Abstract
This study investigated the impact of various light qualities on the growth, photosynthesis, antioxidant capacity, anthocyanin accumulation and associated gene expression in purple lettuce. The results showed that stem diameter, leaf number and dry mass of purple leaves lettuce increased significantly under white light plus red and blue light (WRB) plus 10 µmol·m−2·s−1 UV-A (SUV1), plus 20 µmol·m−2·s−1 UV-A (SUV2) and plus 30 µmol·m−2·s−1 UV-A (SUV3) treatments compared to white light plus red and blue light (WRB). Leaf expansion decreased with increasing UV-A doses, while fresh leaf mass was higher under SUV1 and SUV2 treatments. Photosynthesis parameters were improved under WRB, SUV1 and SUV2 treatments, with an increase in net photosynthetic rate (Pn), stomatal conductance (Gs) and transpiration rate (Tr) and a decrease in intercellular carbon dioxide concentration (Ci) under SUV3 treatment. Superoxide radical generation rate, hydrogen peroxide and malondialdehyde (MDA) content and relative conductivity increased significantly under SUV3 treatment. Anthocyanin content increased significantly with increasing doses of UV-A treatment, while related structural gene expression levels were upregulated more significantly by SUV2 and SUV3 treatments than WRB treatment. In summary, moderate UV-A supplementation can enhance the antioxidant system and promote anthocyanin accumulation in purple lettuce. Specifically, WRB plus 20 µmol·m−2·s−1 UV-A (SUV2) is recommended as an optimal light recipe for cultivating purple lettuce in protected horticulture.
1. Introduction
Lettuce (Lactuca sativa L.) is one of the most widely cultivated vegetables under controlled environmental conditions due to its raw consumption as food, rapid growth rate, and varietal diversity. Compared to common green lettuce, purple lettuce is more popular among consumers, which is mainly related to its higher nutrition value and bright coloration. Light from ultraviolet (UV) radiation to the far-red spectrum has a significant effect on vegetable yield and quality [1,2]. Although there are many papers on the effects of various monochromatic light qualities on vegetable plant height, thick stems and anthocyanin [3,4,5], including green light, the mixed light of various monochromatic spectra seems to make plants grow better [6]. Johkan et al. [7] found that the leaf area and above-ground dry and fresh weight of lettuce were significantly increased under red and blue light treatments [8]. Furthermore, several studies have confirmed that the addition of red or blue wavelengths to white light facilitates the accumulation of pigments and biomass, and white light supplementing green wavelengths was efficient for soluble sugar accumulation in lettuce stem and leaf [9]. However, these studies mainly focus on the effect of the visible spectrum on plant growth, less information is known about the influence of UV light environments on vegetable quality. On the one hand, the lack of UV radiation in a controlled facility environment could cause a significant reduction in the anthocyanin content of purple lettuce [10]. On the other hand, the concentrations of quercetin and anthocyanin increased with the increase in UV radiation [11]. Generally, UV radiation can be divided to UV-A, UV-B, and UV-C according to the wavelength range. To date, plant responses to UV radiation have been the subject of intensive research as atmospheric ozone depletion has raised concerns about plant production and human health, while less attention has been devoted to the effects of UV-A on plants. Therefore, the light environment suitable for the growth of purple lettuce was studied.
UV light can act as a regulation factor that activates downstream signal pathways, but it is also a stress signal that induces reactive oxygen species (ROS), increases antioxidant enzyme activity, and promotes non-enzymatic antioxidant content [12]. Additionally, it has been found that the activity of the enzymes catalase (CAT) and glutathione peroxidase (GPX) catalyze the H2O2 scavenging reaction and increase SOD activity, while total phenolic content and DPPH radical scavenging capacity were also significantly improved after UV-B radiation supplementation [13,14]. Anthocyanins, which are one of the most important secondary metabolites in plants, are related to vegetable coloration development and flavor quality, but also have cardiovascular protection and antioxidant effects [15]. Several studies have indicated UV radiation is an effective light quality that induces anthocyanin synthesis, especially UV-A, which significantly promotes anthocyanin accumulation in hypocotyls by increasing phenylalanine ammonia lyase (PAL) and flavonoid galactosidase (UFGT) enzyme activity and upregulating the expression of the regulatory gene MYB75 and structural genes PAL (phenylalanine ammonia lyase), CHS (chalconesynthase), ANS (anthocyanin synthase) and UFGT (flavonoid galactosidase) [16].
At present, research on UV-LED chips and light sources has not yet reached commercial level, and there are still some problems with artificially supplementing ultraviolet light applied to greenhouse production, especially UV-B and UV-C. The damaging effects of UV-B and UV-C are well documented [17,18]. Higher doses of UV-B easily produce ROS in a short period of time [19] that exceeds the plant’s antioxidant capacity, leading to programmed cell death and yield reduction [12,20]. In addition, UV-B is liable to damage human skin, eyes, and the immune system [21]. However, UV-A is a relatively “milder” stress for plants, it is less damaging to vegetables, while also promoting plant growth and improving secondary metabolites and antioxidant capacity [22]. UV-A radiation supplementation may be more suitable than UV-B and UV-C in controlled environmental conditions, including artificial UV-A light sources in plant factories. In previous research, it was found that the addition of 4:1 red and blue light significantly promoted the growth of purple lettuce and increased carbohydrate accumulation [23], while the change in lettuce quality and the appropriate UV-A dose is unclear. Therefore, the growth, antioxidant system and anthocyanin synthesis of purple lettuce was investigated under 4:1 red and blue background light substitution with varying UV-A doses. This study aimed to identify the optimal spectra for purple lettuce production in controlled environment condition.
2. Materials and Methods
2.1. Plant Materials and Treatments
We used purple lettuce (Lactuca sativa L. cv ‘Zhongshu Purple Lettuce’) as plant material, an LED intelligent light control console (UH-TGT300B, Unihero, Guangzhou, China) as the light source, containing red light, blue light, ultraviolet light (380–400 nm) and white light. The light beads of each light quality were evenly distributed, and the light intensity and photoperiod could be separately regulated.
The experimental design was a random block design, including five light recipe treatments: W is white light, WRB is white light plus red and blue light, SUV1 is WRB plus 10 µmol·m−2·s−1 UV-A, SUV2 is WRB plus 20 µmol·m−2·s−1 UV-A, and SUV3 is WRB plus 30 µmol·m−2·s−1 UV-A (as shown in Table S1 and S2). The experiment was carried out in the Science and Technology Innovation Park of Shandong Agricultural University from June to December 2018. The seeds were sown in plastic trays (55 × 27 × 5 cm) containing a mixture of peat, perlite and vermiculite, which were subsequently placed in a greenhouse. When the seedlings grew to three true leaves, they were transplanted into a plastic pot with a diameter of 12 cm and a height of 10 cm. When the seedlings grew to 5 true leaves, they were moved to the phytotron (Qiushi Environment, Hangzhou, China) for treatment, and 12 plants were placed under each light control console with a total photosynthetic photon flux density (PPFD) of 300 μmol·m−2·s−1. After 25 days of different light quality treatments, random samples were taken to determine the photosynthesis and fluorescence indices, each treatment was repeated five times and the average value was taken. Samples were taken after 30 days of different light quality treatments to determine the content of substances and related enzyme activities. Five plants were randomly selected from each treatment, and the 5th leaf was taken from the core outward as sample material. The PPFD was measured using a luminometer (3415FX, Spectrum Technologies, Aurora, IL, USA). The spectrum of each treatment was measured using a spectrum analyzer (UNISPECTM-DC, PP-Systems, Amesbury, MA, USA). The environmental conditions of the phytotron: day temperature (25 ± 1) °C, night temperature (18 ± 1) °C, air relative humidity 70–80%, and photoperiod 12 h·d−1, DLI was 12.96 mol·m−2·d−1.
2.2. Growth and Photosynthesis Parameters
Stem diameter was measured at the base of the stem using a caliper. Plant expansion was measured above the widest canopy using a ruler. The fresh mass of leaves was measured by an electronic scale, then they were dried at 70 °C in an oven for 3 days to determine the dry mass.
Scanning electron microscopy of paraffin sections made from lettuce leaves: the leaves were cut into 0.5 cm × 0.5 cm pieces and placed in formalin–acetic acid–ethanol (FAA) fixative for more than 24 h, dehydrated and embedded using a microtome (RM2016, Leica, Shanghai, China). A semi-thin section was prepared and fixed on a glass slide, then sealed with a neutral resin. Finally, a stereoscopic examination was performed using an upright scientific microscope (Eclipse Ci-E, Nikon, Tokyo, Japan), and image acquisition was performed using a digital microscope imaging system (DS-Ri1-U3, Nikon, Japan).
After 25 days of different light quality treatments, the fourth or fifth leaf counted outward from the heart was selected to determine leaf photosynthesis parameters using the LI-6400 portable photosynthesis system (Li-Cor 6400XT, Lincoln, NE, USA).
2.3. ROS Accumulation and Stability of Membranes
The superoxide radical (O2·−) generation rate was determined by monitoring the formation of NO2− from hydroxylamine in the presence of O2·− following the method of Wang and Luo (1990). The contents of H2O2 production were measured colorimetrically, and the absorbance at 410 nm was measured using an ultraviolet spectrophotometer (F-100, Metash, Shanghai, China), and the amount of H2O2 was calculated using a prepared standard curve. The degree of membrane damage was determined by measuring the MDA content. The difference in absorbance at 532 and 600 nm was measured and calculated, and the MDA concentration in the extract was determined in terms of MDA’s millimolar extinction coefficient of 155. The relative conductivity was determined when the sample was immersed for 12 h, the conductance of the extract (R1) was measured by a conductivity meter (DDBJ-350, INESA, Shanghai, China), then heated in a boiling water bath for 30 min, cooled to room temperature, shaken, and the conductance of the extract was again measured (R2). Relative conductivity = R1/R2 × 100%.
2.4. Enzymatic Antioxidant Measurement
The photoreduction of nitro blue tetrazolium (NBT) was used to estimate superoxide dismutase (SOD; EC 1.15.1.1) activity. One unit (U) of SOD was defined as the amount of enzyme required to inhibit photochemical reduction of NBT to 50%. Peroxidase (POD; EC 1.11.1.7) activity was measured, because POD can decompose hydrogen peroxide, and the absorbance (A240) of the reaction solution decreases with the reaction time. A240 decreases by 1 in 1 min as a POD activity unit. Catalase (CAT; EC 1.11.1.6) activity was also determined. In the presence of H2O2, peroxidase can oxidize guaiacol to form quinonoids, A470 increases by 1 in 1 min as a CAT activity unit. Ascorbate peroxidase (APX; EC 1.11.1.11) activity was assayed by monitoring the decrease in absorbance at 290 nm using an extinction coefficient of 2.8 mM−1cm−1. One unit of enzyme activity is defined as 1 µmol AsA reduced min−1. Dehydroascorbate reductase (DHAR; EC 2.5.1.18) activity assay was performed. The enzyme activity was calculated by measuring dehydroascorbate (DHA) reduction rate and one unit (U) of DHAR activity is defined as 1 nmol ascorbate restored min−1. Glutathione reductase (GR; EC 1.6.4.2) activity was measured using modification of the procedure of Foyer and Halliwel (1976) by following the decrease in absorption at 340 nm due to NADPH oxidation. One unit (U) of GR activity is defined as 1 nmol NADPH oxidized min−1.
2.5. Non-Enzymatic Antioxidant and Free Radical Scavenging Activity Measurement
Reduced ascorbate (AsA) and oxidized ascorbate (DHA) were determined. AsA can reduce Fe3+ into Fe2+, and Fe2+ reacts with 2,2′-bipyridyl to form red chelate. The intensity of red color developed was recorded at 534 nm, and the amount of AsA was calculated using a standard curve prepared with (AsA), and the results are expressed in µg (AsA) g−1 FW. Dithiothreitol (DTT) reduces DHA to produce AsA and calculates DHA content by measuring AsA production rate. Reduced (GSH) and oxidized glutathione (GSSG) contents were determined by the method of Brehe and Burch [24]. 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) reacts with GSH to form a complex with a characteristic absorption peak at 412 nm to calculate the GSH and GSSG content. Total phenol and flavonoid contents were quantified according to the methodology reported by [25]. Under alkaline conditions, the phenolic substance reduces the tungsten molybdic acid to form a blue compound with a characteristic absorption peak at 760 nm, and the total phenol content is calculated based on the absorbance. In an alkaline nitrite solution, the flavonoid forms a red complex with aluminum ions with a characteristic absorption peak at 502 nm, and the flavonoid content is calculated based on the absorbance. DPPH radical scavenging capacity was measured according to [26] with some modifications. The absorbance was measured at 517 nm with ethanol as the blank, the absorbance (Ao) of the DPPH solution without the sample, the absorbance (Ai) of the DPPH solution after the sample was added, the absorbance (Aj) of the sample itself, and the radical scavenging rate = [1 − (Ai − Aj)/Ao] × 100.
2.6. Anthocyanin Content
Determination of anthocyanin content: accurately weigh 0.2 g of the shredded tissue leaves, add 10 mL of 2% hydrochloric acid methanol solution, immerse in the dark at room temperature for 2 h, until the leaf tissue is completely whitened and filtered. The volume was adjusted to a 50 mL volumetric flask with a 2% hydrochloric acid methanol solution, and the absorbance was measured at a wavelength of 530 nm to calculate the anthocyanin content.
2.7. Gene Expression
Total RNA was extracted using the Trizol reagent (Invitrogen, Gaithersburg, MD, USA) based on the manufacturer’s instructions. Agarose gel electrophoresis was used to determine the integrity of the RNA and then reverse transcribed into cDNA. Because the target gene sequence information in purple lettuce has not been published, each gene sequence was queried in a near-source species lettuce, and the bHLH and DFR genes were selected for amplification verification, and to design and synthesize primers, as shown in Table S3. Using CK1 cDNA as a template, high-fidelity enzyme was used for amplification, and the PCR products were sequenced.
RT-qPCR with three technical repetitions was performed using a LightCycler® 96 real-time PCR system (Roche, Basel, Switzerland). The relative expression level of the genes was calculated using the 2−ΔΔCT method. The gene-specific primers used are shown in Table S4.
2.8. Statistical Analyses
The statistical analyses were carried out using DPS software 17.10 (Zhejiang University, Hangzhou, China). All the data were tested for significant treatment differences using Duncan’s multiple range test (α = 0.05) and were plotted using OriginPro 2017 software. The data in the graphs are mean ± standard deviation. All experiments were performed with three biological replicates.
3. Results
3.1. Growth
Purple lettuce growth was significantly affected by the different light quality treatments (Table 1). Compared to the WRB (white light mixed with red and blue light), stem diameter and dry mass of purple leaves lettuce increased significantly under the SUV1 and SUV2 treatments, and stem diameter was significantly higher under the SUV2 treatment than under the other treatments. Leaf expansion decreased progressively with increasing UV-A dose, while it was greatest under the WRB treatment. Fresh leaf mass was significantly higher under SUV1 and SUV2 treatments than under the SUV3 treatment. Using scanning electron microscopy to observe paraffin sections with different treatments, UV-A substitution significantly increased leaf thickness compared to WRB, and thickness increased with increasing UV-A doses, as can be seen from Figure 1B.

Table 1.
Effects of different doses of UV-A on the growth of purple lettuce.
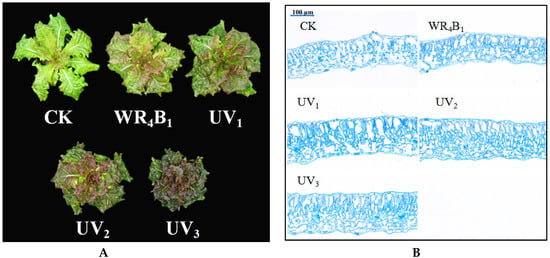
Figure 1.
Effects of light recipes on morphology and leaf thickness of purple lettuce. Pictures in (A) were taken at 25 d from the start of light treatments. (A) Growth of purple lettuce; (B) leaf thickness of purple lettuce (bar scales of leaf thickness of all treatments were 100 μm).
3.2. Photosynthesis Parameters
Photosynthesis parameters are important indexes for assessing photosynthesis capacity in plant. From Figure 2, compared with the W (CK), the stomatal conductance (Gs) and transpiration rate (Tr) increased significantly under WRB, SUV1 and SUV2 treatments, and the net photosynthetic rate (Pn) increased by 21%, 17% and 18%, respectively, while the (Ci) decreased. Additionally, the Gs, Ci and Tr increased significantly under WRB, SUV1, and SUV2 compared with those of W and SUV3. The SUV3 treatment decreased significantly the Gs and Ci, while they were same in the Pn and Tr compared with the W.
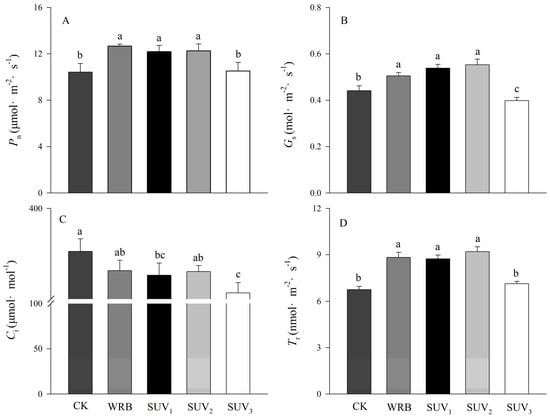
Figure 2.
Effects of light recipes on gas exchange parameters of purple lettuce. (A) Net photosynthetic rate (Pn); (B) stomatal conductance (Gs); (C) intercellular carbon dioxide concentration (Ci); (D) transpiration rate (Tr). Data are means ± SD (n = 3). Different lower-case letters above bars indicate significant differences between treatments (Duncan’s multiple range test, p < 0.05).
3.3. ROS Accumulation and Stability of Cell
Superoxide radical (O2·−) generation rate, hydrogen peroxide (H2O2) content, malondialdehyde (MDA) content and relative conductivity in purple lettuce leaves are described in Figure 3. The O2·− generation rate and H2O2 content under WRB were similar to those under the W, while the addition of medium and high doses of UV-A significantly increased the O2·− generation rate and H2O2 content compared with those of the WRB treatment. In addition, the MDA content and the relative conductivity under SUV2 and SUV3 were higher than the WRB, while there was no different significantly under the WRB and SUV1. Superoxide radical (O2·−) generation rate, hydrogen peroxide (H2O2) content, malondialdehyde (MDA) content and relative conductivity in purple lettuce leaves increased with increasing UV-A dose.
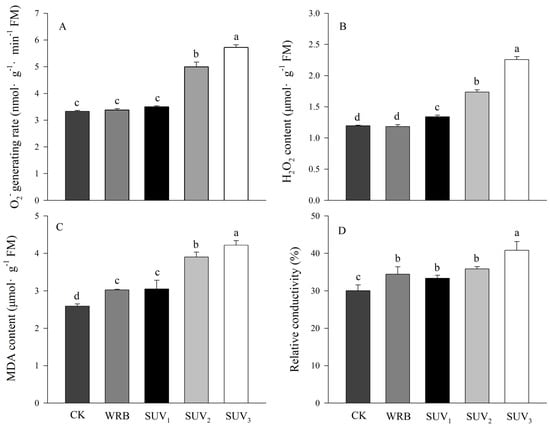
Figure 3.
Effects of light recipes on ROS accumulation and stability of membranes of purple lettuce. (A) Superoxide radical generation rate (O2·−); (B) hydrogen peroxide (H2O2); (C) malondialdehyde (MDA); (D) relative conductivity. Different lower-case letters above bars indicate significant differences between treatments (Duncan’s multiple range test, p < 0.05).
3.4. Enzymatic Antioxidant Mechanism
The antioxidant enzyme activity of the WRB treatment was not significantly different from the W, except CAT and GR activities, which were significantly higher than those of the W. Compared with the WRB, except APX activity, SUV2 and SUV3 treatments significantly stimulated the improvement of antioxidant enzyme activity in purple lettuce (Figure 4A–F). Additionally, compared with the WRB, SOD activity increased by 26% and 36% (Figure 4A), POD activity increased by 24% and 41% (Figure 4B), CAT activity increased by 31% and 53% (Figure 4C), DHAR activity increased by 29% and 107% (Figure 4D), GR activity increased by 30% and 56% under SUV2 and SUV3 treatments (Figure 4E). It is worth mentioning that the POD, CAT, APX activity under SUV1 were significantly higher that the WRB treatments; however, the trend was not consistent with the SOD and DHAR.
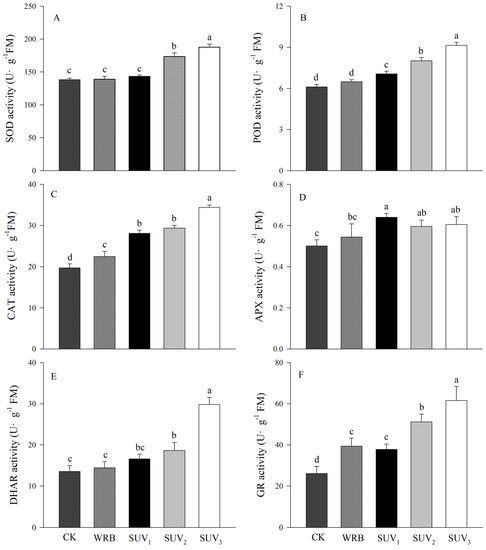
Figure 4.
Effects of light recipes on antioxidase activity of purple lettuce. (A) Superoxide dismutase (SOD); (B) peroxidase activity (POD); (C) catalase activity (CAT); (D) ascorbate peroxidase activity (APX); (E) dehydroascorbate reductase activity (DHAR); (F) glutathione reductase activity (GR). Different lower-case letters above bars indicate significant differences between treatments (Duncan’s multiple range test, p < 0.05).
3.5. Non-Enzymatic Antioxidant Mechanism and Free Radical Scavenging Activity
As shown in Table 2, the addition of UV-A light significantly enhanced the ASA and GSH content in purple lettuce. The ASA content of SUV1, SUV2 and SUV3 treatments increased by 32%, 40% and 67%, respectively, compared with WRB treatment, and the GSH content increased by 3%, 31% and 46%, respectively. The ASA/DHA content was also improved compared with WRB treatment. The GSH/GSSG content was similar to that of the WRB treatment, but significantly higher than that of the W. Compared to WRB, the total phenol and flavonoids content and DPPH radical scavenging capacity were significantly improved under SUV2 and SUV3, and increased with increasing UV-A dose.

Table 2.
Effects of light recipes on non-enzymatic antioxidant mechanism and free radical scavenging activity of purple lettuce.
3.6. Anthocyanin Content and Synthesis-Related Structural Gene Expression
The determination of anthocyanin content in purple lettuce showed that the anthocyanin content increased significantly with increasing UV-A dose (Figure 5). Compared to the W, the WRB treatment increased the expression of related structural genes to varying degrees, and the addition of UV-A treatment further upregulated the related genes. SUV2 and SUV3 treatments increased the expression of related structural genes more significantly. The expression levels of PAL, CHS, CHI, DFR, ANS and UFGT under SUV2 treatment increased by 46%, 65%, 61%, 52%, 102%, 86% and 29%, respectively, compared to WRB treatment.
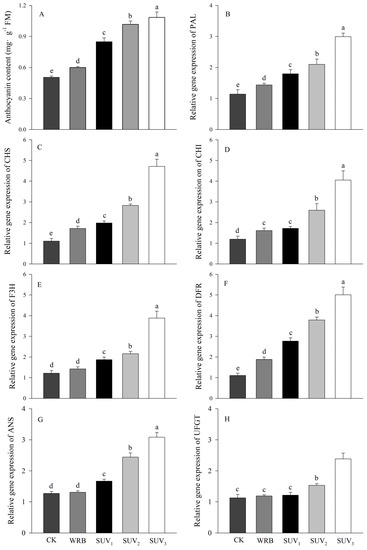
Figure 5.
Effects of light recipes on anthocyanin content and synthesis related structural gene expression of purple lettuce. (A) Anthocyanin content; (B) phenylalanine ammonia lyase (PAL); (C) chalconesynthase (CHS); (D) chalcone isomerase (CHI); (E) key enzymes of flavonoid synthesis pathway (F3H); (F) dihydroflavonol reductase (DFR); (G) anthocyanin synthase (ANS); (H) flavonoid galactosidase (UFGT). Different lower-case letters above bars indicate significant differences between treatments (Duncan’s multiple range test, p < 0.05).
4. Discussion
4.1. UV-A Could Improve Growth via Enhancing Photosynthesis
The crop growth and photosynthesis capacity play important roles in the accumulation of matter and production, which are affected by internal signals and environmental factors [27]. In this study, the growth characteristics and photosynthesis parameters were measured under different light wavelengths in purple lettuce. The results suggested that WRB, SUV1 and SUV2 treatments could significantly increase the dry weight and stem diameter, and also Pn, Gs, and Tr in purple lettuce compared with the W (Table 1; Figure 2). Therefore, the substitution of red and blue light and UV-A radiation based on white light was beneficial to lettuce growth. Kang et al. [28] showed that substituting 8 and 16 h of UV-A to visible radiation (400–700 nm) stimulated plant biomass production by 29% and 33%, respectively, compared with that of control in tomato seedlings. However, the leaf coloration of purple lettuce was brighter under UV-A substitution than WRB (Figure 1), which is more popular among consumers.
It is worth noting that lettuce plant expansion was significantly decreased with different doses of UV-A substitution compared to with WRB (Table 1), and dry weight, fresh weight, Gs and Ci were lowest under the SUV3 treatment in lettuce. This indicates that medium and low doses of UV-A increase the photosynthetic capacity of leaves, but photosynthetic capacity and growth could be inhibited at high UV-A doses. Some studies have shown that UV-A impairs photosynthesis in plants, as it induces degradation of the D1 and D2 protein subunits of the PSII reaction center, thereby reducing Rubisco activity and affecting photosynthetic efficiency [29,30].
4.2. UV-A Could Promote Antioxidant Capacity and Increase Anthocyanin Content
There is ample evidence that the addition of UV radiation induces the accumulation of ROS. However, in order to restore normal cell homeostasis and function, plants can reduce ROS levels through enzymatic and non-enzymatic mechanisms [31]. In the present study, we noted an increase in H2O2 content after enhanced UV-A radiation, with correlated increases in SOD activity. This indicated that under UV-A radiation, hydrogen peroxide accumulation is formed significantly and intensively as a result of enzymatic dismutation of superoxide anion. In recent studies, there has been a growing tendency to determine the role of H2O2 as a signaling molecule that mediates plant responses to abiotic stress [32]. The accumulation of H2O2 induced an increase in the antioxidant enzyme activity of the catalytic hydrogen peroxide scavenging reactions such as POD and CAT. In addition, AsA-GSH cycle enzymes and its metabolites play a key role in scavenging ROS, thus preventing oxidative damage to biomolecules. The addition of UV-A radiation significantly increased ASA and GSH levels, thereby alleviating oxidative stress. AsA/DHA and GSH/GSSG can reflect the redox state of cells. Under UV-A radiation, AsA/DHA is higher in purple lettuce, while GSH/GSSG is relatively stable, which is beneficial for ROS removal. The increase in DHAR and GR enzyme activity is also a key factor in promoting ASA and GSH levels. However, we also found that, although the APX activity under the high-dose UV-A treatment was higher than that without the addition of UV light, it did not increase with increasing H2O2 content. This may be explained by the fact that the unique circular structure of chloroplast APX makes it very sensitive to H2O2, while excess H2O2 inactivates APX [33]. In general, however, the addition of a suitable dose of UV-A radiation ensures that the cells are in a good redox state, the ASA-GSH circulatory system is stable, the efficiency of the antioxidant defense system is enhanced. Phenolic compounds are produced by plant secondary metabolism and enhance in antioxidant resistance by acting directly as an active ROS scavenger or as a complement to antioxidant enzymes. Flavonoids are the main phenolic substances involved in the response of plants to ultraviolet light, and most flavonoids have a high absorption rate in the UV-A range (315–400 nm) and can absorb free radicals generated by ultraviolet radiation [34,35]. In this study, UV-A radiation induced an increase in the total phenolic and flavonoid content of lettuce, which is consistent with previous results from research on pakchoi microgreens and blueberries [16,36], which was also a positive addition to the antioxidant capacity of purple lettuce. The structural genes PAL and CHS encoding the phenylalanine and flavonoid pathways are upregulated by UV radiation (Figure 5). This is the same result as in the study by [37]. Transcription factors related to structural genes involved in the synthesis of phenolic substances are activated by UV radiation.
4.3. UV-A Supplementation Induces Upregulation of Structural Genes Related to Anthocyanin Biosynthesis in Purple Lettuce
Compared to common lettuce, purple lettuce is characterized by its high anthocyanin content in leaves. The increase in anthocyanin content in purple lettuce under 4:1 red-to-blue light supplementation can be attributed mainly to the sucrose content. Red light enhances sucrose anabolism, which is a crucial raw material for anthocyanin synthesis and promotes the accumulation of anthocyanins by regulating the expression of synthesis-related genes such as CHS and DFR (Figure 5) [38,39]. Furthermore, UV-A irradiation significantly improves both the anthocyanin content and synthetic structural gene expression in purple lettuce. Studies have confirmed that UV light induces the accumulation of anthocyanin in many species [16,40,41], but there are different regulation mechanisms between UV-B and UV-A on anthocyanin, with this discussion focusing mainly on UV-A induction reaction. Most research has shown that cryptochrome plays a role in UV-induced anthocyanin synthesis. The working model in [42] established photoinduced anthocyanin synthesis under cryptochrome during eggplant research, suggesting that interaction with activated cryptochromes represses COP1 function allowing downstream transcription factors HY5 and MYB1 to accumulate. The promoters of structural genes CHS and DFR directly bind to HY5 and MYB1 inducing biosynthesis of anthocyanins. Moreover, a recent study in blueberries found that UV-A irradiation significantly promoted their accumulation of anthocyanin contents [36]. In addition, a study on gene expression profiles of radish root epidermis under exposure to both Blue Light and UV-A radiation found that UV-A specifically induced CHS, DFR, and ANS expression [43]. These findings suggest the possibility of a new UV-A specifically induced light signal transduction pathway regulating anthocyanin synthesis. Additionally, reactive oxygen species may play an important role in UV-induced anthocyanin synthesis. For instance, 340 nm of UV-A can induce ROS and anthocyanin accumulation in radish sprouts; exogenous hydrogen peroxide significantly promotes anthocyanin content in radish sprouts under UV-B radiation [44,45], which is consistent with our findings, indicating that as a light signal, UV can not only induce the expression of structural genes producing anthocyanin but also produce ROS together with ROS signals to regulate their accumulation.
5. Conclusions
In conclusion, the growth and quality indicators of purple lettuce significantly improved under white, red, and blue ratios of light quality treatments compared to white light treatment. Furthermore, WRB plus UV-A did not significantly impair vegetative growth, but instead promoted an increase in antioxidant enzyme activity and non-enzymatic antioxidant content by promoting ROS production. The UV-A light signal can regulate the expression of anthocyanin biosynthesis structural genes and significantly promote the accumulation of anthocyanins in purple lettuce. Thus, it can be concluded that WRB plus 20 µmol·m−2·s−1 UV-A can be recommended as a special recipe for growing purple lettuce in protected horticulture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9060634/s1, Table S1: Light recipes and their photosynthetic photon flux density (PPFD, µmol·m-2·s-1); Table S2: Analysis of the LED light spectrum under different intensities of UV-A (%); Table S3: Design and synthesize primers; Table S4: Primer sequences of RT-qPCR.
Author Contributions
Conceptualization, Y.Z. and Q.L.; investigation, H.Q., B.L. and Y.G.; writing, H.Q., Y.X. and Y.G.; supervision, project administration, Y.Z. and Q.L.; funding acquisition, Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agricultural Science and Technology Innovation Program (ASTIP-CAAS, 34-IUA-03); the Elite Youth Program of the Chinese Academy of Agricultural Sciences (NKYCQN-2021-059); the Sichuan Province Key Technology R&D Program (22ZDYF0234), Local Financial Funds of National Agricultural Science and Technology Center, Chengdu (NASC2022KR01; NASC2023ST06).
Data Availability Statement
The associated data set for the study is available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ilic, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Olle, M.; Virsile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kjaer, K.H.; Adnan, M.; Naznin, M.T.; Lim, J.D.; Sung, I.J.; Park, C.H.; Lim, Y.S. The Evaluation of Growth Performance, Photosynthetic Capacity, and Primary and Secondary Metabolite Content of Leaf Lettuce Grown under Limited Irradiation of Blue and Red LED Light in an Urban Plant Factory. Agriculture 2020, 10, 28. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, J.E.; Oh, M.M. Growth and phenolic compounds of Lactuca sativa L. grown in a closed-type plant production system with UV-A,-B, or-C lamp. J. Sci. Food Agric. 2014, 94, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Yang, Q.; Li, T.; Cheng, R.; Barnett, Y.; Lu, C. Study of the beneficial effects of green light on lettuce grown under short-term continuous red and blue light-emitting diodes. Physiol. Plant. 2018, 164, 226–240. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-n.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Claypool, N.; Lieth, J. Physiological responses of pepper seedlings to various ratios of blue, green, and red light using LED lamps. Sci. Hortic. 2020, 268, 109371. [Google Scholar] [CrossRef]
- Chen, X.-l.; Xue, X.-z.; Guo, W.-z.; Wang, L.-c.; Qiao, X.-j. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Shioshita, R.; Enoka, J.; Aiona, D.K.; Wall, M. Coloration and growth of red lettuce grown under UV-radiation transmitting and non-transmitting covers. In Proceedings of the XXVII International Horticultural Congress-IHC2006: International Symposium on Advances in Environmental Control, Automation, Seoul, Republic of Korea, 13–19 August 2006; Volume 761, pp. 221–225. [Google Scholar]
- García-Macías, P.; Ordidge, M.; Vysini, E.; Waroonphan, S.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Wagstaffe, A. Changes in the flavonoid and phenolic acid contents and antioxidant activity of red leaf lettuce (Lollo Rosso) due to cultivation under plastic films varying in ultraviolet transparency. J. Agric. Food Chem. 2007, 55, 10168–10172. [Google Scholar] [CrossRef]
- Hideg, É.; Jansen, M.A.; Strid, Å. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Balakumar, T.; Gayathri, B.; Anbudurai, P. Oxidative stress injury in tomato plants induced by supplemental UV-B radiation. Biol. Plant. 1997, 39, 215–221. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Mendes, R.; Sario, S.; De Oliveira, J.F.; Melo, P.; Santos, C. Tomato plants use non-enzymatic antioxidant pathways to cope with moderate UV-A/B irradiation: A contribution to the use of UV-A/B in horticulture. J. Plant Physiol. 2018, 221, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, M.; Liu, Y.; Ye, Q.; Gu, J.; Luo, G. Isolation and identification of antioxidant compounds from gynura bicolor stems and leaves. Int. J. Food Prop. 2016, 19, 233–241. [Google Scholar] [CrossRef]
- Mao, P.; Duan, F.; Zheng, Y.; Yang, Q. Blue and UV-A light wavelengths positively affected accumulation profiles of healthy compounds in pak-choi. J. Sci. Food Agric. 2021, 101, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.R.; Lee, M.H.; Tovuu, A.; Lee, C.H.; Chung, B.Y.; Park, Y.I.; Kim, J.H. Acute exposure to UV-B sensitizes cucumber, tomato, and Arabidopsis plants to photooxidative stress by inhibiting thermal energy dissipation and antioxidant defense. J. Radiat. Res. 2011, 52, 238–248. [Google Scholar] [CrossRef]
- Gao, C.; Xing, D.; Li, L.; Zhang, L. Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta 2008, 227, 755–767. [Google Scholar] [CrossRef]
- Kalbina, I.; Strid, A. The role of NADPH oxidase and MAP kinase phosphatase in UV-B-dependent gene expression in Arabidopsis. Plant Cell Environ. 2006, 29, 1783–1793. [Google Scholar] [CrossRef]
- Jenkins, G.I.; Long, J.C.; Wade, H.K.; Shenton, M.R.; Bibikova, T.N. UV and blue light signalling: Pathways regulating chalcone synthase gene expression in Arabidopsis. New Phytol. 2001, 151, 121–131. [Google Scholar] [CrossRef]
- Lucas, R.M.; Norval, M.; Neale, R.E.; Young, A.R.; de Gruijl, F.R.; Takizawa, Y.; van der Leun, J.C. The consequences for human health of stratospheric ozone depletion in association with other environmental factors. Photochem. Photobiol. Sci. 2015, 14, 53–87. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.H. Growth and Anthocyanins of Lettuce Grown under Red or Blue Light-emitting Diodes with Distinct Peak Wavelength. Korean J. Hortic. Sci. Technol. 2014, 32, 330–339. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.Y.; Piao, F.Z.; Sun, Z.Q. Effects of different LED sources on the growth and nitrogen metabolism of lettuce. Plant Cell Tissue Organ Cult. 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Brehe, J.E.; Burch, H.B. Enzymatic assay for glutathione. Anal. Biochem. 1976, 74, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Harkat-Madouri, L.; Asma, B.; Madani, K.; Said, Z.B.-O.S.; Rigou, P.; Grenier, D.; Allalou, H.; Remini, H.; Adjaoud, A.; Boulekbache-Makhlouf, L. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Ind. Crops Prod. 2015, 78, 148–153. [Google Scholar] [CrossRef]
- Mitchell, C.A. History of Controlled Environment Horticulture: Indoor Farming and Its Key Technologies. Hortscience 2022, 57, 247–256. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, Y.T.; Zhang, Y.Q.; Zou, J.; Yang, Q.C.; Li, T. Ultraviolet-A Radiation Stimulates Growth of Indoor Cultivated Tomato (Solanum lycopersicum) Seedlings. Hortscience 2018, 53, 1429–1433. [Google Scholar] [CrossRef]
- Nayak, L.; Biswal, B.; Ramaswamy, N.; Iyer, R.; Nair, J.; Biswal, U. Ultraviolet-A induced changes in photosystem II of thylakoids: Effects of senescence and high growth temperature. J. Photochem. Photobiol. B Biol. 2003, 70, 59–65. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Saxena, I.; Srikanth, S.; Chen, Z. Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 2016, 7, 570. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, S.; Kitamura, M.; Koja, N. Triple mutation of Cys26, Trp35, and Cys126 in stromal ascorbate peroxidase confers H2O2 tolerance comparable to that of the cytosolic isoform. Biochem. Biophys. Res. Commun. 2008, 372, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Martins, S.; Goncalves, A.; Correia, C.M.; Ribeiro, C.; Dias, M.C.; Santos, C. The potential use of the UV-A and UV-B to improve tomato quality and preference for consumers. Sci. Hortic. 2019, 246, 777–784. [Google Scholar] [CrossRef]
- Yang, J.; Shi, W.; Li, B.; Bai, Y.; Hou, Z. Preharvest and postharvest UV radiation affected flavonoid metabolism and antioxidant capacity differently in developing blueberries (Vaccinium corymbosum L.). Food Chem. 2019, 301, 125248. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Honda, C.; Hatsuyama, Y.; Igarashi, M.; Bessho, H.; Moriguchi, T. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol. 2007, 48, 958–970. [Google Scholar] [CrossRef]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, L.; Liu, H.; Pan, Q.; Zhan, J.; Huang, W. Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul. 2009, 58, 251–260. [Google Scholar] [CrossRef]
- Arakawa, O.; Hori, Y.; Ogata, R. Relative effectiveness and interaction of ultraviolet-B, red and blue light in anthocyanin synthesis of apple fruit. Physiol. Plant. 1985, 64, 323–327. [Google Scholar] [CrossRef]
- Fernandes de Oliveira, A.; Nieddu, G. Accumulation and partitioning of anthocyanins in two red grape cultivars under natural and reduced UV solar radiation. Aust. J. Grape Wine Res. 2016, 22, 96–104. [Google Scholar] [CrossRef]
- Jiang, M.; Ren, L.; Lian, H.; Liu, Y.; Chen, H. Novel insight into the mechanism underlying light-controlled anthocyanin accumulation in eggplant (Solanum melongena L.). Plant Sci. 2016, 249, 46–58. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.; Sun, M.; Li, Y.; Kawabata, S. UV-A light induces anthocyanin biosynthesis in a manner distinct from synergistic blue plus UV-B light and UV-A/blue light responses in different parts of the hypocotyls in turnip seedlings. Plant Cell Physiol. 2012, 53, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Wu, Q.; Qi, N.; Liu, Y.; Li, N.; Cui, J. Effect of partial shading treatments on anthocyanin synthesis in the hypocotyls of soybean sprouts under UV-A irradiation. J. Plant Growth Regul. 2017, 36, 50–59. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Zhang, X.; Liu, Y.; Cui, J.; Liang, Y. Hydrogen peroxide, nitric oxide and UV RESISTANCE LOCUS8 interact to mediate UV-B-induced anthocyanin biosynthesis in radish sprouts. Sci. Rep. 2016, 6, 29164. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).