Chemical Profiling and Antioxidant Activity of Wild and Cultivated Sage (Salvia officinalis L.) Essential Oil
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Growing Conditions
2.2. Clevenger-Hydrodistillation
2.3. Antioxidative Activity
DPPH Assay
2.4. Gas Chromatography-Mass Spectrometry (GC/MS) and Gas Chromatography-Flame Ionization Detection (GC/FID) Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Essential Oil Yield
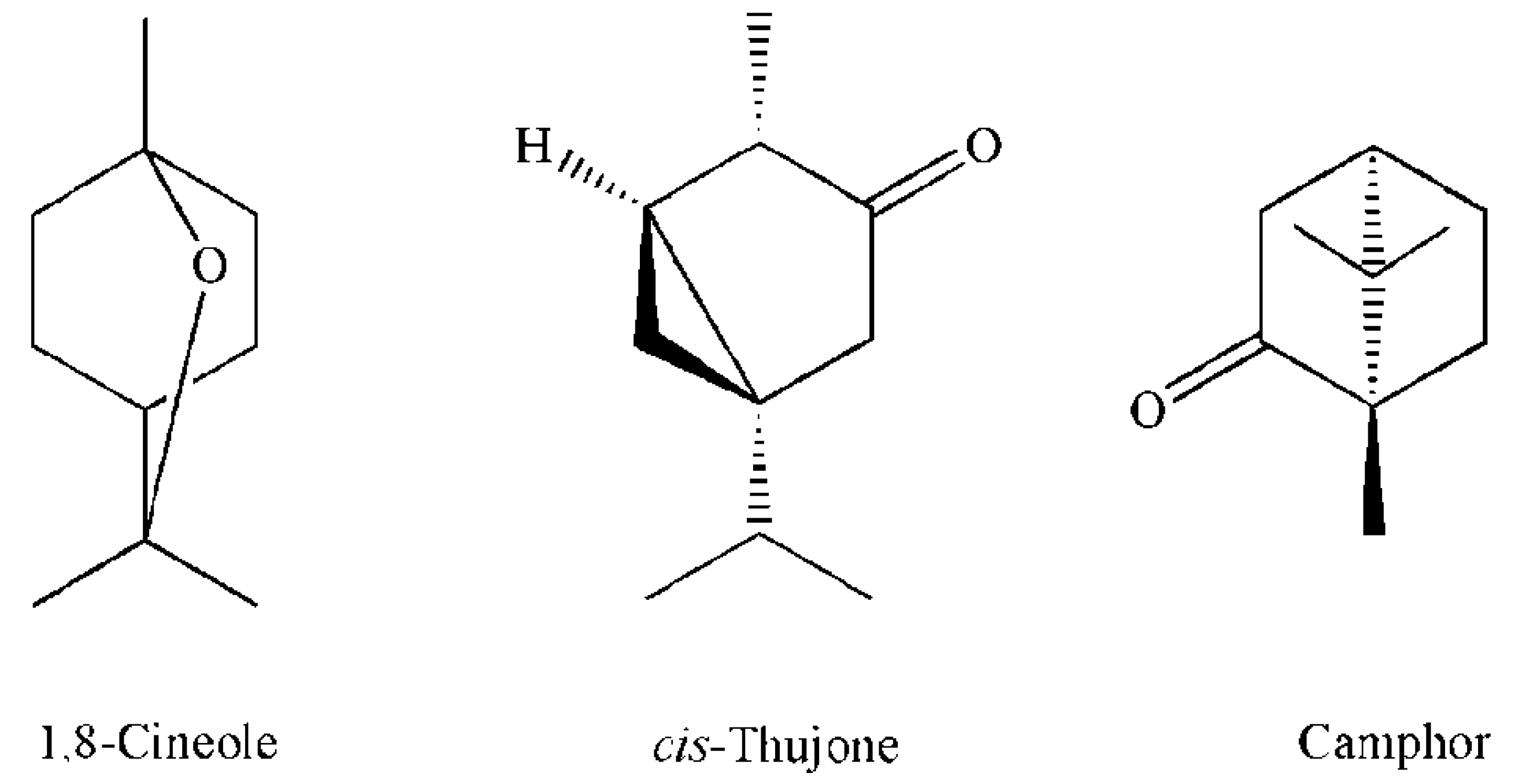
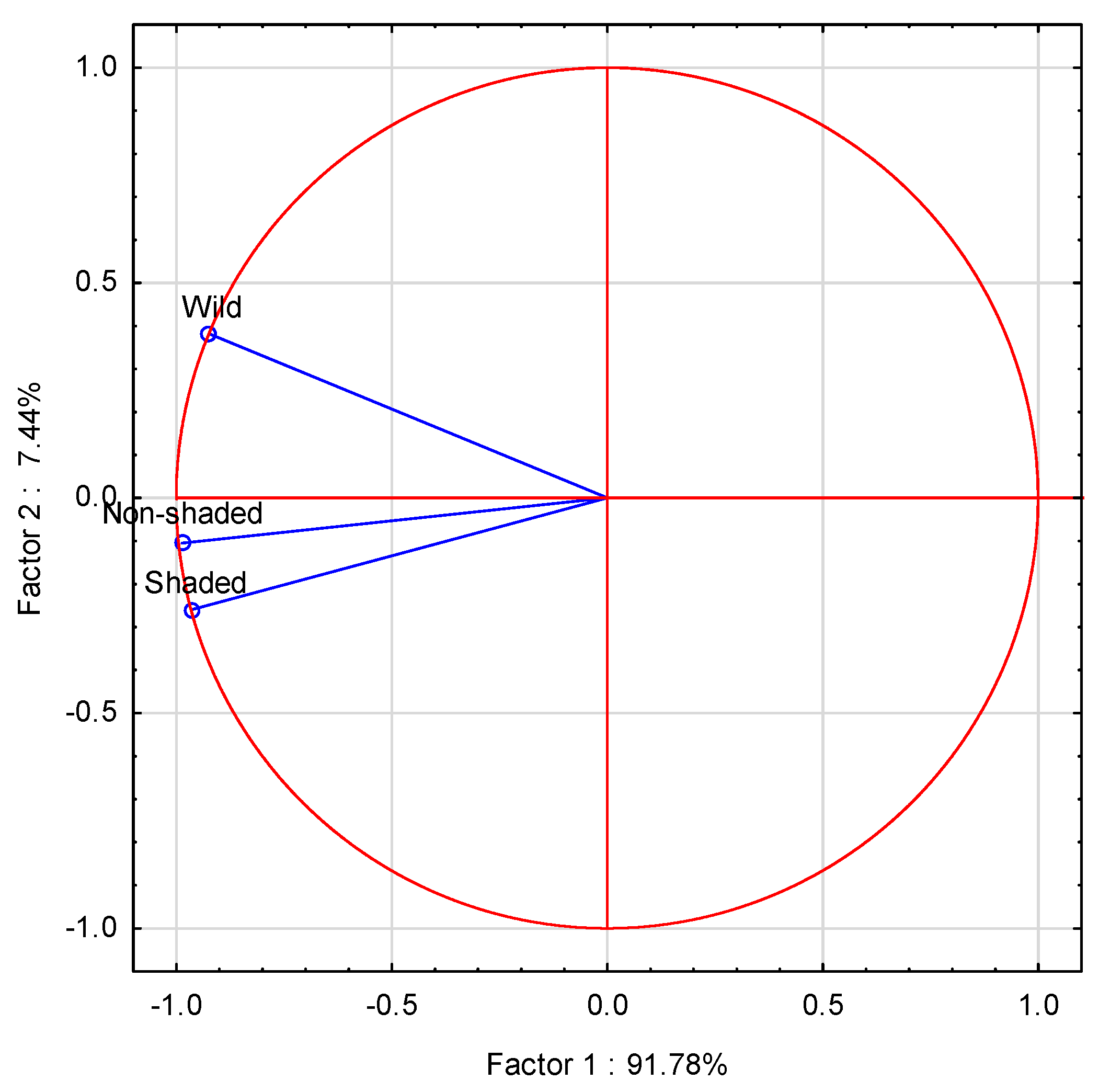
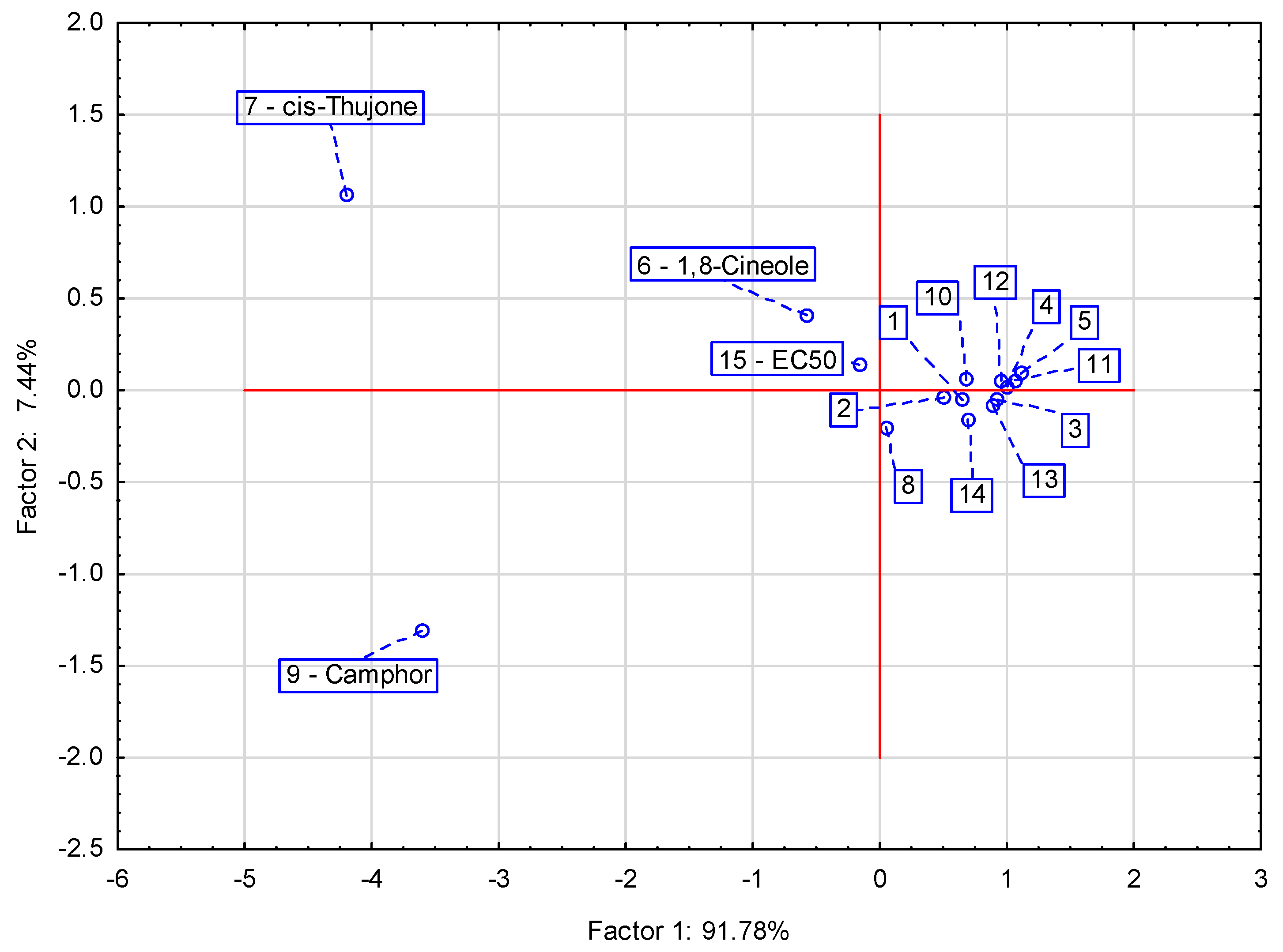
3.2. Essential Oil Composition
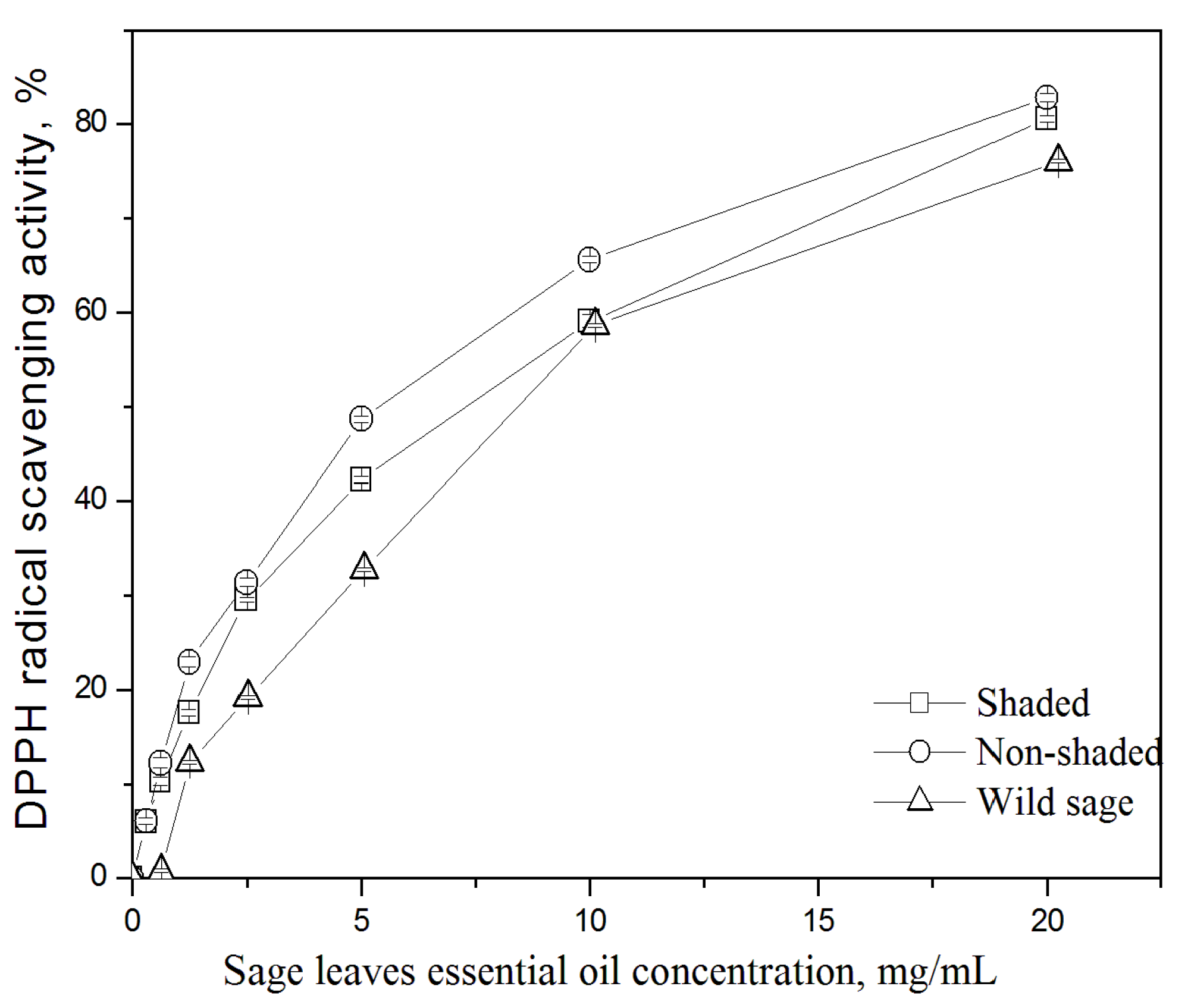
3.3. Antioxidative Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Poulios, E.; Giaginis, C.; Vasios, G.K. Current state of the art on the antioxidant activity of sage (Salvia spp.) and its bioactive components. Planta Med. 2020, 86, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulou, E.; Marugán-Hernández, V.; Skoufos, I.; Giannenas, I.; Bonos, E.; Aguiar-Martins, K.; Lazari, D.; Papagrigoriou, T.; Fotou, K.; Grigoriadou, K.; et al. In Vitro antioxidant, antimicrobial, anticoccidial, and anti-inflammatory study of essential oils of oregano, thyme, and sage from Epirus, Greece. Life 2022, 12, 1783. [Google Scholar] [CrossRef] [PubMed]
- Dogan, G.; Hayta, S.; Yuce, E.; Bagci, E. Composition of the essential oil of two Salvia taxa (Salvia sclarea and Salvia verticillate subsp. verticillata) from Turkey. Nat. Sci. Discov. 2015, 1, 62–67. [Google Scholar]
- Aćimović, M.; Todosijević, M.; Varga, A.; Kiprovski, B.; Tešević, V.; Čabarkapa, I.; Sikora, V. Bioactivity of essential oils from cultivated winter savory, sage and hyssop. Lek. Sirov. 2019, 39, 11–17. [Google Scholar] [CrossRef]
- Kostić, E.; Kitić, D.; Vujović, M.; Marković, M.; Pavlović, A.; Stojanović, G. Chemometric approach to the headspace sampled volatiles of selected Salvia species from Southeastern Serbia. Bot. Serbica 2022, 46, 285–294. [Google Scholar] [CrossRef]
- Duletić-Laušević, S.; Alimpić-Aradski, A.; Živković, J.; Gligorijević, N.; Šavikin, K.; Radulović, S.; Ćoćić, D.; Marin, D.P. Evaluation of bioactivities and phenolic composition of extracts of Salvia officinalis L. (Lamiaceae) collected in Montenegro. Bot. Serbica 2019, 43, 47–58. [Google Scholar] [CrossRef]
- Karalija, E.; Dahija, S.; Tarkowski, P.; Ćavar-Zeljković, S. Influence of climate-related environmental stresses on economically important essential oils of Mediterranean Salvia sp. Front. Plant Sci. 2022, 13, 864807. [Google Scholar] [CrossRef]
- Dent, M.; Bursić-Kovačević, D.; Bosiljkov, T.; Dragović-Uzelac, V. Polyphenolic composition and antioxidant capacity of indigenous wild Dalmatian sage (Salvia officinalis L.). Croat. Chem. Acta 2017, 90, 451–460. [Google Scholar] [CrossRef]
- Hodaj-Celiku, E.; Tsiftsoglou, S.O.; Shuka, L.; Abazi, S.; Hadipavlou-Litina, D.; Lazari, D. Antioxidant activity and chemical composition of essential oils of some aromatic and medicinal plants from Albania. Nat. Prod. Comm. 2017, 12, 785–790. [Google Scholar] [CrossRef]
- Hasa, E.; Duka, S.; Lika, E.; Nushi, E. Variation of Albanian Salvia officinalis L. Yield and essential oil composition in different harvesting seasons and years. Res. Plant Sci. 2021, 9, 7–12. [Google Scholar] [CrossRef]
- Stefkov, G.; Cvetković, I.; Karapandzova, M.; Kulevanova, S. Essential oil composition of wild growing sage from R. Macedonia. Maced. Pharm. Bull. 2011, 57, 71–76. [Google Scholar] [CrossRef]
- Damyanova, S.; Mollova, S.; Stoyanova, A.; Gubenia, O. Chemical composition of Salvia officinalis L. essential oil from Bulgaria. Ukrain. Food J. 2016, 5, 695–700. [Google Scholar] [CrossRef]
- Karik, U.; Çinar, O.; Tunçtürk, M.; Şekeroğlu, N.; Gezici, S. Essential oil composition of some sage (Salvia spp.) species cultivated in Izmir (Turkey) ecological conditions. Indian J. Pharm. Educ. Res. 2018, 52, 102–107. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Trad. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Šojić, B.; Tomović, V.; Savanović, J.; Kocić-Tanackov, S.; Pavlić, B.; Jokanović, M.; Milidrag, A.; Martinović, A.; Vujadinović, D.; Vukić, M. Sage (Salvia officinalis L.) essential oil as a potential replacement for sodium nitrite in dry fermente sausages. Processes 2021, 9, 424. [Google Scholar] [CrossRef]
- Mapes, C.; Xu, Y. Photosynthesis, vegetative habit and culinary properties of sage (Salvia officinalis) in response to low-light conditions. Can. J. Plant Sci. 2014, 94, 881–889. [Google Scholar] [CrossRef]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. J. Trad. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef]
- Randjelović, M.; Branković, S.; Miladinović, B.; Milutinović, M.; Živanović, S.; Mihajilov-Krstev, T.; Kitić, D. The benefits of Salvia sclarea L. ethanolic extracts on gastrointestinal and respiratory spasms. S. Afr. J. Bot. 2022, 150, 621–632. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No.1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No.1601/91, Regulations (EC) No. 2232/96 and (EC) No.110/2008 and Directive 2000/13/EC. Off. J. Eur. Union L 2008, 354, 34–50. [Google Scholar]
- Dincer, C.; Topuz, A.; Sahin-Nadeem, H.; Ozdemir, K.S.; Cam, I.B.; Tontul, I.; Gokturk, R.S.; Tugrul, A.S. A comparative study on phenolic composition, antioxidant activity and essential oil content of wild and cultivated sage (Salvia fruticosa Miller) as influenced by storage. Ind. Crops Prod. 2012, 39, 170–176. [Google Scholar] [CrossRef]
- Veličković, D.T.; Ranđelović, N.V.; Ristić, M.S.; Veličković, A.S.; Šmelcerović, A.A. Chemical constituents and antimicrobial activity of the ethanol extracts obtained from the flower, leaf and stem of Salvia officinalis L. J. Serb. Chem. Soc. 2003, 68, 17–24. [Google Scholar] [CrossRef]
- Đurović, S.; Micić, D.; Pezo, L.; Radić, D.; Bazarnova, J.G.; Smyatskaya, Y.A.; Blagojević, S. The effect of various extraction techniques on the quality of sage (Salvia officinalis L.) essential oil, expressed by chemical composition, thermal properties and biological activity. Food Chem. X 2022, 13, 100213. [Google Scholar] [CrossRef] [PubMed]
- Kulak, M.; Gul, F.; Sekeroglu, N. Changes in growth parameter and essential oil composition of sage (Salvia officinalis L.) leaves in response to various salt stresses. Ind. Crops Prod. 2020, 145, 112078. [Google Scholar] [CrossRef]
- Delamare, A.P.L.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Craft, J.D.; Satyal, P.; Setzer, W.N. The Chemotaxonomy of common sage (Salvia officinalis) based on the volatile constituents. Medicines 2017, 4, 47. [Google Scholar] [CrossRef]
- Jug-Dujaković, M.; Ristić, M.; Pljevljakušić, D.; Dajić-Stevanović, Z.; Liber, Z.; Hančević, K.; Radić, T.; Šatović, Z. High diversity of indigenous populations of Dalmatian sage (Salvia officinalis L.) in essential-oil composition. Chem. Biodivers. 2012, 9, 2309–2323. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Tmušić, N.; Stanojević, L.J.; Cvetković, D. Essential oils content, composition and antioxidant activity of lemon balm, mint and sweet basil from Serbia. LWT—Food Sci. Technol. 2022, 153, 112210. [Google Scholar] [CrossRef]
- Stanojević, J.S.; Stanojević, L.P.; Cvetković, D.J.; Danilović, B.R. Chemical composition, antioxidant and antimicrobial activity of the turmeric essential oil (Curcuma longa L.). Adv. Technol. 2015, 4, 19–25. [Google Scholar] [CrossRef]
- Milenković, L.; Ilić, Z.; Šunić, L.; Tmušić, N.; Lalević, D.; Stanojević, L.J.; Stanojević, L.; Cvetković, D. Modification of light intensity influence essential oils content, composition and antioxidant activity of thyme, marjoram and oregano. Saudi. J. Biol. Sci. 2021, 28, 6532–6543. [Google Scholar] [CrossRef]
- Cvetkovik, I.; Stefkov, G.; Karapandzova, M.; Kulevanova, S.; Šatović, Z. Essential oils and chemical diversity of southeast European populations of Salvia officinalis L. Chem. Biodivers. 2015, 12, 1025–1039. [Google Scholar] [CrossRef]
- Soltanbeigi, A.; Sakartepe, E. Chemical specification of wild Salvia tomentosa Mill. collected from inner Aegean region of Turkey. J. Med. Spice Plants 2020, 24, 31–35. [Google Scholar]
- Marić, S.; Maksimović, M.; Miloš, M. The impact of the locality altitudes and stages of development on the volatile constituents of Salvia officinalis L. from Bosnia and Herzegovina. J. Essen. Oil Res. 2006, 18, 178–180. [Google Scholar] [CrossRef]
- Zawiślak, G. Yield and chemical composition of essential oil from Salvia officinalis L. in third year of cultivation. HerbaPolon 2013, 60, 13–22. [Google Scholar] [CrossRef]
- Lalević, D.; Ilić, Z.S.; Stanojević, L.; Milenković, L.; Šunić, L.; Kovač, R.; Kovačević, D.; Danilović, B.; Milenković, A.; Stanojević, J.; et al. Shade-induced effects on essential oil yield, chemical profiling, and biological activity in some Lamiaceae plants cultivated in Serbia. Horticulturae 2023, 9, 84. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Šunić, L.; Tmušić, N.; Mastilović, J.; Kevrešan, Ž.; Stanojević, L.; Danilović, B.; Stanojević, J. Efficiency of basil essential oil antimicrobial agents under different shading treatments and harvest times. Agronomy 2021, 11, 1574. [Google Scholar] [CrossRef]
- Milenković, L.; Stanojević, J.; Cvetković, D.; Stanojević, L.; Lalević, D.; Šunić, L.; Fallik, E.; Ilić, S.Z. New technology in basil production with high essential oil yield and quality. Ind. Crops Prod. 2019, 140, 111718. [Google Scholar] [CrossRef]
- Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Milenković, A.; Stanojević, J.; Cvetković, D. The yield, chemical composition, and antioxidant activities of essential oils from different plant parts of the wild and cultivated oregano (Origanum vulgare L.). Horticulturae 2022, 8, 1042. [Google Scholar] [CrossRef]
- Oliveira, G.C.; Vieira, W.L.; Bertolli, S.C.; Pacheco, A.C. Photosynthetic behavior, growth and essential oil production of Melissa officinalis L. cultivated under colored shade nets. Chil. J. Agric. Res. 2016, 76, 123. [Google Scholar] [CrossRef]
- Li, Y.; Craker, L.E.; Potter, T. Effect of light level on the essential oil production of sage (Salvia officinalis) and thyme (Thymus vulgaris). Acta Hortic. 1996, 426, 419–426. [Google Scholar] [CrossRef]
- Ovidi, E.; Masci, V.L.; Zambelli, M.; Tiezzi, A.; Vitalini, S.; Garzoli, S. Laurus nobilis, Salvia sclarea and Salvia officinalis essential oils and hydrolates: Evaluation of liquid and vapor phase chemical composition and biological activities. Plants 2021, 10, 707. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Compounds by Gas Chromatography and Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2009. [Google Scholar]
- Perry, N.B.; Anderson, R.A.; Brennan, N.J.; Douglas, M.H.; Hesney, A.J.; McGimpsey, J.A. Essential oils from dalmatian S. officinalis (Salvia officinalis L.): Variation among individuals, plant parts, seasons and sites. J. Agric. Food Chem. 1999, 47, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Zeremski, T.; Šovljanski, O.; Lončar, B.; Pezo, L.; Zheljazkov, V.D.; Pezo, M.; Šuput, D.; Kurunci, Z. Seasonal Variations in essential oil composition of immortelle cultivated in Serbia. Horticulturae 2022, 8, 1183. [Google Scholar] [CrossRef]
- Lakušić, B.; Ristić, M.; Slavkovska, V.; Stojanović, D.; Lakušić, D. Variations in essential oil yields and compositions of Salvia officinalis (Lamiaceae) at different developmental stages. Bot. Serbica 2013, 37, 127–139. [Google Scholar]
- Schmiderer, C.; Torres-Londoño, P.; Novak, J. Proof of geographical origin of Albanian sage by essential oil analysis. Biochem. System. Ecol. 2013, 51, 70–77. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Arak, E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat. Prod. Res. 2007, 21, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Tundis, R.; Leporini, M.; Bonesi, M.; Rovito, S.; Passalacqua, N.G. Salvia officinalis L. from Italy: A comparative chemical and biological study of its essential oil in the Mediterranean context. Molecules 2020, 25, 5826. [Google Scholar] [CrossRef]
- Mot, M.D.; Gavrilaș, S.; Lupitu, A.I.; Moisa, C.; Chambre, D.; Tit, D.M.; Bogdan, M.A.; Bodescu, A.M.; Copolovici, L.; Copolovici, D.M.; et al. Salvia officinalis L. essential oil: Characterization, antioxidant properties, and the effects of aromatherapy in adult patients. Antioxidants 2022, 11, 808. [Google Scholar] [CrossRef]
- Porte, A.; Godoy, R.L.O.; Maia-Porte, L.H. Chemical composition of sage (Salvia officinalis L.) essential oil from the Rio de Janeiro State (Brazil). Rev. Bras. Plant Med. 2012, 15, 438–441. [Google Scholar] [CrossRef]
- Nevkrytaya, N.; Novikov, I.; Soboleva, E.; Kashirina, N.; Radchenko, L. Manifestation features of the productivity potential of Salvia officinalis L. in the conditions of the Crimea foothills. E3S Web Conf. 2021, 254, e01006. [Google Scholar] [CrossRef]
- Sotiropoulou, N.S.D.; Kokkini, M.K.; Megremi, S.F.P.; Daferera, D.J.; Skotti, E.P.; Kimbaris, A.C.; Polissiou, M.G.; Tarantilis, P.A. Determination of α- and β-thujone in wormwood and sage infusions of Greek flora and estimation of their average toxicity. Curr. Res. Nutr. Food Sci. 2016, 4, 152–160. [Google Scholar] [CrossRef]
- EMA/HMPC 2008. Final Community Herbal Monograph on Salvia officinalis L., folium. EMA/HMPC/331653/2008. London, UK, 12 November 2009. Available online: http://www.emea.europa.eu (accessed on 1 December 2020).
- Radulović, N.S.; Genčić, M.S.; Stojanović, N.M.; Randjelović, P.J.; Stojanović-Radić, Z.Z.; Stojiljković, N.I. Toxic essential oils. Part V: Behaviour modulating and toxic properties of thujones and thujone-containing essential oils of Salvia officinalis L., Artemisia absinthium L., Thuja occidentalis L. and Tanacetum vulgare L. Food Chem. Toxicol. 2017, 105, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Couladis, M.; Tzakou, O.; Mimica-Dukić, N.; Jančić, R.; Stojanović, D. Essential oil of Salvia officinalis L. from Serbia and Montenegro. Flavour. Fragr. J. 2002, 17, 119–126. [Google Scholar] [CrossRef]
- Damjanovic-Vratnica, B.; Ðakov, T.; Šukovic, D.; Damjanovic, J. Chemical composition and antimicrobial activity of essential oil of wild-growing Salvia officinalis L. from Montenegro. J. Essent. Oil.-Bear. Plants 2008, 11, 79–89. [Google Scholar] [CrossRef]
- Steševic, D.; Ristic, M.; Nikolic, V.; Nedovic, M.; Cakovic, D.; Šatovic, Z. Chemotype diversity of indigenous dalmatian sage (Salvia officinalis L.) populations in Montenegro. Chem. Biodivers. 2014, 11, 101–114. [Google Scholar] [CrossRef]
- Joshi, S.; Pandey, R.D.; Bhattarai, R.; Gharti, B.B. Antimicrobial activity of essential oil and crude organic extracts of Salvia officinalis L. leaves from Nepal. Inter. J. Inn. Sci. Res. Technol. 2021, 6, 676–682. [Google Scholar]
- Şeker, S.; Çakaloğullar, U.; Bayram, E.; Tatar, Ö. Production of sage, oregano and rosemary under shading conditions and the effects of light on growth and essential oil properties. Ind. Crops Prod. 2023, 193, 116254. [Google Scholar] [CrossRef]
- Thakur, M.; Bhatt, V.; Kumar, R. Effect of shade level and mulch type on growth, yield and essential oil composition of damask rose (Rosa damascena Mill.) under mid hill conditions of Western Himalayas. PLoS ONE 2019, 14, e0214672. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Bertolucci, S.K.V.; Carvalho, A.A.; Tostes, W.N.; Coelho, A.D.; Pinto, J.E.B.P. Light intensities alter growth and essential oil of patchouli under shade nets. Cienc Rural Santa Maria 2022, 52, e20210118. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, S.; Ramesh, K.; Pathania, V.; Prasad, R. Irradiance stress and plant spacing effect on growth, biomass and quality of wild marigold (Tagetes minuta L.)—An industrial crop in western Himalaya. J. Essent. Oil Res. 2014, 26, 348–358. [Google Scholar] [CrossRef]
- Katsoulis, G.I.; Kimbaris, A.C.; Anastasaki, E.; Damalas, C.A.; Kyriazopoulos, A.P. Chamomile and anise cultivation in olive agroforestry systems. Forests 2022, 13, 128. [Google Scholar] [CrossRef]
- Lima, M.C.; da-Silva, C.J.; Mariot, M.P.; Freitag, R.A.; Serpa, R.; Ribeiro, G.A.; Amarante, L. Effect of shading and nitrogen fertilization on nitrogen metabolism, essential oil content and antimicrobial activity of Achillea millefolium. Acta Sci. Biol. Sci. 2020, 42, 1–12. [Google Scholar] [CrossRef]
- Stanojević, L.; Stanković, M.; Cvetković, D.; Danilović, B.; Stanojević, J. Dill (Anethum graveolens L.) seeds essential oil as a potential natural antioxidant and antimicrobial agent. Biol. Nyssana 2016, 7, 31–39. [Google Scholar]
- El Euch, S.K.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis essential oil: Chemical analysis and evaluation of antienzymatic and antioxidant bioactivities. S. Afr. J.Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- Bouaziz, M.; Yangui, T.; Sayadi, S.; Dhouib, A. Disinfectant properties of essential oils from Salvia officinalis L. cultivated in Tunisia. Food Chem. Toxicol. 2009, 47, 2755–2760. [Google Scholar] [CrossRef]
- Mervić, M.; BivalŠtefan, M.; Kindl, M.; Blažeković, B.; Marijan, M.; Vladimir-Knežević, S. Comparative antioxidant, anti-acetylcholinesterase and anti-α-glucosidase activities of Mediterranean Salvia species. Plants 2022, 11, 625. [Google Scholar] [CrossRef] [PubMed]
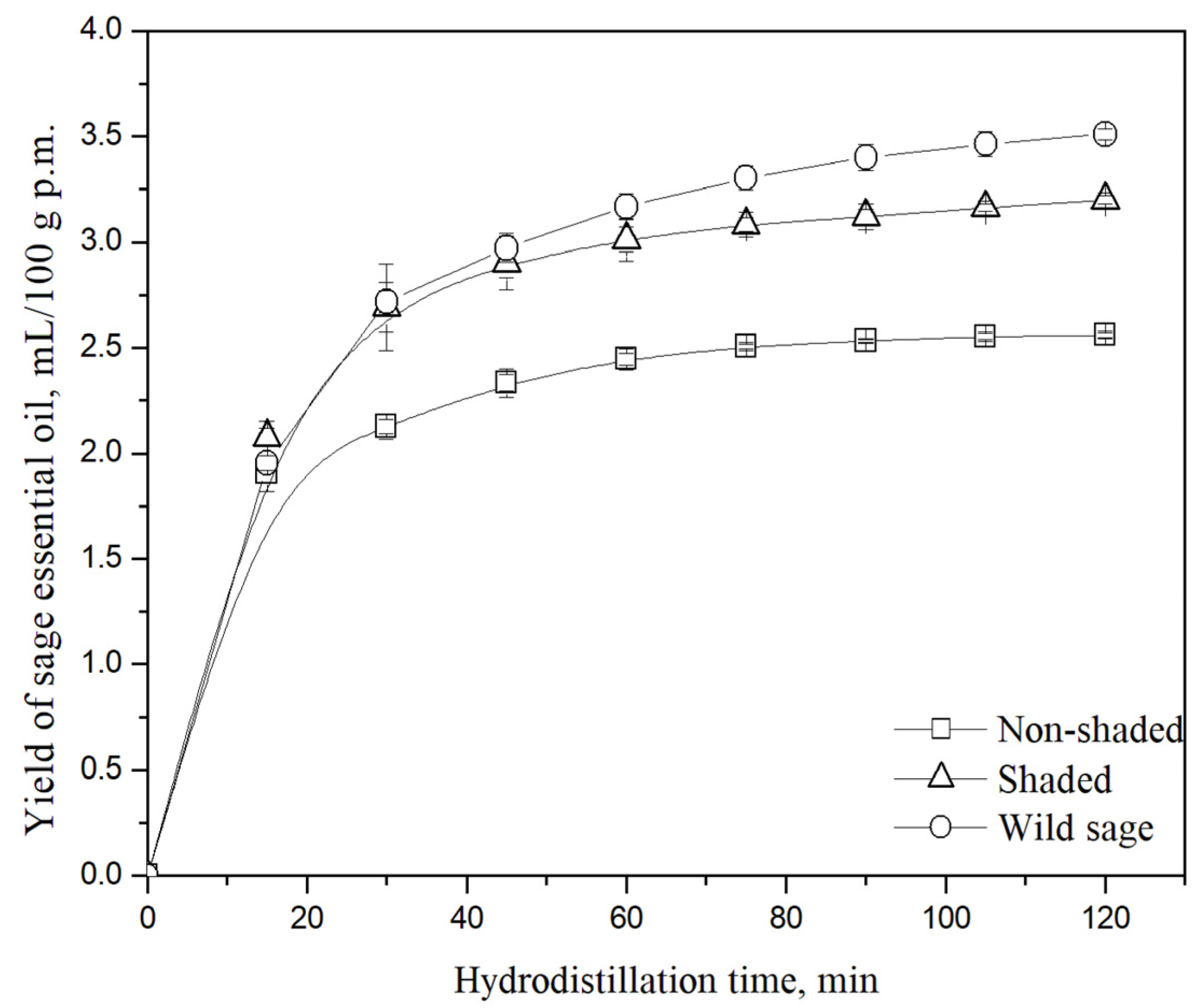
| Sample | Essential Oil Yield, mL/100 g p.m. * |
|---|---|
| Sage leaves | |
| Wild plants | 3.51 ± 0.03 a |
| Cultivated (non-shaded) plants | 2.56 ± 0.02 b |
| Cultivated (shaded) plants | 3.20 ± 0.03 a |
| No. | tret., min | Compound | RIexp | RIlit | Method of Identification | Content % |
|---|---|---|---|---|---|---|
| 1 | 6.60 | Santolina triene | 910 | 906 | RI, MS | tr |
| 2 | 6.70 | α-Thujene | 924 | 924 | RI, MS | tr |
| 3 | 6.94 | α-Pinene | 932 | 932 | RI, MS | 1.9 |
| 4 | 7.41 | Camphene | 947 | 946 | RI, MS | 3.0 |
| 5 | 8.28 | β-Pinene | 976 | 974 | RI, MS, Co-I | 0.5 |
| 6 | 8.61 | 1-Octen-3-ol | 977 | 974 | RI, MS | tr |
| 7 | 8.70 | Myrcene | 980 | 988 | RI, MS | 0.8 |
| 8 | 9.24 | α-Phellandrene | 998 | 1002 | RI, MS | tr |
| 9 | 9.66 | α-Terpinene | 1010 | 1014 | RI, MS | tr |
| 10 | 10.04 | p-Cymene | 1020 | 1020 | RI, MS | 0.8 |
| 11 | 10.14 | Limonene | 1022 | 1024 | RI, MS, Co-I | tr |
| 12 | 10.21 | 1,8-Cineole | 1023 | 1026 | RI, MS, Co-I | 13.8 |
| 13 | 11.27 | γ-Terpinene | 1052 | 1054 | RI, MS | tr |
| 14 | 12.45 | Fenchone | 1083 | 1083 | RI, MS | tr |
| 15 | 12.69 | p-Cymenene | 1090 | 1089 | RI, MS | tr |
| 16 | 13.26 | cis-Thujone | 1104 | 1101 | RI, MS | 43.2 |
| 17 | 13.73 | trans-Thujone | 1115 | 1112 | RI, MS | 5.6 |
| 18 | 14.91 | Camphor | 1144 | 1141 | RI, MS, Co-I | 17.6 |
| 19 | 16.17 | Borneol | 1174 | 1165 | RI, MS, Co-I | 3.4 |
| 20 | 16.51 | Terpinen-4-ol | 1182 | 1174 | RI, MS | 0.8 |
| 21 | 17.27 | α-Terpineol | 1200 | 1196 | RI, MS | tr |
| 22 | 20.75 | Isobornyl acetate | 1282 | 1283 | RI, MS | 1.5 |
| 23 | 21.12 | trans-Sabinyl acetate | 1291 | 1289 | RI, MS | 0.2 |
| 24 | 26.47 | (E)-Caryophyllene | 1421 | 1417 | RI, MS | 0.8 |
| 25 | 27.92 | α-Humulene | 1457 | 1452 | RI, MS | 1.5 |
| 26 | 29.03 | Germacrene D | 1485 | 1484 | RI, MS | tr |
| 27 | 33.11 | Caryophyllene oxide | 1591 | 1582 | RI, MS | tr |
| 28 | 33.66 | Veridiflorol | 1602 | 1592 | RI, MS | 3.8 |
| 29 | 34.15 | Humulene epoxide II | 1618 | 1608 | RI, MS | 0.6 |
| Total identified | 100.0 | |||||
| Grouped components (%) | ||||||
| Monoterpene hydrocarbons (2–5, 7–11, 13,15) | 7.0 | |||||
| Oxygen-containing monoterpenes (12, 14, 16–23) | 86.2 | |||||
| Sesquiterpene hydrocarbons (24–26) | 2.3 | |||||
| Oxygenated sesquiterpenes (27–29) | 4.5 | |||||
| Others (1, 6) | tr | |||||
| No. | tret, min | Compound | RIexp | RIlit | Method of Identification | Content % | |
|---|---|---|---|---|---|---|---|
| Shaded | Non-Shaded | ||||||
| 1 | 4.74 | (Z)-Salvene | 857 | 847 | RI, MS | tr | 0.2 |
| 2 | 4.93 | (E)-Salvene | 868 | 858 | RI, MS | - | tr |
| 3 | 6.41 | Tricyclene | 923 | 921 | RI, MS | tr | 0.1 |
| 4 | 6.51 | α-Thujene | 926 | 924 | RI, MS | 0.2 | 0.2 |
| 5 | 6.73 | α-Pinene | 934 | 932 | RI, MS, Co-I | 2.1 | 4.7 |
| 6 | 7.19 | Camphene | 950 | 946 | RI, MS | 2.9 | 5.4 |
| 7 | 7.90 | Sabinene | 975 | 969 | RI, MS | 0.2 | 0.2 |
| 8 | 8.04 | β-Pinene | 979 | 974 | RI, MS, Co-I | 1.4 | 2.1 |
| 9 | 8.43 | Myrcene | 993 | 988 | RI, MS | 0.9 | 1.1 |
| 10 | 8.94 | α-Phellandrene | 1008 | 1002 | RI, MS | tr | tr |
| 11 | 9.37 | α-Terpinene | 1020 | 1014 | RI, MS | 0.2 | tr |
| 12 | 9.70 | p-Cymenene | 1028 | 1020 | RI, MS | 0.1 | 0.2 |
| 13 | 9.82 | Limonene | 1032 | 1024 | RI, MS, Co-I | 1.9 | tr |
| 14 | 9.89 | 1,8-Cineole | 1034 | 1026 | RI, MS, Co-I | 6.1 | 11.0 |
| 15 | 10.09 | (Z)-β-Ocimene | 1039 | 1032 | RI, MS | tr | tr |
| 16 | 10.91 | γ-Terpinene | 1061 | 1054 | RI, MS, Co-I | 0.6 | 0.4 |
| 17 | 11.44 | cis-Sabinene hydrate | 1075 | 1065 | RI, MS | tr | tr |
| 18 | 12.06 | Terpinolene | 1092 | 1086 | RI, MS | 0.4 | 0.3 |
| 19 | 12.91 | cis-Thujone | 1111 | 1101 | RI, MS | 23.5 | 28.3 |
| 20 | 13.28 | trans-Thujone | 1122 | 1112 | RI, MS | 9.0 | 4.4 |
| 21 | 14.51 | Camphor | 1151 | 1141 | RI, MS, Co-I | 31.5 | 30.9 |
| 22 | 15.57 | Borneol | 1175 | 1165 | RI, MS | 3.0 | 2.1 |
| 23 | 15.95 | Terpinen-4-ol | 1184 | 1174 | RI, MS, Co-I | 0.6 | 0.4 |
| 24 | 16.63 | α-Terpineol | 1192 | 1186 | RI, MS | 0.4 | tr |
| 25 | 19.41 | iso-3-Thujanol acetate | 1267 | 1267 | RI, MS | tr | - |
| 26 | 20.28 | Isobornyl acetate | 1287 | 1283 | RI, MS | 1.3 | 0.9 |
| 27 | 20.61 | trans-Sabinyl acetate* | 1289 | 1295 | RI, MS | tr | - |
| 28 | 21.98 | Myrtenyl acetate* | 1328 | 1324 | RI, MS | tr | - |
| 29 | 25.92 | (E)-Caryophyllene | 1423 | 1417 | RI, MS | 2.5 | 1.3 |
| 30 | 27.32 | α-Humulene | 1458 | 1452 | RI, MS | 4.4 | 2.0 |
| 31 | 32.92 | Khusimone | 1601 | 1604 | RI, MS | 4.5 | 2.1 |
| 32 | 33.46 | Humulene epoxide II | 1616 | 1619 | RI, MS | 0.4 | - |
| 33 | 48.54 | Manool | 2063 | 2056 | RI, MS | 2.1 | 1.4 |
| Total identified (%) | 100.0 | 100.0 | |||||
| Grouped components (%) | |||||||
| Monoterpene hydrocarbons (2–12, 14, 15, 17) | 10.9 | 14.7 | |||||
| Oxygen-containing monoterpenes (13, 16, 18–27) | 75.2 | 78.0 | |||||
| Sesquiterpene hydrocarbons (28, 29, 30) | 6.9 | 3.3 | |||||
| Oxygen-containing sesquiterpenes (32) | 0.4 | - | |||||
| Diterpenoids (33) | 2.1 | 1.4 | |||||
| Others (1, 31) | 4.5 | 2.3 | |||||
| Essential Oil | EC50, mg/mL, 20 min Incubation |
|---|---|
| Wild sage | 9.65 ± 0.01 c |
| Cultivated sage (non-shaded plants) | 7.49 ± 0.13 b |
| Cultivated sage (shaded plants) | 6.16 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilić, Z.S.; Kevrešan, Ž.; Šunić, L.; Stanojević, L.; Milenković, L.; Stanojević, J.; Milenković, A.; Cvetković, D. Chemical Profiling and Antioxidant Activity of Wild and Cultivated Sage (Salvia officinalis L.) Essential Oil. Horticulturae 2023, 9, 624. https://doi.org/10.3390/horticulturae9060624
Ilić ZS, Kevrešan Ž, Šunić L, Stanojević L, Milenković L, Stanojević J, Milenković A, Cvetković D. Chemical Profiling and Antioxidant Activity of Wild and Cultivated Sage (Salvia officinalis L.) Essential Oil. Horticulturae. 2023; 9(6):624. https://doi.org/10.3390/horticulturae9060624
Chicago/Turabian StyleIlić, Zoran S., Žarko Kevrešan, Ljubomir Šunić, Ljiljana Stanojević, Lidija Milenković, Jelena Stanojević, Aleksandra Milenković, and Dragan Cvetković. 2023. "Chemical Profiling and Antioxidant Activity of Wild and Cultivated Sage (Salvia officinalis L.) Essential Oil" Horticulturae 9, no. 6: 624. https://doi.org/10.3390/horticulturae9060624
APA StyleIlić, Z. S., Kevrešan, Ž., Šunić, L., Stanojević, L., Milenković, L., Stanojević, J., Milenković, A., & Cvetković, D. (2023). Chemical Profiling and Antioxidant Activity of Wild and Cultivated Sage (Salvia officinalis L.) Essential Oil. Horticulturae, 9(6), 624. https://doi.org/10.3390/horticulturae9060624








