Mesh Crop Cover Optimizes the Microenvironment in a Tropical Region and Modifies the Physiology and Metabolome in Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
2.2. Crop Establishment and Management
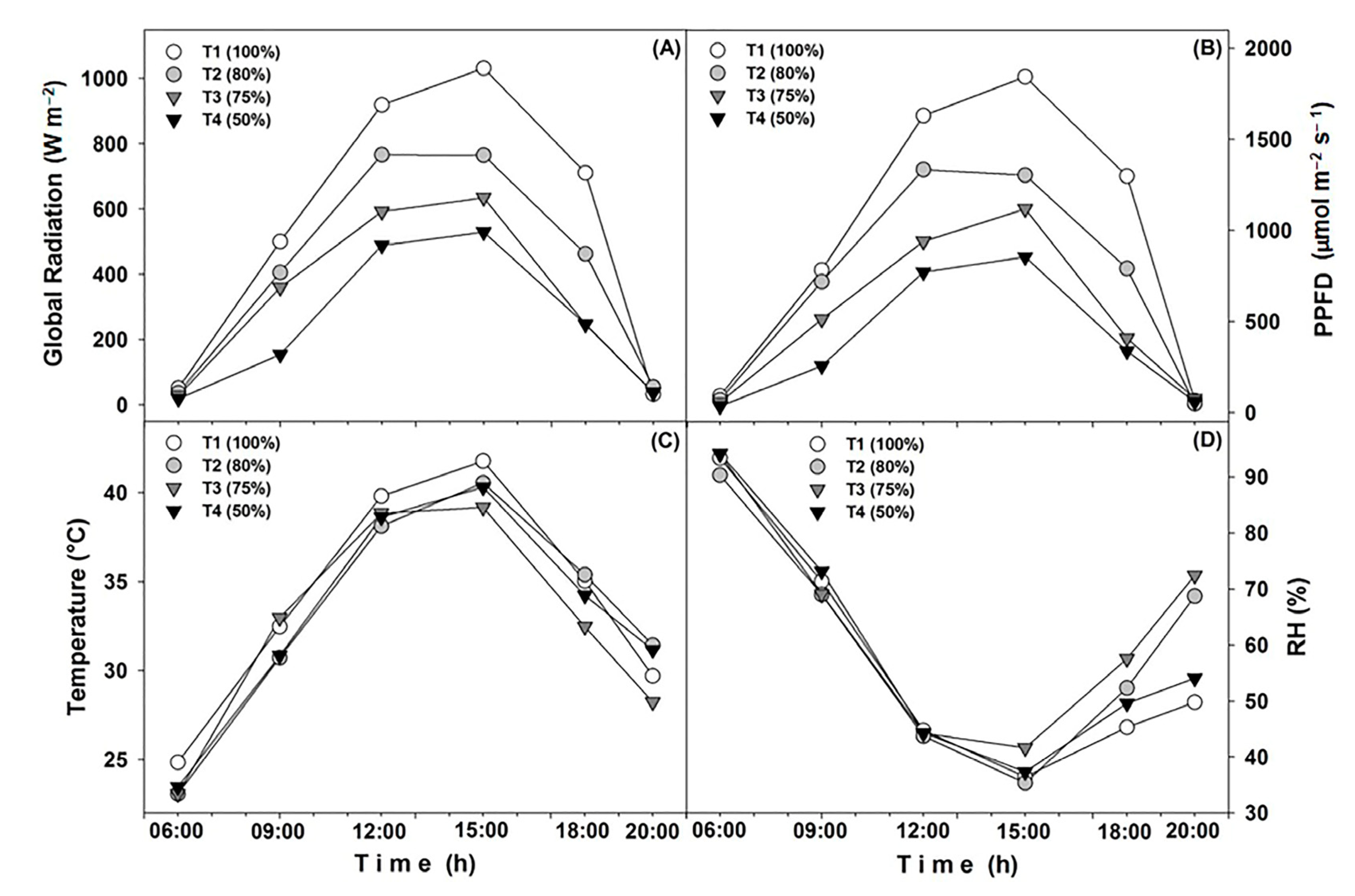
2.3. Treatments and Characterization of Microenvironments
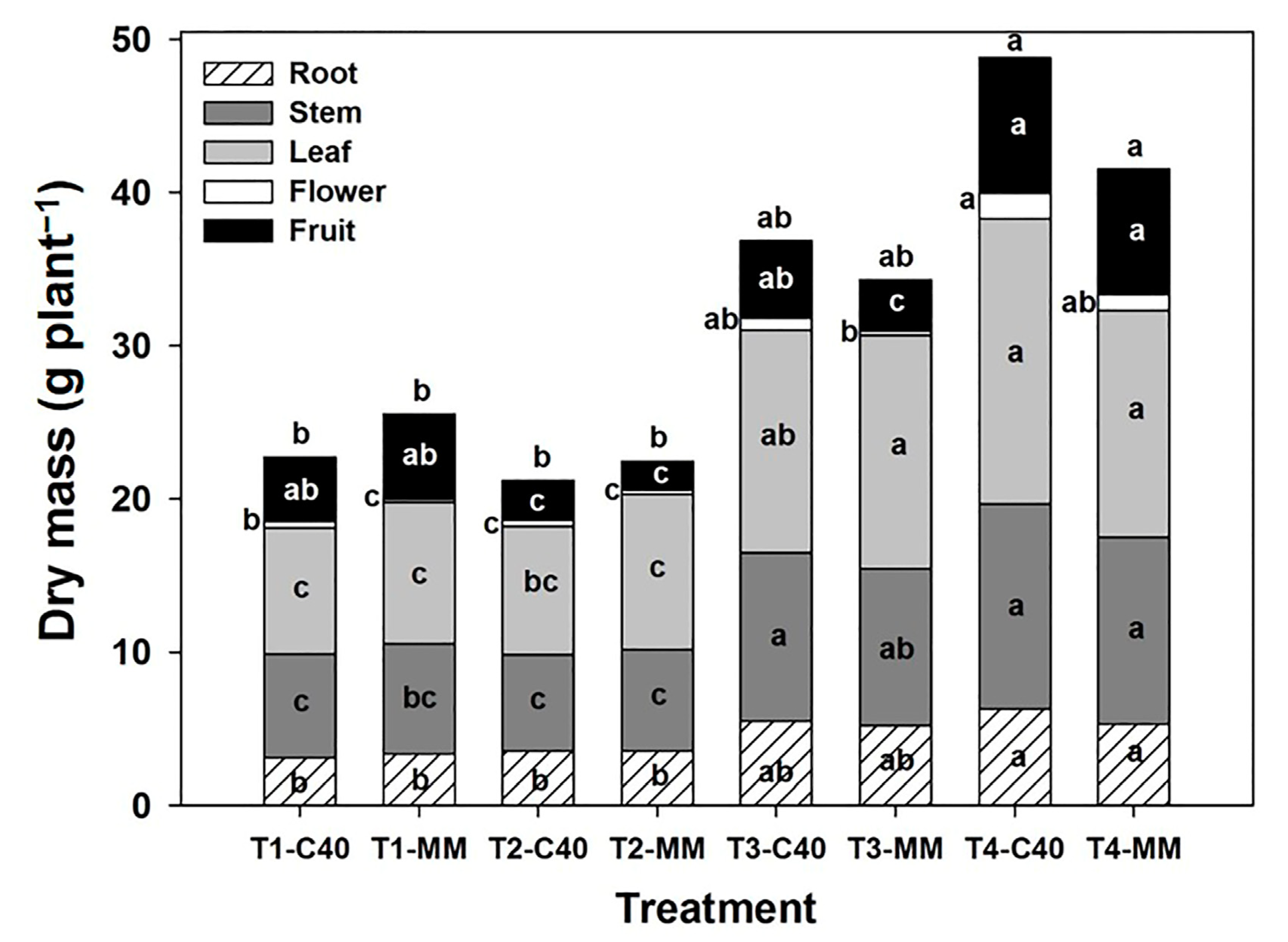
2.4. Morphological Variables and Biomass Distribution
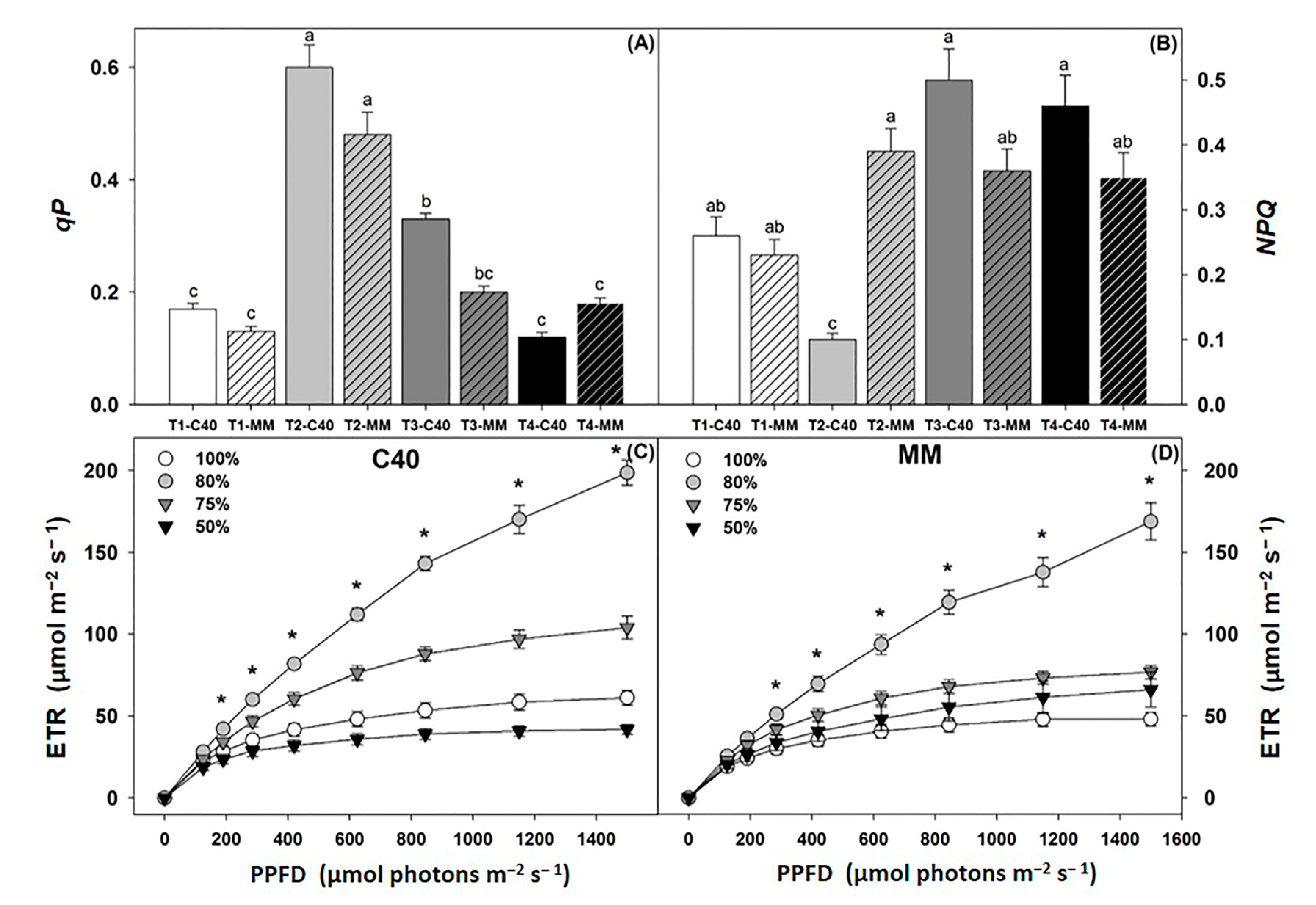
2.5. Leaf Photochemistry and Gas Exchange
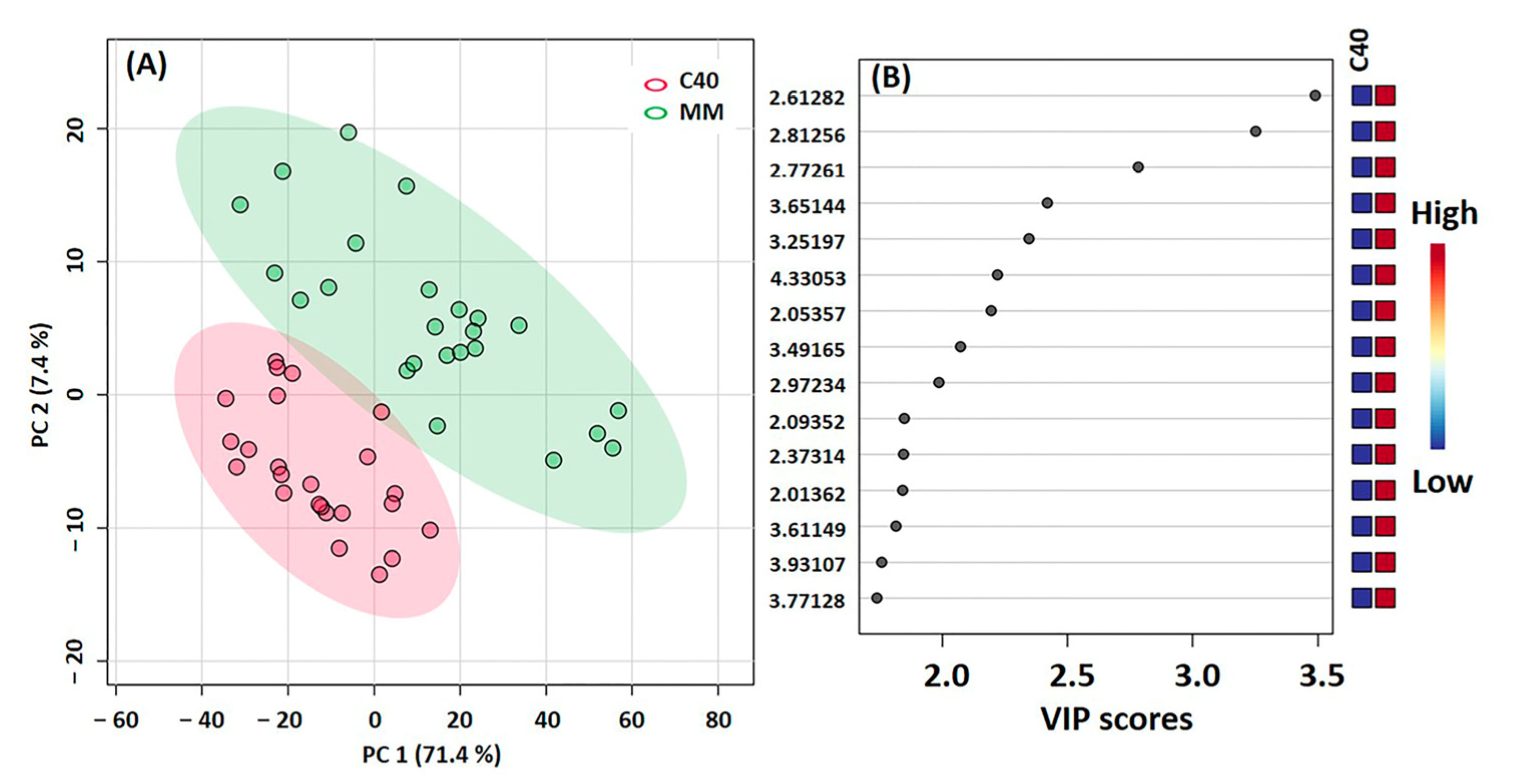
2.6. Metabolic Profile by NMR
2.7. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Microenvironments
3.2. Morphological Variables and Biomass Distribution
3.3. Leaf Photochemistry
3.4. Gas Exchange
3.5. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations Statistics Division. Available online: http://www.fao.org/faostat/en/#data/ (accessed on 10 May 2023).
- Adams, S.R.; Cockshull, K.E.; Cave, C.R.J. Effect of temperature on the growth and development of tomato fruits. Ann. Bot. 2001, 88, 869–877. [Google Scholar] [CrossRef]
- Boote, J.K.; Rybak, M.R.; Scolberg, J.M.; Jones, J.W. Improving the cropgrow-tomato model for predicting growth yield response to temperature. HortScience 2012, 47, 1038–1049. [Google Scholar] [CrossRef]
- Xu, J.; Wolters-Arts, M.; Mariani, C.; Huber, H.; Rieu, I. Heat stress affects vegetative and reproductive performance and trait correlations in tomato (Solanum lycopersicum). Euphytica 2017, 213, 156. [Google Scholar] [CrossRef]
- Orozco, A.J.; Ayala, C.C.; Tatis, H.A. Efecto del cambio climático sobre la fisiología de las plantas cultivadas: Una revisión. Rev. UDCA Actual. Divulg. Científica 2012, 15, 63–76. [Google Scholar] [CrossRef]
- Kubien, D.; Von Caemmerer, S.; Furbank, R.; Sage, R. C4 photosynthesis at low temperature. A study using transgenic plants with reduced amounts of Rubisco. Plant Physiol. 2003, 132, 1577–1585. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Ruíz-Nieves, J.M.; Ayala-Garay, O.J.; Serra, V.; Dumont, D.; Vercambre, G.; Génard, M.; Gautier, H. The effects of diurnal temperature rise on tomato fruit quality. Can the management of the greenhouse climate mitigate such effects. Sci. Hortic. 2021, 278, 109836. [Google Scholar] [CrossRef]
- Kläring, H.P.; Krumbein, A. The effect of constraining the intensity of solar radiation on the photosynthesis, growth, yield and product quality of tomato. J. Agron. Crop. Sci. 2013, 199, 351–359. [Google Scholar] [CrossRef]
- Perin, L.; Nogueira Peil, R.M.; Trentin, R.; Anibele Streck, E.; Bergmann da Rosa, D.S.; Hohn, D.; Silveira Schaun, W. Solar radiation threshold and growth of mini tomato plants in mild autumn/winter condition. Sci. Hortic. 2018, 239, 156–162. [Google Scholar] [CrossRef]
- Karipcin, M.Z.; Dinç, S.; Kara, M.; Kahraman, S.D.; Alp, I.E.; Cicekci, H. High temperature-tolerant tomato lines: Bioactive compounds. J. Verbr. Leb. 2016, 11, 117–125. [Google Scholar] [CrossRef]
- Florido-Bacallao, M.; Alvarez-Gil, M. Aspectos relacionados con el estrés de calor en tomate (Solanum lycopersicum L.). Cultiv. Trop. 2015, 36, 77–95. [Google Scholar]
- Geilfus, C.M. Controlled environment horticulture. In Improving Quality of Vegetables and Medicinal Plants; Springer: Cham, Switzerland, 2019; pp. 1–233. [Google Scholar]
- Valera, D.; Molina, F.; Gil, J. Las mallas como técnica de control climático en invernaderos. Vida Rural 2001, 8, 50–52. [Google Scholar]
- Meza, S.L.; Egea, I.; Massaretto, I.L.; Morales, B.; Purgatto, E.; Egea-Fernández, J.M.; Bolarin, M.C.; Flores, F.B. Traditional tomato varieties improve fruit quality without affecting fruit yield under moderate salt stress. Front. Plant Sci. 2020, 11, 587754. [Google Scholar] [CrossRef]
- Kumar, D. Nuclear magnetic resonance (NMR) spectroscopy: Metabolic profiling of medicinal plants and their products. Crit. Rev. Anal. Chem. 2016, 46, 400–412. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, R.; Choi, Y.; Kim, H. Metabolomics: What’s new? Flavour Fragr. J. 2010, 25, 128–131. [Google Scholar] [CrossRef]
- Ruíz-Nieves, J.M.; Magdaleno-Villar, J.J.; Sánchez-Alonso, M.G.; Delgado-Vargas, V.A.; Gautier, H.; Ayala-Garay, O.J. Parameters of physical and physiological quality in tomato seeds produced under high temperature condition during different periods of development. Agroproductividad 2021, 14, 45–50. [Google Scholar] [CrossRef]
- Delgado-Vargas, V.A.; Magdaleno-Villar, J.J.; Ayala-Garay, Ó.J.; Garfias-Sánchez, D. Calidad de semillas de tres variedades nativas y una comercial de tomate producidas bajo temperaturas altas. Rev. Chapingo Ser. Hortic. 2018, 24, 215–227. [Google Scholar] [CrossRef]
- Guzmán, A.; Corradini, F.; Martínez, P.; Allende, M.; Abarca, P.; Ferlmer, S. Manual de Cultivo Del Tomate al Aire Libre; Instituto de Investigaciones Agropecuarias (INIA): Santiago de Chile, Chile, 2017. [Google Scholar]
- Steiner, A.A. The universal nutrient solution. In Proceedings of the 6th International Congress on Soilless Culture, Lunteren, The Netherlands, 29 April 1984. [Google Scholar]
- Samaniego-Gámez, B.Y.; Garruña, R.; Tun-Suárez, J.M.; Kantun-Can, J.; Reyes-Ramírez, A.; Cervantes-Díaz, L. Bacillus spp. inoculation improves photosystem II efficiency and enhances photosynthesis in pepper plants. Chil. J. Agric. Res. 2016, 76, 409–416. [Google Scholar] [CrossRef]
- Garruña-Hernández, R.; Orellana, R.; Larque-Saavedra, A.; Canto, A. Understanding the physiological responses of a tropical crop (Capsicum chinense Jacq.) at high temperature. PLoS ONE 2014, 9, e111402. [Google Scholar] [CrossRef]
- Gall, G.; Colquhoun, I.J.; Davis, A.L.; Collins, G.J.; Verhoeyen, M.E. Metabolite profiling of tomato (Lycopersicon esculentum) using 1H NMR spectroscopy as a tool to detect potential unintended effects following a genetic modification. J. Agric. Food Chem. 2003, 51, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- Afifah, E.N.; Murti, R.H.; Nuringtyas, T.R. Metabolomics approach for the analysis of resistance of four tomato genotypes (Solanum lycopersicum L.) to root-knot nematodes (Meloidogyne incognita). Open Life Sci. 2019, 14, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.; Sánchez-Guerrero, M.C.; Medrano, E.; García, M.L.; Caparrós, I.; Giménez, M. External greenhouse mobile shading: Effect on microclimate, water use efficiency and yield of a tomato crop grown under different salinity levels of the nutrient solution. Acta Hortic. 2003, 609, 181–186. [Google Scholar] [CrossRef]
- Peet, M.M. Physiological disorders in tomato fruit development. Acta Hortic. 2009, 821, 151–160. [Google Scholar] [CrossRef]
- Ayala-Tafoya, F.; Zatarain-López, D.M.; Valenzuela-López, M.; Partida-Ruvalcaba, L.; Velázquez-Alcaraz, T.D.J.; Díaz-Valdés, T.; Osuna-Sánchez, J.A. Crecimiento y rendimiento de tomate en respuesta a radiación solar transmitida por mallas sombra. Terra Latinoam. 2011, 29, 403–410. [Google Scholar]
- Hang, T.; Lu, N.; Takagaki, M.; Mao, H. Leaf area model based on thermal effectiveness and photosynthetically active radiation in lettuce grown in mini-plant factories under different light cycles. Sci. Hortic. 2019, 252, 113–120. [Google Scholar] [CrossRef]
- Arenas-Corraliza, M.G.; Rolo, V.; López-Díaz, M.L.; Moreno, G. Wheat and barley can increase grain yield in shade through acclimation of physiological and morphological traits in Mediterranean conditions. Sci. Rep. 2019, 9, 9547. [Google Scholar] [CrossRef]
- Li, L.; Aro, E.M.; Millar, H. Mechanisms of photodamage and protein turnover in photoinhibition. Trends Plant Sci. 2018, 23, 667–676. [Google Scholar] [CrossRef]
- Trojak, M.; Skowron, E. Light Quality-Dependent Regulation of Non-Photochemical Quenching in Tomato Plants. Biology 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Yepes, A.; Buckeridge, M.S. Respuestas de las plantas ante los factores ambientales del cambio climático global: Revisión. Colomb. For. 2011, 14, 213–232. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Serrano, R. Salt tolerance in plants and microorganisms: Toxicity targets and defense responses. Int. Rev. Cytol. 1996, 165, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Hüther, C.M.; Martinazzo, E.G.; Rombaldi, C.V.; Bacarin, M.A. Effects of flooding stress in ‘Micro-Tom’ tomato plants transformed with different levels of mitochondrial sHSP23.6. Braz. J. Biol. 2016, 77, 43–51. [Google Scholar] [CrossRef]
- Baracaldo, A.; Carvajal, R.; Romero, A.P.; Prieto, A.M.; García, F.J.; Fischer, G.; Miranda, D. El anegamiento afecta el crecimiento y producción de biomasa en tomate chonto (Solanum lycopersicum L.), cultivado bajo sombrío. Rev. Colomb. De Cienc. Hortícolas 2014, 8, 92–102. [Google Scholar] [CrossRef]
- Carrari, F.; Asis, R.; Fernie, A.R. The metabolic shifts underlying tomato fruit development. Plant Biotechnol. 2007, 24, 45–55. [Google Scholar] [CrossRef]
- Chaves-Barrantes, N.F.; Gutiérrez-Soto, M.V. Respuestas al estrés por calor en los cultivos. I. Aspectos moleculares, bioquímicos y fisiológicos. Agron. Mesoam. 2017, 28, 237–253. [Google Scholar] [CrossRef]
- Kavi-Kishor, P.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Schwacke, R.; Grallath, S.; Breitkreuz, K.E.; Stransky, E.; Stransky, H.; Frommer, W.B.; Rentsch, D. LeProT1, a transporter for proline, glycine betaine, and γ-amino butyric acid in tomato pollen. Plant Cell 1999, 11, 377–391. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- Rodríguez, A.; Campo-Costa, A.; Batista-Ricardo, E.; Morales-Miranda, A.; Camejo-Gálvez, A.I. Influencia del Biobras 16 y Fitomas-E contra el tizón temprano y el geminivirus (TYLCV) en cultivo de tomate (Solanum licopersicum). ICIDCA. Sobre Los Deriv. De La Caña De Azúcar 2017, 51, 3–7. [Google Scholar]



| Treatments | Variety | Plant Height (cm) | Number of Leaves | Leaf Area (cm2) |
|---|---|---|---|---|
| T1 | C40 | 86.5 ± 1.66 b | 13.2 ± 1.65 ab | 882.8 ± 97.75 cd |
| MM | 74.3 ± 3.26 b | 6.5 ± 0.87 b | 358.1 ± 29.75 d | |
| T2 | C40 | 93.0 ± 2.64 b | 12.7 ± 1.31 ab | 716.5 ± 127.97 cd |
| MM | 90.6 ± 2.59 b | 14.3 ± 1.49 a | 879.6 ± 43.90 cd | |
| T3 | C40 | 135.0 ± 3.94 a | 19.8 ± 0.75 a | 2075.4 ± 202.61 ab |
| MM | 158.0 ± 7.40 a | 18.0 ± 1.58 a | 1459.3 ± 61.18 bc | |
| T4 | C40 | 154.8 ± 1.80 a | 19.8 ± 0.75 a | 3100.8 ± 264.90 a |
| MM | 161.3 ± 8.96 a | 14 ± 2.48 a | 1481.7 ± 209.40 bc | |
| LSD | 27.15 | 7.09 | 1051.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Vargas, V.A.; Hernández-Bolio, G.I.; Hernández-Núñez, E.; Gautier, H.; Ayala-Garay, O.J.; Garruña, R. Mesh Crop Cover Optimizes the Microenvironment in a Tropical Region and Modifies the Physiology and Metabolome in Tomato. Horticulturae 2023, 9, 636. https://doi.org/10.3390/horticulturae9060636
Delgado-Vargas VA, Hernández-Bolio GI, Hernández-Núñez E, Gautier H, Ayala-Garay OJ, Garruña R. Mesh Crop Cover Optimizes the Microenvironment in a Tropical Region and Modifies the Physiology and Metabolome in Tomato. Horticulturae. 2023; 9(6):636. https://doi.org/10.3390/horticulturae9060636
Chicago/Turabian StyleDelgado-Vargas, Victoria A., Gloria I. Hernández-Bolio, Emanuel Hernández-Núñez, Hélène Gautier, Oscar J. Ayala-Garay, and René Garruña. 2023. "Mesh Crop Cover Optimizes the Microenvironment in a Tropical Region and Modifies the Physiology and Metabolome in Tomato" Horticulturae 9, no. 6: 636. https://doi.org/10.3390/horticulturae9060636
APA StyleDelgado-Vargas, V. A., Hernández-Bolio, G. I., Hernández-Núñez, E., Gautier, H., Ayala-Garay, O. J., & Garruña, R. (2023). Mesh Crop Cover Optimizes the Microenvironment in a Tropical Region and Modifies the Physiology and Metabolome in Tomato. Horticulturae, 9(6), 636. https://doi.org/10.3390/horticulturae9060636







