Abstract
‘Arrayana’ mandarin is one of the most cultivated citrus species in Colombia, but this fruit has a short postharvest life and is sensitive to chilling injury (CI) during cold storage. Generating strategies that decrease CI to mandarin can reduce quantitative and qualitative losses postharvest. Brassinosteroids (BR) have been used as a sustainable technology to alleviate CI in fruits and improve postharvest quality. This study evaluated the effect of applying the 24-epibrasinolide analogue (EBR), at doses of 5 mg L−1; DI-31 analogue, at 5 and 10 mg L−1; and control, on the main physical and biochemical characteristics of ‘Arrayana’ mandarin stored at 4 °C for 40 days and, subsequently, 7 days at room temperature (shelf life). The application of EBR and DI-31 analogues reduced the appearance of CI in the exocarp of ‘Arrayana’ mandarin fruits by reducing electrolyte leakage, maintaining membrane integrity, and increasing antioxidant activity and phenol content at the end of cold storage and shelf life. This was especially pronounced with 5 mg L−1 of EBR. Similarly, the BR maintained the postharvest quality of mandarins by reducing weight loss, respiratory intensity, and chlorophyll degradation; increasing β-carotene; and maintaining titratable acidity and soluble solids. Our research reports, for the first time, CI tolerance in Arrayana mandarin using natural (EBR) and spirostanic (DI-31) analogues and illustrates the tolerance functionality of the DI-31 analogue on CI in the fruit postharvest.
1. Introduction
Mandarin (Citrus reticulata Blanco) cv. ‘Arrayana’ is one of the most cultivated citrus species in the Colombian tropical regions [1,2]. In the postharvest handling of citrus fruit, cold storage is considered the most efficient tool for extending its useful life, maintaining its postharvest quality, and as a quarantine treatment for the export of different fresh fruit [3]. However, citrus fruits stored at temperatures below 10 °C may present chilling injury (CI) and, depending on the citrus species concerned, present alterations in their organoleptic properties, which restricts the implementation of refrigeration to preserve these fruits [4,5]. In mandarin fruits, this manifests as brown pits, generalized browning and necrosis in the flavedo, accelerated ripening of fruits, and reduction in the integrity of the cell membrane, generating loss of cell stability and increased electrolyte leakage (EL) [6,7]. These symptoms increase in severity and are more visible when the fruits are transferred from storage at low temperatures to higher temperatures, for example, environmental conditions [8,9]. In this regard, ‘Arrayana’ mandarin fruits showed CI at the end of their shelf life after being stored for 40 days at 2 °C [2].
Postharvest CI of horticultural products arises from cellular oxidative stress [10]. The severity of the damage caused by low storage temperatures depends on the antioxidant defense system of the fruits, since these mitigate the negative effects of reactive oxygen species (ROS) through enzymatic and non-enzymatic antioxidant defenses [11]. Recently, efficient strategies have been developed to reduce CI in citrus fruits. The exogenous application of substances, such as plant hormones [12], and physical treatments [2,13] are methods that have been efficient in alleviating CI by maintaining the integrity of the membranes, increasing the antioxidant system, reducing electrolyte leakage, and delaying the ripening of the fruits.
Brassinosteroids (BR) are plant growth regulators that signal molecular, physiological, and biochemical responses in plant organs, including fruits [14]. BR has been used continuously in modern agriculture to increase crop production and generate tolerance to biotic and abiotic stresses [15,16]. Currently, more than 60 natural BR analogues have been identified and synthesized in different plant taxonomic groups, and of these, 24-epibrasinolide is one of the most widely implemented in world agricultural production [17,18]. Moreover, there are spirostane analogues of BR that arise from modifications in the structure of the steroidal nucleus and of the side chain in the natural compounds of BR [19], which can present the same intracellular biological activity at a lower cost in the market [20].
In recent years, various studies have shown the potential of BR in the ripening process and cold storage of horticultural products. Ji et al. [21] found that endogenous BR levels regulate ethylene production in climacteric fruits because BRASSINAZOLE-RESISTANT 1 (BZR1), activated by high BR concentrations, suppresses ACC oxidase (ACO) and ACS synthase (ACS) activity and gene expression of ACO1 and ACS1, which reduces ethylene production and inhibits fruit ripening. In non-climacteric fruits, the application of BR improved postharvest quality by increasing total soluble solids, color, and anthocyanin content in grapes [22]; increased some stress-related metabolites in mandarin [23]; and accelerated strawberry ripening [24]. In fruits stored at stressful low temperatures, the application of 24-epibrasinolide reduced CI, weight, and EL, and increased antioxidant capacity and postharvest life of blood oranges [25], lemons [26], peach [27], pomegranate [28], and zucchini [29]. Therefore, BR may be involved in the metabolic processes of maturation and tolerance to cold stress during the storage of horticultural products.
However, the role of BR in the postharvest life of mandarins under cold storage has not been investigated. Therefore, the objective of this research was to evaluate the effect of the exogenous application of the natural analogue 24-epibrasinolide and the spirostanic analogue DI-31 on chilling injury, postharvest quality, and antioxidant compounds in mandarin fruits (Citrus reticulata Blanco cv. ‘Arrayana’) under cold storage.
2. Materials and Methods
2.1. Plant Material Treatments and Storage Conditions
Mandarin cv. ‘Arrayana’ fruits were extracted from a commercial crop in the municipality of San Juan de Arama (Meta), located at 3°28′53.2920″ N, 73°59′12.2640″ W, at 512 m above sea level (a.s.l.) and with a mean annual temperature of 25 °C. The fruits had reached harvest maturity and were free from physical or phytosanitary problems. Table 1 shows the physicochemical characteristics of the fruits at the beginning of the experiment. The study was carried out in the Laboratory of “Calidad y Poscosecha de Productos Agrícolas” of the Universidad National de Colombia, Bogotá, located at 4°38′13″ N, 74°04′46″ W, at 2630 m a.s.l.

Table 1.
Physicochemical parameters of ‘Arrayana’ mandarin fruits at the beginning of the experiment.
A completely randomized design was used with four treatments: 24-epibrasinolide (EBR, 5 mg L−1); DI-31 (5 and 10 mg L−1); and control without applications. Each treatment had 5 repetitions, for a total of 20 experimental units, each made up of 3 kg of fruit. The fruits were disinfected in a 5% (w/v) sodium hypochlorite solution, washed, and dried at room temperature for 15 min. The EBR treatment fruits (Sigma-Aldrich Co., New York, NY, USA) were submerged for 10 min in an aqueous solution of 5 mg L−1, first dissolving the EBR in ethanol (1:1 ratio). This concentration was selected based on studies carried out on citrus and other fruits [25,26,30]. The spirostanic analogue DI-31 [(25 R)—3β. 5α—dihydroxy-spirostan-6-one] (Biomex DI-31, Minerales exclusivos SA, Bogotá, Colombia) was prepared in a solution with distilled water at concentrations of 5 and 10 mg L−1, immersing the fruits for 10 min. The control treatment fruits were subjected only to distilled water for the same period. After the treatments, all the fruits were dried in the environment and then stored at 4 °C with 80% relative humidity for 40 d. Subsequently, all the fruits were transferred to storage at 19 °C for 7 d to simulate their useful life (40 + 7 d) [2]. The measurements of the variables were made at the end of storage and at the end of the shelf life.
2.2. Evaluation of Chilling Injury Index and Electrolyte Leakage
Chilling injuries were evaluated using the chilling injury index (CII). The CII was estimated by evaluating the severity of browning on the epidermis surface, following the scale used by Balaguera-López et al. [2], where 0 = no injuries, 1 = minor lesion with up to 10% damaged surface, 2 = medium lesion with 10% to 50% of the surface stained, and 3 = severe injury with more than 50% of the surface with major damage, as indicated in Figure 1. The CII was calculated using Equation (1):

Figure 1.
Visual chilling injury classification scale in mandarin fruits ‘Arrayana’. The images were obtained in this experiment.
Electrolyte leakage (EL) was calculated according to the methodology adapted from Wang et al. [31], whereby 10 disks (0.5 cm in diameter) were cut from the epidermis. The epidermis disks were inserted into a falcon tube with 10 mL of deionized water and left at room temperature for 30 min. At the end of this time, the electrical conductivity (EC1) was measured using an EC electrode. After the measurement, the sample was heated at 90 °C in a water bath for 15 min, and the second EC (EC2) was estimated. This value was used as the maximum EL, using Equation (2).
2.3. Determination of Physicochemical Properties
Firmness (N) was determined with an LSI texturometer (Ametek-LLOYD, Berwyn, PA, USA) at three equidistant points from the equatorial section of the fruit.
The total soluble solids (TSS, °Brix) were obtained from approximately 1 mL of juice by carrying out the reading in a HANNA HI96801 digital refractometer (Hanna Instruments, Woonsocket, RI, USA).
The total titratable acidity (TTA) was calculated with a 916 Ti-Touch automatic titrator (Metrohm, Zofingen, Switzerland). Using acid-base titration with NaOH (0.1 N), the total titratable acidity corresponding to citric acid was determined, incorporating 2 mL of juice in 25 mL of distilled water, and proceeding to potentiometric titration until reaching pH 8.2.
2.4. Estimation of Weight Loss and Respiratory Intensity
Weight loss (WL) was calculated by weighing the fruits on a precision semi-analytical balance PA-3102 (Ohaus-Pioneer, Colombia), with an approximation of 0.01 g, and applying Equation (3),
where W1 is the weight at the initial time, and W2 the weight at the final time.
Respiratory intensity (RI) was measured with the Dansensor CheckPoint3 portable gas analyzer (Ametek-Mocon, Berwyn, PA, USA) using hermetic chambers with a volume of 428.94 mL. The same fruit was used for each sample and left for one hour in the chambers. To estimate the RI, Equation (4) was used:
where Vfree is the free volume of the chamber with the fruit, M is the mass of the sample, YCO2(t2) is the numerical value of the final CO2 percentage in the chamber, YCO2(t1) is the numerical value of the initial CO2 percentage, T is the measurement time, and D is the CO2 density.
2.5. Quantifying Pigments and Color Index
The extraction and quantification of β-carotene and total chlorophyll were carried out following the methodology of Gómez et al. [32] and Wellburn [33]. Approximately 300 mg of flavedo was taken from the fruit (M) and macerated in a mortar with 5 mL of acetone (80%), placed in a falcon tube, and vortexed for 1 min. During the extraction, work was carried out under low light conditions to avoid photodegradation of the pigments. Subsequently, the samples were taken to the centrifuge at 4000 rpm for 10 min (19 °C) and the supernatant was deposited in a 20 mL amber flask. This process was repeated twice to obtain a total extraction of pigments until completing a volume of 20 mL (V). Finally, the samples were taken to the spectrophotometer with absorbance (A) at 450, 647, and 663 nm. To calculate the chlorophyll a (Chl a), chlorophyll b (Chl b), and total chlorophyll (Chl Total) content, Equations (5)–(7) were applied, respectively, and the results were expressed in mg g−1 of fresh mass (FW).
For the quantification of β-carotene, a calibration curve was made with different concentrations of 93% β-carotene (Sigma-Aldrich Co., New York, NY, USA), and it was measured in absorbance at 450 nm. Then, Equation (6) was used:
where m and b are the values obtained in the equation of the line. The results were expressed in µg g−1 of FW. The color of the epidermis was determined according to González et al. [34] on the L*, a*, b* scale of the CIELab model at three equidistant equatorial points of the fruit, using the CR-400 digital colorimeter (Minolta Ko., Japan). With these values, the color index (CI*) was calculated using Equation (7).
2.6. Antioxidant Activity and Total Phenolic Content
Total antioxidant activity (TAA) and total phenol content (TPC) were extracted using the methodology proposed by Rey et al. [13]. In total, 500 g of flavedo was cut and macerated in a pre-cooled mortar with 10 mL methanol (80%). The mixture was centrifuged at 4500 rpm for 10 min (4 °C), after which the supernatant was considered for the measurements.
TAA quantification was performed using two different assays: reaction with 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid radical (ABTS) and reaction with 2,2-diphenyl-1-picrylhydrazyl radical (DPPH). ABTS was determined using Tesfay et al.’s method [35]. First, a stock solution of 7 mM ABTS and ammonium persulfate ((NH4)2S2O8) (2.45 mM) was prepared. To prepare the ABTS radical, 3 mL of the ABTS stock solution and 3 mL of the ammonium persulfate solution were taken, stirred until homogeneous, covered with aluminum foil, and incubated for 16 h at room temperature. Once the radical was formed, it was diluted in ethanol until an absorbance value between 0.7 and 754 nm was obtained. A calibration curve was prepared with Trolox (6-hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid) at 200 mM. For quantification, 0.2 mL of the extract was taken, and 3.8 mL of ABTS + (NH4)2S2O8 was added, leaving it to rest for 45 min. Finally, it was measured in the spectrophotometer at 754 nm, and the results were expressed in mM capacity Trolox equivalent antioxidant (TEAC).
TAA using the DPPH radical was carried out according to Rey et al. [13] and Grijalva-Verdugo et al. [36]. A stock solution of DPPH (100 µM) was prepared. Subsequently, 0.1 mL of the extract of each sample was diluted in 2.9 mL of the stock solution and left to settle for 30 min at room temperature in complete darkness. For the blank control, 0.1 mL methanol was diluted. Finally, the samples were taken to the spectrophotometer, and the absorbance (A) at 515 nm was measured. The results were expressed in DPPH radical scavenging capacity (%) using Equation (10):
The TPC was estimated using the Folin-Ciocalteu method proposed by Singleton and Rossi [37]. In total, 0.5 mL of the extract was taken and mixed with 0.75 mL of 1 N Folin-Ciocalteu. Subsequently, it was left to rest at room temperature for 5 min and 0.75 mL of 20% sodium carbonate was added, mixed, and left at rest for 90 min (19 °C), then absorbance at 760 nm was measured for each repetition. The calibration curve was made with gallic acid (GA) as the reference standard, and the equation of the line was established based on the GA standard prepared at different doses. Finally, the wavelength at 760 nm was measured, and the results were expressed in mg GA g−1 of FW.
2.7. Statistical Analysis
Statistical analyses were performed using SAS v.9.2e (SAS Institute Inc., Cary, NC, USA). Once the assumptions of normality and homogeneity of variances were verified, analysis of variance (ANOVA) was performed to determine significant differences between the application of BR and between the measurement times. Subsequently, the Tukey means comparison tests (p < 0.05) were applied between treatments and between storage times.
3. Results
3.1. Chilling Injury Index and Electrolyte Leakage
The chilling injury index (CII) showed significant differences between treatments for each storage moment. When comparing the measurements over time, the EBR was statistically significant when increasing the CII at the end of shelf life, while the other treatments did not change. The application of BR significantly decreased cold damage in the mandarin epidermis, both at the end of storage at 4 °C and in shelf life at 19 °C, with respect to untreated fruits. The CII in the EBR (5 mg L−1) and DI-31 (5 and 10 mg L−1) treatments were 62.6%, 49.4%, and 55.7% lower compared to the control, respectively, at the end of storage at 4 °C (Figure 2A). Seven days after being transferred to environmental conditions, the fruits treated with BR maintained the inhibitory effect of chilling injury, regardless of the analogue and the dose, presenting 45.2% less CII compared to the fruits without BR. CI symptoms in ‘Arrayana’ mandarin fruits appeared as brown pits on the epidermis of BR-treated fruits (Figure 3). In the control treatment, the pitting became a generalized brownish darkening with depressions in the epidermis, affecting fruit quality.
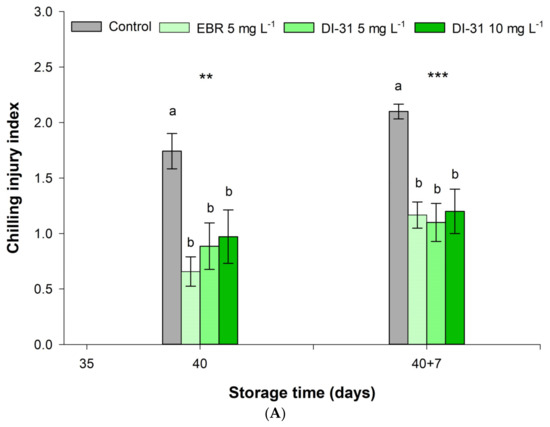
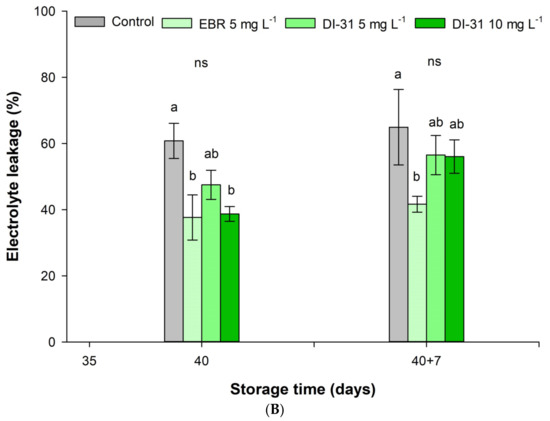
Figure 2.
Effect of BR on the (A) chilling injury index and (B) electrolyte leakage in ‘Arrayana’ mandarin fruits stored at 4 °C for 40 days and later transferred to 19 °C for 7 days. Different letters between columns indicate significant differences between treatments for each Tukey test measurement moment. ns: not significant, ** and *** indicate a significant effect between time according to the ANOVA analysis (p < 0.01 and p < 0.001, respectively). The vertical bars represent the standard error (n = 5).
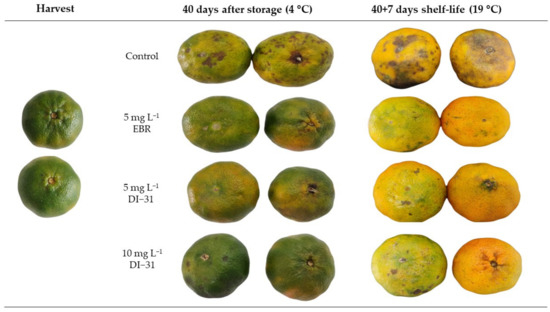
Figure 3.
Epidermis of ‘Arrayana’ mandarins after refrigeration for 40 days at 4 °C and at the end of shelf life (7 days at 19 °C).
The application of 10 mg L−1 of DI-31 significantly increased the EL at the end of shelf life compared to fruits just after refrigerated storage, while the EL in control, EBR, and DI-31 fruits (5 mg L−1) did not vary over time (Figure 2B). In this sense, the BR reduced the EL both at the end of storage and shelf life relative to untreated fruits. During shelf life, the 5 mg L−1 dose of EBR kept the EL constant.
3.2. Firmness, Soluble Solids, and Titratable Acidity
The BRs had a significant effect on fruit firmness 40 days after storage, reducing it by 18.9% compared to the control treatment (Table 2). In contrast, at the end of the shelf life, the firmness values increased in all treatments and did not present significant differences between treatments, reaching a total average of 21.75 ± 5.05 N.

Table 2.
Effect of BRs on firmness, total titratable acidity (TTA), and total soluble solids (TSS) in ‘Arrayana’ mandarin fruits at the end of storage at 4 °C (40 days) and of shelf life at 19 °C (40 + 7 days).
The TTA values did not show significant differences with the application of BR and the measurement time (Table 2), reaching an average of 0.88% ± 0.25 citric acid during the entire experiment.
The fruits treated with the analogue DI-31 (10 mg L−1) presented significant decreases in TSS at the end of storage and shelf life of 11.26% and 22.3%, respectively, compared to the other treatments (Table 2). Likewise, the TSS with the highest values occurred after 7 days at room temperature in the fruits subjected to the application of EBR (5 mg L−1) with respect to the control and DI-31 treatments.
3.3. Weight Loss and Respiratory Intensity
Fruits treated with BR, regardless of the analogue and dose, presented a significant decrease in weight loss (WL) compared to fruits without BR, both at the end of storage and during shelf life (Figure 4B). EBR was the treatment that obtained the lowest PM at the end of the shelf life, with a value of 17.65% ± 4.47.
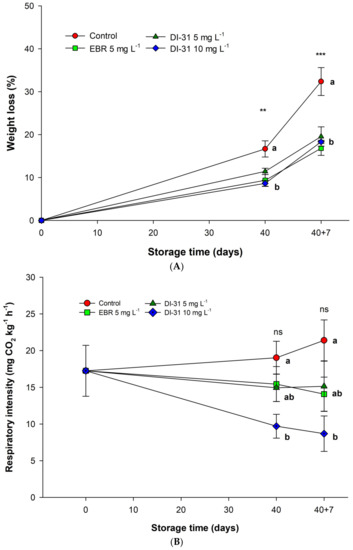
Figure 4.
Effect of BR on (A) weight loss and (B) respiration intensity in ‘Arrayana’ mandarin fruits stored at 4 °C for 40 days and later transferred to 19 °C for 7 days (shelf life). Different letters indicate significant differences between the treatments for each Tukey test measurement moment. ns: not significant, ** and *** indicate a significant effect according to the ANOVA analysis (p < 0.01 and p < 0.001, respectively). The vertical bars represent the standard error (n = 5).
The application of BR significantly reduced respiratory intensity (RI) compared to untreated fruits after storage and at the end of the shelf life (Figure 4A). The fruits subjected to 10 mg L−1 of DI-31 had the lowest RI of all the treatments at the two moments of the measurements, with values of 9.70 ± 3.62 and 8.69 ± 5.39 mg CO2 kg−1 h−1, respectively. Likewise, the EBR and DI-31 (5 mg L−1) fruits showed significant decreases in IR in relation to the control fruits. The results obtained indicate that a higher dose of the DI-31 analogue causes less cellular respiration and a delayed ripening process in mandarin fruits.
3.4. Pigments and Color Index
Chlorophyll a and b values did not present significant differences between the treatments at the end of cold storage and shelf life, but were highly significant between measurement times. However, the treatments with EBR and DI-31 (5 mg L−1) showed the lowest values for these pigments (Table 2). Chlorophyll a decreased by an average of 1.72% and 81.27% at 40 days of refrigeration and 40 + 7 days of shelf life, respectively, which highlights the preservation of color in ‘Arrayana’ mandarin fruits through cold storage. Chlorophyll b decreased by 29.22% and 81.55% at the end of refrigeration and shelf life, respectively, showing a lesser effect of low storage temperatures in attenuating the loss of this pigment. On the other hand, at the end of storage, the total chlorophyll of the fruits treated with EBR presented significantly lower values. Likewise, the application of DI-31 (5 mg L−1) resulted in a higher concentration of chlorophyll compared to all other treatments.
The fruits subjected to 5 mg L−1 of DI-31 had the highest concentration of β-carotene, which was 15.6% higher than the average values obtained with the other treatments after 40 days of storage at 4 °C (Table 2). At the end of the shelf life, none of the treatments had a significant effect on the fruits. These results indicate that DI-31 (5 mg L−1) improved the color change by increasing β-carotenes, but not the chlorophyll concentration.
There were no statistically significant differences between treatments for the color index, but there were differences over time (Table 3). On average, for all fruits, the color index before storage was −12.12 ± 2.51 (Table 1), which subsequently increased to −9.46 ± 2.53 after 40 d of cold storage, and finally increased significantly to −0.35 ± 2.02, indicating a degreening process over time.

Table 3.
Effect of BR on the concentration of pigments in ‘Arrayana’ mandarin fruits at the end of storage at 4 °C (40 days) and shelf life at 19 °C (40 + 7 days).
3.5. Antioxidant Activity and Phenolic Content
The results of the AAT showed highly significant differences between treatments at the end of shelf life, both by the ABTS method and by DPPH. The fruits treated with EBR and 10 mg L−1 of DI-31 had a significantly higher AAT compared to the other treatments, with ABTS values of 0.629 ± 0.07 and 0.609 ± 0.12 mM TEAC (Figure 5A), and capture of DPPH radicals of 34.39% ± 2.84 and 31.06% ± 3.66 (Figure 5B), respectively. These values were 91.8% and 73.2% higher than the control response.
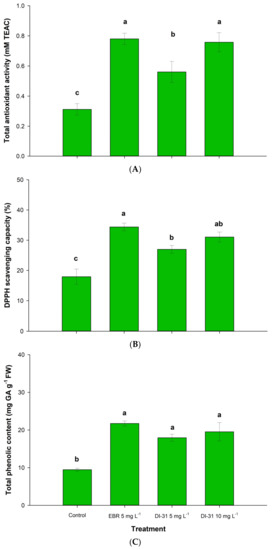
Figure 5.
Effect of BR on (A) total antioxidant activity, (B) DPPH scavenging capacity, and (C) total phenolic content in ‘Arrayana’ mandarin fruits stored at 4 °C for 40 days and later transferred to 19 °C for 7 days. Different letters between columns indicate significant differences between treatments for each Tukey test (p < 0.05). The vertical bars represent the standard error (n = 5).
The application of BR increased the TPC by 108% regardless of the treatment applied, with an average of 19.73 ± 3.59 mg GA g−1 of fresh weight compared to the control (9.45 ± 0.87 mg GA g−1, Figure 5C). These results indicate that the exogenous application of BR increases the antioxidant response of ‘Arrayana’ mandarin fruits.
4. Discussion
Like many other citrus fruits, ‘Arrayana’ mandarin fruits exhibit chilling injuries (CI) when stored at low temperatures. However, the damage is more visible during shelf life than during cold storage [2,9], as found in this research. CI appeared at the end of storage, and subsequently increased at the end of the useful life in all treatments, presenting brown pits and brownish lesions on the epidermis of the fruit (Figure 3). These are common CI symptoms for mandarin fruits [7,13] that result from a loss in the integrity of the cell membrane and phenolic substrates in the cytosol [38]. Electrolyte leakage is a good indicator of membrane integrity and is related to oxidative damage in fruit cold stress [30,39]. Consequently, increased EL was found at the two moments when CI increased in the fruits (Figure 2). However, the postharvest application of BR by immersion significantly reduced CI and EL, which resulted in a better organoleptic appearance of the fruit, highlighting the treatment of the EBR analogue. Similar results were obtained in blood orange [25] and eggplant [40] fruits by reducing CI and EL using concentrations of 10 µM of EBR under cold storage of 5° and 1 °C, respectively. As an alternative, the postharvest application by immersion of the spirostanic analogue DI-31 in two concentrations (5 and 10 mg L−1) before storage at 4 °C proved to be effective in alleviating chilling injury in ‘Arrayana’ mandarin fruits similar to the EBR. This is a pioneering finding in that it shows the functionality of the analogue DI-31 in tolerating CI in fruit postharvest.
Cold storage is the most efficient tool to maintain the postharvest quality of citrus, but at very low temperatures, quality can be affected by the appearance of CI and alterations in fruit ripening [3]. The respiratory intensity and weight loss values significantly increased in the fruits that were not subjected to immersion with BR compared to the fruits treated with DI-31 and EBR (Figure 4). Under chilling injury in citrus fruits, increases in ethylene production and respiratory rate occur due to increased activity of the ACC synthase and ACC oxidase enzymes, which raise ATP production and thus activate different stress defense mechanisms, such as was observed in grapefruit fruits [41]. In addition, WL is a non-destructive indicator of CI in cold citrus fruits [42], since it correlates with the appearance of damage in the epidermis of the fruits, which causes microscopic cracks in the cuticular zone and stomatal epidermis, increasing water loss. This causes increases in respiratory cell metabolism and transpiration rate caused by CI, which accelerated the ripening and senescence of ‘Arrayana’ mandarin fruits without BR application. Our results are consistent with previous studies where the application of BR reduced the RI and WL in ‘Tommy Atkins’ mango fruits stored at 5 °C, associated with a lower incidence of CI [43]. Additionally, a lower RI was observed in the fruits treated with 10 mg L−1, which was not the case with 5 mg L−1 DI-31 (Figure 4B), so with a higher dose, the maturation process of ‘Arrayana’ mandarin fruits should be delayed. Ji et al. [21] reported that a high endogenous concentration of BRs in climacteric fruits suppresses ethylene synthesis, which could have happened in this study.
The titratable acidity values were not affected by the application of BR treatments during storage and shelf life, but the total soluble solids and firmness were. Ladaniya [3] mentions that citrus fruits tend to present slight decreases in the total concentration of sugars and organic acids or sometimes do not show changes until the end of their useful life due to the non-climacteric metabolism that these fruits present and the environmental storage conditions. The lowest TSS values were obtained when the fruits were treated with 10 mg L−1 DI-31, which presented the lowest respiration rates (Figure 4B), indicating a lower use of these compounds as respiratory substrates as well as for the synthesis of new molecules [32] during cold stress. Therefore, as significant differences in acidity are not presented, it could be inferred that organic acids are not related to the response to cold stress in mandarin fruits, with sugars being the first affected. Likewise, at the end of the shelf life, the firmness values increased in all treatments and did not present significant differences between treatments. This may be due to the elasticity of the epidermis, since due to the high presence of polysaccharides and proteins during fruit ripening, elasticity increases the cohesive force when the fruit is faced with mechanical stress [44]. The results obtained in this research are different from those obtained in lemon [26] and grape [45], where BRs in non-climacteric fruits achieved reductions in TSS while maintaining TA and firmness compared to untreated fruits under cold stress.
Mandarins grown in the tropics rarely develop a uniform color due to high temperatures during fruit ripening, which produces green fruits with low carotenoid content in the epidermis [46]. Therefore, degreening strategies are sought to degrade chlorophyll and increase the content of carotenoids to provide more attractive fruits to the consumer. The results of this study suggest that EBR application accelerated chlorophyll degradation when fruits were taken out of storage compared to the control, which could indicate faster degreening. However, the application of DI-31 inhibited this degradation but increased the synthesis of carotenoids (Table 3). It has been shown that BR are involved in the synthesis of carotenoids during fruit ripening by increasing the expression of genes in the biosynthesis of these pigments [47]. In addition, carotenoids increase the tolerance of fruits to cold damage during storage, including mandarin [13]. On the other hand, BR inhibits chlorophyll degradation at doses of 15 in green bell pepper [30] and 10 µM in lemon [26], thanks to the ability of BR to inhibit ethylene synthesis [21] and decrease the oxidation of pigments during cold storage [31].
Recently, it has been shown that BR is involved in the endogenous response to toleration of postharvest CI by increasing the synthesis of enzymes and antioxidant molecules, inhibiting protein degradation, and maintaining cellular energy status through signaling cascades with other hormones, which generates a reduction in the CI of the fruit [48,49]. In our experiment, the fruits with immersion in BR, regardless of the analogue, presented increased antioxidant activity (ABTS and DPPH radicals) and total phenol content (TPC) (Figure 5). Despite the fact that BR induces greater DPPH activity, this variable does not explain all the antioxidant capacity of mandarins. It has been reported that under cold stress conditions, the fruits can activate biochemical mechanisms other than phenolic compounds, such as enzymatic and non-enzymatic antioxidants [25,26,27,28]. Similar results were found in lemon [26], orange [25], peach [27], pomegranate [28], and zucchini [29]. In these studies, fruits presented increased enzymatic (SOD, POD, CAT, APX) and non-enzymatic (proline, PAL activity, phenols, ascorbic acid) antioxidant responses when applying BR before cold storage, which meant a reduction in oxidative damage and, therefore, in CI. In satsuma mandarin, the same effect has been seen, but not under cold storage conditions [23]. Therefore, the application of the EBR and DI-31 analogues reduced CI and improved the quality of ‘Arrayana’ mandarin by decreasing EL, WL, and RI; maintaining TTA and TSS; and increasing TAA and TPC, which in turn increased tolerance to chilling injury. Our research reports for the first time CI tolerance in common mandarin using natural analogues (EBR) and spirostanics (DI-31).
5. Conclusions
The exogenous application by immersion of natural analogues (EBR) or spirostanics (DI-31) significantly reduced the incidence and appearance of CI (55–62% approx.) in the epidermis of ‘Arrayana’ mandarin fruits at the end of cold storage and shelf life, due to increased antioxidant activity and phenol content, which decreased EL and maintained cell membrane integrity. The fruits treated with BR, presenting a lower CI, exhibited better quality parameters in terms of reduced WL, RI, and chlorophyll degradation and increased concentration of β-carotene. To the best of our knowledge, this is the first report that BR application increases CI tolerance in common mandarin orange (C. reticulata Blanco, cv. ‘Arrayana’) and illustrates the tolerance functionality of the DI-31 analogue on postharvest CI. Therefore, it is recommended to use BR as an effective strategy to maintain postharvest quality under cold storage of ‘Arrayana’ mandarins, especially with 10 mg L−1 DI-31, which has a more affordable cost in the market than EBR.
Author Contributions
Conceptualization, D.A.G.-V., J.G.Á.-H. and H.E.B.-L.; methodology, D.A.G.-V., J.G.Á.-H. and H.E.B.-L.; formal analysis, D.A.G.-V., J.G.Á.-H. and H.E.B.-L.; investigation, D.A.G.-V., J.G.Á.-H. and H.E.B.-L.; writing—original draft preparation, D.A.G.-V. and J.G.Á.-H.; writing—review and editing, D.A.G.-V., J.G.Á.-H. and H.E.B.-L.; visualization, D.A.G.-V.; project administration, D.A.G.-V., J.G.Á.-H. and H.E.B.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Sandra Mendoza y Yaneth Alberto Rojas, support staff of the Laboratory of “Calidad y poscosecha de productos agrícolas” of the Universidad Nacional de Colombia, and the members of the Grupo de Investigaciones Agrícolas of the UPTC for support in laboratory material and data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Orduz-Rodríguez, J.O.; Monroy, J.; Barrera, S.; Núñez, V.; Ligarreto, G.A. Caracterización morfo-agronómica y molecular de mandarina ‘Arrayana‘ en el piedemonte del Meta (Colombia). Corpoica Cienc. Tecnol. Agropecu. 2012, 13, 5–12. [Google Scholar] [CrossRef]
- Balaguera-López, H.E.; Ortega, E.A.P.; Consuegra, S.A.L. Effects of thermal treatments on chilling injury and shelf life time of Citrus reticulata Blanco1. Pesqui Agropecu. Trop. 2019, 49, e56821. [Google Scholar] [CrossRef]
- Ladaniya, M. Citrus Fruit: Biology, Technology and Evaluation, 2nd ed.; Academic Press: San Diego, CA, USA, 2022. [Google Scholar] [CrossRef]
- Lado, J.; Cronje, P.; Rodrigo, M.; Zacarías, L. Chilling Injury. In Postharvest Physiological Disorders in Fruits and Vegetables; de Freitas, S.T., Pareek, S., Eds.; CRC Press: Nueva York, NY, USA, 2019; pp. 377–398. [Google Scholar] [CrossRef]
- Strano, M.C.; Altieri, G.; Allegra, M.; Di Renzo, G.C.; Paterna, G.; Matera, A.; Genovese, F. Postharvest Technologies of Fresh Citrus Fruit: Advances and Recent Developments for the Loss Reduction during Handling and Storage. Horticulturae 2022, 8, 612. [Google Scholar] [CrossRef]
- Lafuente, M.; Zacarías, L.; Sala, J.; Sanchez-Ballesta, M.; Gosalbes, M.; Marcos, J.; González-Candelas, L.; Lluch, Y.; Granell, A. Understanding the basis of chilling injury in citrus fruit. Acta Hortic. 2005, 682, 831–842. [Google Scholar] [CrossRef]
- Zacarias, L.; Cronje, P.J.; Palou, L. Postharvest technology of citrus fruits. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 421–446. [Google Scholar] [CrossRef]
- Chalutz, E.; Waks, J.; Schiffmann-Nadel, M. A comparison of the response of different citrus fruit cultivars to storage temperature. Sci. Hortic. 1985, 25, 271–277. [Google Scholar] [CrossRef]
- Balaguera-López, H.E.; Palacios, O. Comportamiento poscosecha de frutos de mandarina (Citrus reticulata Blanco) var. Arrayana: Efecto de diferentes tratamientos térmicos. Rev. Colomb. Cienc. Hortic. 2018, 12, 369–378. [Google Scholar] [CrossRef]
- Albornoz, K.; Zhou, J.; Yu, J.; Beckles, D.M. Dissecting postharvest chilling injury through biotechnology. Curr. Opin. Biotechnol. 2022, 78, 102790. [Google Scholar] [CrossRef]
- Valenzuela, J.L.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative Stress Associated with Chilling Injury in Immature Fruit: Postharvest Technological and Biotechnological Solutions. Int. J. Cell. Sci. Mol. Biol. 2017, 18, 1467. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Martínez-Romero, D.; Giménez, M.J.; Serrano, M.; García-Martínez, S.; Valero, D.; Valverde, J.M.; Zapata, P.J. Enhancing antioxidant systems by preharvest treatments with methyl jasmonate and salicylic acid leads to maintain lemon quality during cold storage. Food Chem. 2021, 338, 128044. [Google Scholar] [CrossRef]
- Rey, F.; Zacarías, L.; Rodrigo, M.J. Carotenoids, Vitamin C, and Antioxidant Capacity in the Peel of Mandarin Fruit in Relation to the Susceptibility to Chilling Injury during Postharvest Cold Storage. Antioxidants 2020, 9, 1296. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fundamentals of Plant Physiology, 6th ed.; Sinauer Associates: New York, NY, USA, 2018. [Google Scholar]
- Hussain, M.A.; Fahad, S.; Sharif, R.; Jan, M.F.; Mujtaba, M.; Ali, Q.; Ahmad, A.; Ahmad, H.; Amin, N.; Ajayo, B.S.; et al. Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regul. 2020, 92, 141–156. [Google Scholar] [CrossRef]
- Cáceres, J.L.; Eduard, D.A.; Cortés, M.C.; Balaguera-López, H.E. Role of brassinosteroids in fruit trees with emphasis on abiotic stress conditions: A review. Cienc. Agric. 2022, 19, 132–147. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2018, 135, 295–303. [Google Scholar] [CrossRef]
- Zullo, M.A.T.; Bajguz, A. The brassinosteroids family–structural diversity of natural compounds and their precursors. In Brassinosteroids: Plant Growth and Development; Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A., Eds.; Springer: Singapore, 2019; pp. 1–44. [Google Scholar] [CrossRef]
- Duran, M.I.; González, C.; Acosta, A.; Olea, A.F.; Díaz, K.; Espinoza, L. Synthesis of Five Known Brassinosteroid Analogs from Hyodeoxycholic Acid and Their Activities as Plant-Growth Regulators. Int. J. Mol. Sci. 2017, 18, 516. [Google Scholar] [CrossRef]
- Rivera, D.G.; León, F.; Coll, F.; Davison, G.P. Novel 5β-hydroxyspirostan-6-ones ecdysteroid antagonists: Synthesis and biological testing. Steroids 2006, 71, 1–11. [Google Scholar] [CrossRef]
- Ji, Y.; Qu, Y.; Jiang, Z.; Yan, J.; Chu, J.; Xu, M.; Su, X.; Yuan, H.; Wang, A. The mechanism for brassinosteroids suppressing climacteric fruit ripening. Plant Physiol. 2021, 185, 1875–1893. [Google Scholar] [CrossRef]
- Vergara, A.E.; Díaz, K.; Carvajal, R.; Espinoza, L.; Alcalde, J.A.; Pérez-Donoso, A.G. Exogenous Applications of Brassinosteroids Improve Color of Red Table Grape (Vitis vinifera L. Cv. “Redglobe”) Berries. Front. Plant Sci. 2018, 9, 363. [Google Scholar] [CrossRef]
- Zhu, F.; Yun, Z.; Ma, Q.; Gong, Q.; Zeng, Y.; Xu, J.; Cheng, Y.; Deng, X. Effects of exogenous 24-epibrassinolide treatment on postharvest quality and resistance of Satsuma mandarin (Citrus unshiu). Postharvest Biol. Technol. 2015, 100, 8–15. [Google Scholar] [CrossRef]
- Chai, Y.-M.; Zhang, Q.; Tian, L.; Li, C.-L.; Xing, Y.; Qin, L.; Shen, Y.-Y. Brassinosteroid is involved in strawberry fruit ripening. Plant Growth Regul. 2013, 69, 63–69. [Google Scholar] [CrossRef]
- Habibi, F.; Serrano, M.; Zacarías, L.; Valero, D.; Guillén, F. Postharvest Application of 24-Epibrassinolide Reduces Chilling Injury Symptoms and Enhances Bioactive Compounds Content and Antioxidant Activity of Blood Orange Fruit. Front. Plant Sci. 2021, 12, 12. [Google Scholar] [CrossRef]
- Tavallali, V. Vacuum infiltration of 24-epibrassinolide delays chlorophyll degradation and maintains quality of lime during cold stor-age. Acta Sci. Pol. Hortorum Cultus 2018, 17, 35–48. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.; Lv, X.; Cheng, N.; Peng, B.; Cao, W. Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Islam, M.; Ali, S.; Nawaz, A.; Naz, S.; Ejaz, S.; Shah, A.A.; Razzaq, K. Postharvest 24-epibrassinolide treatment alleviates pomegranate fruit chilling injury by regulating proline metabolism and antioxidant activities. Postharvest Biol. Technol. 2022, 188, 111906. [Google Scholar] [CrossRef]
- Massolo, J.F.; Sánchez, R.; Zaro, M.J.; Concellón, A.; Vicente, A.R. Low-dose prestorage 24-epibrassinolide spray enhances postharvest chilling tolerance in zucchini squash (Cucurbita pepo L.) by eliciting peroxidase and phenolic antioxidants. J. Food Process. Preserv. 2022, 46, e16576. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, T.; Gao, L.; Pang, J.; Yang, N. Effect of brassinolide on chilling injury of green bell pepper in storage. Sci. Hortic. 2012, 144, 195–200. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Huang, X.; Yang, K.; Gao, S.; Du, R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 2014, 168, 132–137. [Google Scholar] [CrossRef]
- Gómez, C.A.; Herrera, A.O.; Flórez, V.J. Efecto de 1-metilciclopropeno y temperatura de almacenamiento en la poscosecha de mandarina (Citrus reticulata L.) var. Arrayana. Rev. Fac. Cienc. Agrar. 2015, 47, 27–41. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- González, A.K.; González-Martínez, L.F.; Córdoba, L.D.; Rincón, A.; Balaguera-López, H.E. Regulating the postharvest life of Campomanesia lineatifolia R. & P. fruits through the interaction of ethylene, 1-methylcyclopropene and low temperatures. Rev. Colomb. Cienc. Hortic. 2021, 15, e12499. [Google Scholar] [CrossRef]
- Tesfay, S.; Bertling, I.; Bower, J. Effects of postharvest potassium silicate application on phenolics and other anti-oxidant systems aligned to avocado fruit quality. Postharvest Biol. Technol. 2011, 60, 92–99. [Google Scholar] [CrossRef]
- Grijalva-Verdugo, C.; Rodríguez-Núñez, J.R.; Núñez-Colin, C.A.; Aguirre-Mancilla, C.L.; Montoya-Anaya, D.; Villareal-Fuentes, J.M.; Balois-Morales, R.; Rodríguez-Carrillo, M.G. Total polyphenolic, antioxidants, and cytotoxic activity of infusions from soursop (Annona muricata) leaves from two Mexican regions. Agron. Colomb. 2022, 40, 300–310. Available online: https://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-99652022000200300 (accessed on 5 June 2022).
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Tian, J.; Xie, S.; Zhang, P.; Wang, Q.; Li, J.; Xu, X. Attenuation of postharvest peel browning and chilling injury of banana fruit by Astragalus polysaccharides. Postharvest Biol. Technol. 2022, 184, 111783. [Google Scholar] [CrossRef]
- Hatsugai, N.; Katagiri, F. Quantification of Plant Cell Death by Electrolyte Leakage Assay. Bio-Protocol 2018, 8, e2758. [Google Scholar] [CrossRef]
- Gao, H.; Kang, L.; Liu, Q.; Cheng, N.; Wang, B.; Cao, W. Effect of 24-epibrassinolide treatment on the metabolism of eggplant fruits in relation to development of pulp browning under chilling stress. J. Food Sci. Technol. 2015, 52, 3394–3401. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; Zacarías, L. Analysis of ethylene biosynthesis and perception during postharvest cold storage of Marsh and Star Ruby grapefruits. Food Sci. Technol. Int. 2015, 21, 537–546. [Google Scholar] [CrossRef]
- Cohen, E.; Shapiro, B.; Shalom, Y.; Klein, J. Water Loss: A Nondestructive Indicator of Enhanced Cell Membrane Permeability of Chilling-injured Citrus Fruit. J. Am. Soc. Hortic. Sci. 1994, 119, 983–986. [Google Scholar] [CrossRef]
- El-Eryan, E.E. Improving Tommy Atkins Mango Resistance to Chilling Injury During Cold Storage and Marketing. J. Plant Prod. 2020, 11, 563–573. [Google Scholar] [CrossRef]
- Brownleader, M.D.; Jackson, P.; Mobasheri, A.; Pantelides, A.T.; Sumar, S.; Trevan, M.; Dey, P.M. Molecular Aspects of Cell Wall Modifications during Fruit Ripening. Crit. Rev. Food Sci. Nutr. 1999, 39, 149–164. [Google Scholar] [CrossRef]
- Pakkish, Z.; Ghorbani, B.; Najafzadeh, R. Fruit quality and shelf life improvement of grape cv. Rish Baba using Brassinosteroid during cold storage. J. Food Meas. Charact. 2019, 13, 967–975. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Lado, J.; Zacarías, L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Liu, L.; Chengguo, J.; Min, Z.; Delong, C.; Sixue, C.; Rongfang, G.; Deping, G.; Qiaomei, W. Ectopic expression of a BZR1–1D transcription factor in brassinosteroid signalling enhances carotenoid accumulation and fruit quality attributes in tomato. Plant Biotechnol. J. 2014, 12, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yun, Z.; Wu, Q.; Zhang, Z.; Liu, S.; Shi, X.; Duan, X.; Jiang, Y. Proteomic profiling of 24-epibrassinolide-induced chilling tolerance in harvested banana fruit. J. Proteom. 2018, 187, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, T.; Shao, Z.; Meng, F.; Chen, H.; Wang, Q.; Zheng, J.; Liu, L. Brassinosteroid Biosynthetic Gene SlCYP90B3 Alleviates Chilling Injury of Tomato (Solanum lycopersicum) Fruits during Cold Storage. Antioxidants 2022, 11, 115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).