Differences in Lipid Metabolism, Polar Metabolites, and Phenolics in Persea americana under Two Storage Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Storage Conditions
2.2. Polar Metabolites
2.3. Fatty Acids
2.4. Phenolic Contents
2.5. Statistical Analysis
3. Results and Discussion
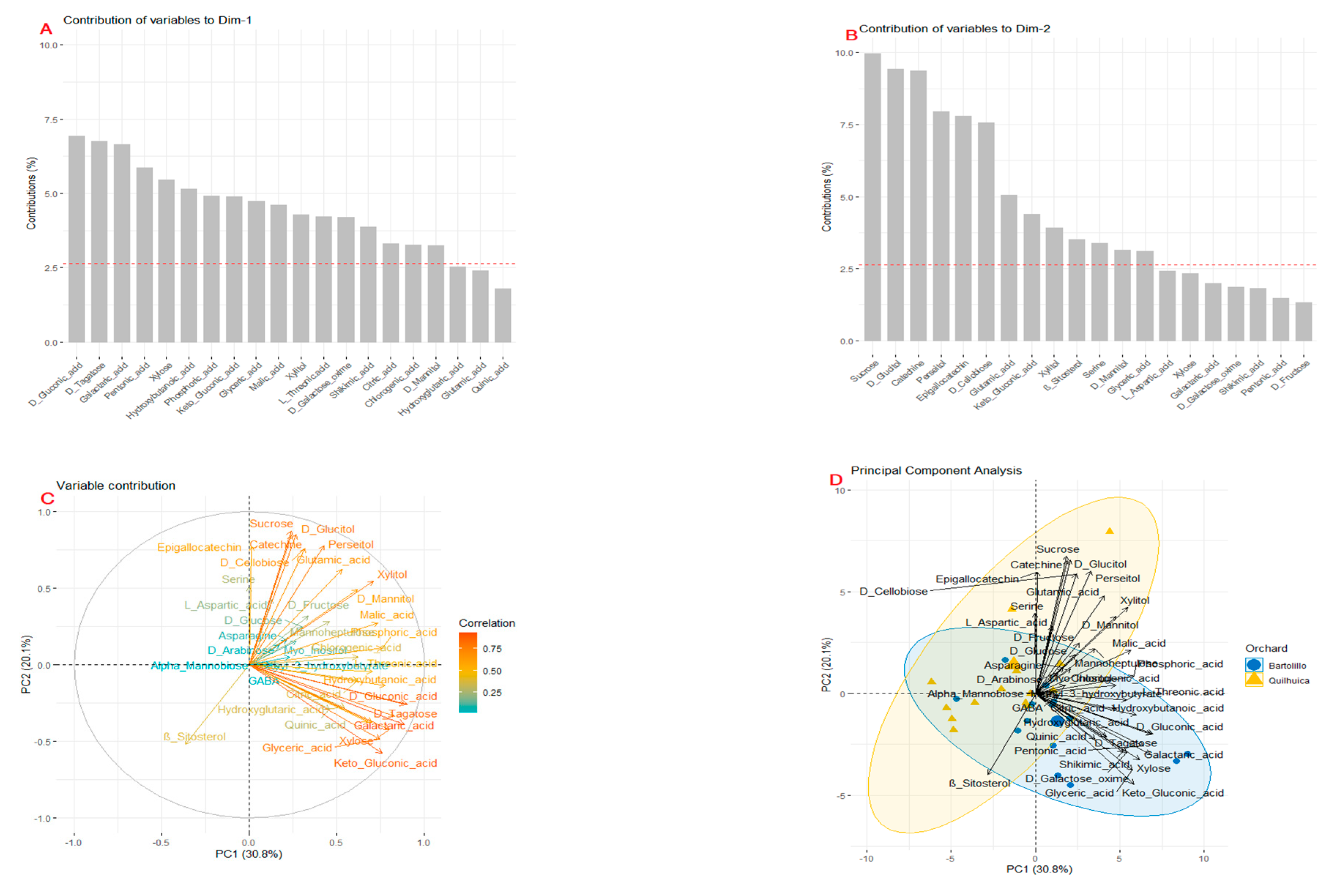
3.1. Polar Metabolites
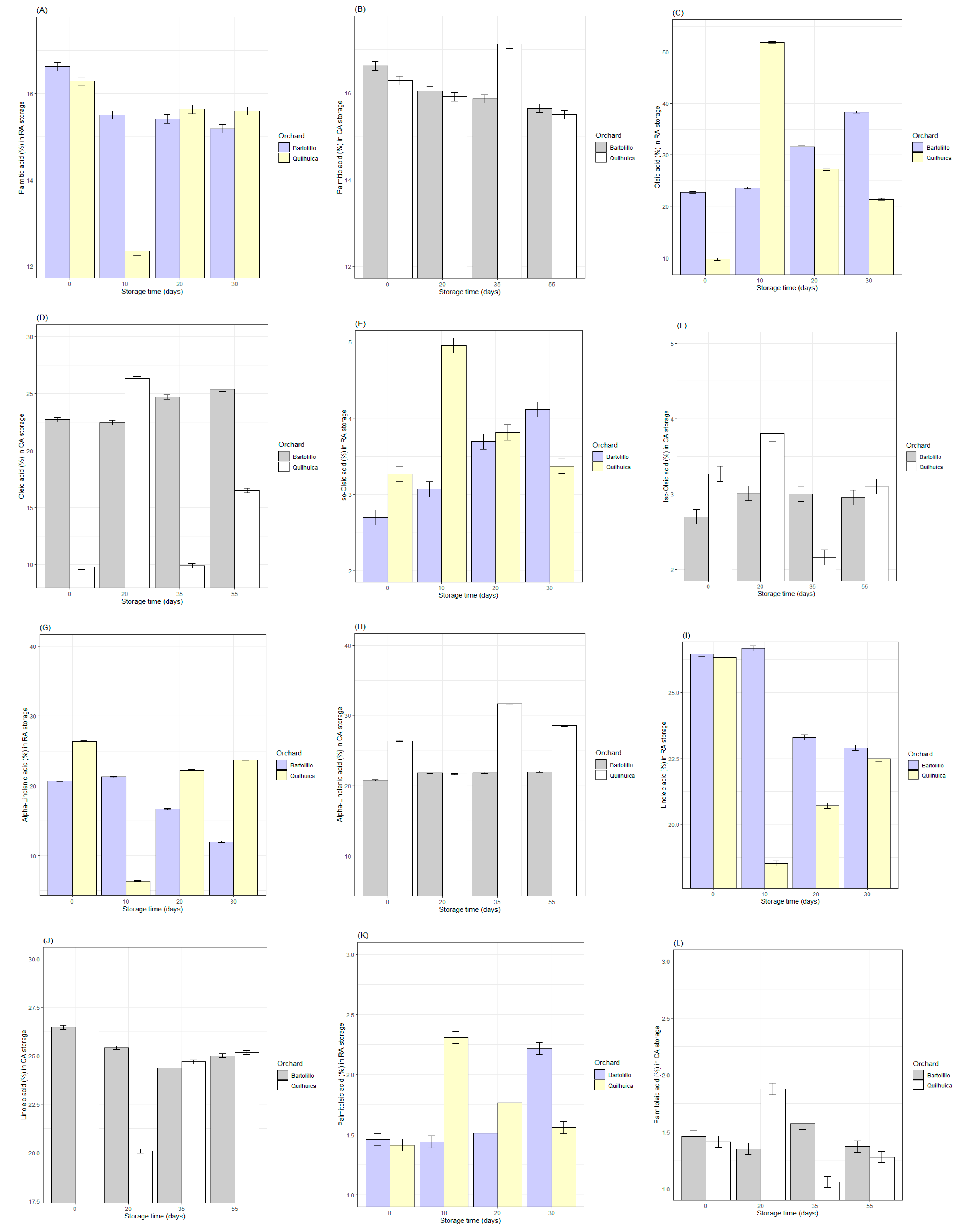
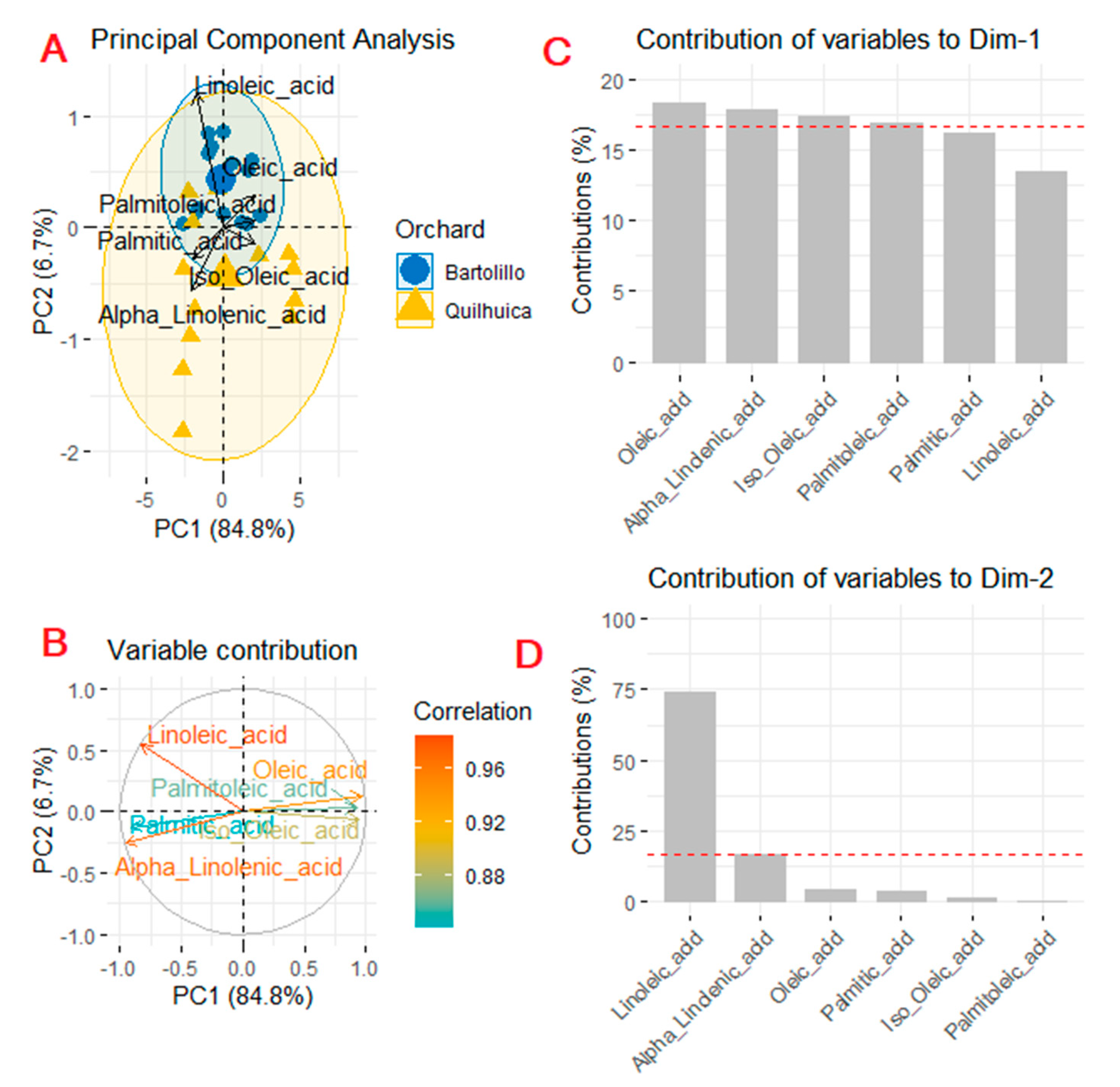
3.2. Fatty Acids
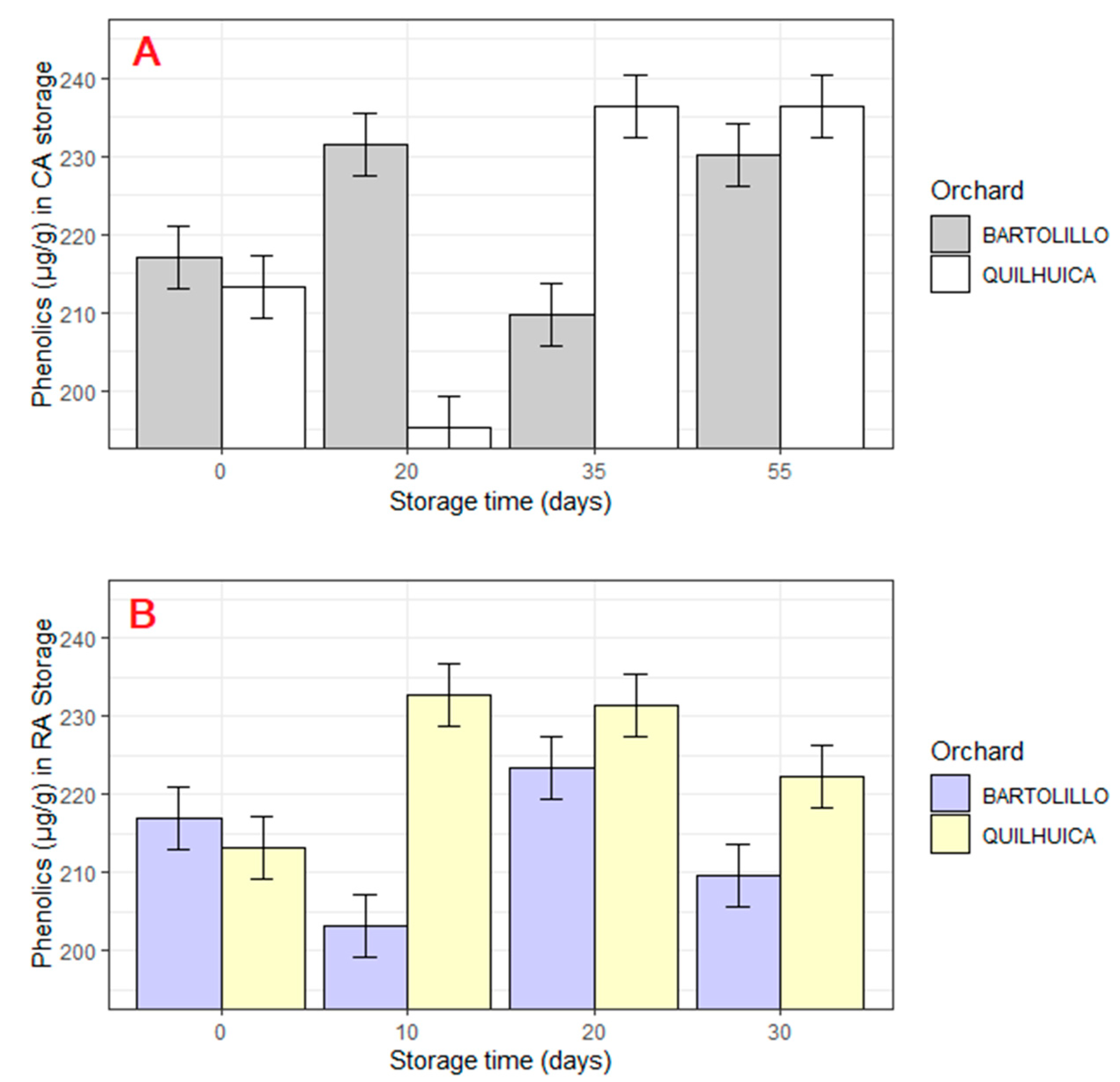
3.3. Phenolic Contents
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uarrota, V.G.; Pedreschi, R. Mathematical modelling of Hass avocado firmness by using destructive and nondestructive devices at different maturity stages and under two storage conditions. Folia Hortic. 2022, 34, 139–150. [Google Scholar] [CrossRef]
- Ramos-Aguilar, A.L.; Ornelas-Paz, J.; Tapia-Vargas, L.M.; Gardea-Béjar, A.A.; Yahia, E.M.; Ornelas-Paz, J.D.J.; Ruiz-Cruz, S.; Rios-Velasco, C.; Ibarra-Junquera, V. Comparative study on the phytochemical and nutrient composition of ripe fruit of Hass and Hass type avocado cultivars. J. Food Compos. Anal. 2021, 97, 103796. [Google Scholar] [CrossRef]
- Hernández, I.; Uarrota, V.; Paredes, D.; Fuentealba, C.; Defilippi, B.G.; Campos-Vargas, R.; Meneses, C.; Hertog, M.; Pedreschi, R. Can metabolites at harvest be used as physiological markers for modelling the softening behaviour of Chilean “Hass” avocados destined to local and distant markets? Postharvest Biol. Technol. 2021, 174, 111457. [Google Scholar] [CrossRef]
- Lindh, V.; Uarrota, V.; Zulueta, C.; Alvaro, J.; Valdenegro, M.; Cuneo, I.; Mery, D.; Pedreschi, R. Image Analysis Reveals That Lenticel Damage Does Not Result in Black Spot Development but Enhances Dehydration in Persea americana Mill. cv. Hass during Prolonged Storage. Agronomy 2021, 11, 1699. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Hernandez, I.; Ponce Guequen, E.; Vidal Cruz, J.; Fuentealba, C.; Defilippi, B.; Lindh, V.; Zulueta, C.; Chirinos, R.; Campos, D.; et al. Unravelling factors associated with ‘blackspot’ disorder in stored Hass avocado (Persea americana Mill) fruit. J. Hortic. Sci. Biotechnol. 2020, 95, 804–815. [Google Scholar] [CrossRef]
- Lu, Q.-Y.; Zhang, Y.; Wang, Y.; Wang, D.; Lee, R.-P.; Gao, K.; Byrns, R.; Heber, D. California Hass Avocado: Profiling of Carotenoids, Tocopherol, Fatty Acid, and Fat Content during Maturation and from Different Growing Areas. J. Agric. Food Chem. 2009, 57, 10408–10413. [Google Scholar] [CrossRef]
- Antunes, M.D.; Guimarães, A.C.; Gago, C.; Guerreiro, A.; Panagopoulos, J.; Vilas Boas, E.; Miguel, M.G. Membrane fatty acids and physiological disorders in cold stored ‘golden delicious’ apples treated with 1-MCP and calcium chloride. Horticulturae 2022, 8, 162. [Google Scholar] [CrossRef]
- Whitaker, B. Membrane lipid metabolism and oxidative stress involved in postharvest ripening, senescence, and storage disorders of fruits. Acta Hortic. 2012, 945, 269–282. [Google Scholar] [CrossRef]
- Calumpang, C.L.F.; Saigo, T.; Watanabe, M.; Tohge, T. Cross-Species Comparison of Fruit-Metabolomics to Elucidate Metabolic Regulation of Fruit Polyphenolics Among Solanaceous Crops. Metabolites 2020, 10, 209. [Google Scholar] [CrossRef]
- Liu, Z.; Rochfort, S. Recent progress in polar metabolite quantification in plants using liquid chromatography-mass spectrometry. J. Integr. Plant Biol. 2014, 56, 816–825. [Google Scholar] [CrossRef]
- Hatoum, D.; Annaratone, C.; Hertog, M.; Geeraerd, A.; Nicolai, B. Targeted metabolomics study of ‘Braeburn’ apples during long-term storage. Postharvest Biol. Technol. 2014, 96, 33–41. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Fuentealba, C.; Hernández, I.; Defilippi-Bruzzone, B.; Meneses, C.; Campos-Vargas, R.; Lurie, S.; Hertog, M.; Carpentier, S.; Poblete-Echeverría, C.; et al. Integration of proteomics and metabolomics data of early and middle season Hass avocados under heat treatment. Food Chem. 2019, 289, 512–521. [Google Scholar] [CrossRef]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Segatto, C.; Voytena, A.; Maraschin, M.; Avila, L.; Kazama, D.; Souza, C.A. Metabolic fingerprinting of water-stressed soybean cultivars by gas chromatography, near-infrared and UV-visible spectroscopy combined with chemometrics. J. Agron. Crop Sci. 2019, 205, 141–156. [Google Scholar] [CrossRef]
- Kosińska, A.; Karamać, M.; Estrella, I.; Hernández, T.; Bartolomé, B.; Dykes, G. Phenolic Compound Profiles and Antioxidant Capacity of Persea americana Mill. Peels and Seeds of Two Varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Drouin, N.; Rudaz, S.; Schappler, J. Sample preparation for polar metabolites in bioanalysis. Analyst 2017, 143, 16–20. [Google Scholar] [CrossRef]
- Nasri, C.; Halabi, Y.; Harhar, H.; Mohammed, F.; Bellaouchou, A.; Guenbour, A.; Tabyaoui, M. Chemical characterization of oil from four Avocado varieties cultivated in Morocco. OCL 2021, 28, 19. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Bajoub, A.; Morales, J.C.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Exploratory analysis of avocado extracts by GC-MS: New insights into the avocado fruit ripening process. Anal. Methods 2015, 7, 7318–7326. [Google Scholar] [CrossRef]
- Dreher, M.L.; Davenport, A.J. Hass Avocado Composition and Potential Health Effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- Yassin, N.; Shaaban, F.; Eletreby, S. Effect of postharvest thermal treatments on reducing external chilling injury in avocado‘fuerte’cv. Fruits. Egypt. J. Agric. Res. 2017, 95, 167–182. [Google Scholar] [CrossRef]
- Nahed, M.M.; Atta, A.A.A.; Awatif, I.I. Changes in the orgnoleptic characteristics and quality of olive oil during fruits storage at low temperature. Minufiya J. Agric. Res. 2011, 36, 829–843. [Google Scholar]
- Salas, J.J.; Martínez-Force, E.; Garcés, R. Biochemical characterization of a high-palmitoleic acid Helianthus annuus mutant. Plant Physiol. Biochem. 2004, 42, 373–381. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef] [PubMed]
- Daiuto, E.R.; Fumes, J.G.F.; Vieites, R.L.; Cabia, N.C.; Castro, R.S. Antioxidant capacity and total phenolic content of hydrothermally-treated Fuerte avocado. Adv. Hortic. Sci. 2011, 25, 75–80. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Yahia, E.M.; González-León, A.; Ifie, I.; Robles-Zepeda, R.E.; Domínguez-Avila, J.D.; Gonzá-lez-Aguilar, G. Ripening of ‘Hass’ avocado mesocarp alters its phytochemical profile and the in vitro cytotoxic ac-tivity of its methanolic extracts. South Afr. J. Bot. 2020, 128, 1–8. [Google Scholar] [CrossRef]
- Golukcu, M.; Ozdemir, F. Changes in phenolic composition of avocado cultivars during harvesting time. Chem. Nat. Compd. 2010, 46, 112–115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uarrota, V.G. Differences in Lipid Metabolism, Polar Metabolites, and Phenolics in Persea americana under Two Storage Conditions. Horticulturae 2023, 9, 234. https://doi.org/10.3390/horticulturae9020234
Uarrota VG. Differences in Lipid Metabolism, Polar Metabolites, and Phenolics in Persea americana under Two Storage Conditions. Horticulturae. 2023; 9(2):234. https://doi.org/10.3390/horticulturae9020234
Chicago/Turabian StyleUarrota, Virgilio Gavicho. 2023. "Differences in Lipid Metabolism, Polar Metabolites, and Phenolics in Persea americana under Two Storage Conditions" Horticulturae 9, no. 2: 234. https://doi.org/10.3390/horticulturae9020234
APA StyleUarrota, V. G. (2023). Differences in Lipid Metabolism, Polar Metabolites, and Phenolics in Persea americana under Two Storage Conditions. Horticulturae, 9(2), 234. https://doi.org/10.3390/horticulturae9020234





