Effectiveness of Natural-Based Coatings on Sweet Oranges Post-Harvest Life and Antioxidant Capacity of Obtained By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Harvest and Treatments
2.2. Pre-Storage Fruit Quality Evaluation
2.3. Fruit Postharvest Quality Assay
2.4. Statistical Assessment
3. Results
3.1. Pre-Storage Fruit Quality Evaluations
3.2. Postharvest Fruit Quality Evaluations
3.2.1. Weight Loss
3.2.2. Fruit Color Index
3.2.3. Juice Quality
3.2.4. Sensory Analysis
4. Discussion
4.1. Pre-Storage Fruit Quality Evaluations
4.2. Postharvest Fruit Quality Evaluations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ladaniya, M.S. Citrus Fruit: Biology, Technology and Evaluation, 2nd ed.; Elsevier Academic Press: San Diego, CA, USA, 2022. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). 2020. Available online: http://www.faostat.fao.org (accessed on 30 November 2022).
- Fund for Citrus Protection (Fundecitrus). Tree Inventory and Orange Crop Forecast for the São Paulo and West-Southwest Minas Gerais Citrus Belt; Fundecitrus: Araraquara, SP, Brazil, 2022. [Google Scholar]
- Lertworasirikul, S.; Saetan, S. Artificial neural network modeling of mass transfer during osmotic dehydration of kaffir lime peel. J. Food Eng. 2010, 98, 214–223. [Google Scholar] [CrossRef]
- Zarina, Z.; Tan, S.Y. Determination of flavonoids in Citrus grandis (Pomelo) peels and their inhibition activity on lipid peroxidation in fish tissue. Int. Food Res. J. 2013, 20, 313–317. [Google Scholar]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: II. Enzyme-assisted extraction method. Sep. Purif. Technol. 2006, 48, 189–196. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chuang, Y.C.; Hsu, H.W. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008, 106, 277–284. [Google Scholar] [CrossRef]
- Ghanem, N.; Mihoubi, D.; Kechaou, N.; Mihoubi, N.B. Microwave dehydration of three citrus peel cultivars: Effect on water and oil retention capacities, color, shrinkage and total phenols content. Ind. Crops Prod. 2012, 40, 167–177. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Chen, J.C.; Liu, D.H.; Ye, X.Q. Simultaneous extraction of phenolic compounds of citrus peel extracts: Effect of ultrasound. Ultrason. Sonochem. 2009, 16, 57–62. [Google Scholar] [CrossRef]
- Castro-Vazquez, L.; Alañón, M.E.; Rodríguez-Robledo, V.; Pérez-Coello, M.S.; Hermosín-Gutierrez, I.; Díaz-Maroto, M.C.; Jordán, J.; Galindo, M.F.; Arroyo-Jimenez, M.D.M. Bioactive flavonoids, antioxidant behaviour, and cytoprotective effects of dried grapefruit peels (Citrus paradisi Macf.). Oxid. Med. Cell. Longev. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Tarancón, P.; Giménez-Sanchis, A.; Aleza, P.; Besada, C. Selection of new late-season mandarin cultivars based on sensory changes and consumer acceptance after fruit cold storage. Agronomy 2021, 11, 116. [Google Scholar] [CrossRef]
- Goedhals-Gerber, L.L.; Khumalo, G. Identifying temperature breaks in the export cold chain of navel oranges: A Western Cape case. Food Control 2020, 110, 107013. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. Polymers for Agri-Food Applications, 1st ed.; Springer International Publishing: Basel, Switzerland, 2019. [Google Scholar]
- Miranda, M.; Sun, X.; Ference, C.; Plotto, A.; Bai, J.; Wood, D.; Assis, O.B.G.; Ferreira, M.D.; Baldwin, E. Nano-and Micro-Carnauba Wax Emulsions versus Shellac Protective Coatings on Postharvest Citrus Quality. J. Am. Soc. Hortic. Sci. 2021, 146, 40–49. [Google Scholar] [CrossRef]
- Motamedi, E.; Nasiri, J.; Malidarreh, T.R.; Kalantari, S.; Naghavi, M.R.; Safari, M. Performance of carnauba wax-nanoclay emulsion coatings on postharvest quality of ‘Valencia’ orange fruit. Sci. Hortic. 2018, 240, 170–178. [Google Scholar] [CrossRef]
- Freitas, C.A.S.; Sousa, P.H.M.; Soares, D.J.; Da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba wax uses in food–A review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Jonglertjunya, W.; Juntong, T.; Pakkang, N.; Srimarut, N.; Sakdaronnarong, C. Properties of lignin extracted from sugarcane bagasse and its efficacy in maintaining postharvest quality of limes during storage. LWT-Food Sci. Technol. 2014, 57, 116–125. [Google Scholar] [CrossRef]
- Fei, T.; Leyva-Gutierrez, F.M.; Wan, Z.; Wang, T. Development of a novel soy-wax containing emulsion with enhanced antifungal properties for the postharvest treatment of fresh citrus fruit. LWT-Food Sci. Technol. 2021, 141, 110878. [Google Scholar] [CrossRef]
- Hagenmaier, R.D. Evaluation of a polyethylene–candelilla coating for ‘Valencia’ oranges. Postharvest Biol. Technol. 2000, 19, 147–154. [Google Scholar] [CrossRef]
- Nasrin, T.A.A.; Rahman, M.A.; Arfin, M.S.; Islam, M.N.; Ullah, M.A. Effect of novel coconut oil and beeswax edible coating on postharvest quality of lemon at ambient storage. J. Sci. Food Agric. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Gaillard, Y.; Mija, A.; Burr, A.; Darque-Ceretti, E.; Felder, E.; Sbirrazzuoli, N. Green material composites from renewable resources: Polymorphic transitions and phase diagram of beeswax/rosin resin. Thermochim. Acta 2011, 521, 90–97. [Google Scholar] [CrossRef]
- Barman, K.; Sharma, S.; Siddiqui, M.W. Emerging Postharvest Treatment of Fruits and Vegetables, 1st ed.; Apple Academic Press Inc.: Waretown, NJ, USA, 2019. [Google Scholar]
- Baswal, A.K.; Dhaliwal, H.S.; Singh, Z.; Mahajan, B.V.C.; Kalia, A.; Gill, K.S. Influence of carboxy methylcellulose, chitosan and beeswax coatings on cold storage life and quality of Kinnow mandarin fruit. Sci. Hortic. 2020, 260, 108887. [Google Scholar] [CrossRef]
- Lorenzi, H.; Noblick, L.; Kahn, F.; Flora, E. Flora Brasileira: Arecaceae (Palmeiras), 1st ed.; Instituto Plantarum: Nova Odessa, SP, Brazil, 2010. [Google Scholar]
- Companhia de Entrepostos e Armazéns Gerais de São Paulo (CEAGESP). Normas de Classificação de Citros de Mesa, 2nd ed.; CEAGESP: São Paulo, SP, Brazil, 2011. [Google Scholar]
- Zhou, J.Y.; Sun, C.D.; Zhang, L.L.; Dai, X.; Xu, C.J.; Chen, K.S. Preferential accumulation of orange-colored carotenoids in Ponkan (Citrus reticulata) fruit peel following postharvest application of ethylene or ethephon. Sci. Hortic. 2010, 126, 229–235. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists (AOAC). Official Methods of Analysis of the AOAC International, 21st ed.; AOAC International: Arlington, VA, USA, 2019. [Google Scholar]
- Rahman, N.F.A.; Shamsudin, R.; Ismail, A.; Shah, N.N.A.K.; Varith, J. Effects of drying methods on total phenolic contents and antioxidant capacity of the pomelo (Citrus grandis (L.) Osbeck) peels. Innov. Food Sci. Emerg. Technol. 2018, 50, 217–225. [Google Scholar] [CrossRef]
- He, J.Z.; Shao, P.; Liu, J.H.; Ru, Q.M. Supercritical carbon dioxide extraction of flavonoids from Pomelo (Citrus grandis (L.) Osbeck) peel and their antioxidant activity. Int. J. Mol. Sci. 2012, 13, 13065–13078. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Mandarin fruit quality: A review. J. Sci. Food Agric. 2018, 98, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tadeo, F.R.; Terol, J.; Rodrigo, M.J.; Licciardello, C.; Sadka, A. Fruit growth and development. In The Genus Citrus, 1st ed.; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 245–269. [Google Scholar] [CrossRef]
- Alferez, F.; Alquezar, B.; Burns, J.K.; Zacarias, L. Variation in water, osmotic and turgor potential in peel of ‘Marsh’ grapefruit during development of postharvest peel pitting. Postharvest Biol. Technol. 2010, 56, 44–49. [Google Scholar] [CrossRef]
- Carvalho, D.U.; Cruz, M.A.; Colombo, R.C.; Tazima, Z.H.; Neves, C.S.V.J. Fruit quality of ‘Salustiana’ seedless oranges during cold storage: Effect of carnauba-based wax and rootstocks. J. Food Meas. Charact. 2020, 14, 3397–3407. [Google Scholar] [CrossRef]
- Cronjé, P.J.; Zacarías, L.; Alferez, F. Susceptibility to postharvest peel pitting in Citrus fruits as related to albedo thickness, water loss and phospholipase activity. Postharvest Biol. Technol. 2017, 123, 77–82. [Google Scholar] [CrossRef]
- Alferez, F.; Zacarias, L.; Burns, J.K. Low relative humidity at harvest and before storage at high humidity influence the severity of postharvest peel pitting in citrus. J. Am. Soc. Hortic. Sci. 2005, 130, 225–231. [Google Scholar] [CrossRef]
- Albrigo, L.G.; Stelinski, L.L.; Timmer, L. Citrus, 1st ed.; CAB International: Wallingford, UK, 2009. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). International Standards for Fruit and Vegetables: Citrus; OECD: Paris, France, 2010. [Google Scholar]
- Pozzan, M.; Triboni, H.R. Colheita e qualidade do fruto. In Citros, 1st ed.; Mattos Junior, D., De Negri, J.D., Pio, R.M., Pompeu Junior, J., Eds.; Fundag: Campinas, SP, Brazil, 2005; pp. 801–822. [Google Scholar]
- Pereira, M.E.C.; Cantillano, F.F.; Gutierez, A.S.D.; Almeida, G.V.B. Procedimentos Pós-Colheita Na Produção Integrada De Citros.; Embrapa Mandioca & Fruticultura Tropical: Cruz Das Almas, BA, Brazil, 2006; 40p. [Google Scholar]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Barros, H.R.M.; Ferreira, T.A.P.C.; Genovese, M.I. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K. Phenols in citrus peel byproducts. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J. Agric. Food Chem. 2001, 49, 3268–3273. [Google Scholar] [CrossRef]
- Ramful, D.; Bahorun, T.; Bourdon, E.; Tarnus, E.; Aruoma, O.I. Bioactive phenolics and antioxidant propensity of flavedo extracts of Mauritian citrus fruits: Potential prophylactic ingredients for functional foods application. Toxicology 2010, 278, 75–87. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids–chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Chang, S.Q.; Azrina, A. Antioxidant content and activity in different parts of pomelo [Citrus grandis (L.) Osbeck] by-products. Acta Hortic. 2017, 1152, 27–34. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Agostini, J.S.; Scalon, S.P.Q.; Lescano, C.H.; Silva, K.E.; Garcete, G.J. Scientific Note: Postharvest conservation of ‘Champagne’ oranges (Citrus reticulata × Citrus sinensis). Braz. J. Food Technol. 2014, 17, 177–184. [Google Scholar] [CrossRef]
- Pereira, G.S.; Machado, F.L.C.; Costa, J.M.C. Aplicação de recobrimento prolonga a qualidade pós-colheita de laranja ‘Valência Delta’ durante armazenamento ambiente. Rev. Cienc. Agron. 2014, 45, 520–527. [Google Scholar] [CrossRef]
- Malgarim, M.B.; Cantillano, R.F.F.; Treptow, R.O. Armazenamento refrigerado de laranjas cv. Navelina em diferentes concentrações de cera à base de carnaúba. Acta Sci. Agron. 2007, 29, 99–105. [Google Scholar] [CrossRef]
- Çandir, E.; Kamiloğlu, M.; Üstün, D.; Kendir, G.T. Comparison postharvest quality of conventionally and organically grown ‘Washington Navel’ oranges. J. Appl. Bot. Food Qual. 2013, 86, 59–65. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, J.M.; Zacarias, L. Maturity indicators and citrus fruit quality. Stewart Postharvest Rev. 2014, 2, 1–6. [Google Scholar]
- Roussos, P.A. Phytochemicals and antioxidant capacity of orange (Citrus sinensis (L.) Osbeck cv. Salustiana) juice produced under organic and integrated farming system in Greece. Sci. Hortic. 2011, 129, 253–258. [Google Scholar] [CrossRef]
- Arpaia, M.; Kader, A.A. Recommendations for Maintaining Postharvest Quality, 1st ed.; University of California: Davis, CA, USA, 2000. [Google Scholar]
- Jones, W.W.; Cree, C.B. Environment factors related to fruiting of Washington navel orange over a 38-year period. Proc. Am. Soc. Hortic. Sci. 1965, 86, 267–271. [Google Scholar]
- Davies, F.S.; Zalman, G. Gibberellic Acid, Fruit Freezing, and Post-freeze Quality of Hamlin’ Oranges. HortTechnology 2006, 16, 301–305. [Google Scholar] [CrossRef]
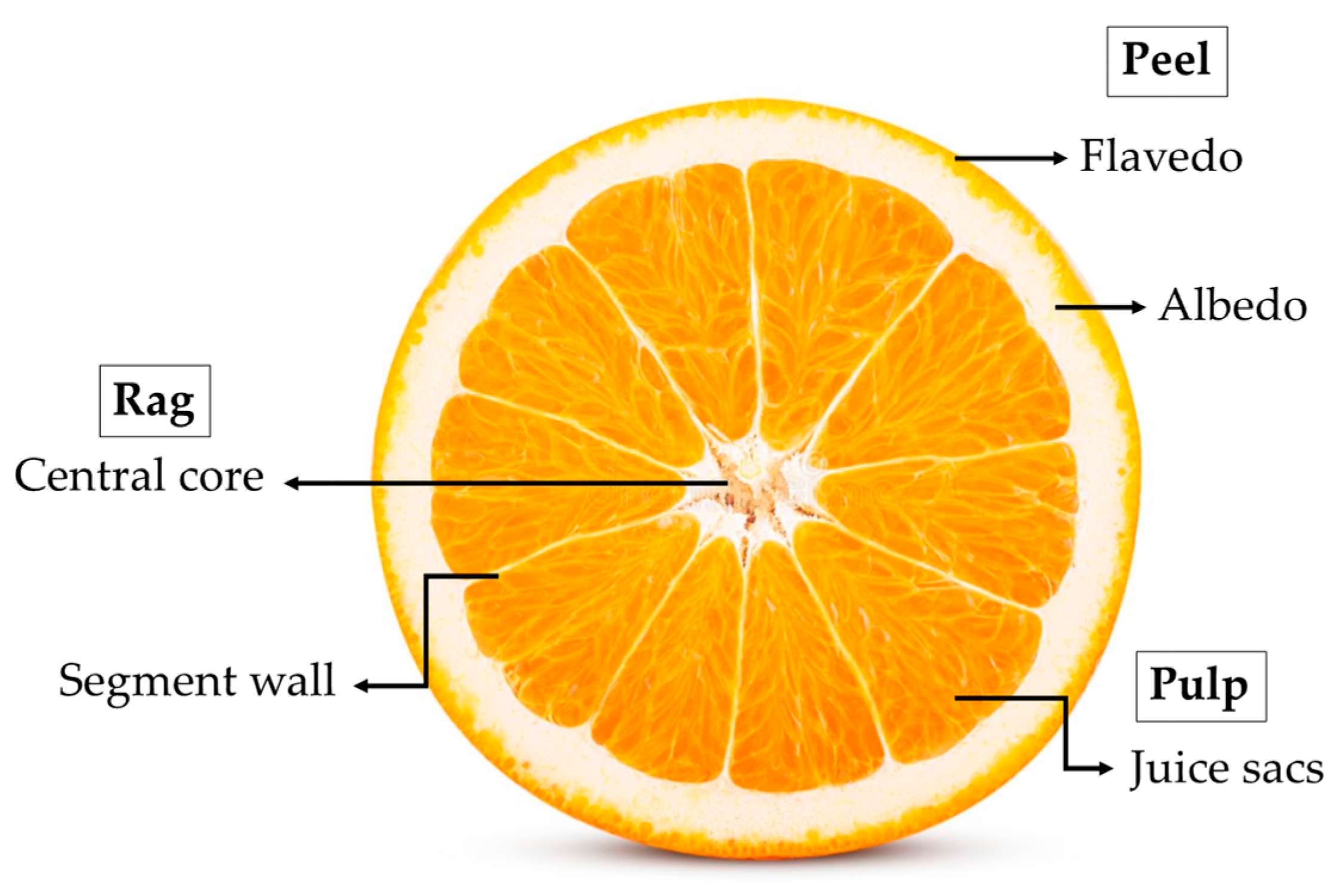




| Fruit Attribute | Valencia Late | Natal IAC | CV (%) 1 | F Value |
|---|---|---|---|---|
| Fruit length—FL (mm) | 70.0 ± 1.17 a 2 | 72.7 ± 2.95 a | 3.15 | 2.21 ns |
| Fruit diameter—FD (mm) | 75.0 ± 1.18 a | 73.4 ± 0.64 a | 1.28 | 4.10 ns |
| Fruit shape index (FL·FD−1) | 0.93 ± 0.01 a | 0.99 ± 0.05 a | 3.49 | 4.36 ns |
| Citrus color index—CCI | 1.57 ± 0.34 b | 2.11 ± 0.06 a | 13.0 | 7.60* |
| Flavedo thickness (mm) | 2.68 ± 0.30 a | 2.74 ± 0.15 a | 8.77 | 0.09 ns |
| Albedo thickness (mm) | 2.36 ± 0.25 a | 2.33 ± 0.11 a | 8.24 | 0.05 ns |
| Fruit weight (g) | 162 ± 2.25 a | 150 ± 8.67 b | 3.99 | 11.8 * |
| Number of seeds | 2 ± 0.92 a | 2 ± 0.40 a | 36.0 | 0.21 ns |
| Juice content (%) | 36.1 ± 2.91 a | 41.3 ± 2.39 a | 6.88 | 5.86 ns |
| Soluble solids content—SST (°Brix) | 11.2 ± 0.95 a | 12.1 ± 0.64 a | 6.95 | 1.98 ns |
| Titratable acidity—TA (g·100 mL−1) | 0.93 ± 0.13 a | 1.08 ± 0.03 a | 9.49 | 3.52 ns |
| Ratio (SST·TA−1) | 12.0 ± 0.65 a | 11.2 ± 0.88 a | 6.62 | 1.61 ns |
| Source of Variance | Total Phenolics (mg 100g−1) | ||
| Valencia Late | Natal IAC | Mean | |
| Flavedo | 246.9 ± 4.1 Ba 1 | 267.9 ± 4.4 Aa | 257.4 ± 4.3 |
| Albedo | 200.0 ± 5.4 Bb | 227.1 ± 6.0 Ab | 213.6 ± 5.7 |
| Central core | 161.3 ± 5.4 Ac | 166.0 ± 7.5 Ac | 163.6 ± 6.5 |
| Juice sacs | 107.7 ± 5.1 Ad | 117.5 ± 6.0 Ad | 112.6 ± 5.6 |
| Segment wall | 85.9 ± 3.9 Ae | 82.7 ± 8.7 Ae | 84.3 ± 6.3 |
| Mean | 160.3 ± 4.8 | 172.3 ± 6.5 | |
| CV (%) 2 | 3.53 | ||
| Fruit section | 876.6 *** | ||
| Variety | 30.8 *** | ||
| Fruit section × Variety | 6.48 *** | ||
| Total flavonoids (mg 100g−1) | |||
| Valencia Late | Natal IAC | Mean | |
| Flavedo | 235.5 ± 18.5 Aa | 241.0 ± 3.6 Aa | 238.6 ± 11.1 |
| Albedo | 167.2 ± 13.2 Bb | 221.9 ± 10.6 Ab | 194.6 ± 18.4 |
| Central core | 129.7 ± 10.2 Ac | 137.6 ± 9.6 Ac | 133.7 ± 9.9 |
| Juice sacs | 38.1 ± 3.7 Bd | 60.2 ± 8.2 Ad | 49.1 ± 6.0 |
| Segment wall | 23.9 ± 1.7 Bd | 51.3 ± 9.6 Ad | 37.6 ± 5.7 |
| Mean | 118.9 ± 9.5 | 142.4 ± 8.3 | |
| CV (%) | 7.72 | ||
| Fruit section | 456.3 *** | ||
| Variety | 40.7 *** | ||
| Fruit section × Variety | 5.75 ** | ||
| DPPH scavenging (%) | |||
| Valencia Late | Natal IAC | Mean | |
| Flavedo | 74.1 ± 3.8 | 74.8 ± 3.6 | 74.4 ± 3.7 a |
| Albedo | 66.5 ± 2.7 | 64.7 ± 1.6 | 65.6 ± 2.2 b |
| Central core | 60.7 ± 3.3 | 54.9 ± 4.8 | 57.8 ± 4.1 c |
| Juice sacs | 51.6 ± 0.5 | 41.4 ± 3.8 | 46.5 ± 2.2 d |
| Segment wall | 37.7 ± 1.4 | 33.2 ± 2.5 | 35.4 ± 2.0 e |
| Mean | 58.1 ± 2.3 A | 53.8 ± 3.3 B | |
| CV (%) | 5.55 | ||
| Fruit section | 147.3 *** | ||
| Variety | 14.4 ** | ||
| Fruit section × Variety | 2.64 ns | ||
| Source of Variance | Total Soluble Solids—TSS (°Brix) | ||||
| Valencia Late + Wax | Natal IAC + Wax | Valencia Late | Natal IAC | Mean | |
| 0 day (harvest) | 10.7 ± 0.70 | 10.6 ± 0.62 | 11.1 ± 0.43 | 11.7 ± 1.47 | 11.1 ± 0.81 |
| 15 days | 11.5 ± 0.32 | 10.6 ± 0.58 | 11.9 ± 0.50 | 11.9 ± 1.41 | 11.5 ± 0.70 |
| 30 days | 11.7 ± 1.38 | 10.5 ± 0.40 | 12.2 ± 1.65 | 11.2 ± 0.55 | 11.4 ± 1.00 |
| 45 days | 12.0 ± 0.50 | 10.0 ± 0.26 | 12.7 ± 0.40 | 11.8 ± 0.55 | 11.6 ± 0.43 |
| 60 days | 11.5 ± 0.10 | 10.1 ± 0.66 | 12.6 ± 1.70 | 11.4 ± 1.11 | 11.4 ± 0.89 |
| Mean | 11.5 ± 0.60 A 1 | 10.4 ± 0.50 B | 12.1 ± 0.94 A | 11.6 ± 1.02 A | |
| CV (%) 2 | 7.97 | ||||
| Storage period | 0.64 ns | ||||
| Treatment | 9.97 *** | ||||
| Storage period × Treatment | 0.73 ns | ||||
| Titratable acidity—TA (g.100 mL−1) | |||||
| Valencia Late + Wax | Natal IAC + Wax | Valencia Late | Natal IAC | Mean | |
| 0 day (harvest) | 0.54 ± 0.05 | 0.68 ± 0.15 | 0.69 ± 0.15 | 0.67 ± 0.11 | 0.64 ± 0.12 |
| 15 days | 0.64 ± 0.11 | 0.67 ± 0.14 | 0.71 ± 0.17 | 0.78 ± 0.22 | 0.70 ± 0.16 |
| 30 days | 0.63 ± 0.09 | 0.65 ± 0.10 | 0.77 ± 0.22 | 0.71 ± 0.13 | 0.69 ± 0.14 |
| 45 days | 0.65 ± 0.03 | 0.77 ± 0.04 | 0.74 ± 0.19 | 0.77 ± 0.09 | 0.73 ± 0.09 |
| 60 days | 0.65 ± 0.04 | 0.79 ± 0.07 | 0.82 ± 0.08 | 0.79 ± 0.22 | 0.76 ± 0.10 |
| Mean | 0.62 ± 0.06 | 0.71 ± 0.10 | 0.75 ± 0.16 | 0.74 ± 0.15 | |
| CV (%) | 19.60 | ||||
| Storage period | 1.21 ns | ||||
| Treatment | 2.74 ns | ||||
| Storage period × Treatment | 0.18 ns | ||||
| TSS.TA−1ratio | |||||
| Valencia Late + Wax | Natal IAC + Wax | Valencia Late | Natal IAC | Mean | |
| 0 day (harvest) | 20.1 ± 1.88 | 16.3 ± 5.06 | 16.5 ± 3.36 | 17.7 ± 2.74 | 17.7 ± 3.26 |
| 15 days | 18.3 ± 3.09 | 16.3 ± 4.55 | 17.5 ± 4.02 | 16.2 ± 5.13 | 17.1 ± 4.19 |
| 30 days | 18.6 ± 1.14 | 16.3 ± 2.22 | 16.4 ± 2.78 | 16.2 ± 2.67 | 16.9 ± 2.20 |
| 45 days | 18.6 ± 1.12 | 13.0 ± 0.68 | 18.0 ± 4.69 | 15.7 ± 1.22 | 16.2 ± 1.92 |
| 60 days | 17.8 ± 1.30 | 13.0 ± 2.03 | 15.4 ± 1.86 | 15.4 ± 6.68 | 15.5 ± 2.96 |
| Mean | 18.7 ± 1.71 A | 15.0 ± 2.91 B | 16.8 ± 3.34 AB | 16.2 ± 3.67 AB | |
| CV (%) | 18.89 | ||||
| Storage period | 0.77 ns | ||||
| Treatment | 3.24 * | ||||
| Storage period × Treatment | 0.28 ns | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, D.U.d.; Neves, C.S.V.J.; Cruz, M.A.d.; Colombo, R.C.; Alferez, F.; Leite Junior, R.P. Effectiveness of Natural-Based Coatings on Sweet Oranges Post-Harvest Life and Antioxidant Capacity of Obtained By-Products. Horticulturae 2023, 9, 635. https://doi.org/10.3390/horticulturae9060635
Carvalho DUd, Neves CSVJ, Cruz MAd, Colombo RC, Alferez F, Leite Junior RP. Effectiveness of Natural-Based Coatings on Sweet Oranges Post-Harvest Life and Antioxidant Capacity of Obtained By-Products. Horticulturae. 2023; 9(6):635. https://doi.org/10.3390/horticulturae9060635
Chicago/Turabian StyleCarvalho, Deived Uilian de, Carmen Silvia Vieira Janeiro Neves, Maria Aparecida da Cruz, Ronan Carlos Colombo, Fernando Alferez, and Rui Pereira Leite Junior. 2023. "Effectiveness of Natural-Based Coatings on Sweet Oranges Post-Harvest Life and Antioxidant Capacity of Obtained By-Products" Horticulturae 9, no. 6: 635. https://doi.org/10.3390/horticulturae9060635
APA StyleCarvalho, D. U. d., Neves, C. S. V. J., Cruz, M. A. d., Colombo, R. C., Alferez, F., & Leite Junior, R. P. (2023). Effectiveness of Natural-Based Coatings on Sweet Oranges Post-Harvest Life and Antioxidant Capacity of Obtained By-Products. Horticulturae, 9(6), 635. https://doi.org/10.3390/horticulturae9060635









