Combined Effect of Biostimulants and Mineral Fertilizers on Crop Performance and Fruit Quality of Watermelon Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Experimental Treatments
2.3. Fruit Quality Parameters
2.4. Chemical Analysis
2.4.1. Proximate Composition and Energetic Value
2.4.2. Hydrophilic Compounds
Sugars
Organic Acids
2.4.3. Lipophilic Compounds
Fatty Acids
Tocopherols
2.4.4. Carotenoid and Chlorophylls Content
2.5. Bioactive Properties
2.5.1. Antioxidant Activity
2.5.2. Cytotoxicity Assays
2.5.3. Anti-Inflammatory Activity
2.5.4. Antimicrobial Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Yield Parameters
3.2. Fruit Quality Parameters
3.3. Chemical Composition
3.3.1. Proximate Composition and Energetic Value
3.3.2. Free Sugars
3.3.3. Organic Acids
3.3.4. Lipophilic Compounds (Fatty Acids and Tocopherols)
3.3.5. Carotenoid and Chlorophyll Content
3.4. Bioactive Properties
3.4.1. Antioxidant Activity
3.4.2. Cytotoxicity
3.4.3. Anti-Inflammatory Activity
3.4.4. Antimicrobial Properties
Antibacterial Activity
Antifungal Activity
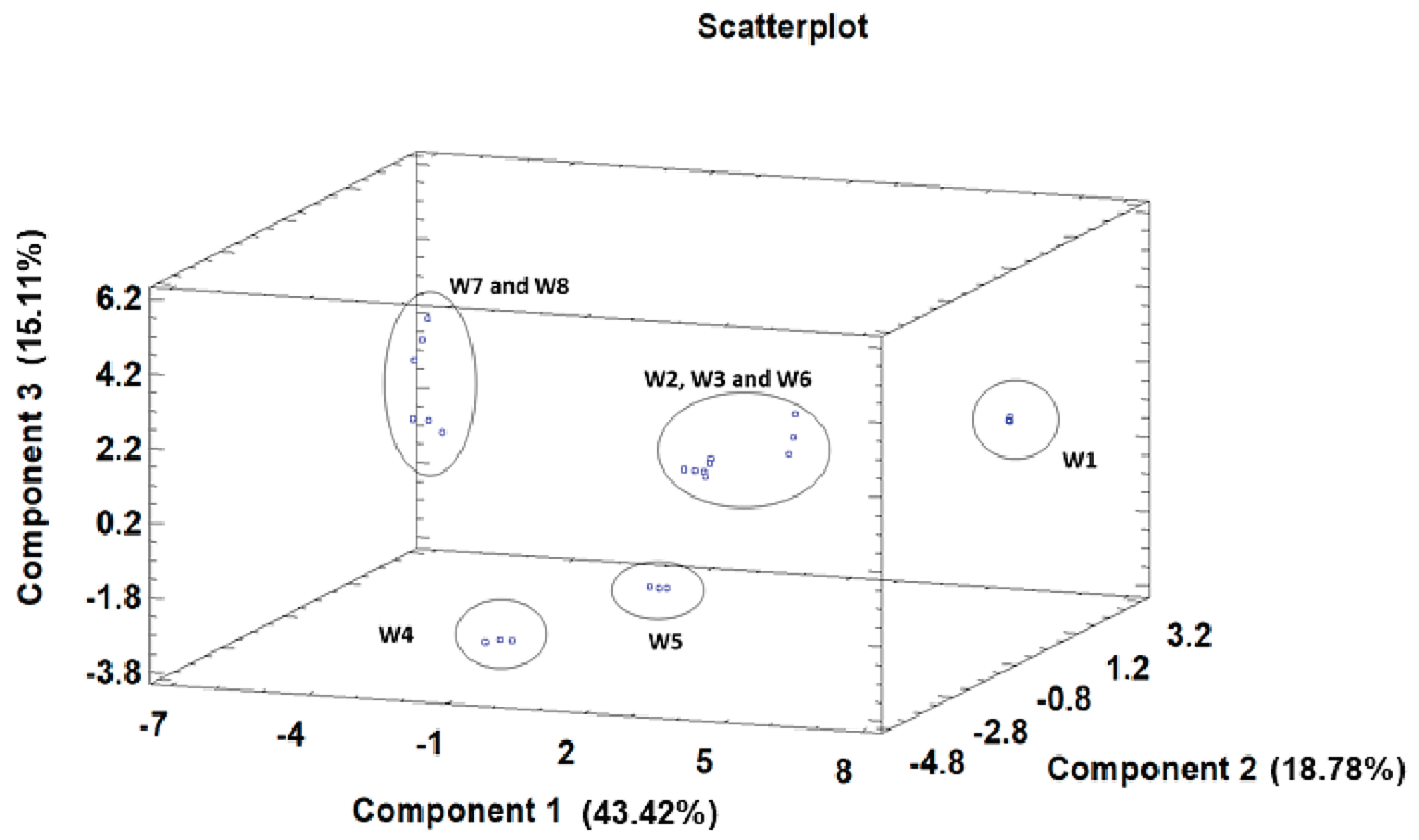
3.5. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simpson, M. Plant Systematics, 2nd ed.; Academic Press: London, UK, 2010; ISBN 9780080922089. [Google Scholar]
- Vaughan, J.; Geissler, C. The New Oxford Book of Food Plants; Oxford University Press: Jericho, VT, USA, 2009; ISBN 978-0199549467. [Google Scholar]
- FAO FAOSTAT Online Database. Available online: https://www.fao.org/food-agriculture-statistics/en/ (accessed on 1 June 2023).
- Touhami, M.; Laroubi, A.; Elhabazi, K.; Loubna, F.; Zrara, I.; Eljahiri, Y.; Oussama, A.; Grases, F.; Chait, A. Lemon juice has protective activity in a rat urolithiasis model. BMC Urol. 2007, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Tlili, I.; Hdider, C.; Lenucci, M.S.; Riadh, I.; Jebari, H.; Dalessandro, G. Bioactive compounds and antioxidant activities of different watermelon (Citrullus lanatus (Thunb.) Mansfeld) cultivars as affected by fruit sampling area. J. Food Compos. Anal. 2011, 24, 307–314. [Google Scholar] [CrossRef]
- Siddiqui, W.A.; Shahzad, M.; Shabbir, A.; Ahmad, A. Evaluation of anti-urolithiatic and diuretic activities of watermelon (Citrullus lanatus) using in vivo and in vitro experiments. Biomed. Pharmacother. 2018, 97, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Alemika, T.E.; Ojerinde, O.S.; Samali, A.; Mustapha, B.K.; Gamaniel, K.S. Nutriceutical potentials of Nigerian grown Citrullus lanatus (Watermelon) seed. J. Pharm. Bioresour. 2018, 14, 253. [Google Scholar] [CrossRef]
- Jibril, M.M.; Abdul-Hamid, A.; Ghazali, H.M.; Sabri, M.; Dek, P.; Ramli, N.S.; Haniff Jaafar, A.; Karrupan, J.; Mohammed, A.S.; Ghazali, M.; et al. Antidiabetic Antioxidant and Phytochemical Profile of Yellow-Fleshed Seeded Watermelon (Citrullus lanatus) Extracts. J. Food Nutr. Res. 2019, 7, 82–95. [Google Scholar] [CrossRef]
- Chaparro, L.; Dhuique-Mayer, C.; Castillo, S.; Vaillant, F.; Servent, A.; Dornier, M. Concentration and purification of lycopene from watermelon juice by integrated microfiltration-based processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 153–160. [Google Scholar] [CrossRef]
- Zhao, W.; Lv, P.; Gu, H. Studies on carotenoids in watermelon flesh. Agric. Sci. 2013, 4, 13–20. [Google Scholar] [CrossRef]
- Montesano, D.; Fallarino, F.; Cossignani, L.; Bosi, A.; Simonetti, M.S.; Puccetti, P.; Damiani, P. Innovative extraction procedure for obtaining high pure lycopene from tomato. Eur. Food Res. Technol. 2008, 226, 327–335. [Google Scholar] [CrossRef]
- Fattore, M.; Montesano, D.; Pagano, E.; Teta, R.; Borrelli, F.; Mangoni, A.; Seccia, S.; Albrizio, S. Carotenoid and flavonoid profile and antioxidant activity in “Pomodorino Vesuviano” tomatoes. J. Food Compos. Anal. 2016, 53, 61–68. [Google Scholar] [CrossRef]
- Suwanaruang, T. Analyzing Lycopene Content in Fruits. Agric. Agric. Sci. Procedia 2016, 11, 46–48. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.S.; Gullón, B.; Rocchetti, G.; Montesano, D.; Lorenzo, J.M. Citrullus lanatus as source of bioactive components: An up-to-date review. Trends Food Sci. Technol. 2021, 111, 208–222. [Google Scholar] [CrossRef]
- Adetutu, A.; Olorunnisola, O.S.; Owoade, O.A. Nutritive Values and Antioxidant Activity of Citrullus lanatus Fruit Extract. Food Nutr. Sci. 2015, 6, 1056–1064. [Google Scholar] [CrossRef]
- Bello, H.S.; Ismail, H.Y.; Mangga, H.K. Antimicrobial Activity of Citrullus lanatus (Watermelon) Seeds on Some Selected Bacteria. J. Biotechnol. Res. 2016, 2, 39–43. [Google Scholar]
- Adelani-Akande, T.A.; Ajiba, L.C.; Dahunsi, S.O.; Oluyori, A.P. Antibacterial activity of watermelon (Citrullus lanatus) seed against selected microorganisms. Afr. J. Biotechnol. 2015, 14, 1224–1229. [Google Scholar] [CrossRef]
- Sathya, J.; Shoba, F.G.; College, V. Assessment of antimicrobial efficacy of Citrullus lanatus methanolic seed extract. J. Chem. Pharm. Res. 2014, 6, 640–643. [Google Scholar]
- Hassan, L.E.A.; Sirat, H.M.; Yagi, S.M.A.; Koko, W.S.; Abdelwahab, S.I. In vitro Antimicrobial activities of Chloroformic, hexane and Ethanolic extracts of Citrullus lanatus var. Citroides (Wild melon). J. Med. Plants Res. 2011, 5, 1338–1344. [Google Scholar]
- Cemaluk, C. Comparative Investigation of the Antibacterial and Antifungal Potentials of the Extracts of Watermelon (Citrullus lanatus) Rind and Seed. Eur. J. Med. Plants 2015, 9, 1–7. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants application: A low input cropping management tool for sustainable farming of vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant substances for sustainable agriculture: Origin, operating mechanisms and effects on cucurbits, leafy greens, and nightshade vegetables species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Cozzolino, E.; Ottaiano, L.; Petropoulos, S.A.; Nocerino, S.; Pelosi, M.E.; Rouphael, Y.; Mori, M.; Mola, I. Di Effect of Biostimulant Application on Plant Growth, Chlorophylls and Hydrophilic Antioxidant Activity of Spinach (Spinacia oleracea L.) Grown under Saline Stress. Horticulturae 2022, 8, 971. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Buono, D. Del Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Fernandes, Â.; Chaski, C.; Pereira, C.; Kostić, M.; Rouphael, Y.; Soković, M.; Barros, L.; Petropoulos, S.A. Water Stress Alleviation Effects of Biostimulants on Greenhouse-Grown Tomato Fruit. Horticulturae 2022, 8, 645. [Google Scholar] [CrossRef]
- Petropoulos, S.A. Practical applications of plant biostimulants in greenhouse vegetable crop production. Agronomy 2020, 10, 1569. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Taofiq, O.; Fernandes, Â.; Tzortzakis, N.; Ciric, A.; Sokovic, M.; Barros, L.; Ferreira, I.C.F.R. Bioactive properties of greenhouse-cultivated green beans (Phaseolus vulgaris L.) under biostimulants and water-stress effect. J. Sci. Food Agric. 2019, 99, 6049–6059. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R. Biostimulants application alleviates water stress effects on yield and chemical composition of greenhouse green bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 181. [Google Scholar] [CrossRef]
- Chaski, C.; Petropoulos, S.A. The Effects of Biostimulant Application on Growth Parameters of Lettuce Plants Grown under Deficit Irrigation Conditions. Horticulturae 2022, 8, 89. [Google Scholar] [CrossRef]
- Harizanova, A.; Koleva-Valkova, L. Effect of silicon on photosynthetic rate and the chlorophyll fluorescence parameters at hydroponically grown cucumber plants under salinity stress. J. Cent. Eur. Agric. 2019, 20, 953–960. [Google Scholar] [CrossRef]
- Celletti, S.; Astolfi, S.; Guglielmo, N.; Colla, G.; Cesco, S.; Mimmo, T. Evaluation of a legume-derived protein hydrolysate to mitigate iron deficiency in plants. Agronomy 2020, 10, 1942. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Sakai, N.; Zhang, L.; Hassanien, H.A.; Gaber, A.; Ammarr, G.A.G. Impact of seaweed liquid extract biostimulant on growth, yield, and chemical composition of cucumber (Cucumis sativus). Agriculture 2021, 11, 320. [Google Scholar] [CrossRef]
- Ozdamar Unlu, H.; Unlu, H.; Karakurt, Y.; Padem, H. Changes in fruit yield and quality in response to foliar and soil humic acid application in cucumber. Sci. Res. Essays 2011, 6, 2800–2803. [Google Scholar] [CrossRef]
- Karakurt, Y.; Ozdamar-Unlu, H.; Unlu, H.; Tonguc, M. Antioxidant compounds and activity in cucumber fruit in response to foliar and soil humic acid application. Eur. J. Hortic. Sci. 2015, 80, 76–80. [Google Scholar] [CrossRef]
- Bijalwan, P.; Jeddi, K.; Saini, I.; Sharma, M.; Kaushik, P.; Hessini, K. Mitigation of saline conditions in watermelon with mycorrhiza and silicon application. Saudi J. Biol. Sci. 2021, 28, 3678–3684. [Google Scholar] [CrossRef]
- Toresano-Sánchez, F.; Díaz-Pérez, M.; Diánez-Martínez, F.; Camacho-Ferre, F. Effect of the application of monosilicic acid on the production and quality of triploid watermelon. J. Plant Nutr. 2010, 33, 1411–1421. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, J.; Li, M.; Fang, F.; Hu, J.; Sun, Z.; Zhang, A.; Gao, X.; Li, J. Synergistic effect of Bacillus subtilis and Paecilomyces lilacinus in alleviating soil degradation and improving watermelon yield. Front. Microbiol. 2023, 13, 1101975. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Deng, K.; Song, Y.; Wu, Y.; Zhao, J.; Raza, W.; Huang, Q.; Shen, Q. Variation of rhizosphere bacterial community in watermelon continuous mono-cropping soil by long-term application of a novel bioorganic fertilizer. Microbiol. Res. 2014, 169, 570–578. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A. Ascophyllum nodosum and Silicon-Based Biostimulants Differentially Affect the Physiology and Growth of Watermelon Transplants under Abiotic Stress Factors: The Case of Salinity. Plants 2023, 12, 433. [Google Scholar] [CrossRef]
- Soteriou, G.A.; Rouphael, Y.; Emmanouilidou, M.G.; Antoniou, C.; Kyratzis, A.C.; Kyriacou, M.C. Biostimulatory action of vegetal protein hydrolysate and the configuration of fruit physicochemical characteristics in grafted watermelon. Horticulturae 2021, 7, 313. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Olympios, C.; Ropokis, A.; Vlachou, G.; Ntatsi, G. Fruit volatiles, quality, and yield of watermelon as affected by grafting. J. Agric. Sci. Technol. 2014, 16, 873–885. [Google Scholar]
- Petropoulos, S.A.; Fernandes, Â.; Barros, L.; Ferreira, I.C.F.R.; Ntatsi, G. Morphological, nutritional and chemical description of “Vatikiotiko”, an onion local landrace from Greece. Food Chem. 2015, 182, 156–163. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists; Horwitz, W., Latimer, G., Eds.; AOAC International: Gaithersburg, MD, USA, 2016; ISBN 0935584773. [Google Scholar]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from Portugal by Ultra-Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Katsoulas, N.; Barros, L.; Ferreira, I.C.F.R. The effect of covering material on the yield, quality and chemical composition of greenhouse-grown tomato fruit. J. Sci. Food Agric. 2019, 99, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Essoh, A.P.; Liberal, Â.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Mandim, F.; Moldão-Martins, M.; Cravo, P.; Duarte, M.P.; Moura, M.; et al. Evaluation of the Polyphenolic Composition and Bioactivities of Three Native Cabo Verde Medicinal Plants. Pharmaceuticals 2022, 15, 1162. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Soteriou, G.A.; Rouphael, Y.; Siomos, A.S.; Gerasopoulos, D. Configuration of watermelon fruit quality in response to rootstock-mediated harvest maturity and postharvest storage. J. Sci. Food Agric. 2016, 96, 2400–2409. [Google Scholar] [CrossRef]
- da Luz Neto, C.A.; da Silva, E.M.; Fonseca, W.L.; de Araújo Moreira, I.; Pessoa, K.D.; Feitoza, M.A. Fertigated cultivation of mini watermelon subjected to salinity levels and foliar application of silicon. Rev. Caatinga 2023, 36, 445–455. [Google Scholar] [CrossRef]
- Song, X.; Ying, L.; JingJing, W.; TingTing, Y.; YiFan, H.; XingBiao, W.; ZhiYong, H. Isolation and potential of Ochrobactrum sp. NW-3 to increase the growth of cucumber. Int. J. Agric. Policy Res. 2015, 3, 341–350. [Google Scholar]
- Zhu, Y.X.; Xu, X.B.; Hu, Y.H.; Han, W.H.; Yin, J.L.; Li, H.L.; Gong, H.J. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jiang, X.; Zhang, J.; He, Y.; Zhu, X.; Zhou, X.; Gong, H.; Yin, J.; Liu, Y. Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins. Plant Physiol. Biochem. 2020, 156, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Toresano, F.; Díaz, M.; Pérez, L.; Camacho, F. Effect of the application of monosilicic acid fertilizer on yield and quality of greenhouse triploid watermelon. Acta Hortic. 2012, 927, 373–378. [Google Scholar] [CrossRef]
- Peris-Felipo, F.J.; Benavent-Gil, Y.; Hernández-Apaolaza, L. Silicon beneficial effects on yield, fruit quality and shelf-life of strawberries grown in different culture substrates under different iron status. Plant Physiol. Biochem. 2020, 152, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Salim, B.B.M.; Abou El-Yazied, A.; Salama, Y.A.M.; Raza, A.; Osman, H.S. Impact of silicon foliar application in enhancing antioxidants, growth, flowering and yield of squash plants under deficit irrigation condition. Ann. Agric. Sci. 2021, 66, 176–183. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Skodra, C.; Molassiotis, A.; Tanou, G. Silicon influenced ripening metabolism and improved fruit quality traits in apples. Plant Physiol. Biochem. 2021, 166, 270–277. [Google Scholar] [CrossRef]
- González-Terán, G.E.; Gómez-Merino, F.C.; Trejo-Téllez, L.I. Effects of silicon and calcium application on growth, yield and fruit quality parameters of cucumber established in a sodic soil. Acta Sci. Pol. Hortorum Cultus 2020, 19, 149–158. [Google Scholar] [CrossRef]
- Abidi, W.; Akrimi, R.; Hajlaoui, H.; Rejeb, H.; Gogorcena, Y. Foliar Fertilization of Potassium Silicon Improved Postharvest Fruit Quality of Peach and Nectarine [Prunus persica (L.) Batsch] Cultivars. Agriculture 2023, 13, 195. [Google Scholar] [CrossRef]
- Prasad Lamsal, B.; Kumar Jindal, V. Variation in Electrical Conductivity of Selected Fruit Juices During Continuous Ohmic Heating. KMUTNB Int. J. Appl. Sci. Technol. 2014, 7, 47–56. [Google Scholar] [CrossRef]
- Fernando, D.; Milagrosa, S.; Francisco, C.; Francisco, M. Biostimulant activity of Trichoderma saturnisporum in melon (Cucumis melo). HortScience 2018, 53, 810–815. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Nasrallah, A.K.; Kheder, A.A.; Kord, M.A.; Fouad, A.S.; El-Mogy, M.M.; Atia, M.A.M. Mitigation of Salinity Stress Effects on Broad Bean Productivity Using Calcium Phosphate Nanoparticles Application. Horticulturae 2022, 8, 75. [Google Scholar] [CrossRef]
- Emad, A.-A.; Yousry, B.; Elmahdy, M.; Mohamed, R. Silicon supplements affect yield and fruit quality of cucumber (Cucumis sativus L.) grown in net houses. Afr. J. Agric. Res. 2017, 12, 2518–2523. [Google Scholar] [CrossRef]
- Costan, A.; Stamatakis, A.; Chrysargyris, A.; Petropoulos, S.A.; Tzortzakis, N. Interactive effects of salinity and silicon application on Solanum lycopersicum growth, physiology and shelf-life of fruit produced hydroponically. J. Sci. Food Agric. 2020, 100, 732–743. [Google Scholar] [CrossRef]
- Munaretto, L.M.; Botelho, R.V.; Resende, J.T.V.; Schwarz, K.; Sato, A.J. Productivity and quality of organic strawberries pre-harvest treated with silicon. Hortic. Bras. 2018, 36, 40–46. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Pii, Y.; Mimmo, T.; Savini, G.; Curzel, S.; Cesco, S. Fertilization strategies as a tool to modify the organoleptic properties of raspberry (Rubus idaeus L.) fruits. Sci. Hortic. 2018, 240, 205–212. [Google Scholar] [CrossRef]
- Jȩdrszczyk, E.; Kopeć, A.; Bucki, P.; Ambroszczyk, A.M.; Skowera, B. The enhancing effect of plants growth biostimulants in garlic cultivation on the chemical composition and level of bioactive compounds in the garlic leaves, stems and bulbs. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 81–91. [Google Scholar] [CrossRef]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The Application of a Plant Biostimulant Based on Seaweed and Yeast Extract Improved Tomato Fruit Development and Quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Jawad, U.; Gao, L.; Gebremeskel, H.; Safdar, L.B.; Yuan, P.; Zhao, S.; Xuqiang, L.; Nan, H.; Hongju, Z.; Liu, W. Expression pattern of sugars and organic acids regulatory genes during watermelon fruit development. Sci. Hortic. 2020, 265, 109102. [Google Scholar] [CrossRef]
- Umer, M.J.; Bin Safdar, L.; Gebremeskel, H.; Zhao, S.; Yuan, P.; Zhu, H.; Kaseb, M.O.; Anees, M.; Lu, X.; He, N.; et al. Identification of key gene networks controlling organic acid and sugar metabolism during watermelon fruit development by integrating metabolic phenotypes and gene expression profiles. Hortic. Res. 2020, 7, 193. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Dai, Z.; Liu, X.; Liu, Y.; Deng, X.; Chen, F.; Xu, J. Volatile chemical and carotenoid profiles in watermelons [Citrullus vulgaris (Thunb.) Schrad (Cucurbitaceae)] with different flesh colors. Food Sci. Biotechnol. 2012, 21, 531–541. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Álvarez-Martínez, F.J.; Borrás, F.; Pérez, D.; Herrero, N.; Ruiz, J.J.; Micol, V. Metabolomic analysis of the effects of a commercial complex biostimulant on pepper crops. Food Chem. 2020, 310, 125818. [Google Scholar] [CrossRef]
- Alfosea-Simón, M.; Simón-Grao, S.; Zavala-Gonzalez, E.A.; Cámara-Zapata, J.M.; Simón, I.; Martínez-Nicolás, J.J.; Lidón, V.; Rodríguez-Ortega, W.M.; García-Sánchez, F. Application of biostimulants containing amino acids to tomatoes could favor sustainable cultivation: Implications for tyrosine, lysine, and methionine. Sustainability 2020, 12, 9729. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars-metabolism, sensing and abiotic stress. A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Berni, R.; Hausman, J.F.; Guerriero, G. A review on the beneficial role of silicon against salinity in non-accumulator crops: Tomato as a model. Biomolecules 2020, 10, 1284. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First fruit in season: Seaweed extract and silicon advance organic strawberry (Fragaria × ananassa Duch.) fruit formation and yield. Sci. Hortic. 2018, 242, 103–109. [Google Scholar] [CrossRef]
- Hu, W.; Su, Y.; Zhou, J.; Zhu, H.; Guo, J.; Huo, H.; Gong, H. Foliar application of silicon and selenium improves the growth, yield and quality characteristics of cucumber in field conditions. Sci. Hortic. 2022, 294, 110776. [Google Scholar] [CrossRef]
- Yaghubi, K.; Vafaee, Y.; Ghaderi, N.; Javadi, T. Potassium Silicate Improves Salinity Resistant and Affects Fruit Quality in Two Strawberry Cultivars Grown Under Salt Stress. Commun. Soil Sci. Plant Anal. 2019, 50, 1439–1451. [Google Scholar] [CrossRef]
- Albishri, H.M.; Almaghrabi, O.A.; Moussa, T.A. Characterization and chemical composition of fatty acids content of watermelon and muskmelon cultivars in Saudi Arabia using gas chromatography/mass spectroscopy. Pharmacogn. Mag. 2013, 9, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate composition, mineral contents and fatty acid composition of the different parts and dried peels of tropical fruits cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Bertling, I.; Bower, J.P. Effects of postharvest potassium silicate application on phenolics and other anti-oxidant systems aligned to avocado fruit quality. Postharvest Biol. Technol. 2011, 60, 92–99. [Google Scholar] [CrossRef]
- Kim, Y.N.; Giraud, D.W.; Driskell, J.A. Tocopherol and carotenoid contents of selected Korean fruits and vegetables. J. Food Compos. Anal. 2007, 20, 458–465. [Google Scholar] [CrossRef]
- Charoensiri, R.; Kongkachuichai, R.; Suknicom, S.; Sungpuag, P. Beta-carotene, lycopene, and alpha-tocopherol contents of selected Thai fruits. Food Chem. 2009, 113, 202–207. [Google Scholar] [CrossRef]
- Isabelle, M.; Lee, B.L.; Lim, M.T.; Koh, W.P.; Huang, D.; Ong, C.N. Antioxidant activity and profiles of common fruits in Singapore. Food Chem. 2010, 123, 77–84. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. A Commercial Extract of Brown Macroalga (Ascophyllum nodosum) Affects Yield and the Nutritional Quality of Spinach In Vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; ElSayed, A.I.; Merwad, A.R.M.A.; Rady, M.M. Stimulating antioxidant defenses, antioxidant gene expression, and salt tolerance in Pisum sativum seedling by pretreatment using licorice root extract (LRE) as an organic biostimulant. Plant Physiol. Biochem. 2019, 142, 292–302. [Google Scholar] [CrossRef]
- Galindo, F.S.; Pagliari, P.H.; Rodrigues, W.L.; Fernandes, G.C.; Boleta, E.H.M.; Santini, J.M.K.; Jalal, A.; Buzetti, S.; Lavres, J.; Teixeira Filho, M.C.M. Silicon amendment enhances agronomic efficiency of nitrogen fertilization in maize and wheat crops under tropical conditions. Plants 2021, 10, 1329. [Google Scholar] [CrossRef]
- Abd-Elkader, D.Y.; Mohamed, A.A.; Feleafel, M.N.; Al-Huqail, A.A.; Salem, M.Z.M.; Ali, H.M.; Hassan, H.S. Photosynthetic Pigments and Biochemical Response of Zucchini (Cucurbita pepo L.) to Plant-Derived Extracts, Microbial, and Potassium Silicate as Biostimulants Under Greenhouse Conditions. Front. Plant Sci. 2022, 13, 879545. [Google Scholar] [CrossRef]
- Perkins-Veazie, P. Carotenoids in watermelon and mango. Acta Hortic. 2007, 746, 259–264. [Google Scholar] [CrossRef]
- Mousavi, S.A.A.; Roosta, H.R.; Esmaeilizadeh, M.; Eshghi, S. Alleviating the adverse effects of salinity and alkalinity stresses on some physiological traits by selenium and silicon foliar applications on cucumber (Cucumis sativus L.) plants. J. Plant Nutr. 2022, 46, 556–573. [Google Scholar] [CrossRef]
- Pilon, C.; Soratto, R.P.; Moreno, L.A. Effects of soil and foliar application of soluble silicon on mineral nutrition, gas exchange, and growth of potato plants. Crop Sci. 2013, 53, 1605–1614. [Google Scholar] [CrossRef]
- Mostafa, G.G. Improving the growth of fennel plant grown under salinity stress using some biostimulants. Am. J. Plant Physiol. 2015, 10, 77–83. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ansary, M.M.U.; Keya, S.S.; Abdelrahman, M.; Miah, M.G.; Phan Tran, L.S. Silicon in mitigation of abiotic stress-induced oxidative damage in plants. Crit. Rev. Biotechnol. 2021, 41, 918–934. [Google Scholar] [CrossRef]
- Nkoana, D.K.; Mashilo, J.; Shimelis, H.; Ngwepe, R.M. Nutritional, phytochemical compositions and natural therapeutic values of citron watermelon (Citrullus lanatus var. citroides): A Review. S. Afr. J. Bot. 2022, 145, 65–77. [Google Scholar] [CrossRef]
- Cruz, R.C.R.; Neto, F.R.; Furtado, R.A.; Souza, L.M.; de Sousa, F.D.; Ozelin, S.D.; Bastos, J.K.; Magalhães, G.M.; Tavares, D.C.; de Oliveira, P.F. Watermelon Reduces the Toxicity of Cisplatin Treatment in C57BL/6 Mice with Induced Melanoma. Nutr. Cancer 2022, 74, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Gu, I.; Balogun, O.; Brownmiller, C.; Kang, H.W.; Lee, S.O. Bioavailability of Citrulline in Watermelon Flesh, Rind, and Skin Using a Human Intestinal Epithelial Caco-2 Cell Model. Appl. Sci. 2023, 13, 4882. [Google Scholar] [CrossRef]
- El Gizawy, H.A.; El-Haddad, A.E.; Attia, Y.M.; Fahim, S.A.; Zafer, M.M.; Saadeldeen, A.M. In Vitro Cytotoxic Activity and Phytochemical Characterization (UPLC/T-TOF-MS/MS) of the Watermelon (Citrullus lanatus) Rind Extract. Molecules 2022, 27, 2480. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Shi, J.; Feng, S.X.; Xue, L.; Tian, L.P. Two new phenolic glycosides from the seeds of Citrullus lanatus. Nat. Prod. Res. 2020, 34, 398–404. [Google Scholar] [CrossRef]
- Atolani, O.; Omere, J.; Otuechere, C.A.; Adewuyi, A. Antioxidant and cytotoxicity effects of seed oils from edible fruits. J. Acute Dis. 2012, 1, 130–134. [Google Scholar] [CrossRef]
- Kikuchi, T.; Ikedaya, A.; Toda, A.; Ikushima, K.; Yamakawa, T.; Okada, R.; Yamada, T.; Tanaka, R. Pyrazole alkaloids from watermelon (Citrullus lanatus) seeds. Phytochem. Lett. 2015, 12, 94–97. [Google Scholar] [CrossRef]
- Itoh, T.; Muramatsu, M.; Miyazono, D.; Koketsu, M.; Fujita, S.; Hashizume, T. Phenolic Glycosides Citrulluside H and Citrulluside T Isolated from Young Watermelon (Citrullus lanatus) Fruit Have Beneficial Effects Against Cutibacterium acnes-Induced Skin Inflammation. Nat. Prod. Commun. 2023, 18, 1–11. [Google Scholar] [CrossRef]
- Rafi, M.M.; Yadav, P.N.; Reyes, M. Lycopene inhibits LPS-induced proinflammatory mediator inducible nitric oxide synthase in mouse macrophage cells. J. Food Sci. 2007, 72, S069–S074. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Effects of divergent ultrasound pretreatment on the structure of watermelon seed protein and the antioxidant activity of its hydrolysates. Food Chem. 2019, 299, 125165. [Google Scholar] [CrossRef]
- Fetni, S.; Bertella, N. Cytotoxic and antioxidant effect of the ethanolic extract of Citrullus colocynthis L. plant fruit. Genet. Biodivers. J. 2021, 5, 93–102. [Google Scholar] [CrossRef]
- Darwish, R.S.; Abdulmunem, O.A.; Khairy, A.; Ghareeb, D.A.; Yassin, A.M.; Abdulmalek, S.A.; Shawky, E. Comparative metabolomics reveals the cytotoxic and anti-inflammatory discriminatory chemical markers of raw and roasted colocynth fruit (Citrullus colocynthis L.). RSC Adv. 2021, 11, 37049–37062. [Google Scholar] [CrossRef]
- Neglo, D.; Tettey, C.O.; Essuman, E.K.; Kortei, N.K.; Boakye, A.A.; Hunkpe, G.; Amarh, F.; Kwashie, P.; Devi, W.S. Comparative antioxidant and antimicrobial activities of the peels, rind, pulp and seeds of watermelon (Citrullus lanatus) fruit. Sci. Afr. 2021, 11, e00582. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Macieja, S.; Śliwiński, M.; Bartkowiak, A.; Roy, S.; Sobolewski, P. Alginate Biofunctional Films Modified with Melanin from Watermelon Seeds and Zinc Oxide/Silver Nanoparticles. Materials 2022, 15, 2381. [Google Scholar] [CrossRef]
- Elsayed, D.A.; Yousof, S.M.; Khalil, I.A.; Kolieb, E.; Zayed, M.A. Citrullus lanatus (Watermelon) Wastes: Maximizing the Benefits and Saving the Environment. In Mediterranean Fruits Bio-Wastes: Chemistry, Functionality and Technological Applications; Ramada, M.F., Farag, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 627–646. ISBN 9783030844363. [Google Scholar]
- Ali, O.M.; Hasanin, M.S.; Suleiman, W.B.; Helal, E.E.H.; Hashem, A.H. Green biosynthesis of titanium dioxide quantum dots using watermelon peel waste: Antimicrobial, antioxidant, and anticancer activities. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Mohammed, M.F.; Kannan, H.B.; Abdalqader, M.; Alwan, M.R.; Alhoot, M.A.; Ghazi, H.F. Antibacterial activities of watermelon (Citrullus lanatus) rind and seed extracts against selected gram-positive and gram-negative bacteria. Int. J. Med. Toxicol. Leg. Med. 2020, 23, 95–100. [Google Scholar] [CrossRef]
- Babaiwa, U.F.; Erharuyi, O.; Falodun, A.; Akerele, J.O. Antimicrobial activity of ethyl acetate extract of Citrullus lanatus seeds. Trop. J. Pharm. Res. 2017, 16, 1631–1636. [Google Scholar] [CrossRef]
- Dash, P.; Ghosh, G. Fractionation, amino acid profiles, antimicrobial and free radical scavenging activities of Citrullus lanatus seed protein. Nat. Prod. Res. 2017, 31, 2945–2947. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.; Azhar, N.; Megat Abdul Rani, P.S.; Mohamed Zaini, H. Metal Removal and Antimicrobial Properties of Watermelon rind modified with clove. MATEC Web Conf. 2016, 78, 01028. [Google Scholar] [CrossRef]
- Harith, S.S.; Mazlun, M.H.; Mydin, M.M.; Nawi, L.; Saat, R. Studies on phytochemical constituents and antimicrobial properties of Citrullus lanatus peels. Malays. J. Anal. Sci. 2018, 22, 151–156. [Google Scholar] [CrossRef]
- Velmurugan, P.; Hong, S.C.; Aravinthan, A.; Jang, S.H.; Yi, P.I.; Song, Y.C.; Jung, E.S.; Park, J.S.; Sivakumar, S. Comparison of the Physical Characteristics of Green-Synthesized and Commercial Silver Nanoparticles: Evaluation of Antimicrobial and Cytotoxic Effects. Arab. J. Sci. Eng. 2017, 42, 201–208. [Google Scholar] [CrossRef]
- Aktepe, N.; Baran, A. Biosynthesis of AgNPs by extract from waste leaves of Citrullus lanatus sp. (watermelon); Characterization, antibacterial and antifungal effects. Prog. Nutr. 2021, 23, e2021243. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Liu, G.; Yang, X. Antifungal properties of a thaumatin-like protein from watermelon. Acta Physiol. Plant. 2018, 40, 186. [Google Scholar] [CrossRef]



| Formulation | Composition |
|---|---|
| A | 92% SiO2 w/w |
| B | 35% w/v SiO2 + 35% w/v CaO |
| C | Mixture of natural metabolic catalysts, amino acids, and trace elements |
| D | 15-65-15 (N-P-K) + trace elements |
| E | 15-10-50 (N-P-K) + 3MgO + trace elements |
| Treatment | Application | Dose | Time Schedule |
|---|---|---|---|
| W1 | One application of formulation A | 20 kg/ha/application | 15 DAT * |
| W2 | One application of formulation B | 20 L/ha/application | 15 DAT |
| W3 | Three applications of formulation A | 20 kg/application | 15 DAT 35 DAT 55 DAT |
| W4 | Three applications of formulation B | 20 L/ha/application | 15 DAT 35 DAT 55 DAT |
| W5 | Three applications of formulation B | 20 L/ha/application (B) | 15 DAT 35 DAT 55 DAT |
| One application of formulation C | 10 L/ha (C) | 15 DAT At flowering initiation | |
| Two applications of formulation D | 30 L/ha/application | At fruit setting | |
| Three applications of formulation E | 30 L/ha/application | At fruit enlargement and two more applications at 10-day intervals after the first application | |
| W6 | Six applications of formulation A | 20 kg/application | 15 DAT 25 DAT 35 DAT 45 DAT 55 DAT 65 DAT |
| W7 | Six applications of formulation B | 20 L/application | 15 DAT 25 DAT 35 DAT 45 DAT 55 DAT 65 DAT |
| W8 | Control | - | - |
| Treatment | Yield (kg/ha) | No of Fruit/ha | Mean Fruit Weight (kg) | Fruit Perimeter 1 (P1) (cm) | Fruit Perimeter 2 (P2) (cm) | P1/P2 | Rind Thicness (cm) |
|---|---|---|---|---|---|---|---|
| W1 | 78,226 ± 1523 c | 8299 ± 235 d | 9.43 ± 0.42 a | 96.5 ± 4.3 a | 77.3 ± 3.5 b | 1.24 ± 0.03 a | 1.23 ± 0.13 d |
| W2 | 76,555 ± 1274 d | 8299 ± 367 d | 9.20 ± 0.76 a | 91.48 ± 5.27 c | 75.38 ± 4.61 d | 1.21 ± 0.05 b | 1.33 ± 0.11 c |
| W3 | 81,521 ± 897 b | 9479 ± 668 b | 8.55 ± 1.08 c | 89.7 ± 4.11 d | 75.6 ± 3.1 d | 1.19 ± 0.02 c | 1.27 ± 0.22 cd |
| W4 | 69,455 ± 1158 f | 7812 ± 777 e | 8.85 ± 0.54 b | 95 ± 4 b | 77.7 ± 3.2 b | 1.21 ± 0.05 b | 1.52 ± 0.28 a |
| W5 | 84,042 ± 1897 a | 9930 ± 395 a | 8.47 ± 0.85 c | 96.48 ± 3.97 a | 79.12 ± 2.78 a | 1.22 ± 0.03 b | 1.42 ± 0.21 b |
| W6 | 75,226 ± 956 e | 8785 ± 459 c | 8.55 ± 1.09 c | 87.84 ± 7.05 e | 75.22 ± 3.43 d | 0.86 ± 0.10 d | 1.17 ± 0.12 e |
| W7 | 74,267 ± 1101 e | 8368 ± 332 d | 8.67 ± 1.55 c | 89.04 ± 5.61 d | 76.05 ± 8.69 c | 1.19 ± 0.09 c | 1.42 ± 0.18 b |
| W8 | 76,090 ± 1024 d | 8333 ±551 d | 8.64 ± 2.02 c | 94.49 ± 6.41 b | 77.82 ± 3.71 b | 1.21 ± 0.06 b | 1.33 ± 0.12 c |
| Treatment | Flesh Color | ||||
|---|---|---|---|---|---|
| L* | a* | b* | C* | h* | |
| W1 | 30.2 ± 1.7 a | 21.55 ± 1.65 b | 9.59 ± 0.72 a | 23.59 ± 1.79 b | 23.99 ± 0.43 a |
| W2 | 31.88 ± 4.16 a | 22.48 ± 2.59 ab | 9.97 ± 1.01 a | 24.59 ± 2.75 ab | 23.96 ± 0.92 a |
| W3 | 31.81 ± 2.53 a | 22.55 ± 1.53 ab | 9.93 ± 0.55 a | 24.64 ± 1.61 ab | 23.79 ± 0.59 a |
| W4 | 30.49 ± 3.44 a | 23.65 ± 1.74 a | 10.10 ± 0.9 a | 25.71 ± 1.95 a | 23.10 ± 0.65 b |
| W5 | 31.66 ± 2.35 a | 21.95 ± 1.58 ab | 9.74 ± 0.77 a | 24.02 ± 1.73 ab | 23.92 ± 0.68 a |
| W6 | 31.3 ± 2.4 | 22.5 ± 2.9 ab | 10.10 ± 1.17 a | 24.69 ± 3.11 ab | 24.17 ± 0.57 a |
| W7 | 31.56 ± 4.02 a | 22.21 ± 2.82 ab | 9.94 ± 1.38 a | 24.33 ± 3.12 ab | 24.08 ± 0.82 a |
| W8 | 30.43 ± 3.01 a | 22.75 ± 2.59 ab | 10 ± 1 a | 24.85 ± 2.78 ab | 23.8 ± 0.7 a |
| Treatment | Flesh Firmness (N) | ΤSS (° Brix) | pH | ΕC (mS/cm) | Titratable Acidity (g of Citric Acid/100 mL of Juice) |
|---|---|---|---|---|---|
| W1 | −0.49 ± 0.01 a | 12.49 ± 0.67 a | 5.49 ± 0.09 a | 2.34 ± 0.14 b | 0.09 ± 0.01 bc |
| W2 | −0.48 ± 0.08 a | 12.56 ± 0.72 a | 5.41 ± 0.09 ab | 2.34 ± 0.07 b | 0.09 ± 0.01 bc |
| W3 | −0.55 ± 0.12 a | 12.22 ± 0.41 a | 5.46 ± 0.13 ab | 2.43 ± 0.14 ab | 0.09 ± 0.01 bc |
| W4 | −0.48 ± 0.15 a | 12.09 ± 0.78 ab | 5.38 ± 0.08 b | 2.40 ± 0.18 ab | 0.10 ± 0.01 ab |
| W5 | −0.46 ± 0.09 a | 12.31 ± 0.83 a | 5.50 ± 0.14 a | 2.53 ± 0.06 a | 0.10 ± 0.01 ab |
| W6 | −0.52 ± 0.13 a | 11.60 ± 0.82 b | 5.47 ± 0.06 ab | 2.53 ± 0.09 ab | 0.08 ± 0.02 c |
| W7 | −0.55 ± 0.15 a | 12.15 ± 0.42 a | 5.48 ± 0.09 ab | 2.59 ± 0.17 a | 0.10 ± 0.01 ab |
| W8 | −0.46 ± 0.06 a | 12.08 ± 0.43 ab | 5.51 ± 0.07 a | 2.55 ± 0.33 a | 0.11 ± 0.02 a |
| W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | |
|---|---|---|---|---|---|---|---|---|
| Proximal Composition | (g/100 g dw) | |||||||
| Fat | 0.46 ± 0.01 a | 0.36 ± 0.01 c | 0.31 ± 0.02 d | 0.25 ± 0.01 f | 0.42 ± 0.01 b | 0.34 ± 0.01 c | 0.28 ± 0.01 e | 0.24 ± 0.01 f |
| Proteins | 7.36 ± 0.06 a | 6.65 ± 0.08 d | 6.27 ± 0.01 e | 5.83 ± 0.02 f | 7.22 ± 0.09 b | 6.86 ± 0.07 c | 5.80 ± 0.01 f | 4.80 ± 0.08 g |
| Ash | 3.42 ± 0.02 a | 2.93 ± 0.08 b | 2.58 ± 0.09 d | 2.38 ± 0.07 ef | 3.02 ± 0.07 b | 2.77 ± 0.06 c | 2.51 ± 0.08 de | 2.25 ± 0.09 f |
| Carbohydrates | 88.75 ± 0.05 f | 90.1 ± 0.1 d | 90.84 ± 0.04 c | 91.54 ± 0.06 b | 89.3 ± 0.1 e | 90.03 ± 0.01 d | 91.41 ± 0.06 b | 92.7 ± 0.1 a |
| Energy (Kcal/100 g dw) | 388.6 ± 0.1 e | 390.1 ± 0.3 d | 391.2 ± 0.3 bc | 391.8 ± 0.2 ab | 390.0 ± 0.2 d | 390.6 ± 0.1 cd | 391.4 ± 0.2 b | 392.2 ± 0.3 a |
| Free sugars | (g/100 g dw) | |||||||
| Fructose | 14.99 ± 0.07 c | 14.97 ± 0.08 c | 15.64 ± 0.02 a | 12.46 ± 0.03 f | 12.72 ± 0.07 e | 15.39 ± 0.06 b | 11.77 ± 0.08 g | 13.76 ± 0.05 d |
| Glucose | 8.54 ± 0.06 a | 7.50 ± 0.08 b | 7.39 ± 0.07 b | 5.34 ± 0.08 g | 6.14 ± 0.07 e | 6.61 ± 0.01 d | 5.88 ± 0.05 f | 7.05 ± 0.01 c |
| Sucrose | 15.77 ± 0.03 e | 14.53 ± 0.08 g | 15.50 ± 0.04 f | 21.85 ± 0.08 a | 21.90 ± 0.04 a | 19.86 ± 0.01 c | 21.24 ± 0.01 b | 19.53 ± 0.01 d |
| Trehalose | 0.27 ± 0.01 d | 0.38 ± 0.01 a | 0.39 ± 0.01 a | 0.30 ± 0.01 c | 0.31 ± 0.03 c | 0.31 ± 0.01 c | 0.30 ± 0.01 c | 0.34 ± 0.01 b |
| Total | 39.6 ± 0.2 e | 37.4 ± 0.2 h | 38.92 ± 0.01 g | 40.0 ± 0.2 d | 41.07 ± 0.01 b | 42.2 ± 0.1 a | 39.19 ± 0.03 f | 40.69 ± 0.07 c |
| Organic acids | (g/100 g dw) | |||||||
| Oxalic acid | 0.067 ± 0.012 d | 0.083 ± 0.001 c | 0.059 ± 0.001 e | 0.050 ± 0.001 f | 0.071 ± 0.001 d | 0.079 ± 0.001 c | 0.098 ± 0.001 a | 0.090 ± 0.001 b |
| Malic acid | 1.39 ± 0.03 d | 1.62 ± 0.02 b | 1.55 ± 0.01 c | 1.75 ± 0.04 a | 1.19 ± 0.02 f | 1.25 ± 0.01 e | 1.16 ± 0.01 g | 1.25 ± 0.01 e |
| Ascorbic acid | tr | tr | tr | tr | tr | tr | tr | tr |
| Citric acid | 1.40 ± 0.01 c | 1.36 ± 0.01 d | 1.43 ± 0.01 b | 1.48 ± 0.01 a | 1.35 ± 0.01 d | 1.24 ± 0.01 e | 1.21 ± 0.01 f | 1.35 ± 0.01 d |
| Fumaric acid | tr | tr | tr | tr | tr | tr | tr | tr |
| Total | 2.86 ± 0.03 c | 3.06 ± 0.03 b | 3.04 ± 0.01 b | 3.28 ± 0.03 a | 2.62 ± 0.02 ef | 2.56 ± 0.01 f | 2.47 ± 0.01 g | 2.69 ± 0.02 d |
| W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | |
|---|---|---|---|---|---|---|---|---|
| Fatty Acids | Relative Percentage (%) | |||||||
| C16:0 | 42.35 ± 0.01 c | 42.7 ± 0.2 bc | 42.4 ± 0.2 c | 43.3 ± 0.3 ab | 43.7 ± 0.1 a | 43.6 ± 0.3 a | 43.7 ± 0.4 a | 43.1 ± 0.8 abc |
| C16:1 | 2.11 ± 0.03 d | 2.38 ± 0.01 c | 2.74 ± 0.04 b | 3.21 ± 0.06 a | 3.1 ± 0.2 a | 3.18 ± 0.01 a | 3.2 ± 0.1 a | 3.2 ± 0.1 a |
| C17:0 | 1.06 ± 0.02 e | 1.18 ± 0.04 cd | 1.12 ± 0.01 de | 1.16 ± 0.06 d | 1.25 ± 0.02 c | 1.19 ± 0.06 cd | 1.55 ± 0.01 b | 1.73 ± 0.07 a |
| C18:0 | 14.3 ± 0.2 ab | 14.3 ± 0.2 ab | 14.2 ± 0.2 b | 14.7 ± 0.2 a | 14.43 ± 0.03 ab | 13.6 ± 0.3 c | 13.3 ± 0.3 c | 13.6 ± 0.2 c |
| C18:1n9c | 15.86 ± 0.08 a | 15.5 ± 0.6 abc | 15.70 ± 0.06 ab | 15.2 ± 0.1 bc | 14.99 ± 0.02 c | 15.2 ± 0.2 bc | 15.2 ± 0.1 bc | 15.4 ± 0.6 abc |
| C18:2n6c | 7.1 ± 0.1 a | 7.34 ± 0.01 a | 7.2 ± 0.1 a | 6.5 ± 0.3 c | 6.6 ± 0.2 bc | 7.05 ± 0.03 ab | 5.6 ± 0.5 d | 5.20 ± 0.04 d |
| C20:0 | 3.68 ± 0.09 cd | 3.86 ± 0.08 ab | 3.79 ± 0.09 bc | 3.51 ± 0.06 e | 3.56 ± 0.04 de | 3.96 ± 0.08 a | 4.00 ± 0.09 a | 3.9 ± 0.1 ab |
| C21:0 | 1.23 ± 0.05 b | 1.18 ± 0.08 bc | 1.47 ± 0.01 a | 1.44 ± 0.01 a | 1.22 ± 0.01 b | 1.03 ± 0.01 e | 1.07 ± 0.02 de | 1.12 ± 0.03 cd |
| C22:0 | 5.38 ± 0.03 b | 5.35 ± 0.03 bc | 5.17 ± 0.03 bc | 5.08 ± 0.01 c | 5.09 ± 0.02 bc | 5.35 ± 0.05 bc | 6.38 ± 0.04 a | 6.4 ± 0.4 a |
| C23:0 | 2.61 ± 0.06 a | 1.72 ± 0.07 c | 1.64 ± 0.05 dde | 1.5 ± 0.2 f | 1.52 ± 0.07 ef | 1.63 ± 0.01 def | 1.13 ± 0.01 g | 1.99 ± 0.07 b |
| C24:0 | 4.25 ± 0.01 cd | 4.47 ± 0.08 bcd | 4.53 ± 0.03 ab | 4.5 ± 0.3 bc | 4.46 ± 0.07 bcd | 4.23 ± 0.02 d | 4.8 ± 0.1 a | 4.3 ± 0.2 bcd |
| SFA | 74.88 ± 0.06 bcd | 74.8 ± 0.5 bcd | 74.37 ± 0.01 d | 75.2 ± 0.4 bc | 75.3 ± 0.4 b | 74.6 ± 0.2 cd | 76.0 ± 0.2 a | 76.2 ± 0.5 a |
| MUFA | 17.98 ± 0.05 b | 17.9 ± 0.6 b | 18.4 ± 0.1 ab | 18.36 ± 0.08 ab | 18.1 ± 0.1 ab | 18.4 ± 0.2 ab | 18.4 ± 0.3 ab | 18.6 ± 0.4 a |
| PUFA | 7.1 ± 0.1 a | 7.34 ± 0.01 a | 7.2 ± 0.1 a | 6.5 ± 0.3 c | 6.6 ± 0.2 bc | 7.05 ± 0.03 ab | 5.6 ± 0.5 d | 5.20 ± 0.04 d |
| Tocopherols | (µg/100 g dw) | |||||||
| α-Tocopherol | 120.8 ± 0.6 d | 128.8 ± 0.6 b | 125.2 ± 0.6 c | 136.2 ± 0.3 a | 105.2 ± 0.6 f | 101.0 ± 0.3 g | 83.4 ± 0.3 h | 114.2 ± 0.8 e |
| β-carotene | Lycopene | Total Carotenoids | Chlorophyll a | Chlorophyll b | Total Chlorophylls | |
|---|---|---|---|---|---|---|
| W1 | 0.100 ± 0.020 bc | 0.123 ± 0.006 de | 0.225 ± 0.017 c | 0.060 ± 0.020 a | 0.080 ± 0.030 a | 0.14 ± 0.04 a |
| W2 | 0.150 ± 0.010 a | 0.140 ± 0.004 a | 0.289 ± 0.017 a | 0.033 ± 0.009 b | 0.040 ± 0.020 b | 0.07 ± 0.02 b |
| W3 | 0.142 ± 0.008 a | 0.116 ± 0.001 e | 0.258 ± 0.007 b | 0.030 ± 0.020 b | 0.040 ± 0.010 b | 0.07 ± 0.03 b |
| W4 | 0.091 ± 0.009 bc | 0.131 ± 0.004 bc | 0.222 ± 0.005 c | 0.030 ± 0.010 b | 0.040 ± 0.020 b | 0.07 ± 0.02 b |
| W5 | 0.090 ± 0.010 bc | 0.129 ± 0.005 bcd | 0.223 ± 0.007 c | 0.020 ± 0.010 b | 0.040 ± 0.020 b | 0.07 ± 0.01 b |
| W6 | 0.100 ± 0.005 bc | 0.125 ± 0.003 cd | 0.226 ± 0.005 c | 0.018 ± 0.001 b | 0.028 ± 0.001 b | 0.05 ± 0.01 b |
| W7 | 0.106 ± 0.009 b | 0.130 ± 0.002 bcd | 0.236 ± 0.011 c | 0.030 ± 0.010 b | 0.040 ± 0.020 b | 0.07 ± 0.03 b |
| W8 | 0.085 ± 0.009 c | 0.136 ± 0.004 ab | 0.221 ± 0.009 c | 0.020 ± 0.007 b | 0.030 ± 0.010 b | 0.05 ± 0.02 b |
| W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | Positive Controls | |
|---|---|---|---|---|---|---|---|---|---|
| Antioxidant activity (IC50; µg/mL) | Trolox | ||||||||
| OxHLIA (Δt = 60 min) | 1729 ± 168 d | 907 ± 65 e | 894 ± 85 e | 4326 ± 163 b | 3845 ± 153 bc | 3738 ± 102 c | 6435 ± 393 a | 902 ± 34 e | 21.8 ± 0.2 |
| Cytotoxicity to tumor cell lines (GI50; µg/mL) | Ellipticine | ||||||||
| AGS | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | 0.90 ± 0.07 |
| CaCo | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | 0.83 ± 0.05 |
| MCF-7 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | 1.02 ± 0.01 |
| NCI-H460 | 14 ± 1 f | 112 ± 9 d | 55 ± 5 e | 178 ± 6 bc | 233 ± 7 a | 166 ± 7 c | 190 ± 12 b | 162 ± 13 c | 1.01 ± 0.01 |
| Cytotoxicity to non-tumor cell lines (GI50; µg/mL) | Ellipticine | ||||||||
| Vero | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | 0.64 ± 0.05 |
| Anti-inflammatory activity (EC50; µg/mL) | Dexamethasone | ||||||||
| RAW 264.7 | 27 ± 2 c | 31 ± 2 bc | 30 ± 2 bc | 32 ± 3 bc | 35 ± 3 b | 32 ± 2 bc | 42 ± 2 a | 45 ± 4 a | 16 ± 1 |
| S. aureus (ATCC 11632) | B. cereus (Clinical Isolate) | L. monocytogenes (NCTC 7973) | S. typhimurium (ATCC 13311) | E. coli (ATCC 25922) | E. cloacae (ATCC 35030) | ||
|---|---|---|---|---|---|---|---|
| W1 | MIC | 1.00 | 1.00 | 1.00 | 0.50 | 1.00 | 1.00 |
| MBC | 2.00 | 2.00 | 2.00 | 1.00 | 2.00 | 2.00 | |
| W2 | MIC | 4.00 | 1.00 | 2.00 | 1.00 | 1.00 | 1.00 |
| MBC | 8.00 | 2.00 | 4.00 | 2.00 | 2.00 | 2.00 | |
| W3 | MIC | 2.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| MBC | 4.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
| W4 | MIC | 2.00 | 1.00 | 2.00 | 0.50 | 0.50 | 2.00 |
| MBC | 4.00 | 2.00 | 4.00 | 1.00 | 1.00 | 4.00 | |
| W5 | MIC | 2.00 | 1.00 | 2.00 | 0.50 | 1.00 | 1.00 |
| MBC | 4.00 | 2.00 | 4.00 | 1.00 | 2.00 | 2.00 | |
| W6 | MIC | 2.00 | 1.00 | 2.00 | 1.00 | 1.00 | 1.00 |
| MBC | 4.00 | 2.00 | 4.00 | 2.00 | 2.00 | 2.00 | |
| W7 | MIC | 2.00 | 1.00 | 2.00 | 1.00 | 1.00 | 2.00 |
| MBC | 4.00 | 2.00 | 4.00 | 2.00 | 2.00 | 4.00 | |
| W8 | MIC | 4.00 | 1.00 | 2.00 | 1.00 | 0.50 | 2.00 |
| MBC | 8.00 | 2.00 | 4.00 | 2.00 | 1.00 | 4.00 | |
| E211 | MIC | 4.00 | 0.50 | 1.00 | 1.00 | 1.00 | 2.00 |
| MBC | 4.00 | 0.50 | 2.00 | 2.00 | 2.00 | 4.00 | |
| E224 | MIC | 1.00 | 2.00 | 0.50 | 1.00 | 0.50 | 0.50 |
| MBC | 1.00 | 4.00 | 1.00 | 1.00 | 1.00 | 0.50 |
| A. fumigatus (ATCC 9197) | A. ochraceus (ATCC 12066) | A. niger (ATCC 6275) | P. funiculosum (ATCC 36839) | P. v. var. cyclopium (Food Isolate) | T. viride (IAM 5061) | ||
|---|---|---|---|---|---|---|---|
| W1 | MIC | 0.50 | 0.50 | 1.00 | 0.50 | 0.50 | 0.25 |
| MFC | 1.00 | 1.00 | 2.00 | 1.00 | 1.00 | 0.50 | |
| W2 | MIC | 0.50 | 0.50 | 1.00 | 0.50 | 1.00 | 0.50 |
| MFC | 1.00 | 1.00 | 2.00 | 1.00 | 2.00 | 1.00 | |
| W3 | MIC | 1.00 | 0.50 | 1.00 | 0.50 | 1.00 | 0.25 |
| MFC | 2.00 | 1.00 | 2.00 | 1.00 | 2.00 | 0.50 | |
| W4 | MIC | 0.50 | 0.50 | 0.50 | 0.50 | 1.00 | 0.25 |
| MFC | 1.00 | 1.00 | 1.00 | 1.00 | 2.00 | 0.50 | |
| W5 | MIC | 1.00 | 1.00 | 0.50 | 0.50 | 1.00 | 1.00 |
| MFC | 2.00 | 2.00 | 1.00 | 1.00 | 2.00 | 2.00 | |
| W6 | MIC | 1.00 | 1.00 | 1.00 | 0.50 | 0.50 | 0.50 |
| MFC | 2.00 | 2.00 | 2.00 | 1.00 | 1.00 | 1.00 | |
| W7 | MIC | 1.00 | 1.00 | 1.00 | 0.50 | 0.50 | 0.25 |
| MFC | 2.00 | 2.00 | 2.00 | 1.00 | 1.00 | 0.50 | |
| W8 | MIC | 1.00 | 0.50 | 1.00 | 0.50 | 0.50 | 0.25 |
| MFC | 2.00 | 1.00 | 2.00 | 1.00 | 1.00 | 0.50 | |
| E211 | MIC | 1.00 | 1.00 | 1.00 | 1.00 | 2.00 | 1.00 |
| MFC | 2.00 | 2.00 | 2.00 | 2.00 | 4.00 | 2.00 | |
| E224 | MIC | 1.00 | 1.00 | 1.00 | 0.50 | 1.00 | 0.50 |
| MFC | 1.00 | 1.00 | 1.00 | 0.50 | 1.00 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, Â.; Polyzos, N.; Mandim, F.; Pereira, C.; Petrović, J.; Soković, M.; Petropoulos, S.A. Combined Effect of Biostimulants and Mineral Fertilizers on Crop Performance and Fruit Quality of Watermelon Plants. Horticulturae 2023, 9, 838. https://doi.org/10.3390/horticulturae9070838
Fernandes Â, Polyzos N, Mandim F, Pereira C, Petrović J, Soković M, Petropoulos SA. Combined Effect of Biostimulants and Mineral Fertilizers on Crop Performance and Fruit Quality of Watermelon Plants. Horticulturae. 2023; 9(7):838. https://doi.org/10.3390/horticulturae9070838
Chicago/Turabian StyleFernandes, Ângela, Nikolaos Polyzos, Filipa Mandim, Carla Pereira, Jovana Petrović, Marina Soković, and Spyridon A. Petropoulos. 2023. "Combined Effect of Biostimulants and Mineral Fertilizers on Crop Performance and Fruit Quality of Watermelon Plants" Horticulturae 9, no. 7: 838. https://doi.org/10.3390/horticulturae9070838
APA StyleFernandes, Â., Polyzos, N., Mandim, F., Pereira, C., Petrović, J., Soković, M., & Petropoulos, S. A. (2023). Combined Effect of Biostimulants and Mineral Fertilizers on Crop Performance and Fruit Quality of Watermelon Plants. Horticulturae, 9(7), 838. https://doi.org/10.3390/horticulturae9070838









