Abstract
In order to improve the resistance of apples to stresses (abiotic stresses) during hot and dry summers to enhance their productivity and marketability, Anna apple trees were treated with aminoethoxyvinylglycine (AVG), 1-methlcyclopropene (1-MCP), and naphthalene acetic acid (NAA), alone, or in combination, in two successive seasons. All treatments significantly increased the yield per tree, the average fruit volume, fruit diameter, and fruit weight compared to the control (growing under hot and dry summers without any treatments). All treatments significantly reduced the apple size, total soluble solid (TSS) content, anthocyanin content, carotenoid content, total sugar solids, and sugar percentage, and they led to fruit softening, loss of fruit weight, firmness, breakdown percentage, as well as a decreased polyphenol oxidase and peroxidase activity at one-week post-harvest (except for 20-ppm NAA) in comparison with the control. Trees treated with 320-ppm 1-MCP + 250-ppm AVG exhibited maximum yield per tree, fruit firmness, and no-reducing sugars or starch. On the other hand, the 20-ppm NAA produced the maximum TSS content, total sugars, with a reduced sugar percentage, carotenoid and anthocyanin fruit content, and (TSS)/acidity ratio. The application of 320-ppm 1-MCP + 250-ppm AVG effectively delayed the harvest for 14 days compared to the control. Our results show that the sprays of NAA and inhibitors of ethylene had a significant effect on the productivity and marketability of Anna apples under abiotic stresses.
1. Introduction
With the world’s rapid development, plants serve a variety of environmental, economic, and social purposes, in addition to increasing the production of primary and secondary goods with economic value and positively affecting human health [1]. However, stress factors directly affect all plant properties, so the harmony between the climatic requirements of plants and the climatic conditions of a region is critical [2,3]. Abiotic stresses caused by frequent variations in global climate are responsible for inducing various modifications at the molecular and cellular levels in plants that, in turn, cause irreversible damage to the agricultural yields of several major fruit crops [4]. The changes may be induced by environmental components, including local geo-climatic and seasonal changes, external conditions of temperature, light, humidity, and developmental processes, which impact yield and quality production [5,6]. Abiotic stresses, which are one of the most severe environmental stresses and affect almost all plant functions, are induced by hot and dry summers during the maturity and ripening of fruits. In these conditions, environmental stresses cause serious reductions in the growth and quality of many plants and ensure regular crop yields [7,8].
Apples are one of the most popular fruit crops in the world, with significant economic and nutritional values, covering roughly five million hectares and producing approximately 93,144,358 tons of apples in 2021 [9,10]. The arid climate that has appeared has made apple production challenging; thus, environmental and management skills are required in order to help crops survive and adapt to abiotic stresses in their living conditions. Among different abiotic factors, high temperature is the main factor that develops various disorders in apples [11,12]. Apple cultivars, such as Anna apples that ripen in hot and dry summer conditions, may fail to produce marketable yields and high-quality fruits. This is because the temperature regimen can affect fruit growth and ripening, as well as limit fruit storability, shelf life, and several types of fruit breakdown before and after harvest, e.g., bitter pit, rapid softening, and browning [13,14,15]. Additionally, the risk and economic perspectives require more research, which needs to focus on a priority basis [16]. Although the Anna cultivar has a favorable low-chilling requirement and a fruit development period of almost 120 days [9,17], Anna apple fruits ripen under conditions that cause several types of PFD and reduce marketable yield [9], such as during hot and dry summers, a period of extremely high day temperatures (>40 °C). Increased temperatures could potentially reduce photosynthetic capacity by increasing the air evaporative demand and stomatal closure, which further decreases photosynthesis due to a smaller CO2 flux into the leaves [18]. It has been suggested that higher temperatures reduce the net carbon gain by increasing plant respiration more than photosynthesis. In addition, the productivity of Anna apples under abiotic stresses, such as warmer temperatures that speed up maturation and degree-days, can incur directly due to fruit drops from the spur and lead to smaller-sized and lighter crops, as well as indirectly due to harvesting fruit prior to optimal ripening [9,19,20,21]. For these reasons, in order to ensure regular crop yields and to reduce inter-annual yield variability, the use of chemical products, such as growth regulators, is necessary to reduce the problem. Growth regulators can alleviate abiotic stresses (salinity, extreme temperatures, drought, etc.) by modulating plant growth and development and improving growth for many plants, whether they are naturally occurring in the plant or added to the plant in order to increase its productivity and improve its efficiency under extreme conditions [22]. In addition, it can act separately or coordinate with other signaling pathways in a complex network. Cross-talk between the different hormones results in synergetic or antagonistic interactions that play crucial roles in the response of plants to abiotic stress [23,24]. Currently, the application of synthetic auxins or ethylene inhibitors as plant growth regulators are successfully practiced and mainly used in various countries, including all aspects of modern apple production to control and manipulate vegetative growth and regulation of flowering, reduce immature fruit drop, pre-harvest drops, fruit maturity, firmness, and manage apple harvest; they can also improve fruit quality and development while reducing ripening, which help improve the post-harvest life and final quality of fruit for better marketability [25,26,27,28,29,30,31,32,33]. Additionally, synthetic auxins and ethylene inhibitors are important because harvest windows are short, and these chemicals help anticipate or delay fruit maturation where necessary when applied within one month of pre-harvest [34,35]. Their effectiveness is closely related to application timing and concentrations, crop loads, the availability of nutrients, and biotic stresses. However, they can also be affected by cultivars, years, and locations [36].
Among the auxin-type growth regulators, NAA is a synthetic auxin analogue that may down-regulate abscission-related genes and reduce the sensitivity of the abscission zone to ethylene. It has long been used to reduce or totally prevent pre-harvest fruit drops, to preserve fruit flesh firmness, and to prevent starch degradation in apples [25,26,37,38,39]. It affects fruit formation, cell elongation, apical dominance, the photoperiod, and geotropism, and it has been used by some researchers to control preharvest fruit drops and flower thinning and to improve the quality of fruit in apples [38,40,41,42]. In recent years, it has become quite common to use 1-MCP as an inhibitor of ethylene to extend the shelf life of fruits and to minimize compositional changes, including those involved in respiration, ethylene generation, volatile production, chlorophyll degradation, and other color changes. It also affects protein and membrane modifications, softening, degradation of organic acids and phenolic compounds; in addition, it reduces flesh browning, the rotting of ripe fruits, titratable acidity loss, and sugars, and it controls ripening, delays maturation processes, maintains quality, extends shelf life, and improves the keeping quality, depending on the species being treated [43,44,45,46]. In addition, among the most potent ethylene inhibitors that are widely used for plant physiology studies and agricultural applications are AVG applications, which are used to delay ethylene-mediated processes, such as pre-harvest fruit abscission and fruit maturation on the tree, in addition to reducing the falling of fruit, decreasing preharvest fruit drops, maintaining fruit firmness, delaying fruit harvest, improving storage quality, prolonging the storage life of the fruit, and protecting the fruit firmness by inhibiting ethylene that cause to accelerate the maturation at period before harvest [25,33,47,48,49,50,51]. Previous results have shown that combinations of NAA (a synthetic auxin) and/or AVG (an inhibitor of ethylene biosynthesis), as well as 1-MCP (an inhibitor of ethylene action), were more effective when used in combination than when used alone on some apple varieties. Such applications can help extend fruit marketability and achieve the highest possible return from an orchard in terms of fruit retention, optimal yield, superior fruit quality, and extended harvest [42,47,52,53,54,55,56]. Such applications were used to reduce product losses be-tween producers and consumers [56] and to maintain sales and prices at affordable levels [57]. So, these chemicals could provide significant economic advantages to growers by lowering their losses due to preharvest fruit drops, minimizing the loss of fruit firmness, starch loss, and storage quality loss before harvest, as well as extending the harvest season under conditions of abiotic stress [25,26,39,56].
In Egypt, farmers cannot choose a strategy for planting different varieties with different harvest times, as the Anna apple variety is the most suitable variety for abiotic environmental conditions with which to increase apple production and the farmers’ income. Furthermore, based on a search of the literature, there are no complete studies that have focused on enhancing Anna apples’ productivity, physico-chemical properties, and marketability using sprays of NAA (a synthetic auxin), AVG (an inhibitor of ethylene bi-osynthesis), and 1-MCP (an inhibitor of ethylene action) to alleviate abiotic stresses. Hence, the present investigation aimed to compare the effects of 1-MCP, AVG, and NAA when applied alone or in combination to improve Anna apples’ yield, fruit quality, and marketability by extending the harvest date and fruit shelf life via natural physiological mechanisms and, thus, improving the product quality under abiotic stresses.
2. Materials and Methods
2.1. Experimental Site and Plant Materials
This study was performed in two consecutive seasons in 2020 and 2021 with 7-year-old Anna apple trees that were budded on “MM106” rootstock and grown on the North Coast, Matrouh Governorate, Egypt (GPS coordinates: 30.841852° N, 29.394043° E). The trees were spaced 3 × 4 m apart and grown in sandy loam soil with a drip irrigation system. All selected trees were healthy and nearly uniform in terms of their growth vigor and fruiting. All trees regularly received the same common cultural practices according to the recommendations of the Ministry of Agriculture of Egypt [9]. They were sprayed once at full bloom with Amcotone (0.6 g L−1), which is a commercial product comprising 0.45% naphthalene acetic acid (NAA) + 1.25% naphthalene acetamide (NAD) (Amvac Chemical Corporation, Commerce, CA, USA). The variations in monthly air temperatures and relative humidity during the 2010, 2020, and 2021 seasons are shown in Table 1.

Table 1.
Climate characteristics of the Matrouh Governorate during the 2010, 2020, and 2021 seasons.
In total, 64 trees, which were largely uniform in terms of size, productivity, and appearance, were selected for eight treatments that were organized in a randomized complete block design (RCBD), with four replicates for each treatment and two trees for each replicate (i.e., 8 treatments × 4 replicates × 2 trees per replicate = 64 trees). For each treatment, the trees received the following spraying treatments once before the anticipated harvest date (9 June and 2 June in the first and second seasons, respectively) at 4 weeks preharvest: the control, which was untreated, continued growing naturally under hot and dry summers with the environmental factors naturally (T1); 320 ppm 1-MCP was applied as a sprayable formulation (Rohm and Haas Co., Spring House, PA, USA) (T2); 250 ppm AVG (obtained at 1666.6 mg L−1 from the commercial product ReTain, which contained 15% AVG; Valent BioSciences Corp., Libertyville, IL, USA) (T3); 20 ppm NAA (T4); 320 ppm 1-MCP + 250 ppm AVG (T5); 320 ppm 1-MCP + 20 ppm NAA (T6); 250 ppm AVG + 20 ppm NAA (T7); 320 ppm 1-MCP + 250 ppm AVG + 20 ppm NAA (T8).
Spraying was applied by using a hand pressure sprayer. The surfactant agent Tensotec (produced by BC Fertilize, Valencia, Spain) was added to all of these treatment solutions at 0.05% v/v to obtain the best spraying results on each tree.
2.2. Measurement of the Studied Parameters
2.2.1. The Productivity of Anna Apples
The productivity of Anna apples was evaluated by measuring the following parameters: fruit drop, fruit yield, and fruit yield increment. To determine the drop percentage, from 28 days before the anticipated harvest time, the number of fallen fruits under a tree was counted twice a week until the harvest date. The fruits remaining on the trees were then harvested, and the percentage of fruit drop was calculated according to Yildiz et al. [58] using the following equations:
No. of total fruits = No. of fruits fallen from 28 days before anticipated harvest until harvest time + No. of remaining fruits at harvest date
When fruit coloring reached approximately 75%, the harvest date for each replicate was recorded, and yield was recorded as the number of fruits per tree and the weight of fruits in kilograms per tree. The fruit yield increment percentage compared with the control was calculated using the following equation:
2.2.2. Fruit Physico-Chemical Properties
To determine the effect of different treatments on the physico-chemical fruit properties, twenty fruits were randomly collected from each tree at the harvest date (when fruit coloring reached approximately 75 percent) and transported directly to the Food Science and Technology Department, Faculty of Agriculture, Alexandria University, Alexandria, Egypt.
Fruit samples (20 fruits) for each replicate were used to measure the following: average fruit weight (g); fruit length and diameter, measured using a hand caliper and shape index for calculation of the length/diameter ratio; fruit volume (five fruits), measured using the water displacement method and used to calculate average fruit volume (cm3); and flesh firmness (kPa), measured on two opposite sides of the fruit using a Magness–Taylor pressure tester. The percentage of the total soluble solid (TSS) of fruit juice was determined via a hand refractometer (ATAGO N-1 E, Japan). The percentage of total acidity (TA) as malic acid was determined in fruit juice according to AOAC [59]. The TSS/acidity ratio was calculated for each replicate of the applied treatments. Total sugar content (TSC) percentage was determined in fresh fruit samples based on the method of Malik and Singh [60]. Total and reducing sugars were extracted from 5 g of fresh weight and determined with phenol sulfuric acid and Nelson’s arsenatemolybdate using colorimetric methods, respectively. Non-reducing sugar levels were calculated as the difference between total sugars and reducing sugars. Starch was estimated in 0.1 g of the residue remaining after sugar extraction using hydrolysis with concentrated HCl for 3 h in a reflux condenser [59]. The ascorbic acid content (vitamin C) of the juice was determined by titration with 2,6-dichlorophenolindophenol [59] and calculated as milligrams per 100 mL of juice. Anthocyanin content (mg/100 g fresh weight) was calorimetrically determined according to Rabino et al. [61] at 535 nm in fruit skin. Carotenoid content (mg/100 g fresh weight) was quantified in fruit peel according to the method of Moran and Porath [62].
2.2.3. Fruit Marketability
The marketability effects of treatments were evaluated via the extension of harvest date and the maintenance of fruit shelf life because of the delayed loss of fruit firmness, weight, and breakdown, as well as the control of oxidizing enzyme activity. When fruits in each replicate (64 fruits per treatment) reached the early ripe stage (i.e., fruit coloring reached approximately 75%), 16 fruits were sampled per tree, transported immediately to the laboratory, and then held in a storage room at 21 °C ± 1 °C for 1 week to determine the effect of the treatments on marketability. The harvest date of each treatment was recorded at harvest. Fruit firmness, weight, and incidence of breakdown were recorded every 2 days. The percentages of these variables were calculated as follows:
Oxidizing enzyme activity was assayed for fresh and stored apple samples. Fifty grams of apple tissue were weighed and transferred to a mortar with 10 g of purified sand. Subsequently, 10 mL of 0.1 M sodium phosphate buffer (pH 6.8) were added, and the tissue was ground for 10 min with a pestle. The resultant slurry was added to double-thick cheesecloth and squeezed. The collected liquid was centrifuged at ~3000 rpm for 5 min, and the supernatant was then kept on ice during the enzymatic assay. Polyphenol oxidase (PPO) activity was assayed using a reaction mixture containing 0.2 mL of 0.001 M catechol, 2.3 mL of phosphate buffer (0.1 M, pH 6.8), and 0.5 mL of enzyme extract. The activity was measured spectrophotometrically at 15 s intervals as changes in absorbance occurred at 495 nm. The amount of enzyme that caused a 0.001 absorbance increase at 495 nm in 1 min was taken as the definition of 1 unit of PPO activity. Polyphenol peroxidase (POD) activity was also assayed spectrophotometrically at 420 nm using a reaction mixture containing 0.2 mL pyrogallol, 0.1 mL hydrogen peroxide (1% v/v), 2.2 mL sodium phosphate buffer (0.1 M, pH 6.0), and 0.5 mL enzyme extract. Hydrogen peroxide was replaced with distilled water in the blank cuvette. One unit of POD was defined as forming 1 mg of purpurogallin from pyrogallol in 20 s at pH 6.0 and 20 °C. The enzyme unit (EU) was calculated for 50 g of apple tissue. The percentage of remaining activity for both enzymes was calculated from the following equation:
2.3. Statistical Analysis
The treatments were arranged in an RCBD according to the design of Gomez and Gomez [63]. All data were tested for treatment effects on the analyzed parameters by using one-way ANOVA. A least-significant-difference test was used to separate and compare the treatment means, and a probability level of 0.05 was set according to the method of Snedecor and Cochran [64]. All statistical analyses were performed in SAS, version 9.13 [65].
3. Results
3.1. Productivity
All treatments significantly decreased the fruit drops in comparison with the control during the first and second seasons (Figure 1a). In these seasons, the treatment with 320 ppm 1-MCP + 250 ppm AVG (T5) led to the lowest fruit drop percentages (2.51% and 2.75%). Similarly, all treatments led to a significant increase in yield per tree compared to the control in the first and second seasons (40.13 kg/tree and 38.945 kg/tree, respectively) (Figure 1b). Specifically, T5 produced the maximum yield per tree in each season. As shown in Figure 1c, all treatments significantly affected the yield increment percentage in comparison with the control in both seasons. In addition, the highest mean values of the yield increment percentage were determined in the T5 treatment.
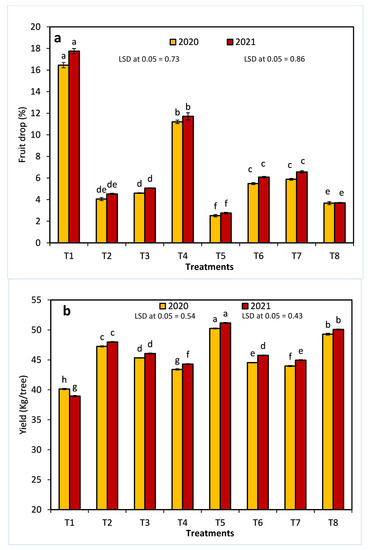
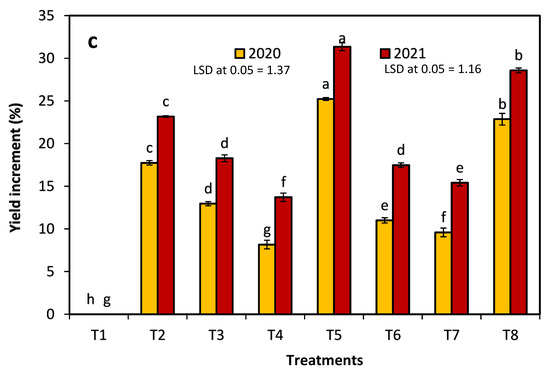
Figure 1.
The effects of 1-methylcyclopropene (1-MCP), aminoethoxyvinylglycine (AVG), naphthalene acetic acid (NAA), and combinations thereof on fruit drop (a), yield (b), and yield increment (c) of Anna apples in the 2020 and 2021 seasons. (T1): control; (T2): 320-ppm 1-MCP; (T3): 250-ppm AVG; (T4): 20-ppm NAA; (T5): 320-ppm 1-MCP + 250-ppm AVG; (T6): 320-ppm 1-MCP + 20-ppm NAA; (T7): 250-ppm AVG + 20-ppm NAA; and (T8): 320-ppm 1-MCP + 250-ppm AVG + 20-ppm NAA. Different letters indicate significant differences at p ≤ 0.05.
3.2. Fruit Physico-Chemical Properties
The data in Table 2 show that all treatments significantly increased the average fruit weight, fruit volume, and fruit diameter relative to these measures in the control fruits. Additionally, the treatment with 20 ppm NAA or 320 ppm 1-MCP + 250 ppm AVG + 20 ppm NAA (T8) significantly increased the fruit diameter, fruit volume, and fruit weight in comparison with these measures in all other treatments for both seasons. All treatments slightly, but often significantly, increased the shape index in comparison with the control (Table 1), and T8 led to the highest values in both seasons. Compared with the control fruits in both seasons, all treatments, except for 20 ppm NAA, significantly reduced apple fruit softening. The treatment of 320 ppm 1-MCP + 250 ppm AVG led to the maximum fruit firmness values.

Table 2.
The effects of 1-methylcyclopropene (1-MCP), aminoethoxyvinylglycine (AVG), naphthalene acetic acid (NAA), and combinations thereof on the physical characteristics of Anna apples in the 2020 and 2021 seasons.
As shown in Table 3, all treatments, except for 20 ppm NAA, significantly reduced the TSS percentage in the Anna apples compared with the control (12.70% and 12.77% in the first and second seasons, respectively). The treatment of 320 ppm 1-MCP + 250 ppm AVG (T5) gave the lowest values for the TSS percentage (11.79% and 11.80% in each season, respectively). In terms of acidity, all treatments, except for 20 ppm NAA (T4), significantly increased the TA percentage compared with that of the control. As with TSS, T5 had the greatest effect on acidity by increasing TA to the greatest extent in both seasons. In contrast, the maximum fruit TSS/acidity ratio was recorded for T4, whereas the 320 ppm 1-MCP + 250 ppm AVG treatment led to the minimum TSS/acidity ratio. Compared with the control, all treatments significantly reduced the contents of anthocyanins and carotenoids in Anna apples, except for 20 ppm NAA. The contents of both carotenoids and anthocyanins in the fruit were the lowest after the 320 ppm 1-MCP + 250 ppm AVG treatment in both seasons. Concerning the total sugar content (TSC), the data in Table 3 reveal that all treatments, except for 20 ppm NAA, significantly decreased the TSC of the Anna apples. The lowest TSC values were recorded after the 320 ppm 1-MCP + 250 ppm AVG treatment in both seasons. Similarly, all treatments significantly decreased the reducing sugar percentage, except for 20 ppm NAA (T4), while the lowest reducing sugar percentage was observed with T5 in both seasons. Concurrently, all treatments, except for T4, significantly increased the non-reducing sugar and starch percentages relative to these measures in the control fruit, with the 320 ppm 1-MCP + 20 ppm NAA treatment producing significantly higher non-reducing sugar and starch values than those of all other treatments.

Table 3.
The effects of 1-methylcyclopropene (1-MCP), aminoethoxyvinylglycine (AVG), naphthalene acetic acid (NAA), and combinations thereof on the chemical characteristics of Anna apples in the 2020 and 2021 seasons.
3.3. Marketability
The treatments’ effects on the extension of the Anna apple harvest date in the 2020 and 2021 seasons are presented in Figure 2. The results revealed that, in both seasons, the fruits treated with 20 ppm NAA (T4) were the first reach to their harvest date, followed by the control fruit, and then the fruit treated with 250 ppm AVG + 20 ppm NAA (T7). Conversely, the latest harvest dates in both seasons were observed in the trees sprayed with 320 ppm 1-MCP + 250 ppm AVG, followed by T8. For the control, in both seasons, T5 effectively delayed the harvest date by 14 days, whereas the application of T4 brought the harvest date forward by two days.
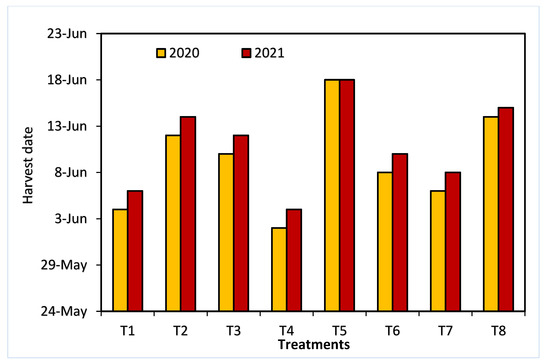
Figure 2.
The effects of 1-methylcyclopropene (1-MCP), aminoethoxyvinylglycine (AVG), naphthalene acetic acid (NAA), and combinations thereof on extending the harvest date of Anna apples in the 2020 and 2021 seasons. (T1): control; (T2): 320-ppm 1-MCP; (T3): 250-ppm AVG; (T4): 20-ppm NAA; (T5): 320-ppm 1-MCP + 250-ppm AVG; (T6): 320-ppm 1-MCP + 20-ppm NAA; (T7): 250-ppm AVG + 20-ppm NAA; and (T8): 320-ppm 1-MCP + 250-ppm AVG + 20-ppm NAA.
As shown in Figure 3, all treatments, except for 20 ppm NAA, significantly decreased the loss of fruit weight and firmness at one week postharvest. In comparison with all other sprayed growth regulators, treatment T5 resulted in the lowest percentages of fruit firmness loss and fruit weight loss in both seasons. Similarly, fruit breakdown was decreased in all treatments, except for 20 ppm NAA in both seasons, and T5 showed the lowest fruit breakdown percentage.
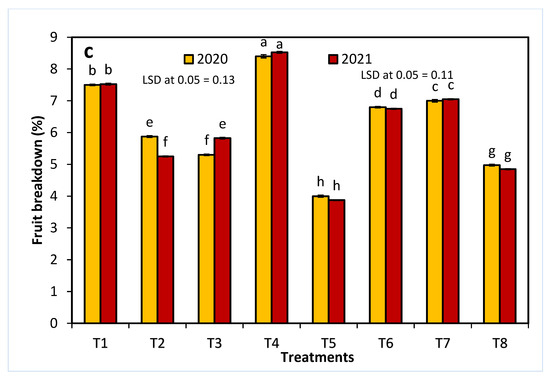
Figure 3.
The effects of 1-methylcyclopropene (1-MCP), aminoethoxyvinylglycine (AVG), naphthalene acetic acid (NAA), and combinations thereof on fruit firmness (a), fruit weight loss (b), and fruit breakdown (c) of Anna apples in the 2020 and 2021 seasons. (T1): control; (T2): 320-ppm 1-MCP; (T3): 250-ppm AVG; (T4): 20-ppm NAA; (T5): 320-ppm 1-MCP + 250-ppm AVG; (T6): 320-ppm 1-MCP + 20-ppm NAA; (T7): 250-ppm AVG + 20-ppm NAA; and (T8): 320-ppm 1-MCP + 250-ppm AVG + 20-ppm NAA. Different letters indicate significant differences at p ≤ 0.05.
With the exception of 20 ppm NAA, all treatments significantly decreased the initial PPO and POD activities in both seasons (Figure 4). The enzymatic activity of both enzymes decreased in the control and treated fruits after one week of storage. The control fruit and the fruit treated with 20 ppm NAA had the lowest percentages of remaining activity for each enzyme, whereas T5 had the highest percentage of remaining activity for both enzymes.
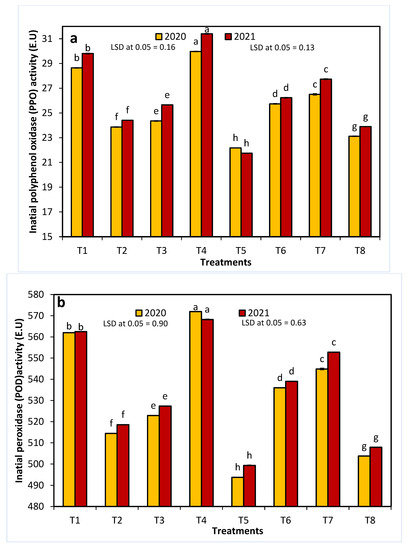
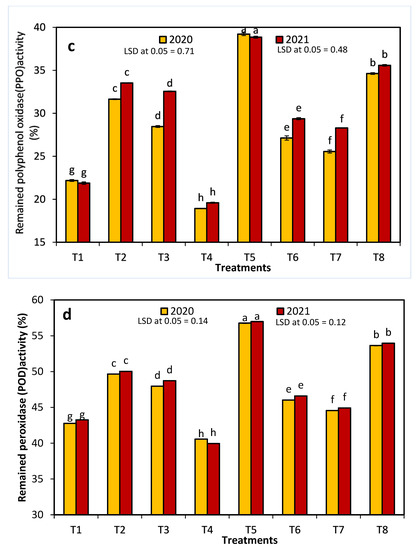
Figure 4.
The effects of 1-methylcyclopropene (1-MCP), aminoethoxyvinylglycine (AVG), naphthalene acetic acid (NAA), and combinations thereof on Anna apple polyphenol oxidase (PPO: (a,b)) and peroxidase (POD: (c,d)) activity in the 2020 and 2021 seasons. (T1): control; (T2): 320-ppm 1-MCP; (T3): 250-ppm AVG; (T4): 20-ppm NAA; (T5): 320-ppm 1-MCP + 250-ppm AVG; (T6): 320-ppm 1-MCP + 20-ppm NAA; (T7): 250-ppm AVG + 20-ppm NAA; and (T8): 320-ppm 1-MCP + 250-ppm AVG + 20-ppm NAA. Different letters indicate significant differences at p ≤ 0.05.
4. Discussion
Generally, all treatments had positive effects on the fruit drop, yield increment percentage, and yield of Anna apple trees. These results are in agreement with those of previous reports on other apple cultivars, such as ‘Arlet’ [66] and Jersey Mac [67]. The reduction in fruit drop, inhibition of fruit ethylene production, and delayed fruit ripening following the uptake of NAA or inhibitors of ethylene into leaves and/or branches could be due to the molecules’ translocation into the fruit or a modification of some leaf–fruit signaling system associated with the onset of fruit ripening [48]. Thus, the preharvest fruit drop is most likely caused by increased biomechanics of internal hormone levels, specifically higher auxin levels and reduced ethylene levels. Besides, AVG and 1-MCP delay preharvest fruit drop by blocking ethylene production and delaying the commencement of apple fruit ethylene climacteric, while NAA reduces mature fruit drop by decreasing ethylene-enhanced cellulase gene expression and de novo cellulase synthesis [28,34,36,58,68]. Hence, treating fruit with NAA reduces the breakdown of fruit cell walls and their subsequent loosening or abscission. Yuan and Carbaugh [25] reported that there is a synergic effect among NAA, 1-MCP, and AVG when controlling preharvest fruit drops. This suggestion is supported by the finding that the combination of AVG, 1-MCP, and NAA resulted in greater fruit ethylene production, but less preharvest fruit drop, in comparison with AVG alone in ‘Golden Supreme’ and ‘Golden Delicious’ apples.
In our study, the treatments with NAA and inhibitors of ethylene delayed PFD and maturity for an additional two to sixteen days; such an effect may increase crop value by increasing the yield, fruit quality, and price. The increase in yield under these growth regulator treatments was likely associated with the increase in fruit numbers, increased fruit retention, low percentage of fruit drop, and increased fruit size and weight via the control of cell division and cell expansion that resulted from increased auxin concentrations in the fruitlet tissues [55,69]. Byers et al. [66] reported that growth regulator treatments—especially those with 1-MCP + AVG—preserved the cortical and vascular regions in the abscission zone, and the entire fruit pedicle was brighter green when compared with the pedicle of a control fruit. Thus, the AVG and 1-MCP contents during the growth and development of fruit are important for ensuring increased fruit retention and, therefore, increased yield [70,71,72]. It was clear from previous studies that the reduction in both fruit drops and the expression of MdACS5B, MdACO1, MdACS5A, MdEG1, and MdPG2 occurred as a result of the application of AVG, whereas a reduction in the expression of MdRCCR2, MdNYC1, MdNYC3, and MdNOL2 occurred when AVG+1-MCP was applied in the abscission zone of the fruit [27,73]
Generally, all measured physical properties were significantly affected by the growth regulator sprays in both the 2020 and 2021 seasons. Inhibited or delayed ethylene generation in 1-MCP- or AVG-treated fruit is associated with lower losses due to preharvest drop and delayed maturation, as well as delayed harvest, thus allowing the fruit size to increase [25,28,29,31,32]. Similarly, in a previous study, the application of NAA, 1-MCP, and AVG increased the fruit diameter, length, and length/diameter ratio; these effects increased the crop value because the fruit was larger and redder [25,38,39]. The increase in fruit dimensions (i.e., length and diameter) and weight due to the application of NAA, AVG, and 1-MCP might be attributable to their roles in activating protein biosynthesis and RNA/DNA mechanisms. Through these processes, fruit grows larger because of the efficiency of cells, i.e., the building blocks of fruit mass, and because these cells can attract more water, minerals, and carbohydrates, which enable the expansion of the fruit to larger sizes [74,75]. Because summer apple cultivars often ripen unevenly and require two to four pickings, a significant reduction in the loss of firmness may be due to the gradual breakdown of proto-pectin into fractions of a lower molecular weight that are more soluble in water, which was found to be directly correlated with the rate of softening of the fruit [76]. The AVG, 1-MCP, and NAA treatments resulted in fruit that was firmer than that of the untreated control. Additionally, the addition of AVG or 1-MCP also delayed the loss of fruit firmness, thus overcoming the adverse effect of fruit softening caused by abiotic stresses [25,34]. The lower rates of ethylene generation and respiration, the reduced activity of ACC oxidase, and the greater flesh firmness observed in fruits sprayed with AVG or 1-MCP may all be contributing factors [42], in addition to their slowing of the impacts of ethylene-induced enzyme activity, such as polygalactrnase and pectine formation [77]. Compared with those that underwent the control treatment, the apples that received preharvest applications of AVG or 1-MCP had greater levels of flesh firmness and texture characteristics. The degradation of cell wall components caused by ethylene leads to a decrease in the hardness of the flesh [51]. Additionally, fruit treated with AVG or 1-MCP may have firmer flesh due to the reduced ACC oxidase activity and ethylene generation rates [45,78,79].
All of the measured chemical attributes of Anna apples were significantly positively affected by the growth regulator treatments in the 2020 and 2021 seasons. The higher TSS content in apples sprayed with AVG or 1-MCP was related to the reduction in flesh firmness caused by the hydrolysis of cell walls and high content of soluble pectins, since those fruits had the lowest pulp firmness and a lower pulp penetration and skin rupture strength [80]. In other studies, starch degradation was increased by an NAA treatment but decreased by 1-MCP and AVG applications, which were similarly shown to delay fruit maturation [25,47,81]. The solubility of cell wall components, such as pectins, was likely responsible for the high acidity observed in the NAA-treated fruit, as they are mainly composed of galacturonic acid and can increase the acidity of the juice [82] (a decrease in titratable acidity could be caused by the utilization of a high rate of oxidation for various organic acids during respiration or sugar conversion [83]). Applications of AVG or 1-MCP have been reported by previous studies to delay the synthesis of anthocyanins in the epidermis of Pink Lady [49], Imperial Gala [84], Royal Gala [34], and Brookfield [42] apples because of the delayed development of red blush and reduced skin redness. In addition, when Brookfield apples were treated with NAA, the red color index was higher, while it was lower with an NAA + AVG treatment, and this delayed the process of the development of skin color to become red [42]. Similarly, Zhang et al. [85] suggested that the synthesis of anthocyanins and carotenoids was reduced by more than 36% by their 1-MCP treatment, whereas the retention of chlorophyll and phenolics was improved by this treatment. These results may be attributable to the use of AVG and 1-MCP, which are known to delay the ripening and senescence of fruits, inhibit enzymes such as ACC synthase, inhibit ethylene, alter the development of ground and over-skin color, and slow down starch degradation [86,87].
Anna apples are a climacteric fruit, and a reduction in respiration is required to delay the climacteric peak in semi-arid regions [9]. Our results indicated that the effect of AVG or 1-MCP on delaying the loss of fruit quality in Anna apples produced under abiotic stresses was very similar to that obtained in studies on Anna apples [88] and Jersey Mac apples [67]. Previous studies have indicated that treating apple trees with 1-MCP or AVG at 30 days before harvest delays the climacteric peak of respiration and ethylene production during the ripening process, which extends the shelf life of climacteric fruits by delaying fruit ripening and senescence [42,53,73,89]. Because ethylene is a plant growth regulator involved in fruit ripening, blocking its activity with AVG or 1-MCP slows down physiological changes by decreasing the respiratory rate, preventing ethylene synthesis, reducing fruit ethylene production, and delaying fruit ripening to increase the storage life of horticultural products [25,88]. Some authors suggested that NAA application stimulated the synthesis of induced ACC oxidase enzyme activity and new ACC synthase enzymes; therefore, it increased ethylene production [27,90]. In addition, preharvest NAA application increased the expression of genes related to ethylene biosynthesis (MdACS1 and MdACO1), perception (MdERS1), and cell wall degradation (MdPG1) [27]. So, NAA accelerated the maturation and ripening of fruit; thus, starch degradation was accelerated by NAA treatment.
Rapid fruit ripening poses a serious problem for quality and limits the shelf life of Anna apples on the market. Although postharvest changes in horticultural crops cannot be prevented, they can be slowed within certain limits [91]. Indeed, the maintenance of the postharvest life of fresh horticultural crops is becoming increasingly important [92]. The activity of PPO and POD was affected by the 1-MCP, NAA, and AVG treatments. These results were confirmed by those of Li et al. [93] and Zhang et al. [85], who noted that 1-MCP inhibited the activities of enzymes (such as POD and PPO) related to cell wall degradation, fruit browning, and ethylene biosynthesis; in particular, 1-MCP was shown to maintain firmness and extend the postharvest life in mango fruit. Additionally, phenolic compounds act as substrates for oxidizing enzymes, which can turn fruit tissues brown during handling, processing, and manipulation. This enzymatic browning leads to deterioration in the color, taste, and texture of the fruit, thus reducing the overall quality and value. Enzymatic browning is a serious issue in apples, especially in the food processing industry; thus, after sulfites were banned, antibrowning agents were required for apple fruits [94]. Treatments with 1-MCP, AVG, and NAA can delay fruit maturity and maintain quality because of the inhibition of fruit-softening enzyme activities; they also offer an alternative method for maintaining fruit appearance and nutritional value during the postharvest period [34,95,96]. Polyphenol oxidases (PPOs) and peroxidases (PODs) use phenolic compounds as substrates in oxidative browning. Apple browning leads to the degradation of flesh color, taste, texture, and flavor, which are disadvantages for apple varieties and their market appraisals [94].
5. Conclusions
The present study concluded that the exogenous application of sprays of NAA, 1-MCP, and AVG could be an effective approach to the improvement of Anna apples’ resistance to the stresses of hot and dry summers. The findings of the present study could be employed in an effective approach for many fruit crops and will be useful in alleviating the stresses of hot and dry summers. The effects of AVG, NAA, and 1-MCP sprays on several apple cultivars could be of great economic benefit to growers by reducing losses due to preharvest fruit drop, reducing the loss of fruit firmness, the loss of starch, and the loss of storage quality before harvest, and extending the harvest season. It can be concluded that foliar spraying of 320 ppm 1-MCP + 250 ppm AVG delays Anna apples’ harvest date, in addition to reducing fruit drop and acidity, improving tree yield, and extending fruit shelf life (by reducing fruit firmness loss, weight loss, and breakdown). Additionally, a spray treatment with 320 ppm 1-MCP + 250 ppm AVG helps maintain starch levels and the remaining enzyme activity at harvest or during storage at room temperature. Finally, the application of 20 ppm NAA along with 320 ppm 1-MCP + 250 ppm AVG improves the volume, length, and diameter of Anna apples.
Author Contributions
Conceptualization, M.A.-S., A.L. and D.H.E.; Data curation, M.A.-S. and D.H.E.; Formal analysis, M.A.-S., R.S.A.-O. and D.H.E.; Methodology, M.A.-S., R.S.A.-O. and D.H.E.; Investigation, M.A.-S. and D.H.E.; Resources, M.A.-S., R.S.A.-O., A.L. and D.H.E.; Software, M.A.-S., R.S.A.-O. and D.H.E.; Validation, M.A.-S., A.L. and D.H.E.; Visualization, M.A.-S. and D.H.E.; Writing—original draft preparation, M.A.-S. and D.H.E.; Writing—review and editing, M.A.-S., R.S.A.-O. and D.H.E.; Supervision, M.A.-S. and A.L.; Funding acquisition, M.A.-S. and R.S.A.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project no. (IFKSUOR3-569-1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project no. (IFKSUOR3-569-1).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sevik, H.; Cetin, M.; Ozel, H.B.; Erbek, A.; Cetin, I.Z. The effect of climate on leaf micromorphological characteristics in some broad-leaved species. Environ. Dev. Sustain. 2021, 23, 6395–6407. [Google Scholar] [CrossRef]
- Cetin, M.; Adiguzel, F.; Gungor, S.; Kaya, E.; Sancar, M.C. Evaluation of thermal climatic region areas in terms of building density in urban management and planning for Burdur, Turkey. Air Qual. Atmos. Health 2019, 12, 1103–1112. [Google Scholar] [CrossRef]
- Cetin, M.; Sevik, H.; Yigit, N. Climate type-related changes in the leaf micromorphological characters of certain landscape plants. Environ. Monit. Assess. 2018, 190, 404. [Google Scholar] [CrossRef] [PubMed]
- Malik, G.; Deveshwar, P. Abiotic stress management in fruit crop Litchi chinensis. In The Lychee Biotechnology; Springer Nature: Singapore, 2017; pp. 243–263. [Google Scholar]
- Ramakrishna, A.; Ravishankar, G.A. Influences of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Berini, J.L.; Brockman, S.A.; Hegeman, A.D.; Reich, P.B.; Muthukrishnan, R.; Montgomery, R.A.; Forester, J.D. Combinations of abiotic factors differentially alter production of plant secondary metabolites in five woody plant species in the boreal-temperate transition zone. Front. Plant Sci. 2018, 9, 1257. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.P.; Romero, P.; Navarro, J.M.; Botia, P. Response of sweet orange cv “Lane late” to deficit irrigation in two rootstocks. I: Water relations, leaf gas exchange and vegetative growth. Irrig. Sci. 2008, 26, 415–4252. [Google Scholar] [CrossRef]
- Bolat, I.; Dikilitas, M.; Ercisli, S.; Ikinci, A.; Tonkaz, T. The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and quince rootstocks. Sci. World J. 2014, 2014, 769732. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Kotb, H.R.M. Nutritional status and productivity of Anna apple trees in the year following autumn irrigation determent. Agric. Water Manag. 2021, 252, 106882. [Google Scholar] [CrossRef]
- FAOSTAT. FAO Statics for Crop Production. 2023. Available online: http://www.fao.org/faostat/ar/#data/QC (accessed on 26 April 2023).
- Shannon, M.C. Adaption of plants to salinity. Adv. Agron. 1997, 60, 75–120. [Google Scholar]
- Johnson, S. Opportunities for Optimal Apple Production Management in Arid Conditions. Master’s Thesis, Utah State University, Logan, UT, USA, 2022. [Google Scholar]
- Hauagge, R.; Cummins, J.N. Pome fruit genetic pool for production in warm climates. In Temperate Fruit Crops in Warm Climates; Kluwer Academic: Dordrecht, The Netherlands, 2001; pp. 267–304. [Google Scholar]
- Leite, G.B.; Petri, J.L.; Basso, C. Promalin effect on ‘Imerial Gala’ and ‘Fuji’ apple trees fructification. Acta Hortic. 2006, 727, 221–226. [Google Scholar]
- Castro, D.C.; Álvarez, N.; Gabriel, P.; Micheloud, N.; Buyatti, M.; Gariglio, N. Crop loading studies on ‘Caricia’ and ‘Eva’ apples grown in a mild winter area. Sci. Agric. 2015, 72, 237–244. [Google Scholar] [CrossRef]
- Nawaz, R.; Abbasi, N.A.; Hafiz, I.A.; Khalid, A.; Ahmad, T.; Aftab, M. Impact of climate change on Kinnow fruit industry of Pakistan. Agrotechnology 2019, 8, 1–6. [Google Scholar] [CrossRef]
- Trejo-Gonzalez, A.; Soto-Valdez, H. Partial characterization of polyphenoloxidase extracted from ‘Anna’ Apple. J. Am. Soc. Hortic. Sci. 1991, 116, 672–675. [Google Scholar] [CrossRef]
- Singh, N.P.; Bal, S.K.; More, N.S.; Singh, Y.; Gudge, A. Adaptation and Intervention in Crops for Managing Atmospheric Stresses. In Climate Change and Agriculture in India: Impact and Adaptation; Springer Nature: Cham, Switzerland, 2018; pp. 111–127. [Google Scholar]
- Atkinson, D.; Porter, J.R. Temperature, plant development and crop yields. Trends Plant Sci. 1996, 1, 119–124. [Google Scholar] [CrossRef]
- Wheeler, T.R.; Craufurda, P.Q.; Ellis, R.H.; Porter, J.R.; Prasad, P.V.V. Temperature variability and the yield of annual crops. Agric. Ecosyst. Environ. 2000, 82, 159–167. [Google Scholar] [CrossRef]
- Chelong, I.; Sdoodee, S. Effect of climate variability and degree-day on development, yield and quality of Shogun (Citrus reticulata Blanco) in Southern Thailand. Agric. Nat. Resour. 2013, 47, 333–341. [Google Scholar]
- Bhattacharya, A. Plant growth hormones in plants under low-temperature stress: A Review. In Physiological Processes in Plants Under Low Temperature Stress; Springer Nature: Singapore, 2022; pp. 517–627. [Google Scholar]
- Williams, K.M.; Fallahi, E. The effects of exogenous bioregulators and environment on regular cropping of apple. HortTechnology 1999, 9, 223–327. [Google Scholar] [CrossRef]
- Morkunas, I.; Mai, V.C.; Waśkiewicz, A.; Formela, M.; Goliński, P. Major Phytohormones under abiotic stress. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Springer: New York, NY, USA, 2013; pp. 87–135. [Google Scholar]
- Yuan, R.; Carbaugh, D.H. Effects of NAA, AVG, and 1-MCP on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Golden Supreme’ and ‘Golden Delicious’ apples. HortScience 2007, 42, 101–105. [Google Scholar] [CrossRef]
- Dal Cin, V.; Danesin, M.; Botton, A.; Boschetti, A.; Dorigoni, A.; Ramina, A. Ethylene and preharvest drop: The effect of AVG and NAA on fruit abscission in apple (Malus domestica L. Borkh). Plant Growth Regul. 2008, 56, 317–325. [Google Scholar] [CrossRef]
- Li, J.; Yuan, R. NAA and ethylene regulate expression of genes related to ethylene biosynthesis, perception, and cell wall degradation during fruit abscission and ripening in ‘delicious’ apples. J. Plant Growth Regul. 2008, 27, 283–295. [Google Scholar] [CrossRef]
- Yuan, R.; Li, J. Effect of sprayable 1-MCP, AVG, and NAA on ethylene biosynthesis preharvest fruit drop, fruit maturity, and quality of ’Delicious’ apples. HortScience 2008, 43, 1454–1460. [Google Scholar] [CrossRef]
- Arseneault, M.H.; Cline, J.A. A review of apple preharvest fruit drop and practices for horticultural management. Sci. Hortic. 2016, 211, 40–52. [Google Scholar] [CrossRef]
- Watkins, C.B. Advances in postharvest handling and storage of apples. In Achieving Sustainable Cultivation of Apples; Chapter 13; Burleigh Dodds Scientific Publishing: Cambridge, UK, 2017; pp. 337–367. [Google Scholar]
- Doerflinger, F.C.; Nock, J.F.; Miller, W.B.; Watkins, C.B. Preharvest aminoethoxyvinylglycine (AVG) and 1-methylcyclopropene (1- MCP) effects on ethylene and starch concentrations of ‘Empire’ and ‘McIntosh’ apples. Sci. Hortic. 2019, 244, 134–140. [Google Scholar] [CrossRef]
- Algul, B.E.; Al Shoffe, Y.; Park, D.; Miller, W.B.; Watkins, C.B. Preharvest 1-methylcyclopropene treatment enhances ‘stress-associated watercore’ dissipation in ‘Jonagold’ apples. Postharvest Biol. Technol. 2021, 181, 111689. [Google Scholar] [CrossRef]
- Cai, W.; Al Shoffe, Y.; Park, D.; Watkins, C.B. Harvest Maturity and Preharvest Aminoethoxyvinylglycine Treatment Effects on Cold-induced Ethylene Production of ‘Gala’ Apples. HortScience 2023, 58, 532–538. [Google Scholar] [CrossRef]
- Scolaro, A.M.T.; Argenta, L.C.; Amarante, C.V.T.D.; Petri, J.L.; Hawerroth, F.J. Preharvest control of ‘Royal gala’ apple fruit maturation by the inhibition of ethylene action or synthesis. Rev. Bras. Frutic. 2015, 37, 38–47. [Google Scholar] [CrossRef]
- Brighenti, A.F.; Würz, D.A.; da Silveira Pasa, M.; Rufato, L. Plant growth regulators to enhance fruit color of ‘Gala’ apples. Pesqui. Agropecuária Bras. 2017, 52, 1118–1122. [Google Scholar] [CrossRef]
- Liu, J.; Islam, M.T.; Sherif, S.M. Effects of Aminoethoxyvinylglycine (AVG) and 1-Methylcyclopropene (1-MCP) on the Pre-Harvest Drop Rate, Fruit Quality, and Stem-End Splitting in ‘Gala’ Apples. Horticulturae 2022, 8, 1100. [Google Scholar] [CrossRef]
- Robinson, T.L.; Hoying, S.; Iungerman, K.; Kviklys, D. AVG combined with NAA control pre-harvest drop of ‘McIntosh’ apples better than either chemical alone. Proc. XI International Symposium on Plant Bioregulators Fruit Production. Acta Hortic. 2010, 884, 343–350. [Google Scholar] [CrossRef]
- Ozkan, Y.; Altuntas, E.; Ozturk, B.; Yildiz, K.; Saracoglu, O. The effect of NAA (1-naphthalene acetic acid) and AVG (aminoethoxyvinylglycine) on physical, chemical, colour and mechanical properties of Braeburn apple. Int. J. Food Eng. 2012, 8, 17. [Google Scholar] [CrossRef]
- Ozkan, Y.; Ozturk, B.; Yıldız, K. Effects of Aminoethoxyvinylglycine and Naphthalene acetic Acid on Ethylene Biosynthesis, Preharvest Fruit Drop and Fruit Quality of Apple. Pak. J. Agri. Sci. 2016, 53, 893–900. [Google Scholar]
- Chaudhari, J.C.; Patel, K.D.; Yadav, L.; Patel, U.I.; Varu, D.K. Effect of plant growth regulators on flowering, fruit set and yield of custard apple (Annona squamosa L.) cv. Sindhan. Adv. Life Sci. 2016, 5, 1202–1204. [Google Scholar]
- Arseneault, M.H.; Cline, J.A. AVG, NAA, boron, and magnesium influence preharvest fruit drop and fruit quality of ‘Honeycrisp’ apples. Can. J. Plant Sci. 2018, 98, 741–752. [Google Scholar] [CrossRef]
- Wendt, L.M.; Brackmann, A.; Both, V.; Thewes, F.R.; Schultz, E.E.; Ludwig, V.; Berghetti, M.R.P. Postharvest quality of ‘Brookfield’ apple field-treated with naphthalene acetic acid alone or combined with other growth regulators. Bragantia 2020, 79, 155–168. [Google Scholar] [CrossRef]
- Tian, M.S.; Prakash, S.; Elgar, H.J.; Young, H.; Burmeister, D.M.; Ross, G.S. Responses of strawberry fruit to 1–MCP and ethylene. Plant Growth Regul. 2000, 32, 83–90. [Google Scholar] [CrossRef]
- Tuna-Gunes, N. Effect of 1-MCP and different ecological conditions on postharvest quality of ‘Eşme’ quince fruit during long term storage. Acta Hortic. 2009, 877, 387–394. [Google Scholar]
- Taş, A.; Berk, S.K.; Orman, E.; Gundogdu, M.; Ercişli, S.; Karatas, N.; Jurikova, T.; Adamkova, A.; Nedomova, S.; Mlcek, J. Influence of Pre-Harvest Gibberellic Acid and Post-Harvest 1-methyl Cyclopropane Treatments on Phenolic Compounds, Vitamin C and Organic Acid Contents during the Shelf Life of Strawberry Fruits. Plants 2021, 10, 121. [Google Scholar] [CrossRef]
- Gunes, N.T.; Poyrazoğlu, E.S. Influence of Hot Water and 1-Methylcyclopropane Treatments on Air-Stored Quince Fruit. Agronomy 2022, 12, 458. [Google Scholar] [CrossRef]
- Greene, D.W.; Schupp, J.R. Effect of aminoethoxyvinylglycine (AVG) on preharvest drop, fruit quality, and maturation of ‘McIntosh’ apples. II. Effect of timing and concentration relationships and spray volume. HortScience 2004, 39, 1036–1041. [Google Scholar] [CrossRef]
- Rath, A.C.; Kang, I.K.; Park, C.H.; Yoo, W.J.; Byun, J.K. Foliar application of aminoethoxyvinylglycine (AVG) delays fruit ripening and reduces pre-harvest fruit drop and ethylene production of bagged “Kogetsu” apples. Plant Growth Regul. 2006, 50, 91–100. [Google Scholar] [CrossRef]
- Whale, S.K.; Singh, Z.; Behboudian, M.H.; Janes, J.; Dhaliwal, S.S. Fruit quality in ‘Cripp’s Pink’ apple, especially colour, as affected by preharvest sprays of aminoethoxyvinylglycine and ethephon. Sci. Hortic. 2008, 115, 342–351. [Google Scholar] [CrossRef]
- Salas, N.A.; Molina-Corral, F.J.; González-Aguilar, G.A.; Otero, A.; Sepulveda, D.R.; Olivas, G.I. Volatile production by ‘Golden Delicious’ apples is affected by preharvest application of aminoethoxyvinylglycine. Sci. Hortic. 2011, 130, 436–444. [Google Scholar] [CrossRef]
- Brackmann, A.; Thewes, F.R.; de Oliveira Anese, R.; Both, V.; Junior, W.L.; Schultz, E.E. Aminoethoxyvinylglycine: Isolated and combined with other growth regulators on quality of ‘Brookfield’ apples after storage. Sci. Agric. 2015, 72, 221–228. [Google Scholar] [CrossRef]
- Unrath, C.R.; Obermiller, J.D.; Green, A.; McArtney, S.J. The effects of aminoethoxyvinylglycine and naphthalene acetic acid treatments on abscission and firmness of ‘Scarletspur Delicious’ apples at normal and delayed harvests. HortTechnology 2009, 19, 620–625. [Google Scholar] [CrossRef]
- Thongkum, M.; Imsabai, W.; Burns, P.; McAtee, P.A.; Schaffer, R.J.; Allan, A.C.; Ketsa, S. The effect of 1-methylcyclopropene (1-MCP) on expression of ethylene receptor genes in durian pulp during ripening. Plant Physiol. Biochem. 2018, 125, 232–238. [Google Scholar] [CrossRef]
- Pratima, P.; Chawla, W. Influence of plant growth regulators on growth and yield of pome and stone fruits. J. Pharmacogn. Phytochem. 2019, 8, 557–565. [Google Scholar]
- Choudhary, S.M.; Chavan, D.L.; Singh, R. Use of plant growth regulators in dry land fruit crops: A Review. Indian Res. J. Genet. Biotechnol. 2020, 12, 128–135. [Google Scholar]
- Tomala, K.; Grzęda, M.; Guzek, D.; Głąbska, D.; Gutkowska, K. The effects of preharvest 1-Methylcyclopropene (1-MCP) treatment on the fruit quality parameters of cold-Stored ‘Szampion’ cultivar apples. Agriculture 2020, 10, 80. [Google Scholar] [CrossRef]
- Russia’s and the EU’s Sanctions: Economic and Trade Effects, Compliance and the Way Forward. Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2017/603847/EXPO_STU(2017)603847_EN.pdf (accessed on 26 September 2021).
- Yildiz, K.; Ozturk, B.; Ozkan, Y. Effects of aminoethoxyvinylglycine (AVG) on preharvest fruit drop, fruit maturity, and quality of ‘Red Chief’ apple. Sci. Hortic. 2012, 144, 121–124. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Method of Analysis, 21st ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Malik, C.P.; Singh, M.B. Plant Engymology and Histo-Engymology; A Text Manual; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Rabino, L.; Alberto, L.; Monrad, M.K. Photocontrol of anthocyanin synthesis. J. Plant Physiol. 1977, 59, 569–573. [Google Scholar] [CrossRef]
- Moran, R.; Porath, D. Carotenoids determination in intact tissues. Plant Physiol. 1980, 65, 479. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1984; 680p. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1990; p. 593. [Google Scholar]
- SAS Institute Inc. The SAS System for Windows, version 9.13; SAS Institute Inc.: Cary, NC, USA, 2008.
- Byers, R.E.; Carbaugh, D.H.; Combs, L.D.; Smith, A.H. Ethylene inhibitors delayfruit drop, maturity, and increase fruit size of ‘Arlet’ apples. HortScience 2005, 40, 2061–2065. [Google Scholar] [CrossRef]
- Aglar, E.; Yildiz, K.; Ozkan, Y.; Ozturk, B.; Erdem, H. The effects of aminoethoxyvinylglycine and foliar zinc treatments on pre-harvest drops and fruit quality attributes of Jersey Mac apples. Sci. Hortic 2016, 213, 173–178. [Google Scholar] [CrossRef]
- Candan, A.P.; Graell, J.; Crisosto, C.; Larrigaudiere, C. Improvement of storability and shelf-life of ‘Blackamber’ plums treated with 1-methylcyclopropene. Food Sci. Technol. Int. 2006, 15, 437–444. [Google Scholar] [CrossRef]
- Ranjan, R.; Purohit, S.S.; Prasad, V. Plant Hormones: Action and Application; Agrobios: Jodhpur, India, 2003; pp. 183–189. [Google Scholar]
- Valdés, H.H.; Pizarro, M.M.; Campos-Vargas, R.; Infante, R.; Defilippi, B.G. Effect of ethylene inhibitors on quality attributes of apricot cv. Modesto and Patterson during storage. Chil. J. Agric. Res. 2009, 69, 134–144. [Google Scholar] [CrossRef]
- D’Aquino, S.; Schirra, M.; Molinu, M.G.; Tedde, M.; Palma, A. Preharvest aminoethoxyvinylglicine treatments reduce internal browning and prolong the Shelf-life of early ripening pears. Sci. Hortic. 2010, 125, 353–360. [Google Scholar] [CrossRef]
- Muñoz-Robredo, P.; Rubio, P.; Infante, R.; Campos-Vargas, R.; Manríquez, D.; González-Agüero, M.; Defilippi, B.G. Ethylene biosynthesis in apricot: Identification of a ripening-related aminocyclopropane-1-carboxylic acid synthase (ACS) gene. Postharvest Biol. Technol. 2012, 63, 85–90. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, M.; Bai, L.; Han, X.; Ge, Y.; Wang, W.; Li, J. Effects of 1-methylcyclopropene (1-MCP) on the expression of genes involved in the chlorophyll degradation pathway of apple fruit during storage. Food Chem. 2020, 308, 125707. [Google Scholar] [CrossRef]
- Kano, Y. Effect of GA and CPPU treatments on cell size and types of sugars accumulated in Japanese pear fruit. J. Hortic. Sci. Biotechnol. 2003, 78, 331–334. [Google Scholar] [CrossRef]
- Öztürk, B.; Özkan, Y.; Kılıç, K.; Uçar, M.; Karakaya, O.; Karakaya, M. The effects of pre-harvest plant growth regulators treatments on pre-harvest drop and fruit quality of Braeburn apple (Malus domestica Borkh.). J. Agric. Fac. Gaziosmanpasa Univ. 2015, 32, 68–76. [Google Scholar] [CrossRef]
- Erkan, M.; Dogan, A. Chapter 5—Harvesting of Horticultural Commodities. In Postharvest Technology of Perishable Horticultural Commodities; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 129–159. ISBN 978-0-12-813276-0. [Google Scholar]
- Öztürk, B.; Özkan, Y.; Yıldız, K.; Özkan, A.; Kılıç, K.; Uçar, M.; Karakaya, M.; Karakaya, O. The Role of Pre-Harvest Aminoethoxyvinylglycine Treatments on Fruit Quality of Braeburn Apple During Cold Storage. In Proceedings of the International Mesopotamia Agriculture Congress, Diyarbakır, Turkey, 22–25 September 2014. [Google Scholar]
- Tomala, K.; Małachowska, M.; Guzek, D.; Głąbska, D.; Gutkowska, K. The effects of 1-methylcyclopropene treatment on the fruit quality of ‘Idared’ apples during storage and transportation. Agriculture 2020, 10, 490. [Google Scholar] [CrossRef]
- Soethe, C.; Steffens, C.A.; Hawerroth, F.J.; Moreira, M.A.; do Amarante, C.V.T.; Stanger, M.C. Quality of ‘Baigent’ apples as a function of pre-harvest application of aminoethoxyvinylglycine and ethephon stored in controlled atmosphere. Appl. Food Res. 2022, 2, 100117. [Google Scholar] [CrossRef]
- Soethe, C.; Steffens, C.A.; Hawerroth, F.J.; do Amarante, C.V.T.; Heinzen, A.S. Maturation of ‘Baigent’ apples protected by anti-hail nets and sprayed with aminoethoxyvinylglycine and ethephon. Pesqui. Agropecuária Bras. 2021, 56, e02439. [Google Scholar] [CrossRef]
- Byers, R.E. Effects of Aminoethoxyvinylglycine (AVG) on preharvest fruit drop, maturity, and cracking of several apple cultivars. J. Tree Fruit Prod. 2016, 2, 77–97. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ramachandraiah, K.; Hasan, R.; Chowdhury, R.I.; Kanan, K.A.; Ahmed, S.; Ali, M.A.; Islam, M.T.; Ahmed, M. Application of Oxalic Acid and 1-Methylcyclopropane (1-Mcp) with Low and High-Density Polyethylene on Post-Harvest Storage of Litchi Fruit. Sustainability 2021, 13, 3703. [Google Scholar] [CrossRef]
- Petri, J.L.; Hawerroth, F.J.; Leite, G.B. Maturação, qualidade e queda pré-colheita de maçãs ‘Imperial Gala’ em função da aplicação de aminoetoxivinilglicina. Bragantia Camp. 2010, 69, 599–608. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Dong, C.; Terry, L.A.; Watkins, C.B.; Yu, Z.; Cheng, Z.M. Meta-analysis of the effects of 1-Methylcyclopropene (1-MCP) treatment on climacteric fruit ripening. Hortic. Res. 2020, 7, 208. [Google Scholar] [CrossRef]
- Drake, S.R.; Elfving, D.C.; Drake, M.A.; Eisele, T.A.; Drake, S.L.; Visser, D.B. Effects of aminoethoxyvinylglycine, ethephon, and 1-methylcyclopropene on apple fruit quality at harvest and after storage. HortTechnology 2006, 16, 16–23. [Google Scholar] [CrossRef]
- Amarante, C.V.T.; Steffens, C.A.; Blum, L.E.B. Fruit color, physiological disorders and diseases of ‘Gala’ and ‘Fuji’ apples sprayed with aminoethoxyvinylglycine. Rev. Bras. Frutic. 2010, 31, 9–18. [Google Scholar] [CrossRef]
- Pre-Aymard, C.; Weksler, A.; Lurie, S. Responses of ‘Anna’, a rapidly ripening summer apple, to 1-methylcyclopropene. Postharvest Biol. Technol. 2003, 27, 163–170. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Trainotti, L.; Tadiello, A.; Casadoro, G. The involvement of auxin in the ripening of climacteric fruits comes of age: The hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J. Exp. Bot. 2007, 58, 3299–3308. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.A. Postharvest Technology of Horticultural Crops, 2nd ed.; University of California Agriculture and Natural Resources: Davis, CA, USA, 1992; p. 3311. [Google Scholar]
- Imahori, Y. Postharvest stress treatments in fruits and vegetables. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 347–358. [Google Scholar]
- Li, L.; Li, C.; Sun, J.; Sheng, J.; Zhou, Z.; Xin, M.; Yi, P.; He, X.; Zheng, F.; Tang, Y.; et al. The effects of 1-methylcyclopropene in the regulation of antioxidative system and softening of mango fruit during storage. J. Food Qual. 2020, 2020, 6090354. [Google Scholar] [CrossRef]
- Serra, S.; Anthony, B.; Boscolo Sesillo, F.B.; Masia, A.; Musacchi, S. Determination of post-harvest biochemical composition, enzymatic activities, and oxidative browning in 14 apple cultivars. Foods 2021, 10, 186. [Google Scholar] [CrossRef]
- Razzaq, K.; Singh, Z.; Khan, A.S.; Khan, S.A.K.U.; Ullah, S. Role of 1-MCP in regulating ‘Kensington Pride’ mango fruit softening and ripening. Plant Growth Regul. 2016, 78, 401–411. [Google Scholar] [CrossRef]
- Falagán, N.; Terry, L.A. 1-Methylcyclopropene maintains postharvest quality in Norwegian apple fruit. Food Sci. Technol. Int. 2019, 26, 420–429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).