Induced Defense in Avocado Fruits Mediated by Secondary Metabolites Produced by Bacillus atrophaeus B5
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Fruit Material
2.2. Preparation of Suspension of Fungal-Spore-Cell-Free Supernatant
2.3. Effect of CFS on Mycelial Growth
2.4. Efficacy of CFS for Control of Avocado Anthracnose
2.5. Assay of Enzyme Activity
2.6. Analysis of the Expression of Defense-Related Genes
2.7. Semiquantitative Analysis of Gene Expression
2.8. Effects of Metabolites on Storage Quality of Avocado Fruit
2.9. Statistical Analysis
3. Results
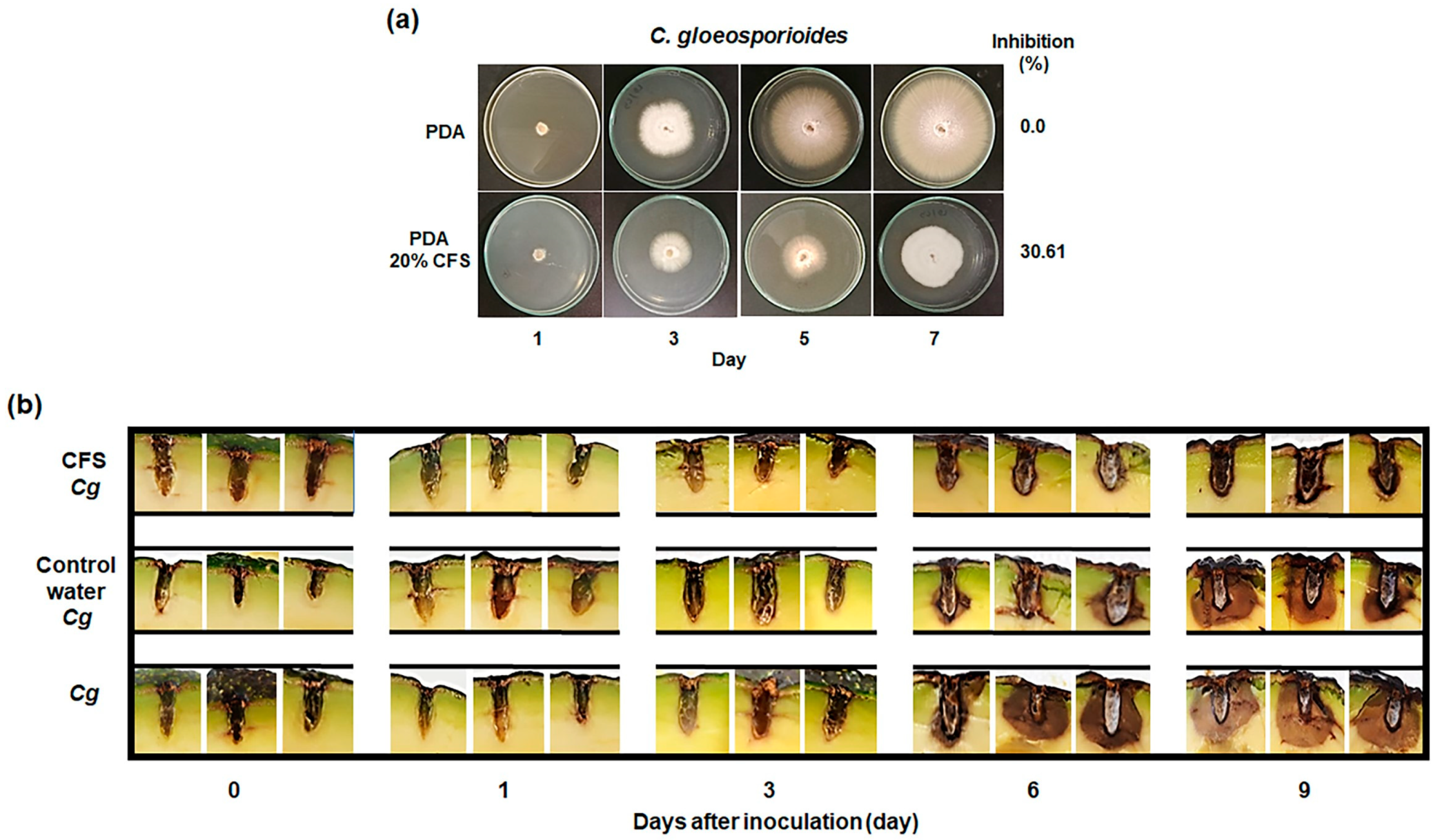
3.1. Suppression of Mycelial Growth by CFS
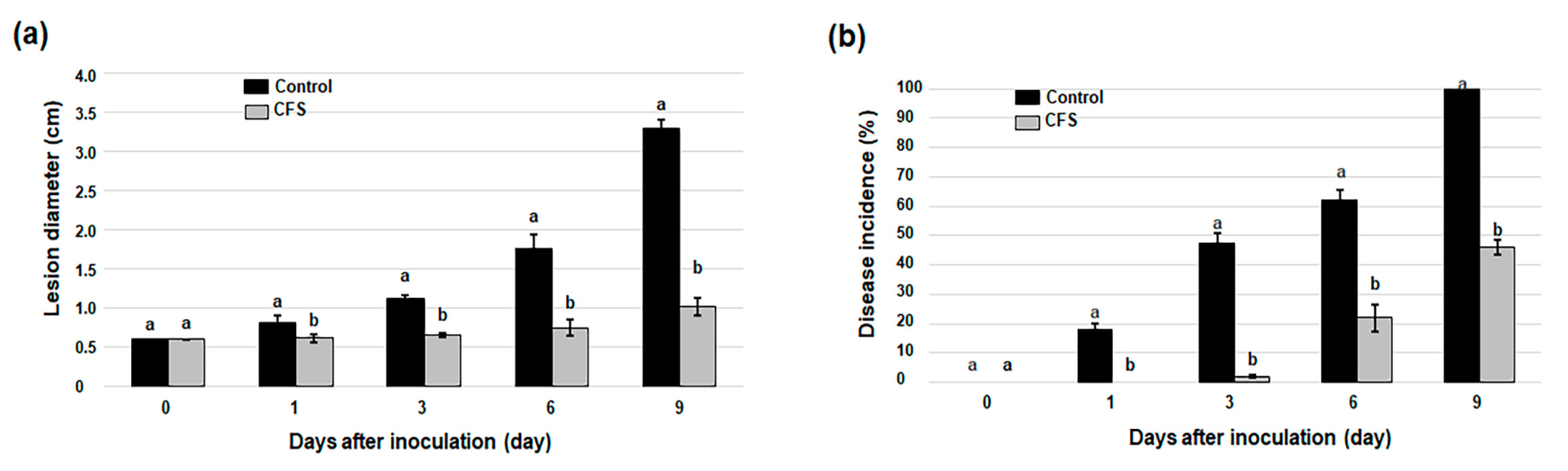
3.2. Biocontrol Effects of CFS from B. atrophaeus B5 on Anthracnose in Avocado Fruit
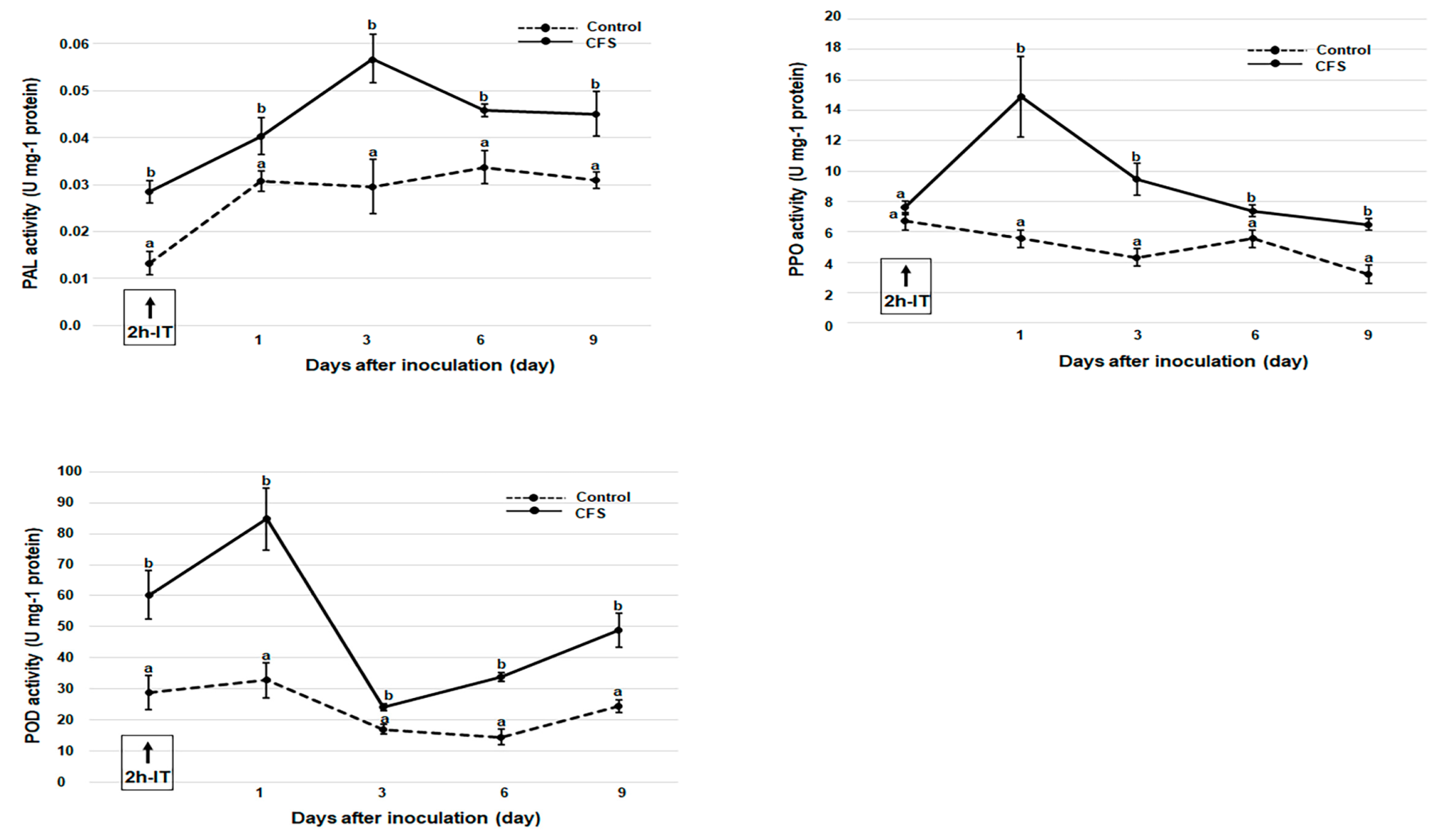
3.3. Effects of CFS Treatment on PPO, POD, and PAL Activities
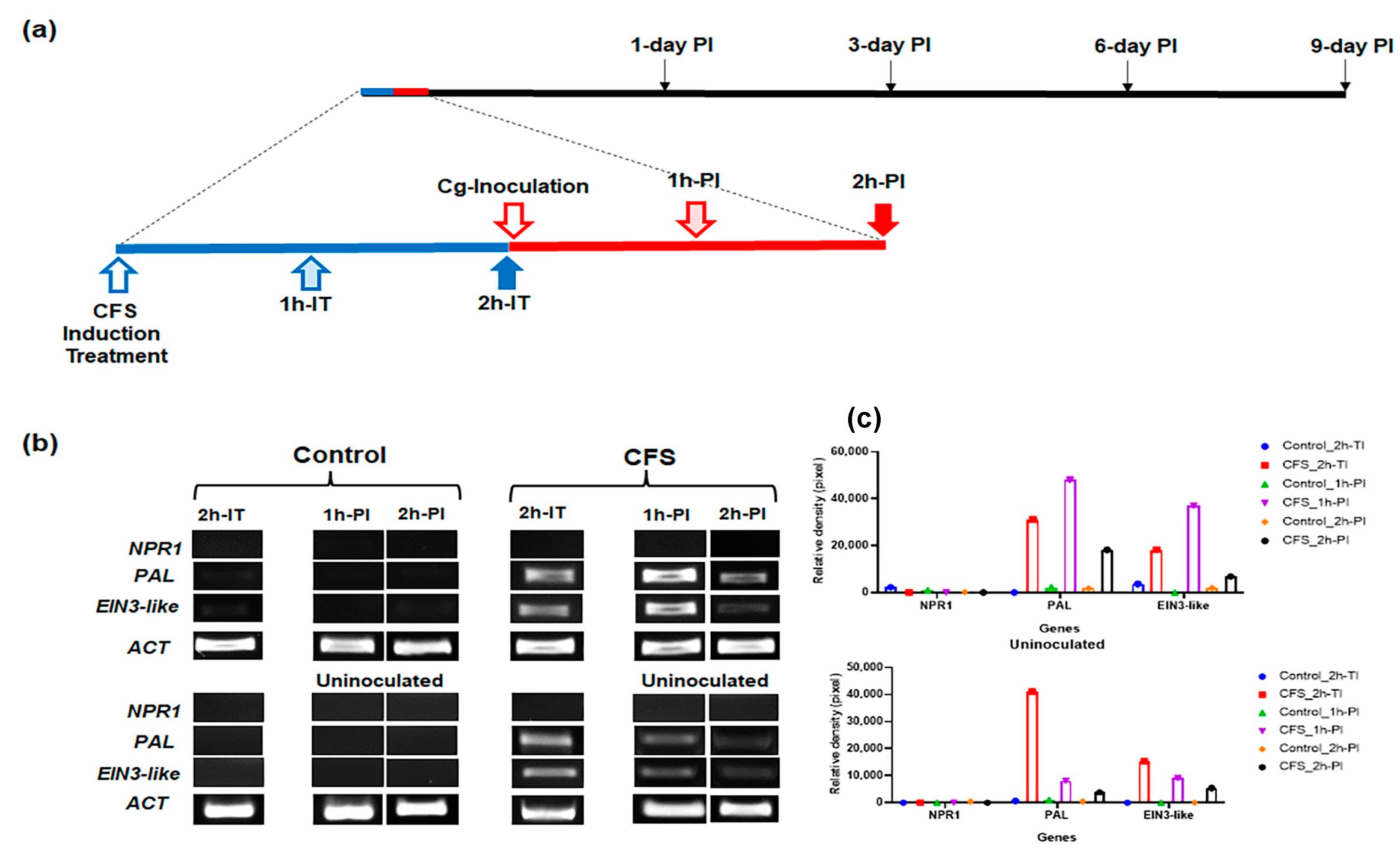
3.4. In Fruit, CFS Treatment Induced Higher Expression of Genes Related to Defense
3.5. Effects of CFS on Storage Quality of Avocado Fruit
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araujo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Pintado, M.M.E.; Aguilar, C.N. Avocado by-products: Nutritional and functional properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Rodríguez-López, E.S.; González-Prieto, J.M.; Mayek-Pérez, N. Infection of avocado (Persea americana Mill.) by Colletotrichum gloeosporioides (Penz.) Penz. y Sacc.: Biochemical and genetics aspects. Rev. Mex. Fitopatol. 2009, 27, 53–63. [Google Scholar]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Bruhl, C.A.; Imfeld, G.; Knabel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmuller, A.; et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Frances, J.; Rosello, G.; Montesinos, E. Bacteria as biological control agents of plant diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant. Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Wang, Y.; Guo, Q.; Lu, X.; Li, S.; Ma, P. Lipopeptides, a novel protein, and volatile compounds contribute to the antifungal activity of the biocontrol agent Bacillus atrophaeus CAB-Appl. Microbiol. Biotechnol. 2013, 97, 9525–9534. [Google Scholar]
- Ni, M.; Wu, Q.; Wang, J.; Liu, W.C.; Ren, J.H.; Zhang, D.P.; Zhao, J.; Liu, W.; Rao, Y.H.; Lu, C.G. Identification and comprehensive evaluation of a novel biocontrol agent Bacillus atrophaeus JZBJ Environ. Sci. Health B 2018, 53, 777–785. [Google Scholar]
- Guardado-Valdivia, L.; Tovar-Perez, E.; Chacon-Lopez, A.; Lopez-Garcia, U.; Gutierrez-Martinez, P.; Stoll, A.; Aguilera, S. Identification and characterization of a new Bacillus atrophaeus strain B5 as biocontrol agent of postharvest anthracnose disease in soursop (Annona muricata) and avocado (Persea americana). Microbiol. Res. 2018, 210, 26–32. [Google Scholar] [CrossRef]
- Chacon-Lopez, A.; Guardado-Valdivia, L.; Banuelos-Gonzalez, M.; Lopez-Garcia, U.; Montalvo-Gonzalez, E.; Arvizu-Gomez, J.; Stoll, A.; Aguilera, S. Effect of metabolites produced by Bacillus atrophaeus and Brevibacterium frigoritolerans strains on postharvest biocontrol of Alternaria alternata in tomato (Solanum lycopersicum L.). Biocontrol Sci. 2021, 26, 67–74. [Google Scholar] [CrossRef]
- Han, J.H.; Shim, H.; Shin, J.H.; Kim, K.S. Antagonistic activities of Bacillus spp. strains isolated from tidal flat sediment towards anthracnose pathogens Colletotrichum acutatum and C. gloeosporioides in South Korea. Plant. Pathol. J. 2015, 31, 165–175. [Google Scholar] [CrossRef]
- Wang, K.; Jin, P.; Cao, S.; Rui, H.; Zheng, Y. Biological control of green mould decay in postharvest chinese bayberries by Pichia membranaefaciens. J. Phytopathol. 2011, 159, 417–423. [Google Scholar] [CrossRef]
- Tian, S.; Wan, Y.; Qin, G.; Xu, Y. Induction of defense responses against Alternaria rot by different elicitors in harvested pear fruit. Appl. Microbiol. Biotechnol. 2006, 70, 729–734. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P.; Korsten, L. Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol. Technol. 2013, 81, 66–72. [Google Scholar] [CrossRef]
- Healey, A.; Furtado, A.; Cooper, T.; Henry, R.J. Protocol: A simple method for extracting next-generation sequencing quality genomic DNA from recalcitrant plant species. Plant. Methods 2014, 10, 21. [Google Scholar] [CrossRef]
- Antiabong, J.F.; Ngoepe, M.G.; Abechi, A.S. Semiquantitative digital analysis of polymerase chain reaction electrophoresis gel: Potential applications in low-income veterinary laboratories. Vet. World. 2016, 9, 935–939. [Google Scholar] [CrossRef]
- Kassim, A.; Workneh, T.S. Influence of postharvest treatments and storage conditions on the quality of Hass avocados. Heliyon 2020, 6, e04234. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; Almasoudi, N.M.; Abdelmagid, A.W.M.; Roberto, S.R.; Youssef, K. Plant extract treatments induce resistance to bacterial spot by tomato plants for a sustainable system. Horticulturae 2020, 6, 36. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; Ali, E.F.; Sallam, N.M.A. Alternative control of tomato wilt using the aqueous extract of Calotropis procera. Horticulturae 2022, 8, 197. [Google Scholar] [CrossRef]
- Petriacq, P.; Lopez, A.; Luna, E. Fruit decay to diseases: Can induced resistance and priming help? Plants 2018, 7, 77. [Google Scholar] [CrossRef]
- Wang, B.; Yang, B. The role of signal production and transduction in induced resistance of harvested fruits and vegetables. Food Qual. Saf. 2021, 5, 1–8. [Google Scholar] [CrossRef]
- Blaschek, L.; Pesquet, E. Phenoloxidases in plants-how structural diversity enables functional specificity. Front. Plant. Sci. 2021, 12, 754601. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Gomez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barcelo, A.; Pedreno, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Jiang, W.B.; Bi, Y.; Luo, Y.B. Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269. [Google Scholar] [CrossRef]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zheng, Y.; Tang, S.; Wang, K. Improved control of anthracnose rot in loquat fruit by a combination treatment of Pichia membranifaciens with CaCl(2). Int. J. Food Microbiol. 2008, 126, 216–220. [Google Scholar] [CrossRef]
- Arrebola, E.; Sivakumar, D.; Bacigalupo, R.; Korsten, L. Combined application of antagonist Bacillus amyloliquefaciens and essential oils for the control of peach postharvest diseases. Crop Prot. 2010, 29, 369–377. [Google Scholar] [CrossRef]
- Wang, X.; Xu, F.; Wang, J.; Jin, P.; Zheng, Y. Bacillus cereus AR156 induces resistance against Rhizopus rot through priming of defense responses in peach fruit. Food Chem. 2013, 136, 400–406. [Google Scholar] [CrossRef]
- Backer, R.; Naidoo, S.; van den Berg, N. The nonexpressor of pathogenesis-related genes 1 (NPR1) and related family: Mechanistic insights in plant disease resistance. Front. Plant. Sci. 2019, 10, 102. [Google Scholar] [CrossRef]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A.R. The evolution of phenylpropanoid metabolism in the green lineage. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 123–152. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wang, J.; Jin, P.; Liu, H.; Zheng, Y. Bacillus cereus AR156-induced resistance to Colletotrichum acutatum is associated with priming of defense responses in loquat fruit. PLoS ONE 2014, 9, e112494. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, L.; Romero, D.; Zeriouh, H.; Cazorla, F.M.; Torés, J.A.; de Vicente, A.; Pérez-García, A. Isolation and selection of plant growth-promoting rhizobacteria as inducers of systemic resistance in melon. Plant. Soil 2012, 358, 201–212. [Google Scholar] [CrossRef]
- Kashyap, A.S.; Manzar, N.; Nebapure, S.M.; Rajawat, M.V.S.; Deo, M.M.; Singh, J.P.; Kesharwani, A.K.; Singh, R.P.; Dubey, S.C.; Singh, D. Unraveling microbial volatile elicitors using a transparent methodology for induction of systemic resistance and regulation of antioxidant genes at expression levels in chili against bacterial wilt disease. Antioxidants 2022, 11, 404. [Google Scholar] [CrossRef]
- Waewthongrak, W.; Leelasuphakul, W.; McCollum, G. Cyclic lipopeptides from Bacillus subtilis ABS-S14 elicit defense-related gene expression in citrus fruit. PLoS ONE 2014, 9, e109386. [Google Scholar] [CrossRef]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J. Chem. Ecol. 2013, 39, 869–878. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Z.; Tian, C.; Liang, Y.; You, C.; Chen, L. Identification and characterization of the endophytic bacterium Bacillus atrophaeus XW2, antagonistic towards Colletotrichum gloeosporioides. Ann. Microbiol. 2015, 65, 1361–1371. [Google Scholar] [CrossRef]
- Jing, M.; Huang, B.; Li, W.; Zeng, J.; Shao, Y. Biocontrol of Cladosporium cladosporioides of mango fruit with Bacillus atrophaeus TE7 and effects on storage quality. Curr. Microbiol. 2021, 78, 765–774. [Google Scholar] [CrossRef]
- Jiang, M.; Pang, X.; Liu, H.; Lin, F.; Lu, F.; Bie, X.; Lu, Z.; Lu, Y. Iturin A induces resistance and improves the quality and safety of harvested cherry tomato. Molecules 2021, 26, 6905. [Google Scholar] [CrossRef]
| Primer Name | Primer Sequence | Gene |
|---|---|---|
| Npr1 Fw | GTGGAAAGGTGAGACAGCCGCCTG | NPR1 |
| Npr1 Rv | CGACATCATCAGAATCAAGAGCCCTG | |
| Pal Fw | CCTTGCACTTGTCAACGGCACTG | PAL |
| Pal Rv | GCTCCCTTGAACCCATAATCCAAGC | |
| Ein3 Fw | GATTATGCAGCACTGTGACCCACCG | EIN3 |
| Ein3 Rv | GCCTTGGCTGTCATTTTGTCTTGG | |
| Act Fw | CCAAAAGCCAACAGGGAGAAGATGAC | ACT |
| Act Rv | ATCAGCAATGCCTGGGAACATGG |
| Day | Pulp Color (°Hue) | Weight Loss (%) | Firmness (kgf) | |||
| Control | CFS | Control | CFS | Control | CFS | |
| 0 | 95.56 ± 4.30a | 93.56 ± 4.30a | - | - | 9.33 ± 0.28a | 9.66 ± 0.76a |
| 1 | 95.48 ± 3.25a | 95.85 ± 2.13a | 0.85 ± 0.65a | 0.74 ± 0.60a | 9.53 ± 0.15a | 9.63 ± 0.15a |
| 3 | 94.92 ± 2.77a | 99.45 ± 5.46a | 2.77 ± 0.38a | 2.50 ± 0.92a | 6.93 ± 0.40a | 7.43 ± 0.15a |
| 6 | 96.94 ± 3.70a | 95.59 ± 3.78a | 4.86 ± 0.73a | 4.61 ± 1.16a | 6.16 ± 0.92a | 6.90 ± 0.18a |
| 9 | 94.66 ± 1.46a | 96.91 ± 1.63a | 7.19 ± 0.97a | 6.78 ± 1.76a | - | 4.10 ± 0.33b |
| Day | Titratable Acidity (%) | pH | TSS (°Brix) | |||
| Control | CFS | Control | CFS | Control | CFS | |
| 0 | 0.04 ± 0.10a | 0.04 ± 0.10a | 6.96 ± 0.17a | 6.96 ± 0.17a | 8.00 ± 0.44a | 8.00 ± 0.44a |
| 1 | 0.04 ± 0.01a | 0.04 ± 0.00a | 7.42 ± 0.19a | 7.24 ± 0.15a | 6.66 ± 0.60a | 7.25 ± 0.98a |
| 3 | 0.07 ± 0.01a | 0.09 ± 0.03a | 7.08 ± 0.17a | 6.86 ± 0.13a | 8.05 ± 0.77a | 7.75 ± 0.68a |
| 6 | 0.10 ± 0.03a | 0.09 ± 0.03a | 6.94 ± 0.10a | 6.83 ± 0.18a | 8.50 ± 0.77a | 8.50 ± 1.34a |
| 9 | 0.11 ± 0.01a | 0.10 ± 0.01a | 6.89 ± 0.14a | 6.82 ± 0.03a | 8.00 ± 1.00a | 7.58 ± 0.73a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bañuelos-González, M.d.C.; Cuéllar-Torres, E.A.; López-García, U.M.; Montalvo-González, E.; Ortiz-Basurto, R.I.; Aguilera-Aguirre, S.; Chacón-López, A. Induced Defense in Avocado Fruits Mediated by Secondary Metabolites Produced by Bacillus atrophaeus B5. Horticulturae 2023, 9, 714. https://doi.org/10.3390/horticulturae9060714
Bañuelos-González MdC, Cuéllar-Torres EA, López-García UM, Montalvo-González E, Ortiz-Basurto RI, Aguilera-Aguirre S, Chacón-López A. Induced Defense in Avocado Fruits Mediated by Secondary Metabolites Produced by Bacillus atrophaeus B5. Horticulturae. 2023; 9(6):714. https://doi.org/10.3390/horticulturae9060714
Chicago/Turabian StyleBañuelos-González, Miriam del Carmen, Esther Angélica Cuéllar-Torres, Ulises Miguel López-García, Efigenia Montalvo-González, Rosa Isela Ortiz-Basurto, Selene Aguilera-Aguirre, and Alejandra Chacón-López. 2023. "Induced Defense in Avocado Fruits Mediated by Secondary Metabolites Produced by Bacillus atrophaeus B5" Horticulturae 9, no. 6: 714. https://doi.org/10.3390/horticulturae9060714
APA StyleBañuelos-González, M. d. C., Cuéllar-Torres, E. A., López-García, U. M., Montalvo-González, E., Ortiz-Basurto, R. I., Aguilera-Aguirre, S., & Chacón-López, A. (2023). Induced Defense in Avocado Fruits Mediated by Secondary Metabolites Produced by Bacillus atrophaeus B5. Horticulturae, 9(6), 714. https://doi.org/10.3390/horticulturae9060714








