 | 422 | 0.63 | 3.6 | 19 | 3.4 | 3.3 | |
| Conv 20–40 | 5.6 | 25 | 49 | 5.5 | 66 | 8 | 107 | 74 | 305 | 0.58 | 4.0 | 26 | 3.9 | 2.8 | |
| Mar. 2020 | Org 0–20 | 5.0 | 25 | 56 | 3.4 | 39 | 11 | 77 | 70 | 71 | 1.00 | 4.7 | 19 | 5.1 | 3.8 |
| Org 20–40 | 4.8 | 19 | 30 | 2.2 | 23 | 10 | 70 | 50 | 52 | 0.90 | 5.4 | 17 | 3.0 | 1.7 | |
| Conv 0–20 | 4.9 | 19 | 16 | 2.6 | 20 | 5 | 64 | 42 | 42 | 1.09 | 6.1 | 19 | 3.2 | 0.9 | |
| Conv 20–40 | 4.4 | 15 | 6 | 1.4 | 13 | 4 | 63 | 29 | 148 | 0.86 | 5.3 | 16 | 3.1 | 0.4 | |
References
- Denby, C.M.; Li, R.A.; Vu, V.T.; Costello, Z.; Lin, W.; Chan LJ, G.; Keasling, J.D. Industrial brewing yeast engineered for the production of primary flavor determinants in hopped beer. Nat. Commun. 2018, 9, 965. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, L.; Sahpaz, S.; Hilbert, J.L.; Rambaud, C.; Rivière, C. Humulus lupulus L., a very popular beer ingredient and medicinal plant: Overview of its phytochemistry, its bioactivity, and its biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Bamforth, W.C. Beer is Proof God Loves Us; FT Press: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Pavolovič, V.; Čerenak, A.; Pavolovič, M.; Košir, I.J.; Rozman, Č.; Bohanec, M.; Naglič, B. Modelling of Quality Prediction for Hops (Humulus lupulus L.) in Relation to Meteorological Variables. Balwois 2010, 25–29. Available online: https://balwois.com/wp-content/uploads/old_proc/ffp-1920.pdf (accessed on 1 June 2023).
- Boulton, C. Encyclopedia of Brewing, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gerhauser, C.; Alt, A.; Heiss, E.; Gamal-Eldeen, A.; Klimo, K.; Knauft, J.; Becker, H. Cancer chemopreventive activity of Xanthohumol, a natural product derived from hop. Mol. Cancer Ther. 2002, 1, 959–969. Available online: https://www.researchgate.net/publication/10992279_Cancer_chemopreventive_activity_of_Xanthohumol_a_natural_product_derived_from_hop (accessed on 15 July 2021).
- COMEXSTAT. Dados Estatísticos do Comércio Exterior Brasileiro. Available online: http://comexstat.mdic.gov.br/pt/home (accessed on 20 February 2021).
- Kavalier, A.R.; Litt, A.; Ma, C.; Pitra, N.J.; Coles, M.C.; Kennelly, E.J.; Matthews, P.D. Phytochemical and Morphological Characterization of Hop (Humulus lupulus L.) Cones over Five Developmental Stages Using High Performance Liquid Chromatography Coupled to Time-of-Flight Mass Spectrometry, Ultrahigh Performance Liquid Chromatography Photodiode Array Detection, and Light Microscopy Techniques. J. Agric. Food Chem. 2011, 59, 4783–4793. [Google Scholar] [CrossRef]
- Savithramma, N.; Linga Rao, M.; Suhrulatha, D. Screening of medicinal plants for secondary metabolites. Middle East J. Sci. Res. 2011, 8, 579–584. [Google Scholar]
- Matsui, H.; Inui, T.; Ishimaru, M.; Hida, Y. The influence of the age of a hop plant on the quality of hop aromas in beer. Acta Hortic. 2013, 1010, 171–182. [Google Scholar] [CrossRef]
- Van Holle, A.; Van Landschoot, A.; Roldán-Ruiz, I.; Naudts, D.; De Keukeleire, D. The brewing value of Amarillo hops (Humulus lupulus L.) grown in northwestern USA: A preliminary study of terroir significance. J. Inst. Brew. 2017, 123, 312–318. [Google Scholar] [CrossRef]
- Rettberg, N.; Biendl, M.; Garbe, L. Hop Aroma and Hoppy Beer Flavor: Chemical Backgrounds and Analytical Tools—A. Review, J. Am. Soc. Brew. Chem. 2018, 76, 1402574. [Google Scholar] [CrossRef]
- Rodolfi, M.; Chiancone, B.; Liberatore, C.M.; Fabbri, A.; Cirlini, M.; Ganino, T. Changes in chemical profile of Cascade hop cones accordingto the growing area. J. Sci. Food Agric. 2019, 99, 6011–6019. [Google Scholar] [CrossRef]
- Campos, O.P.; Leme, F.M.; Fortuna, G.C.; Gomes, J.A.O.; Neves, C.S.; Arruda, R.C.O.; Bonfim, F.P.G. Morphological characteristics, trichomes, and phytochemistry of inflorescences of Humulus lupulus L: Comparison of cropping systems and varieties. Aust. J. Crop Sci. 2023, 17, 263–274. [Google Scholar] [CrossRef]
- Grzyb, Z.S.; Piotrowski, W.; Bielicki, P.; Sas Paszt, L. Quality of Apple Maidens as Influenced by the Frequency of Application of Different Fertilizers in the Organic Nursery—Preliminary Results. J. Fruit Ornam. Plant Res. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Solarska, E.; Sosnowska, B. The impact of plant protection and fertilization on content of bioactive substances in organic hops. Acta Sci. Pol. Hortorum Cultus 2015, 14, 93–101. Available online: https://www.cabdirect.org/cabdirect/abstract/20153213020 (accessed on 25 July 2021).
- Jastrombek, J.M.; Faguerazzi, M.M.; de Cássio Pierezan, H.; Rufato, L.; Sato, A.J.; da Silva Ricce, W.; Marques, V.V.; Leles, N.R.; Roberto, S.R. Hop: An Emerging Crop in Subtropical Areas in Brazil. Horticulturae 2022, 8, 393. [Google Scholar] [CrossRef]
- Köppen, W. Climatologia: Con Un Studio De Los Climas De La Tierra; Fundo de Cultura Econômica: Mexico City, México, 1948. [Google Scholar]
- Gingrich, C.; Hart, J.; Christensen, N. Hops Fertilizer Guide; OSU Extension Catalog, Oregon State University: Corvallis, OR, USA, 2018. [Google Scholar]
- Healey, J. The Hops List: 265 Beer Hop Varieties from Around The World; 2016; pp. 94–95, 109–110, 124–125, 205–206, 303–304. Available online: https://www.melkkobrew.fi/the-hops-list-by-j-healey (accessed on 1 June 2023).
- Su, X.; Yin, Y. Aroma characterization of regional Cascade and Chinook hops (Humulus lupulus L.). Food Chem. 2021, 364, 130410. [Google Scholar] [CrossRef]
- Alves, F.; Fandiño, M.; Mirás-Avalos, J.M.; Rey, B.J.; Mota, M.; Cancela, J.J. Effects of irrigation on hop (Humulus lupulus L.) cv. Nugget in Galicia: Yield and quality aspects. In Proceedings of the Scientific-Techinical Comission of International Hop Growers Convention, Bischoffsheim, Alsace, France, 7–11 July 2019. [Google Scholar]
- Forteschi, M.; Porcu, M.C.; Fanari, M.; Zinellu, M.; Secchi, N.; Buiatti, S.; Passaghe, P.; Bertoli, S.; Pretti, L. Quality assessment of Cascade Hop (Humulus lupulus L.) grown in Sardinia. Eur. Food Res. Technol. 2019, 245, 863–871. [Google Scholar] [CrossRef]
- Katsiotis, S.T.; Langezaal, C.R.; Scheffer, J.J.C.; Verpoorte, R. Comparative study of the essential oils from hops of various Humulus lupulus L. cultivars. Flavour Fragr. J. 1989, 4, 187–191. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/ Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Prencipe, F.P.; Brighenti, V.; Rodolfi, M.; Mongelli, A. Development of a new high-performance liquid chromatography method with diode array and electrospray ionization-mass spectrometry detection for the metabolite fingerprinting of bioactive compounds in Humulus lupulus L. J. Chromatogr. A 2014, 1349, 50–59. [Google Scholar] [CrossRef]
- Minitab, LLC. 2021. Available online: www.minitab.com (accessed on 1 June 2023).
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência E Agrotecnologia Lavras 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Durello, R.S.; Silva, L.M.; Bogusz, S., Jr. Química do lúpulo. Quim Nova. 2019, 42, 900–919. [Google Scholar] [CrossRef]
- Yan, D.; Wong, Y.F.; Tedone, L.; Shellie, R.; Marriott, P.; Whittock, S.; Koutoulis, A. Chemotyping of new hop (Humulus lupulus L.) genotypes using comprehensive two-dimensional gas chromatography with quadrupole accurate mass-time-of-flight mass spectrometry. J. Chromatogr. A 2017, 1536, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Biendl, M.; Engelhard, B.; Forster, A.; Gahr, A.; Lutz, A.; Mitter, W.; Schönberger, C. Hops: Their Cultivation, Composition and Usage; Fachverlag Hans Carl: Nuremberg, Germany, 2014. [Google Scholar]
- Variety manual. In: Hop Growers of America Website. [Yakima, WA: Hop Growers of America]. 2018. Available online: https://www.usahops.org/ (accessed on 10 August 2021).
- Kishimoto, T. Investigations of hop-derived odor-active compounds in beer, hop flavor and aroma. In Proceedings of the 1st International Brewers Symposium; American Society of Brewing Chemists, Master Brewers Association of the Americas, UEA: St. Paul, Brazil, 2009. [Google Scholar]
- Holopainen, J.K. Multiple functions of inducible plant volatiles. Trends Plant Sci. 2004, 9, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wong, Y.F.; Shellie, R.; Marriott, P.; Whittock, S.; Koutoulis, A. Assessment of the phytochemical profiles of novel hop (Humulus lupulus L.) cultivars: A potential route to beer crafting. Food Chem. 2018, 275, 15–23. [Google Scholar] [CrossRef]
- Krofta, K.; Kučera, J. Mathematical model for prediction of alpha acid contents from meteorological data for ‘Saaz’ aroma variety. Acta Hortic. 2009, 848, 131–139. [Google Scholar] [CrossRef]
- Sharp, D.; Townsend, M.; Qian, Y.; Shellhammer, T.H. Harvest Maturity on the Chemical Composition of Cascade and Willamette Hops. J. Am. Soc. Brew. Chem. 2014, 72, 231–238. [Google Scholar] [CrossRef]
- Prado, M.R. Nutrição de Plantas; UNESP: São Paulo, Brazil, 2008. [Google Scholar]
- De Keukeleire, J.; Janssens, I.; Heyerick, A.; Ghekiere, G.; Cambie, J.; Roldán-Ruiz, I.; De Keukeleire, D. (Relevance of organic farming and effect of climatological conditions on the formation of α-acids, β-acids, desmethylxanthohumol, and xanthohumol in hop (Humulus lupulus L.). J. Agric. Food Chem. 2007, 55, 61–66. [Google Scholar] [CrossRef]
- McAdam, E.L.; Vaillancourt, R.E.; Koutoulis, A.; Whittock, S.P. Quantitative genetic parameters for yield, plant growth and cone chemical traits in hop (Humulus lupulus L.). BMC Genet. 2014, 15, 22. [Google Scholar] [CrossRef]
- Sirrine, R.; Lizotte, E.; Brown, D.; O’Brien, T.; Leach, A. Estimated Costs of Producing Hops in Michigan. Available online: https://www.canr.msu.edu/uploads/resources/pdfs/estimated_costs_of_producing_hops_in_michigan_(e3236).pdf (accessed on 15 June 2023).
- Mozny, M.; Tolasz, R.; Nekovar, J.; Sparks, T.; Trnka, M.; Zalud, Z. The impact of climate change on the yield and quality of Saaz hops in the Czech Republic. Agric. For. Meteorol. 2009, 149, 913–919. [Google Scholar] [CrossRef]
| Substance (%) | LRI Cal | LRI Lit | Org Cas | Con Cas | Org Nug | Con Nug | Org Chi | Con Chi | Org Hal | Con Hal | Org Col | Con Col | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | β-Pinene | 976 | 974 | 0.90 ± 0.06 | 0.68 ± 0.32 | 0.48 ± 0.30 | 0.52 ± 0.37 | 0.57 ± 0.39 | 0.82 ± 0.02 | 0.68 ± 0.06 | 0.23 ± 0.00 | 0.50 ± 0.18 | 0.50 ± 0.19 |
| 2 | Myrcene | 991 | 988 | 55.92 ± 7.21 | 51.63 ± 19.08 | 41.40 ± 13.49 | 43.20 ± 16.43 | 43.97 ± 19.06 | 57.65 ± 2.68 | 39.26 ± 3.15 | 14.83 ± 5.65 | 38.27 ± 11.82 | 44.84 ± 3.83 |
| 3 | Methylheptanone | 1021 | 1021 | -- | -- | -- | -- | -- | -- | 0.38 ± 0.11 | 0.27 ± 0.07 | -- | -- |
| 4 | 2-Nonanal | 1089 | 1087 | -- | -- | -- | -- | -- | -- | 0.43 ± 0.14 | 0.28 ± 0.10 | -- | -- |
| 5 | Linalool | 1098 | 1095 | 0.65 ± 0.08 | 0.79 ± 0.27 | 0.62 ± 0.13 | 0.91 ± 0.26 | 0.52 ± 0.19 | 0.68 ± 0.04 | 0.62 ± 0.12 | 0.66 ± 0.00 | 0.59 ± 0.25 | 0.72 ± 0.11 |
| 6 | n-Nonanal | 1102 | 1100 | -- | -- | -- | -- | -- | -- | 0.63 ± 0.34 | 0.54 ± 0.13 | -- | -- |
| 7 | Methyl octanoate | 1123 | 1123 | -- | -- | -- | -- | -- | -- | 0.24 ± 0.04 | 0.23 ± 0.02 | -- | -- |
| 8 | 2-Decanone | 1191 | 1192 | 0.24 ± 0.01 | 0.32 ± 0.18 | -- | -- | -- | -- | 1.09 ± 0.92 | 0.49 ± 0.05 | -- | -- |
| 9 | Methyl nonanoate | 1222 | 1223 | 0.60 ± 0.26 | 1.03 ± 0.60 | 0.40 ± 0.19 | 1.39 ± 0.60 | 0.45 ± 0.15 | 0.69 ± 0.32 | 0.56 ± 0.22 | 1.16 ± 0.18 | 0.23 ± 0.00 | 0.23 ± 0.00 |
| 10 | 2-Undecanone | 1191 | 1293 | -- | -- | -- | -- | -- | -- | 0.64 ± 0.55 | 2.42 ± 0.31 | -- | -- |
| 11 | Undecanal | 1307 | 1305 | -- | -- | -- | -- | -- | -- | 0.56 ± 0.22 | 1.16 ± 0.18 | -- | -- |
| 12 | Undec-9E-em-1-al | 1312 | 1311 | -- | -- | -- | -- | -- | -- | 0.60 ± 0.25 | 1.47 ± 0.30 | -- | -- |
| 13 | (E)-Caryophyllene | 1417 | 1417 | 4.99 ± 1.85 | 3.82 ± 1.24 | 7.49 ± 1.85 | 3.89 ± 0.88 | 7.20 ± 4.07 | 2.81 ± 0.15 | 2.13 ± 0.34 | 2.60 ± 0.18 | 8.37 ± 1.52 | 5.85 ± 1.05 |
| 14 | α-(E)-Bergamotene | 1434 | 1432 | 0.78 ± 0.10 | 0.93 ± 0.40 | 1.00 ± 0.23 | 1.11 ± 0.37 | 0.97 ± 0.30 | 0.80 ± 0.05 | 1.43 ± 0.27 | 1.89 ± 0.15 | 1.04 ± 0.22 | 0.98 ± 0.11 |
| 15 | (E)-β-Farnesene | 1456 | 1452 | 17.24 ± 1.16 | 22.85 ± 9.31 | 23.98 ± 5.52 | 26.16 ± 8.78 | 22.69 ± 6.75 | 19.16 ± 1.10 | 25.84 ± 2.12 | 38.43 ± 2.33 | 25.29 ± 5.66 | 24.01 ± 2.65 |
| 16 | β-Charmigrene | 1474 | 1476 | 1.23 ± 0.25 | 1.23 ± 0.49 | 1.67 ± 0.40 | 1.63 ± 0.45 | 1.63 ± 0.59 | 1.09 ± 0.09 | 1.23 ± 0.20 | 1.87 ± 0.19 | 1.76 ± 0.35 | 1.56 ± 0.11 |
| 17 | β-Selinene | 1485 | 1489 | 7.37 ± 1.45 | 7.40 ± 2.95 | 10.05 ± 2.43 | 9.73 ± 2.77 | 9.72 ± 3.42 | 6.63 ± 0.62 | 6.77 ± 1.15 | 10.56 ± 1.22 | 10.66 ± 2.07 | 9.43 ± 0.75 |
| 18 | Ledene | 1494 | 1496 | 8.08 ± 1.57 | 8.12 ± 3.24 | 11.09 ± 2.57 | 10.65 ± 2.99 | 10.72 ± 3.86 | 7.41 ± 0.67 | 6.70 ± 1.03 | 9.26 ± 0.57 | 11.72 ± 2.31 | 10.19 ± 0.65 |
| 19 | 2-Methyl-lavandula butanoate | 1513 | 1511 | -- | -- | -- | -- | -- | -- | 0.74 ± 0.17 | 0.77 ± 0.13 | -- | -- |
| 20 | α-Cadinene | 1532 | 1537 | 0.30 ± 0.08 | -- | 0.46 ± 0.16 | 0.33 ± 0.20 | 0.37 ± 0.11 | 0.81 ± 0.14 | 1.22 ± 0.13 | 0.27 ± 0.08 | -- | |
| 21 | mi | 1554 | 1559 | -- | 0.81 ± 0.13 | 1.20 ± 0.09 | |||||||
| 22 | α-Eudesmol | 1650 | 1652 | -- | -- | -- | -- | -- | -- | 0.88 ± 0.20 | 1.49 ± 0.20 | -- | -- |
| 23 | 6-Z-Pentadecen-2-o | 1667 | 1667 | 0.63 ± 0.32 | 0.68 ± 0.23 | 1.04 ± 0.27 | 0.82 ± 0.25 | 1.00 ± 0.43 | 0.55 ± 0.04 | 0.90 ± 0.26 | 1.47 ± 0.23 | 0.94 ± 0.17 | 0.87 ± 0.11 |
| Monoterpene hydrocarbons | 56.82 | 52.31 | 41.88 | 43.72 | 44.54 | 57.68 | 39.94 | 15.06 | 38.77 | 45.34 | |||
| Oxygenated monoterpenes | 0.65 | 0.79 | 0.62 | 0.91 | 0.52 | 0.68 | 0.62 | 0.66 | 0.59 | 0.72 | |||
| Sesquiterpene hydrocarbons | 39.81 | 44.35 | 55.28 | 53.17 | 53.27 | 38 | 44.10 | 64.61 | 59.94 | 52.02 | |||
| Oxygenated sesquiterpenes | -- | -- | -- | -- | -- | -- | 0.88 | 1.49 | -- | -- | |||
| Esters | 0.60 | 1.03 | 0.40 | 1.39 | 0.45 | 0.69 | 1.18 | 1.66 | 0.23 | 0.23 | |||
| Ketones | -- | -- | -- | -- | -- | -- | 2.22 | 3.45 | -- | -- | |||
| Aldehydes | 0.24 | 0.32 | -- | -- | -- | -- | 1.73 | 2.91 | -- | -- | |||
| Substance | Org Cas | Con Cas | Org Nug | Con Nug | Org Chi | Con Chi | Org Hal | Con Hal | Org Col | Con Col |
|---|---|---|---|---|---|---|---|---|---|---|
| n-Humulone (mg g−1) | 0.77 ± 1.42 | 0.98 ± 1.26 | 8.17 ± 9.53 | 2.98 ± 1.81 | 1.38 ± 1.51 | 0.87 ± 1.56 | 2.39± 0.60 | 3.19 ± 2.33 | 0.69 ± 1.49 | 2.22 ± 3.09 |
| Cohumulone (mg g−1) | 2.86 ± 0.92 | 3.46 ± 1.72 | 28.78 ± 32.47 | 10.13 ± 7.79 | 3.45 ± 0.13 | 3.16 ± 0.78 | 8.38± 3.88 | 10.43 ± 5.02 | 2.34 ± 0.32 | 6.62 ± 7.64 |
| Adhumulone (mg g−1) | 0.68 ± 0.21 | 0.91 ± 0.35 | 6.08 ± 2.78 | 2.04 ± 1.66 | 0.85 ± 0.03 | 0.76 ± 0.19 | 1.84 ± 0.90 | 2.38 ± 1.24 | 0.58 ± 0.10 | 2.06 ± 1.75 |
| Colupulone (mg g−1) | 4.79 ± 0.94 | 6.78 ± 2.58 | 8.41 ± 6.31 | 6.27 ± 3.50 | 4.38 ± 0.39 | 4.41 ± 1.70 | 2.48 ± 0.95 | 3.18 ± 1.41 | 4.10 ± 0.92 | 6.31 ± 3.57 |
| n-Lupulone (mg g−1) | 2.68 ± 1.09 | 3.64 ± 0.88 | 7.48 ± 5.50 | 3.70 ± 0.84 | 2.37 ± 1.00 | 2.41 ± 0.87 | 2.46± 0.47 | 3.03 ± 1.97 | 2.08 ± 1.31 | 3.31 ± 1.60 |
| Adlupulone (mg g−1) | 1.26 ± 0.39 | 1.84 ± 0.47 | 2.69 ± 1.80 | 1.67 ± 0.68 | 1.19 ± 0.37 | 1.20 ± 0.39 | 0.89± 0.18 | 1.12 ± 0.73 | 1.01 ± 0.58 | 1.63 ± 0.87 |
| Alpha acids (%) | 0.43 ± 0.68 | 0.54 ± 0.59 | 4.31 ± 4.85 | 1.51 ± 0.70 | 0.53 ± 0.73 | 0.48 ± 0.78 | 1.26± 0.24 | 1.60 ± 1.15 | 0.36 ± 0.72 | 1.06 ± 1.39 |
| Beta acids (%) | 0.87 ± 0.26 | 1.23 ± 0.33 | 1.86 ± 1.23 | 1.16 ± 0.45 | 0.79 ± 0.27 | 0.80 ± 0.26 | 0.58± 0.11 | 0.73 ± 0.49 | 0.72 ± 0.38 | 1.12 ± 0.56 |
| Alpha + beta acids (%) | 1.31 ± 0.33 | 1.76 ± 0.69 | 6.16 ± 6.93 | 2.68 ± 1.25 | 1.32 ± 0.03 | 1.28 ± 0.17 | 1.84 ± 0.82 | 2.33 ± 1.11 | 1.08 ± 0.21 | 2.28 ± 1.84 |
| Xanthohumol (mg g−1) | 0.38 ± 0.03 | 0.56 ± 0.20 | 1.57 ± 1.40 | 0.66 ± 0.08 | 0.79 ± 0.97 | 0.52 ± 0.23 | 0.72± 0.27 | 1.05 ± 0.31 | 0.44 ± 0.21 | 0.40 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortuna, G.C.; Neves, C.S.; Campos, O.P.; Gomes, J.A.O.; Silva, J.C.R.L.; Souza, A.A.; Funari, C.S.d.; Marques, M.O.M.; Bonfim, F.P.G. Hop Tropicalization: Chemical Compositions of Varieties Grown under Organic and Conventional Systems in Subtropical Conditions. Horticulturae 2023, 9, 855. https://doi.org/10.3390/horticulturae9080855
Fortuna GC, Neves CS, Campos OP, Gomes JAO, Silva JCRL, Souza AA, Funari CSd, Marques MOM, Bonfim FPG. Hop Tropicalization: Chemical Compositions of Varieties Grown under Organic and Conventional Systems in Subtropical Conditions. Horticulturae. 2023; 9(8):855. https://doi.org/10.3390/horticulturae9080855
Chicago/Turabian StyleFortuna, Gabriel Cássia, Caio Scardini Neves, Olivia Pak Campos, Jordany Aparecida Oliveira Gomes, Júlio César Rodrigues Lopes Silva, Amauri Alves Souza, Cristiano Soleo de Funari, Márcia Ortiz Mayo Marques, and Filipe Pereira Giardini Bonfim. 2023. "Hop Tropicalization: Chemical Compositions of Varieties Grown under Organic and Conventional Systems in Subtropical Conditions" Horticulturae 9, no. 8: 855. https://doi.org/10.3390/horticulturae9080855
APA StyleFortuna, G. C., Neves, C. S., Campos, O. P., Gomes, J. A. O., Silva, J. C. R. L., Souza, A. A., Funari, C. S. d., Marques, M. O. M., & Bonfim, F. P. G. (2023). Hop Tropicalization: Chemical Compositions of Varieties Grown under Organic and Conventional Systems in Subtropical Conditions. Horticulturae, 9(8), 855. https://doi.org/10.3390/horticulturae9080855






