Quantification and Prediction with Near Infrared Spectroscopy of Carbohydrates throughout Apple Fruit Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Near Infrared Spectroscopy and Carbohydrate Quantification
2.3. Statistical Analyses
3. Results and Discussion
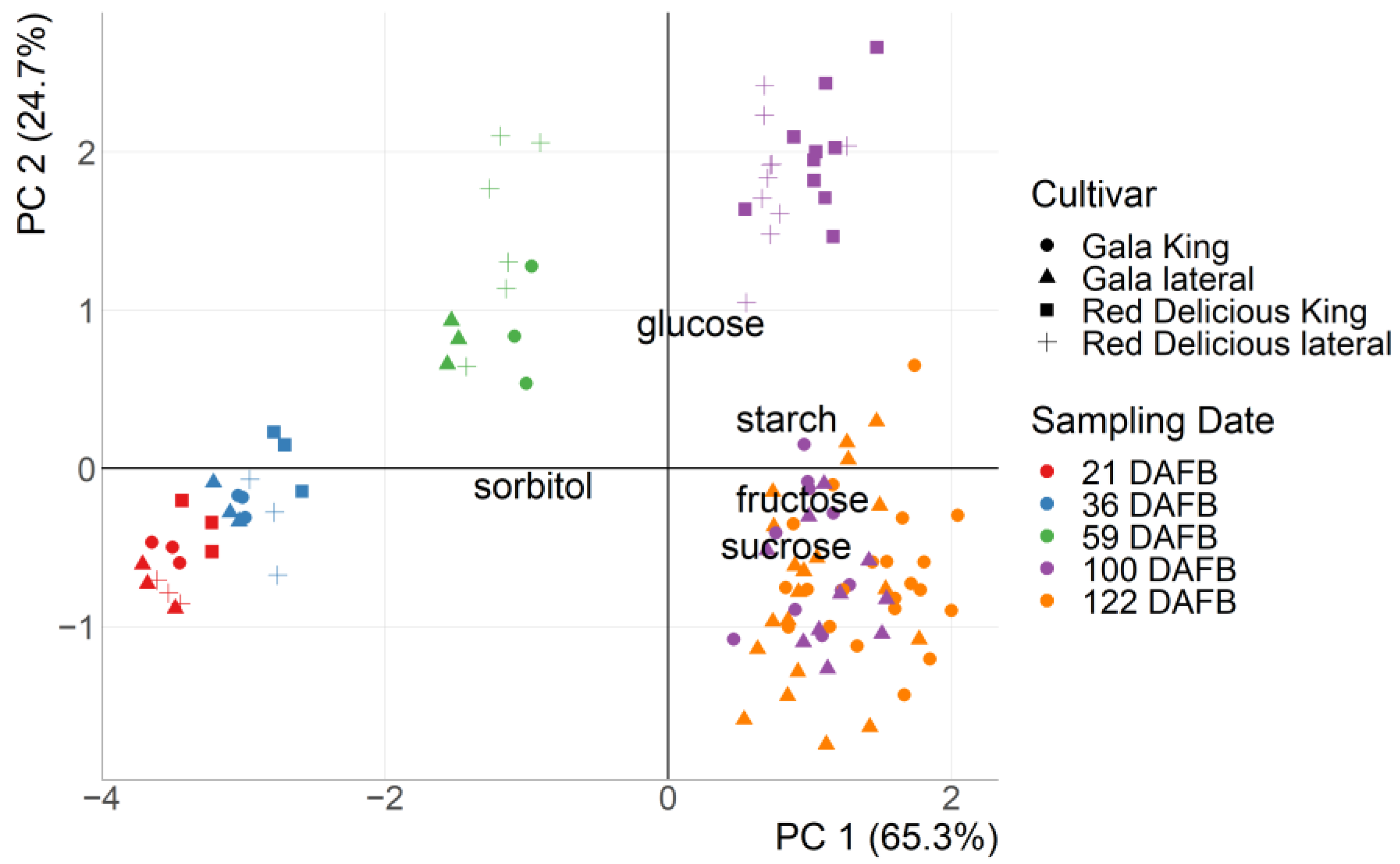
3.1. Measured Carbohydrate Content with HPLC
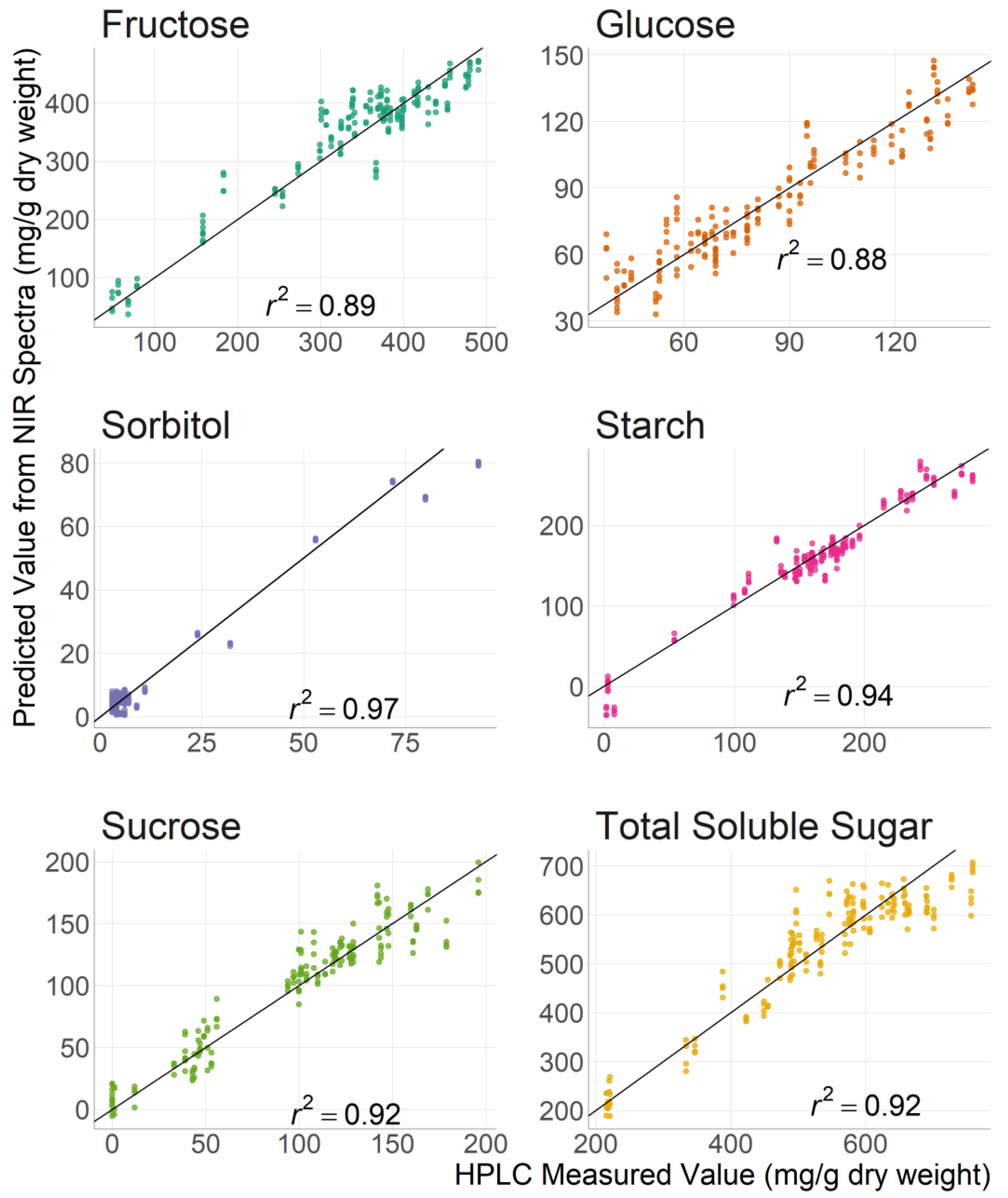
3.2. Carbohydrate Prediction with Near Infrared Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dash, M.; Johnson, L.K.; Malladi, A. Reduction of Fruit Load Affects Early Fruit Growth in Apple by Enhancing Carbohydrate Availability, Altering the Expression of Cell Production-related Genes, and Increasing Cell Production. J. Am. Soc. Hortic. Sci. 2013, 138, 253–262. [Google Scholar] [CrossRef]
- Jing, S.; Malladi, A. Higher growth of the apple (Malus × domestica Borkh.) fruit cortex is supported by resource intensive metabolism during early development. BMC Plant Biol. 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Botton, A.; Eccher, G.; Forcato, C.; Ferrarini, A.; Begheldo, M.; Zermiani, M.; Moscatello, S.; Battistelli, A.; Velasco, R.; Ruperti, B.; et al. Signaling Pathways Mediating the Induction of Apple Fruitlet Abscission. Plant Physiol. 2011, 155, 185–208. [Google Scholar] [CrossRef]
- McGlone, A.; Jordan, R.B.; Seelye, R.; Clark, C. Dry-matter—A better predictor of the post-storage soluble solids in apples? Postharvest Biol. Technol. 2003, 28, 431–435. [Google Scholar] [CrossRef]
- Larson, J.E.; Kon, T.M.; Malladi, A. Apple Fruitlet Abscission Mechanisms. Hortic. Rev. 2022, 49, 243–274. [Google Scholar] [CrossRef]
- DeJong, T.M. Concepts for Understanding Fruit Trees; CABI: Wallingford, UK, 2021. [Google Scholar]
- Priestly, C.A. The importance of autumn foliage to carbohydrate status and root growth of apple trees. Annu. Rep. East Malling Res. Stn. 1964, 104–106. [Google Scholar]
- Oliveira, C.M.; Priestley, C.A. Carbohydrate Reserves in Deciduous Fruit Trees. Hortic. Revies 1988, 10, 403–430. [Google Scholar] [CrossRef]
- Lakso, A.N.; Goffinet, M.C. Advances in understanding apple fruit development. In Achieving Sustainable Cultivation of Apples; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; pp. 127–158. [Google Scholar]
- Breen, K.; Tustin, S.; Palmer, J.; Boldingh, H.; Close, D. Revisiting the role of carbohydrate reserves in fruit set and early-season growth of apple. Sci. Hortic. 2020, 261, 109034. [Google Scholar] [CrossRef]
- Blanke, M.M.; Lenz, F. Fruit photosynthesis. Plant Cell Environ. 1989, 12, 31–46. [Google Scholar] [CrossRef]
- Tijero, V.; Girardi, F.; Botton, A. Fruit Development and Primary Metabolism in Apple. Agronomy 2021, 11, 1160. [Google Scholar] [CrossRef]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. Effects of location within the tree canopy on carbohydrates, organic acids, amino acids and phenolic compounds in the fruit peel and flesh from three apple (Malus× domestica) cultivars. Hortic. Res. 2014, 1, 14019. [Google Scholar] [CrossRef]
- Ackerman, M.; Samach, A. Doubts regarding carbohydrate shortage as a trigger toward abscission of specific Apple (Malus domestica) fruitlets. New Negat. Plant Sci. 2015, 1–2, 46–52. [Google Scholar] [CrossRef]
- Suni, M.; Nyman, M.; Eriksson, N.A.; Björk, L.; Björck, I. Carbohydrate composition and content of organic acids in fresh and stored apples. J. Sci. Food. Agric. 2000, 80, 1538–1544. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Posada, J.M.; Handa, I.T.; Hoch, G.; Vohland, M.; Messier, C.; Reu, B. Near-infrared spectroscopy (NIRS) predicts non-structural carbohydrate concentrations in different tissue types of a broad range of tree species. Methods Ecol. Evol. 2015, 6, 1018–1025. [Google Scholar] [CrossRef]
- Leopold, L.F.; Leopold, N.; Diehl, H.-A.; Socaciu, C. Quantification of carbohydrates in fruit juices using FTIR spectroscopy and multivariate analysis. Spectrosc. 2011, 26, 93–104. [Google Scholar] [CrossRef]
- Rustioni, L.; Ciacciulli, A.; Zulini, L.; Zuliani, E.; Sivilotti, P.; Herrera, J.C. Starch quantification in woody tissues by reflectance spectroscopy and on-solid iodine complexation. Sci. Hortic. 2017, 226, 117–121. [Google Scholar] [CrossRef]
- De Bei, R.; Fuentes, S.; Sullivan, W.; Edwards, E.; Tyerman, S.; Cozzolino, D. Rapid measurement of total non-structural carbohydrate concentration in grapevine trunk and leaf tissues using near infrared spectroscopy. Comput. Electron. Agric. 2017, 136, 176–183. [Google Scholar] [CrossRef]
- Baianu, I.; Guo, J. NIR calibrations for soybean seeds and soy food composition analysis: Total carbohydrates, oil, proteins and water contents. Nat. Proc. 2011, 661, 1. [Google Scholar]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Pissard, A.; Fernández Pierna, J.A.; Baeten, V.; Sinnaeve, G.; Lognay, G.; Mouteau, A.; Dupont, P.; Rondia, A.; Lateur, M. Non-destructive measurement of vitamin C, total polyphenol and sugar content in apples using near-infrared spectroscopy. J. Sci. Food Agric. 2013, 93, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Eisenstecken, D.; Panarese, A.; Robatscher, P.; Huck, C.W.; Zanella, A.; Oberhuber, M. A near infrared spectroscopy (NIRS) and chemometric approach to improve apple fruit quality management: A case study on the cultivars “Cripps Pink” and “Braeburn”. Molecules 2015, 20, 13603–13619. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S. Quantification of starch in plant tissues. Nat. Protoc. 2006, 1, 1342–1345. [Google Scholar] [CrossRef]
- Fall, L.A.; Perkins-Veazie, P.; Ma, G.; McGregor, C. QTLs associated with flesh quality traits in an elite × elite watermelon population. Euphytica 2019, 215, 30. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 8 September 2022).
- Li, M.; Feng, F.; Cheng, L. Expression Patterns of Genes Involved in Sugar Metabolism and Accumulation during Apple Fruit Development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.W.; Chong, C.; Taper, C.D. Sorbitol and other carbohydrate variation during growth and cold storage of mcintosh apple fruits. Can. J. Plant Sci. 1972, 52, 743–750. [Google Scholar] [CrossRef]
- Lombardo, V.A.; Osorio, S.; Borsani, J.; Lauxmann, M.; Bustamante, C.A.; Budde, C.O.; Andreo, C.S.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Metabolic Profiling during Peach Fruit Development and Ripening Reveals the Metabolic Networks That Underpin Each Developmental Stage. Plant Physiol. 2011, 157, 1696–1710. [Google Scholar] [CrossRef]
- Dai, Z.; Léon, C.; Feil, R.; Lunn, J.E.; Delrot, S.; Gomès, E. Metabolic profiling reveals coordinated switches in primary carbohydrate metabolism in grape berry (Vitis vinifera L.), a non-climacteric fleshy fruit. J. Exp. Bot. 2013, 64, 1345–1355. [Google Scholar] [CrossRef]
- Williams, P. Observations on the use, in prediction of functionality in cereals, of weights derived during development of partial least squares regression. J. Near Infrared Spectrosc. 1996, 4, 175. [Google Scholar] [CrossRef]
- Zhang, Y.; Nock, J.F.; Al Shoffe, Y.; Watkins, C.B. Non-destructive prediction of soluble solids and dry matter contents in eight apple cultivars using near-infrared spectroscopy. Postharvest Biol. Technol. 2019, 151, 111–118. [Google Scholar] [CrossRef]



| ‘Gala’ | ‘Red Delicious’ | |||
|---|---|---|---|---|
| Sampling Date | King z | Lateral z | King z | Lateral z |
| Sample 1 | 21 DAFB y | 21 DAFB | 21 DAFB | 21 DAFB |
| Sample 2 | 36 DAFB | 36 DAFB | 36 DAFB | 36 DAFB |
| Sample 3 | 59 DAFB | 59 DAFB | - | 59 DAFB |
| Sample 4 | 100 DAFB | 100 DAFB | 100 DAFB | 100 DAFB |
| Sample 5 | 122 DAFB | 122 DAFB | - | - |
| ‘Gala’ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fructose z | Glucose z | Sorbitol z | Starch z | Sucrose z | ||||||
| DAFB y | King x | Lateral | King | Lateral | King | Lateral | King | Lateral | King | Lateral |
| 21 | 68.0 | 56.2 | 58.5 | 49.3 | 93.3 | 93.6 | 7.86 | 7.3 | 0.7 | 0.4 |
| 36 | 78.6 | 78.3 | 67.7 | 68.2 | 67.1 | 72.0 | 4.4 * | 1.8 * | 0 | 0 |
| 59 | 176.7 | 158.3 | 106.4 | 105.3 | 31.7 | 36.9 | 108.3 ** | 65.2 ** | 33.4 * | 20.2 * |
| 100 | 386.6 | 392.5 | 70.5 | 62.4 | 5.7 | 5.6 | 164.8 * | 183.5 * | 106.9 | 117.9 |
| 122 | 422.1 | 391.2 | 79.3 | 70.4 | 4.1 | 4.0 | 157.2 | 140.3 | 150.9 * | 128.8 * |
| ‘Red Delicious’ | ||||||||||
| 21 | 72.2 * | 48.7 * | 63.9 * | 46.4 * | 80.3 | 85.2 | 3.3 * | 5.3 * | 0 | 0 |
| 36 | 88.1 | 67.5 | 78.4 | 60.9 | 53.3 | 53.2 | 3.0 | 3.8 | 0 | 0 |
| 59 | - | 162.6 | - | 128.7 | - | 21.6 | - | 68.4 | - | 13.5 |
| 100 | 306.4 | 282.5 | 134.3 | 131.5 | 3.8 | 4.4 | 264.1 ** | 229.9 ** | 45.9 | 45.4 |
| Sugar | Mean z | Minimum z | Maximum z |
|---|---|---|---|
| Fructose | 291 | 43 | 496 |
| Glucose | 85 | 37 | 169 |
| Sorbitol | 21 | 2 | 101 |
| Sucrose | 78 | 0 | 207 |
| Starch | 133 | 1 | 299 |
| Total Soluble Sugar | 475 | 136 | 785 |
| r2 z | RMSEP y | RPD x | ||||
|---|---|---|---|---|---|---|
| Sugar | Cortex w | Peel, Cortex, Pith | Cortex | Peel, Cortex, Pith | Cortex | Peel, Cortex, Pith |
| Fructose | 0.89 | 0.87 | 36.70 | 39.40 | 3.12 | 2.80 |
| Glucose | 0.88 | 0.60 | 10.40 | 22.40 | 2.92 | 1.60 |
| Sorbitol | 0.97 | 0.96 | 3.62 | 3.97 | 5.72 | 5.47 |
| Sucrose | 0.92 | 0.84 | 15.00 | 21.30 | 3.54 | 2.47 |
| Starch | 0.94 | 0.85 | 16.90 | 26.80 | 4.05 | 2.63 |
| Total Soluble Sugar | 0.92 | 0.87 | 49.60 | 61.90 | 3.58 | 2.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larson, J.E.; Perkins-Veazie, P.; Ma, G.; Kon, T.M. Quantification and Prediction with Near Infrared Spectroscopy of Carbohydrates throughout Apple Fruit Development. Horticulturae 2023, 9, 279. https://doi.org/10.3390/horticulturae9020279
Larson JE, Perkins-Veazie P, Ma G, Kon TM. Quantification and Prediction with Near Infrared Spectroscopy of Carbohydrates throughout Apple Fruit Development. Horticulturae. 2023; 9(2):279. https://doi.org/10.3390/horticulturae9020279
Chicago/Turabian StyleLarson, James E., Penelope Perkins-Veazie, Guoying Ma, and Thomas M. Kon. 2023. "Quantification and Prediction with Near Infrared Spectroscopy of Carbohydrates throughout Apple Fruit Development" Horticulturae 9, no. 2: 279. https://doi.org/10.3390/horticulturae9020279
APA StyleLarson, J. E., Perkins-Veazie, P., Ma, G., & Kon, T. M. (2023). Quantification and Prediction with Near Infrared Spectroscopy of Carbohydrates throughout Apple Fruit Development. Horticulturae, 9(2), 279. https://doi.org/10.3390/horticulturae9020279






