SO2-Generating Pads and Packaging Materials for Postharvest Conservation of Table Grapes: A Review
Abstract
1. The World Grape Industry
2. Factors That Impact the Table Grape Quality
3. Postharvest Conservation of Table Grapes
3.1. Cold Storage
3.2. Gray Mold Disease
3.3. Packaging Materials
3.4. SO2-Generating Pads and SO2 Fumigation
3.5. Perforated Plastic Liners
3.6. Biodegradable and Edible Coatings
3.7. Alternatives to the Use of SO2 and New Trends
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO—Food and Agriculture Organization of the United Nations. Available online: https://www.agricultura.pr.gov.br/arquivos/File/deral/Prognosticos/2017/Fruticultura_2016_17.pdf (accessed on 3 September 2022).
- OIV—International Organisation of Vine and Wine. Available online: https://www.oiv.int/sites/default/files/documents/OIV_Annual_Assessment_of_the_World_Vine_and_Wine_Sector_in_2021.pdf (accessed on 22 March 2023).
- Anese, R.O.; Fronza, D. Fisiologia Pós-Colheita em Fruticultura; UFSM: Santa Maria, Brazil, 2015. [Google Scholar]
- Champa, H. Pre and postharvest practices for quality improvement of table grapes (Vitis vinifera L.). J. Natl. Sci. Found. Sri Lanka 2015, 43, 3–9. [Google Scholar] [CrossRef]
- Choudhury, M.M.; Costa, T.S. Colheita e pós-colheita. In Cultivo da Videira; Leão, P.C.S., Ed.; Embrapa Semiárido: Petrolina, Brazil, 2004; pp. 61–66. [Google Scholar]
- Chitarra, M.I.F.; Chitarra, A.B. Pós-Colheita de Frutos e Hortaliças: Fisiologia e Manuseio, 2nd ed.; UFLA: Lavras, Brazil, 2005; 785p.
- Piazzolla, F.; Pati, S.; Amodio, M.L.; Colelli, G. Effect of harvest time on table grape quality during on vine storage. J. Sci. Food Agric. 2016, 96, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Creasy, G.L.; Creasy, L.L. Grapes—Crop Production Science in Horticulture; CABI: Oxfordshire, UK, 2009. [Google Scholar]
- European Commission. Council Directive 2000/29/EC. Official Journal of the European Union. 2000; 112p. [Google Scholar]
- Artés-Hernández, F.; Tomás-Barberán, F.A. Modified atmosphere packaging preserves quality of SO2-free ‘Superior seedless’ table grapes. Postharvest Biol. Technol. 2006, 39, 146–154. [Google Scholar] [CrossRef]
- Sen, F.; Altun, A.; Kesgin, M.; Inan, M.S. Effect of different shading practices used in the pre-harvest stage on quality and storage life of Sultana seedless grapes. J. Agric. Sci. Technol. 2012, 2, 1234–1240. [Google Scholar]
- Cerqueira, T.S.; Jacomino, A.G.; Sasaki, F.F.; Alleoni, C.C. Recobrimento de goiabas com filmes proteicos e de quitosana. Bragantia 2011, 70, 2016–2221. [Google Scholar] [CrossRef]
- Crisosto, C.H. Grapes, Fumigation with Sulfur Dioxide (SO2). WFLO Commodity Storage Manual. 2008. Available online: http://ucanr.edu/datastoreFiles/234-2689.pdf (accessed on 12 May 2023).
- FAO—Food and Agriculture Organization of the United Nations. GRAPE Post-Harvest Operations; FAO: Rome, Italy, 2005; 43p. [Google Scholar]
- SeaRates. Distances and Time—Online Tool for Calculation Distances and Shipping Rates between Air and Sea Ports. Available online: https://www.searates.com/de/reference/portdistance/?A=ChIJ1-4miA9QzB0Rh6ooKPzhf2g&D=19956&G=10666&shipment=1&container=20st&weight=1& (accessed on 12 May 2023).
- Saunders, C.; Hayes, P. Airfreight Transport of Fresh Fruit and Vegetables. Research Report No. 299; Lincoln University: Lincoln, New Zealand, 2007; 63p. [Google Scholar]
- Cenci, S.A.; Soares, A.G.; Freire Junior, M. Manual de Perdas Pós-Colheita em Frutos e Hortaliças; Documentos n. 27; Embrapa-CTAA: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- De Castro, J.V.; Park, K.J.; Honório, S.L. Determinação de curvas de resfriamento de uvas ‘Itália’ em dois sistemas de acondicionamento. Ver. Eng. Agríc. 2000, 20, 34–44. [Google Scholar]
- Brackmann, A.; Ceretta, M.; Pinto, J.A.V.; Venturini, T.L.; Lucio, A.D.L. Tolerância de maçãs ‘Gala’ a baixas temperaturas durante o armazenamento. Ciênc. Rural. 2010, 40, 1909–1915. [Google Scholar] [CrossRef]
- Romanazzi, G.; Joseph, L.S.; Erica, F.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Benato, E.A. Tecnologia, fisiologia e doenças pós-colheita de uvas de mesa. In Uva: Tecnologia de Produção, Pós-Colheita, Mercado; Pommer, C.V., Ed.; Cinco Continentes: Porto Alegre, Brazil, 2003; pp. 635–723. [Google Scholar]
- Zoffoli, J.P.; Latorre, B.A. Table grapes: (Vitis vinifera L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E., Ed.; Woodhead Publishing: Cambridge, UK, 2011; Volume 3, pp. 179–207. [Google Scholar]
- Ribeiro, I.J.A. Doenças e pragas. In Uva: Tecnologia de Produção, Pós-Colheita, Mercado; Pommer, C.V., Ed.; Cinco Continentes: Porto Alegre, Brazil, 2003; pp. 225–633. [Google Scholar]
- Tessmann, D.J.; Vida, J.B.; Genta, W.; Roberto, S.R.; Kishino, A.Y. Doenças e seu manejo. In Viticultura Tropical: O Sistema de Produção de Uvas de Mesa do Paraná; Kishino, A.Y., Carvalho, S.L.C., Roberto, S.R., Eds.; IAPAR: Londrina, Brazil, 2019; pp. 453–584. [Google Scholar]
- Grabke, A. Fungicide Resistance in Botrytis cinerea from Strawberry—Molecular Mechanisms and Management. Ph.D. Thesis, University of Clemson, Clemson, SC, USA, 2014. [Google Scholar]
- Michailides, T.J.; Elmer, P.A.G. Botrytis gray mold of kiwifruit caused by Botrytis cinerea in the United States and New Zealand. Plant Dis. 2000, 84, 208–223. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Leroch, M.; Plesken, C.; Weber, R.W.S.; Kauff, F.; Scalliet, G.; Hahn, M. Gray mold populations in German strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Appl. Environ. Microbiol. 2013, 79, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Vivier, M.; Fillinger, S. Botrytis: The good, the bad and the ugly. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Vivier, M., Eds.; Springer: Heidelberg, Germany, 2015; pp. 1–15. [Google Scholar]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.C.; Carvalho, D.U.; Da Cruz, M.A.; Sumida, C.H.; Ahmed, S.; Bassoli, P.A.; De Souza, R.T.; Roberto, S.R. Cold storage and biocontrol agents to extend the storage period of ‘BRS Isis’ seedless table grapes. Horticulturae 2018, 4, 18. [Google Scholar] [CrossRef]
- Saito, S.; Michailides, T.J.; Xiao, C.L. Fungicide-resistant phenotypes in Botrytis cinerea populations and their impact on control of gray mold on stored table grapes in California. Eur. J. Plant Pathol. 2019, 154, 203–213. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Mansour, M.F.; Mlikota Gabler, F.; Margosan, D.A.; Hashim-Buckey, J. Control of postharvest gray mold of table grapes in the San Joaquin Valley of California by fungicides applied during the growing season. Plant Dis. 2010, 94, 250–257. [Google Scholar] [CrossRef]
- Hashim, A.F.; Youssef, K.; Abd-Elsalam, K.A. Ecofriendly nanomaterials for controlling gray mold of table grapes and maintaining postharvest quality. Eur. J. Plant Pathol. 2019, 154, 377–388. [Google Scholar] [CrossRef]
- Melgarejo-Flores, B.G.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Miranda, M.R.A.; Ayala-Zavala, J.F. Antifungal protection and antioxidant enhancement of table grapes treated with emulsions, vapors, and coatings of cinnamon leaf oil. Postharvest Biol. Technol. 2013, 86, 321–328. [Google Scholar] [CrossRef]
- Youssef, K.; Roberto, S.R. Applications of salt solutions before and after harvest affect the quality and incidence of postharvest gray mold of ‘Italia’ table grapes. Postharvest Biol. Technol. 2014, 87, 95–102. [Google Scholar] [CrossRef]
- Yamashita, F. Embalagem pós-colheita para frutos. In Manual Pós-Colheita da Fruticultura Brasileira; Neves, L.C., Ed.; EDUEL: Londrina, Brazil, 2009; pp. 163–187. [Google Scholar]
- Liguori, G. Effects of modified atmosphere packaging on quality parameters of minimally processed table grapes during cold storage. Adv. Hortic. Sci. 2015, 29, 152–154. [Google Scholar]
- Bordin, M.R. Embalagem Para Frutas e Hortaliças. Boletim de Tecnologia e Desenvolvimento de Embalagens. Informativo CETEA: Campinas, Brazil, 1998; Volume 10, 4p. [Google Scholar]
- Motoike, S.; Borem, A. Uva: Do Plantio à Colheita; UFV: Viçosa, Brazil, 2018; 185p. [Google Scholar]
- De Lima, M.A.C. Tecnologias pós-colheita para conservação de uvas apirênicas produzidas no submédio do Vale do São Francisco. In Livro Técnico-Científico; Carvalho, J.M.M., Ed.; Embrapa Semiárido: Petrolina, Brazil, 2009; pp. 191–210. [Google Scholar]
- Karaca, H.; Smilanick, J.L. The influence of plastic composition and ventilation area on ozone diffusion through some food packaging materials. Postharvest Biol. Technol. 2011, 62, 85–88. [Google Scholar] [CrossRef]
- Chaves, O.J., Jr.; Youssef, K.; Koyama, R.; Ahmed, S.; Domingues, A.R.; Mühlbeier, D.T.; Roberto, S.R. Control of gray mold on clamshell-packaged ‘Benitaka’ table grapes using sulphur dioxide pads and perforated liners. Pathogens 2019, 8, 271. [Google Scholar]
- Crisosto, C.H.; Mitchell, F.G. Postharvest handling systems: Table grapes. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California, Agriculture & Natural Resources: Oakland, CA, USA, 2002; pp. 357–363. [Google Scholar]
- Dantas, B.C.; Higuchi, M.T.; de Aguiar, A.C.; Bosso, B.E.; Roberto, S.R. Postharvest conservation of ‘BRS Nubia’ hybrid table grape subjected to field ultra-fast SO2-generating pads before packaging. Horticulturae 2022, 8, 285. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Higuchi, M.T.; Ribeiro, L.T.M.; Leles, N.R.; Bosso, B.E.C.; Shimizu, G.D.; Da Silva, M.J.R.; Marques, V.V.; Yamashita, F.; Youssef, K.; et al. Bio-based and SO2-generating plastic liners to extend the shelf life of ‘Benitaka’ table grapes. Postharvest Biol. Technol. 2023, 197, 112217. [Google Scholar] [CrossRef]
- Fernández-Trujillo, J.P.; Obando-Ulloa, J.M.; Baró, R.; Martínez, J.A. Quality of two table grape guard cultivars treated with single or dual-phase release SO2 generators. J. Appl. Bot. Food Qual. 2012, 82, 1–8. [Google Scholar]
- Zutahy, Y.; Lichter, A.; Kaplunov, T.; Lurie, S. Extended storage of ‘Red Globe’ grapes in modified SO2 generating pads. Postharvest Biol. Technol. 2008, 50, 12–17. [Google Scholar] [CrossRef]
- Quimas. SmartPac Soluciones Para Uvas. 2022. Available online: https://www.quimas.cl/uva (accessed on 12 May 2023).
- Saito, S.; Obenland, D.; Xiao, C.L. Influence of sulfur dioxide-emitting polyethylene packaging on blueberry decay and quality during extended storage. Postharvest Biol. Technol. 2020, 160, 111045. [Google Scholar] [CrossRef]
- Ngcobo, M.E.K.; Opara, U.L.; Thiart, G.D. Effects of packaging liners on cooling rate and quality attributes of table grape (cv. Regal Seedless). Packag. Technol. Sci. 2011, 25, 73–84. [Google Scholar] [CrossRef]
- Henríquez, J.L.; Pinochet, S. Impact of ventilation area of the liner bag, in the performance of SO2 generator pads in boxed table grapes. Acta Hortic. 2016, 1144, 267–272. [Google Scholar] [CrossRef]
- Halonen, N.; Pálvolgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-based smart materials for food packaging and sensors—A review. Front. Mater. 2020, 7, 82. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Dhull, S.B.; Siroha, A.K.; Purewal, S.S.; Kaur, M.; Kidwai, M.K. Oat starch: Physico-chemical, morphological, rheological characteristics and its applications—A review. Int. J. Biol. Macromol. 2020, 154, 493–498. [Google Scholar] [CrossRef]
- Antoniolli, L.; de Lima, M.A.C. Boas Práticas de Fabricação e Manejo na Colheita e Pós-Colheita de Uvas Finas de Mesa; Circular Técnico; Embrapa Uva e Vinho: Bento Gonçalves, Brazil, 2008; 13p.
- Camili, E.C.; Benato, E.A.; Pascholati, S.F.; Cia, P. Avaliação de quitosana, aplicada em pós-colheita, na proteção de uva ‘Itália’ contra Botrytis cinerea. Summa Phytopathol. 2007, 33, 215–221. [Google Scholar] [CrossRef]
- Gabler, F.M.; Mercier, J.; Jimenez, J.I.; Smilanick, J.L. Integration of continuous biofumigation with Muscodor albus with pre-cooling fumigation with ozone or sulfur dioxide to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2010, 55, 78–84. [Google Scholar] [CrossRef]
- Chervin, C.; Westercamp, P.; Monteils, G. Ethanol vapours limit Botrytis development over the postharvest life of table grapes. Postharvest Biol. Technol. 2005, 36, 319–322. [Google Scholar] [CrossRef]
- Muñoz, V.; Benato, E.A.; Sigrist, J.M.M.; Oliveira, J.D.V.; Corrêa, A.C.C. Effect of SO2 for controlling Botrytis cinerea in Italia and Red Globe grapes stored at different temperatures. Rev. Bras. Frutic. 2000, 22, 100–105. [Google Scholar]
- Sarantópulos, C.I.G.L.; Moraes, B.B. Embalagens Ativas e Inteligentes para Frutas e Hortaliças; Boletim de Tecnologia e Desenvolvimento de Embalagens; Instituto de Tecnologia de Alimentos: Campinas, Brazil, 2009.
- Crisosto, C.H.; Garner, D.; Crisosto, G. Carbon dioxide-enriched atmospheres during cold storage limit losses from Botrytis but accelerate rachis browning of ‘Redglobe’ table grapes. Postharvest Biol. Tecnol. 2002, 26, 181–189. [Google Scholar] [CrossRef]
- Retamales, J.; Defilippi, B.G.; Arias, M.; Castillo, P.; Manríquez, D. High-CO2 controlled atmospheres reduce decay incidence in ‘Thompson Seedless’ and ‘Red Globe’ table grapes. Postharvest Biol. Technol. 2003, 29, 177–182. [Google Scholar] [CrossRef]
- Nelson, K.E. Fumigation with sulfur dioxide to control decay of table grapes. In Harvesting and Handling California Table Grapes for Market; Nelson, K.E., Ed.; ANR Publications, University of California: Oakland, CA, USA, 1985; pp. 52–53. [Google Scholar]
- Crisosto, C.H.; Palou, L.; Garner, D.; Armson, D.A. Concentration by Time Product and Gas Penetration after Marine Container Fumigation of Table Grapes with Reduced Doses of Sulfur Dioxide. HortTechnology 2002, 12, 241–245. [Google Scholar] [CrossRef]
- Pires, J.C.M.; Sousa, S.I.V.; Pereira, M.C.; Alvim-Ferraz, M.C.M.; Martins, F.G. Management of air quality monitoring using principal component and cluster analysis Part I: SO2 and PM10. Atmos. Environ. 2008, 42, 1249–1260. [Google Scholar] [CrossRef]
- Food and Drug Administration. Sulfites: An Important Food Safety Issue. 2003. Available online: http://vm.cfsan.fda.gov/~dms/fssulfit.html (accessed on 22 March 2023).
- Mustonen, H.M. The efficacy of a range of sulfur dioxide generating pads against Botrytis cinerea infection & on out-turn quality of Calmeria table grapes. Aust. J. Exp. Agric. 1992, 32, 389–393. [Google Scholar]
- Smilanick, J.L.; Harvey, J.M.; Hartsell, P.L.; Hensen, D.J.; Harris, C.M.; Fouse, D.C.; Assemi, M. Factors influencing sulfite residues in table grapes after sulfur dioxide fumigation. Am. J. Enol. Vitic. 1990, 41, 131–136. [Google Scholar] [CrossRef]
- Lurie, S.; Pesis, E.; Gadiyeva, O.; Feygenberg, O.; Ben-Arie, R.; Kaplunov, T.; Zutachi, Y.; Lichter, A. Modified ethanol atmosphere to control decay of table grapes during storage. Postharvest Biol. Pathol. 2006, 42, 222–227. [Google Scholar] [CrossRef]
- Jamieson, A.J.; Brooks, L.S.R.; Reid, W.D.K.; Piertney, S.B.; Narayanaswamy, B.E.; Linley, T.D. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A review of contemporary development from conventional plastics to polylactic acid-based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Olivas, G.I.; Dávila-Aviña, J.; Molina, F. Use of edible coatings to preserve the quality of fruits and vegetables during storage. Stewart Postharvest Rev. 2008, 4, 1–10. [Google Scholar]
- Palou, L.; Serrano, M.; Martínez-Romero, D.; Valero, D. New Approaches for Postharvest Quality Retention of Table Grapes. Fresh Prod. 2010, 4, 103–110. [Google Scholar]
- González-Barrio, R.; Beltrán, D.; Cantos, E.; Gil, M.I.; Espín, J.C.; Tomás-Barberán, F.A. Comparison of ozone and UV-C treatments on the postharvest stilbenoid monomer, dimer, and trimer induction in var. ‘Superior’ white table grapes. J. Agric. Food Chem. 2006, 54, 4222–4228. [Google Scholar] [CrossRef]
- Palou, L.; Smilanick, J.L.; Crisosto, C.H.; Mansour, M. Effect of gaseous ozone exposure on the development of green and blue molds on cold stored citrus fruit. Plant Dis. 2001, 85, 632–638. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Gabler, F.M.; Mansour, M.; Smilanick, J.L. Postharvest ethanol and hot water treatments of table grapes to control gray mold. Postharvest Biol. Technol. 2004, 34, 169–177. [Google Scholar] [CrossRef]
- Romero, I.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Individual anthocyanins and their contribution to total antioxidant capacity in response to low temperature and high CO2 in stored Cardinal table grapes. Postharvest Biol. Technol. 2008, 49, 1–9. [Google Scholar] [CrossRef]
- Youssef, K.; de Oliveira, A.G.; Tischer, C.A.; Hussain, I.; Roberto, S.R. Synergistic effect of a novel chitosan/silica nanocomposites-based formulation against gray mold of table grapes and its possible mode of action. Int. J. Biol. Macromol. 2019, 141, 247–258. [Google Scholar] [CrossRef] [PubMed]


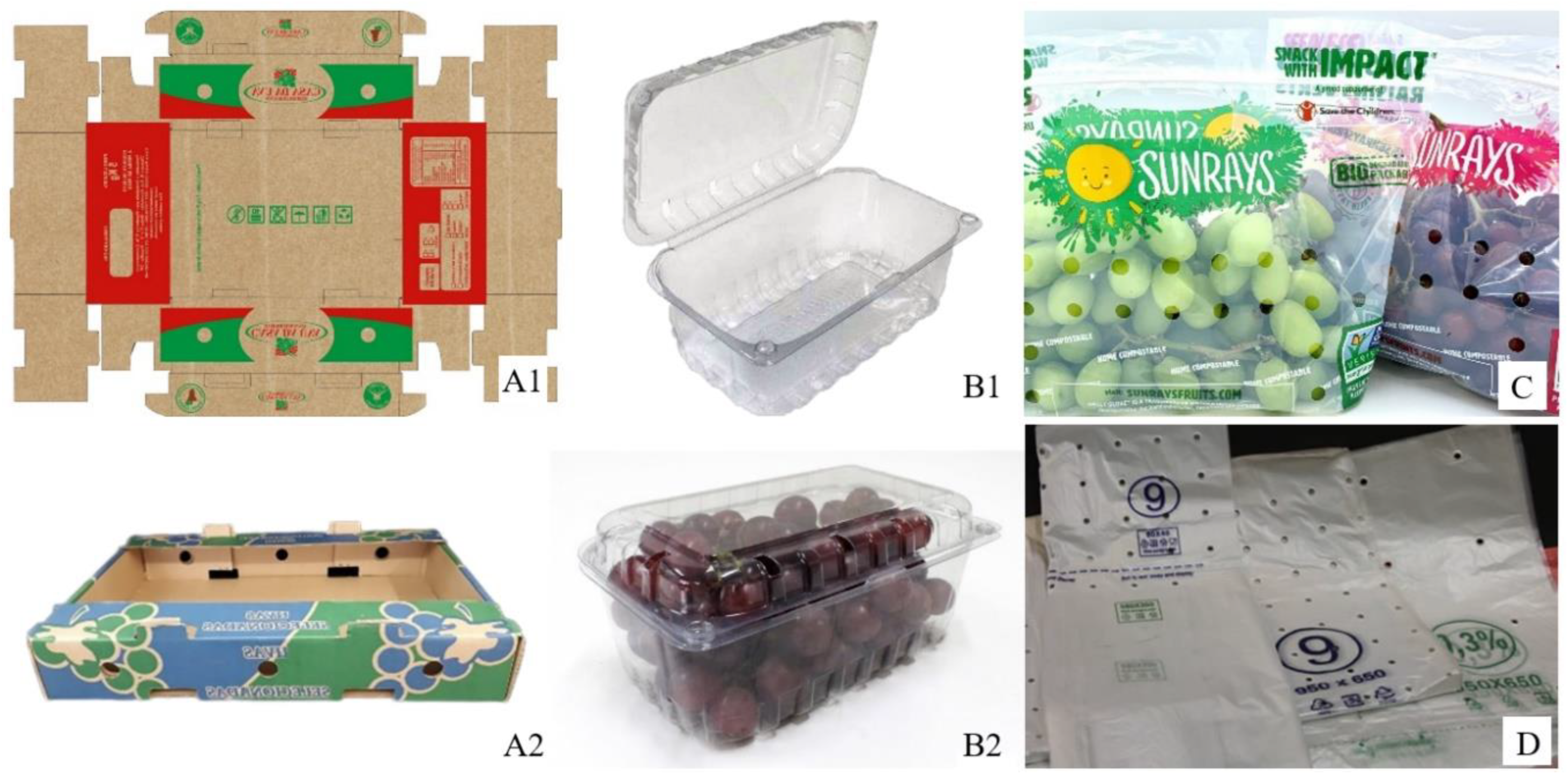





| Postharvest Materials | References |
|---|---|
| Packaging | |
| Cardboard boxes | [4,7,10,22,31,33,36,38,42,43,44,45,46] |
| Vented plastic clamshells | |
| Perforated plastic bags | |
| SO2-generatings pads | |
| Slow-release pads during cold storage | [22,45,46,47,48] |
| Dual release pads (fast and slow-release phases) during cold storage | |
| Field ultrafast release pads before packaging | |
| Perforated SO2-generating plastic liners | |
| Perforated slow-release plastic liners | [22,46,49,50] |
| Perforated dual release plastic liners | |
| Perforated plastic liners | |
| Perforated plastic liners with 0.2–2.6% of ventilation area | [43,46,51,52] |
| Laser perforated bio-based liners | |
| Starch-based liners (GMO-free) with microscopic holes | [46,53,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Aguiar, A.C.; Higuchi, M.T.; Yamashita, F.; Roberto, S.R. SO2-Generating Pads and Packaging Materials for Postharvest Conservation of Table Grapes: A Review. Horticulturae 2023, 9, 724. https://doi.org/10.3390/horticulturae9060724
de Aguiar AC, Higuchi MT, Yamashita F, Roberto SR. SO2-Generating Pads and Packaging Materials for Postharvest Conservation of Table Grapes: A Review. Horticulturae. 2023; 9(6):724. https://doi.org/10.3390/horticulturae9060724
Chicago/Turabian Stylede Aguiar, Aline Cristina, Maíra Tiaki Higuchi, Fábio Yamashita, and Sergio Ruffo Roberto. 2023. "SO2-Generating Pads and Packaging Materials for Postharvest Conservation of Table Grapes: A Review" Horticulturae 9, no. 6: 724. https://doi.org/10.3390/horticulturae9060724
APA Stylede Aguiar, A. C., Higuchi, M. T., Yamashita, F., & Roberto, S. R. (2023). SO2-Generating Pads and Packaging Materials for Postharvest Conservation of Table Grapes: A Review. Horticulturae, 9(6), 724. https://doi.org/10.3390/horticulturae9060724







