Abstract
Ascorbic acid (AsA) is the most abundant antioxidant in plants and is an important nutritional index for agricultural products. Some plants, such as Rosa roxburghii Tratt., contain exceptionally high levels of AsA, but are relatively unpalatable. In view of its role in human health, as well as plant growth and development, we examined the effects of two important AsA regulatory genes from R. roxburghii in tomato, with the aim of producing a crop of higher nutritional quality. RrGGP2 and RrDHAR were cloned from R. roxburghii fruit. The overexpression vectors were made using 35S promoters and mediated by Agrobacterium tumefaciens to obtain the overexpression lines. A PCR and qRT-PCR verified that the two genes had been inserted and overexpressed in the tomato leaves and fruits. The results showed that the overexpression of RrGGP2 increased tomato leaf and fruit AsA content by 108.5% and 294.3%, respectively, while the overexpression of RrDHAR increased tomato leaf and fruit AsA content by 183.9% and 179.9%. The overexpression of RrGGP2 and RrDHAR further changed the expression of genes related to AsA metabolism, and the upregulation of one such gene, SlGGP, may have contributed greatly to the increase in AsA. Results here indicate that RrGGP2 contributes more towards fruit AsA accumulation in tomato than RrDHAR.
1. Introduction
Ascorbic acid (AsA, Vitamin C) is an essential antioxidant in plants that scavenges reactive oxygen species (ROS) generated during exposure to biotic or abiotic stresses and those produced during normal growth and development [1,2,3]. Studies have also found that AsA regulates genes which control plant development [4,5], as well as flowering time regulation, premature senescence, and programmed cell death [6]. Unlike plants, humans cannot synthesize AsA independently due to the absence of a crucial enzyme called L-gulonolactone oxidase, and insufficient dietary AsA intake can cause a deficiency and related diseases in humans [7]. The predominant source of AsA is plant products such as fresh fruits or vegetables [8]; therefore, genetic engineering techniques have been extensively studied to enhance the AsA content of horticultural plant products [9].
Several pathways for AsA biosynthesis have been identified, including the D-mannose/L-galactose pathway [10], the D-galacturonate pathway [11], the myoinositol pathway [12], and the L-gulose pathway [13]. Among them, the L-galactose pathway has been identified as the principal pathway for AsA biosynthesis in many higher plants, such as apple [14], kiwifruit [15], R. roxburghii [16], blueberry [17], and tomato [18], and is the only pathway present in Arabidopsis [19]. In this pathway, D-glucose-6-phosphate is used as a substrate, which sequentially passes through glucose-6-phosphate isomerase (GPI), phosphomannose isomerase (PMI) [20], phosphomannose mutase (PMM) [21], GDP-mannose pyrophosphorylase (GMP) [22], GDP-mannose-3′,5′-phenotypic isomerase (GME) [13], GDP-L-galactose phosphorylase (GGP) [19,23], L-galactose-1-phosphatase (GPP) [24,25], L-galactose dehydrogenase (GDH) [26], and L-galactose-1,4-lactone dehydrogenase (GLDH) [27]. As a vital antioxidant and electron donor, AsA is metabolized to monodehydroascorbic acid (MDHA) and dehydroascorbic acid (DHA) by the reactions of ascorbate oxidase (AO) and ascorbate peroxidase (APX) [28], and hydrogen peroxide (H2O2) is consumed to form malonyl H2O and O2. MDHA and DHA are catalyzed by monodehydroascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR) [29,30], respectively.
All genes in this pathway have been cloned, with several identified as key genes for AsA accumulation in various plants. Interestingly, studies on the same plant have shown inconsistent findings regarding the key genes for AsA accumulation. For instance, in kiwifruit, GGP was originally believed to be the key gene [15,31], but later, GPP was found to play this role [32]. In apple, initial evidence suggested that GDH, GPP, and GME expression were closely linked to AsA accumulation [14], but later studies showed that GGP was the key gene [33]. In tomato, GME was initially believed to be the key rate-limiting gene for AsA regulation [34,35], but recent research indicates that GGP is the actual key gene [33,36]. These variations in conclusions could be due to varietal differences or caused by experimental methods [33], and this controversy also exists in R. roxburghii. One research study has suggested that RrDHAR plays a pivotal role in achieving the high levels of AsA accumulation seen in R. roxburghii fruit [37], while another study has indicated that RrGGP2 plays a more significant regulatory role in R. roxburghii AsA biosynthesis [38,39]. In this study, we aim to explore the roles of RrGGP2 and RrDHAR in AsA biosynthesis and determine which makes the greatest contribution to the accumulation of AsA in R. roxburghii.
R. roxburghii belongs to the Rosaceae family, which is cultivated as a functional fruit rich in a variety of nutrients and health-promoting compounds [40,41,42]. It is primarily found in Southwest China and referred to as the ‘King of Vitamin C’ because of its high vitamin C content [43,44]. Therefore, understanding the mechanism behind this exceptional AsA accumulation and identifying the key genes responsible for its regulation would be significant. However, due to the absence of effective genetic transformation and regeneration systems, RrGGP2 and RrDHAR’s functional validation is best conducted in model organisms, such as Arabidopsis and tobacco [37,39]. Such an approach, however, has its limitations, as they are not food crop plants and hence do not produce fruits for human consumption. As a consequence, this study aims to shed light on the functions of RrGGP2 and RrDHAR by performing genetic transformation experiments in tomato, a food crop plant that bears fruit, to provide more impactful and informative evidence regarding the mechanism of high AsA accumulation in R. roxburghii.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The donor material used was a three-year-old asexual line of R. roxburghii ‘Guinong 5’, while tomato plants (‘Ailsa Craig’) were selected as the receiver plants. Wild and subsequently obtained transgenic plants were grown in a greenhouse under specific conditions, including a temperature range of 25–28 °C, a humidity level of 60–80%, a light intensity between 30,000–50,000 lux, and a light/dark alternation time of approximately 13/11 h. The tomato plants were irrigated with Hoagland’s nutrient solution every five days and watered every 2–3 days, according to standard production practices. The tomatoes were managed until maturity, which typically occurred after around 50 days of self-pollination. The 4th–6th leaves and mature fruits were then harvested and snap-frozen using liquid nitrogen, before being stored in an ultra-low temperature refrigerator at −80 °C for subsequent experiments.
2.2. Total DNA and RNA Extraction, cDNA Synthesis
The Genome DNA Extraction Kit (BioTeke, Wuxi, China) was used to extract total genomic DNA from the leaves of both ‘Guinong 5’ and ‘Ailsa Craig’. Moreover, total RNA was extracted from the fruits of ‘Guinong 5’ and the leaves and fruits of ‘Ailsa Craig’ using the RNAprep Pure Plant Plus Kit (TIANGEN, Beijing, China). To synthesize the first strand of cDNA, the PrimeScript™ RT reagent Kit (Takara, Dalian, China) was used.
2.3. Cloning of Target Gene and Constructing of Overexpression Vector
The transformation vector was constructed using pcambia1301-ky (Figure 1), and the target gene was integrated into the vector by homologous recombination. To achieve this, homologous cloning primers were designed based on the sequences of the target gene [45] and vector. The forward and reverse cloned primers both included the KpnI restriction enzyme site at the 5′ end of each. Subsequently, the full-length RrGGP2 and RrDHAR were amplified via PCR from ‘Guinong 5′ cDNA using homologous recombination clone primers (Table 1), while the vector was digested using the KpnI enzyme. Finally, RrGGP2 and RrDHAR were introduced into the KpnI polyclonal site located between the 35S promoter and the nos terminator of the vector through the action of recombinase (ClonExpress II One Step Cloning Kit, Vazyme). The resultant recombinant vector containing the RrGGP2 or RrDHAR gene sequence was then transferred into the Agrobacterium tumefaceiens strain LBA4404 and saved for transformation into tomato plants.
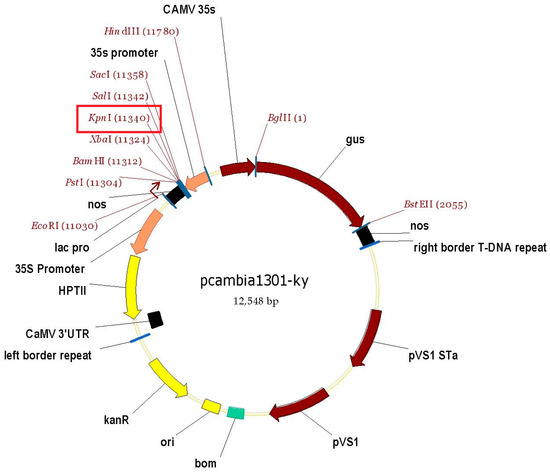
Figure 1.
A map of the pcambia1301-ky overexpression vector, which is regulated by the 35S promoter and the nos terminator. The vector includes a reporter gene (gus) and is designed with both kanamycin (KanR) and hygromycin (HPTII) resistance genes for screening transformed plants. The restriction enzyme site is at the red rectangle.

Table 1.
Primer names and sequences used in this experiment.
2.4. Plant Transformation
The transgenic tomato was created through genetic transformation mediated by Agrobacterium tumefaciens. Initially, tomato cotyledons were cultured under light for 7 days, following which they were infected with Agrobacterium tumefaciens for 10 min and cocultured for 2 days. A screening culture was then carried out to encourage the growth of adventitious buds using the MS medium supplemented with 2.0 mg/L zeatin (ZT), 1 mg/L indole-3-acetic acid (IAA), 300 mg/L timentin, and 10 mg/L hygromycin. Finally, the adventitious buds were transferred to a 1/2 MS medium containing 150 mg/L timentin and 10 mg/L hygromycin to induce root growth and recover the whole plant.
2.5. Transgenic Plants Identification and qRT-PCR Analysis
To identify transgenic tomato plants resulting from plant transformation, we used a PCR with genomic DNA from leaves and a qRT-PCR with cDNA from both leaves and fruits. Additionally, a qRT-PCR was utilized to analyze the gene expression related to AsA biosynthesis and metabolism in the leaves and fruits of both wild-type and transgenic plants. The genes analyzed included GPI, PMI, PMM, GMP, GME, GGP, GPP, GDH, and GLDH in the L-galactose pathway, AO and APX in the catabolism pathway, as well as DHAR and MDHAR in the recycling pathway. Previous studies [46,47] were referenced for primer design. The Acitin gene of Solanum lycopersicum (Solyc11g005330) was utilized as an internal reference gene to normalize expression data. All the primers were synthesized through Sangon Bioengineering Co., Ltd., (Shanghai, China) (Table 1). The qRT-PCR was performed on an ABI ViiA 7 DX system (Applied Biosystems, Waltham, MA, USA) using TB Green® Premix Ex Taq™ II kit (Takara). The qRT-PCR reaction system was 20 μL: SYBR Premix Ex Taq (2×) 10 μL, 10 μmol/L forward and reverse primers 1 μL each, c-DNA 1 μL, ddH2O 7 μL. Reaction procedure was 95 °C 30 s; 95 °C 5 s, 55~60 °C 20 s, 40 cycles; 72 °C 20 s. Melting curve procedure was 95 °C 15 s; 55~95 °C 1 min in 0.3 °C increments; 95 °C 15 s. All reactions were performed in three replicates and the relative expression was calculated using the 2−ΔΔCT method.
2.6. Determination of AsA and DHA Content
The concentration of AsA and DHA were determined according to a previous method with minor modifications [48]. An amount of 0.5 g of leaf or fruit was ground into a homogenate in 5% metaphosphoric acid, centrifuged at 7500 rpm for 20 min at 4 °C, and the supernatant was collected. A total of 100 μL of supernatant was pipetted with 2.9 mL of 100 mmol/L potassium phosphate buffer (pH 6.8), and the change of absorbance value at 265 nm was recorded when 1 U of AAO was added. For the determination of DHA, 100 μL of the extract was added to 1.9 mL of 100 mmol/L potassium phosphate buffer (pH 6.8), and the absorbance at 265 nm was recorded after the addition of 2 mmol/L DTT. At the same time, standard curves were made with known concentrations of AsA and DHA solutions using the same method to calculate the AsA and DHA contents of the samples. Each tomato line was measured in triplicate.
2.7. Statistical Analysis
All data were determined in three independent biological replicates for each experiment. Data were counted and graphs were made using Excel 2019. Significant differences were tested by Duncan’s method and correlation analysis was performed using Pearson’s method in SPSS 26.0 software. The significance levels remained p < 0.05 and p < 0.01, respectively.
3. Results
3.1. Positive Identification of Transgenic Tomato Lines
A total of four tomato lines each overexpressing RrGGP2 and RrDHAR were obtained by genetic transformation. The genome DNA of the transgenic tomato was used as the template, wild-type tomato DNA was utilized as a negative control, and R. roxburghii DNA was used as a positive control, using the specific primers (Table 1) to determine the presence of RrGGP2 and RrDHAR in the transgenic tomato lines. After agarose gel electrophoresis, the electrophoretic bands of the four lines overexpressing RrGGP2 (Figure 2A) and the four lines overexpressing RrDHAR (Figure 2B) were of the same intensity as the positive control. However, in the case of the wild-type tomato, the target fragments were not amplified (Figure 2A,B), indicating that the RrGGP2 and RrDHAR genes of R. roxburghii have been successfully integrated into the tomato genome. The results of the qRT-PCR further confirmed that the expression levels of RrGGP2 and RrDHAR in the leaves and fruits were significantly higher than the control (Figure 2C,D), thus proving that the two genes were successfully overexpressed in the tomato lines.
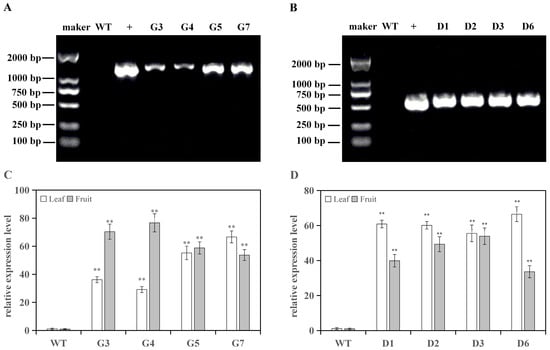
Figure 2.
Positive identification and overexpression verification of transgenic tomato lines. (A) confirmation of RrGGP2 by PCR analysis; (B) confirmation of RrDHAR by PCR analysis; (C) relative expression of RrGGP2 in transgenic tomato plants; (D) relative expression of RrDHAR in transgenic tomato plants. RrGGP2 transgenic lines are represented by G3, G4, G5, and G7, and RrDHAR transgenic lines are represented by D1, D2, D3, and D6. M = marker; WT = wild-type plant; + = positive control (PCR amplification products of the GGP2 and DHAR gene of R. roxburghii); bp—number of bases. The relative quantification of RrGGP2 and RrDHAR expression was calculated using the comparative Ct (2−ΔΔCT) method; three replicate experiments were performed; error bars represent standard error, means ± SE. The asterisks above the bar chart represent significant differences from WT (** p < 0.01).
3.2. Analysis of Ascorbate Levels in Transgenic Lines
3.2.1. Analysis of Ascorbate Levels in Transgenic Tomato Fruits
The determination results indicate that the overexpression of RrGGP2 and RrDHAR genes substantially augmented the levels of AsA and DHA in tomato fruits (Figure 3A,B). The content of AsA increased significantly from 209.7% to 384.3% in all the four tomato lines that overexpressed RrGGP2, and there was an increase in DHA that ranged from 69.2% to 309.0% (Figure 3A). Similarly, in the four tomato lines overexpressing RrDHAR, AsA content was increased by 175.4% to 213.6%, and DHA content was increased from 116.8% to 261.2% (Figure 3B). On average, the overexpression of RrGGP2 in the tomato lines resulted in an increase of 294.3% for AsA and 190.8% for DHA, compared to an increase of 179.9% for AsA and 182.6% for DHA in the tomato lines overexpressing RrDHAR (Figure 3A,B). Hence, it is clear that the overexpression of RrGGP2 is more effective in enhancing the AsA content of tomato fruits than the overexpression of RrDHAR.
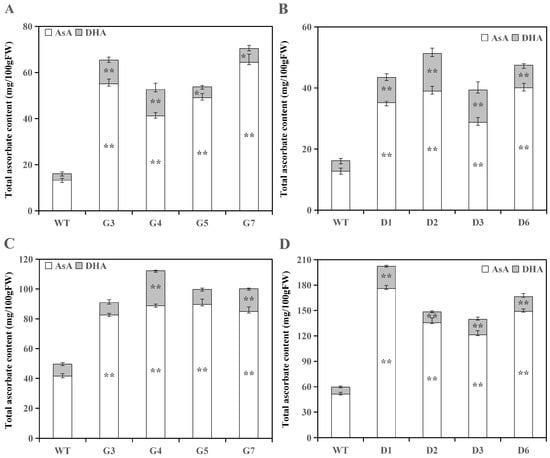
Figure 3.
Determination of AsA and DHA contents in tomato lines overexpressing RrGGP2 and RrDHAR. (A) AsA and DHA contents in transgenic RrGGP2 fruits; (B) AsA and DHA contents in transgenic RrDHAR fruits; (C) AsA and DHA contents in transgenic RrGGP2 leaves; (D) AsA and DHA contents in transgenic RrDHAR leaves. Three replicate experiments were performed; error bars represent standard error, means ± SE; FW—fresh weight. The asterisks represent significant differences from WT (* p < 0.05; ** p < 0.01).
3.2.2. Analysis of Ascorbate Levels in Transgenic Tomato Leaves
The overexpression of RrGGP2 and RrDHAR genes also significantly increased AsA and DHA contents of transgenic tomato leaves (Figure 3C,D). Notably, the AsA content in the leaves of the four tomato lines overexpressing RrGGP2 was significantly increased, ranging between 99.1% and 116.3%, while DHA content increased from 2.6% to 188.9%; however, only two lines (G4 and G7) of transgenic lines showed a significant increase (Figure 3C). In contrast, the AsA content in the leaves of the four overexpressing RrDHAR tomato lines was significantly increased between 136.6% and 243.5%, and DHA content increased from 55.8% to 220.5% (Figure 3D). On average, the AsA and DHA levels in the leaves of tomato lines overexpressing RrGGP2 increased by 108.5% and 75.7%, respectively. Similarly, in the tomato plants overexpressing RrDHAR, both the AsA and DHA levels in the leaves increased by 183.9% and 127.3%, respectively (Figure 3C,D). Therefore, it is evident that the overexpression of RrDHAR leads to a more pronounced increase in the AsA and DHA content in tomato leaves than RrGGP2.
3.3. Analysis of Genes Expression in Transgenic Lines
3.3.1. Analysis of Genes Expression in Transgenic Tomato Fruits
The overexpression of RrGGP2 and RrDHAR affected the expression of a range of genes related to the biosynthesis, catabolism, and recycling of AsA in transgenic tomato line fruits. In four tomato lines overexpressing RrGGP2, the expression of SlPMI, SlGME, SlGGP, SlAO, SlDHAR, and SlMDHAR were upregulated, and SlGPI, SlPMM, SlGMP, SlGPP, SlGLDH, and SlAPX were downregulated; SlGDH was significantly upregulated in three lines, while in line G7 it was significantly downregulated (Figure 4A). Furthermore, in the four tomato lines overexpressing RrDHAR, the expression of SlGMP, SlGME, SlGLDH, SlAO, SlAPX, SlDHAR, and SlMDHAR was upregulated, while SlGPI, SlPMI, SlGGP, and SlGPP were downregulated. The expression of SlPMM and SlGDH was not significantly different from the control (Figure 4B).
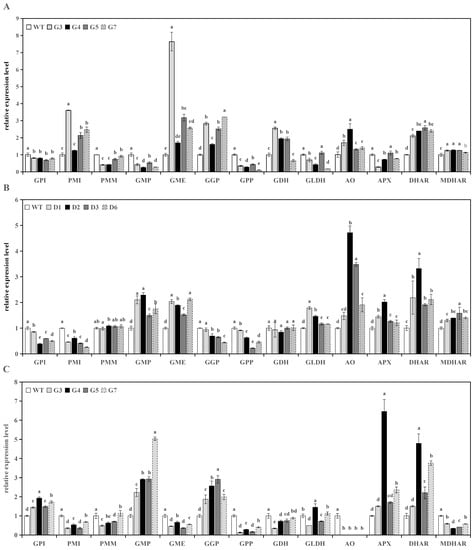
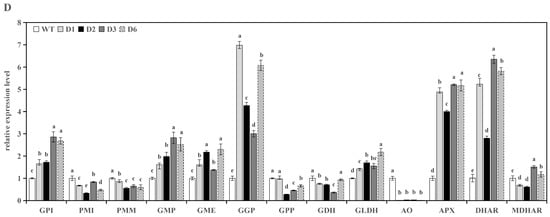
Figure 4.
Relative expression of AsA biosynthesis, metabolism, and recycling-related genes in the WT and transgenic lines. (A) Expression of transgenic RrGGP2 fruits; (B) expression of transgenic RrDHAR fruits; (C) expression of transgenic RrGGP2 leaves; (D) expression of transgenic RrDAHR leaves. The relative expression levels of each gene were obtained using the comparative Ct (2−ΔΔCt) method; three replicate experiments were performed; error bars represent standard error, means ± SE; the same letter above bars indicates a nonsignificant difference at the p < 0.05 probability level.
3.3.2. Analysis of Genes Expression in Transgenic Tomato Leaves
The overexpression of RrGGP2 and RrDHAR also significantly affected the expression of genes related to AsA biosynthesis and metabolism in transgenic tomato line leaves. In the leaves of the four tomato lines overexpressing RrGGP2, the expressions of SlGPI, SlGMP, SlGGP, SlAPX, and SlDHAR were upregulated, and SlPMI, SlPMM, SlGME, SlGPP, SlGDH, SlAO, and SlMDHAR were downregulated (Figure 4C). SlGLDH was upregulated in G4 and G7 and downregulated in G3 and G5. Similarly, the expression of SlGPI, SlGMP, SlGME, SlGGP, SlGLDH, SlAPX, and SlDHAR was upregulated, and SlPMI, SlPMM, SlGPP, SlGDH, and SlAO were downregulated in the four tomato lines overexpressing RrDHAR. In addition, the expression of SlMDHAR was upregulated in G5 and G7, but downregulated in G3 and G4 (Figure 4D).
3.4. Correlation Analysis between Gene Expression and AsA Content
3.4.1. Correlation Analysis between Gene Expression and AsA Content in Transgenic Tomato Fruits
There was a significant correlation between the expression of SlGGP and the content of AsA in the fruits of tomato lines overexpressing RrGGP2; additionally, positive correlations were observed with SlPMI, SlGME, and SlDHAR. The expression of SlAO was significantly correlated with DHA (Table 2). In the RrDHAR overexpressing tomato lines, SlGME was significantly correlated with AsA content, while SlGMP and SlDHAR expression were also highly correlated with AsA levels. Furthermore, SIAO was observed to have a significant correlation with DHA (Table 2).

Table 2.
Correlation analysis between gene expression and AsA content in transgenic tomato fruits.
3.4.2. Correlation Analysis between Gene Expression and AsA Content in Transgenic Tomato Leaves
In the leaves of tomato lines overexpressing RrGGP2, the expression of SlGGP was significantly correlated with AsA content, and SlGPI, SlGMP, SlAPX, and SlDHAR were also positively correlated with AsA. Notably, SlAO had very low expression but showed a highly significant negative correlation with AsA, which matches its function of AsA catabolism. Regarding the concentration of DHA, both SlAPX and SlDHAR showed a significant correlation (Table 3). In the transgenic RrDHAR tomato lines, the expression of SlGGP was significantly correlated with AsA content, and SlGPI, SlGMP, SlGME, SlGLDH, SlAPX, and SlDHAR were also positively correlated with AsA, whilst SlAO showed a significant negative correlation with AsA content. For DHA, SlAPX and SlDHAR were correlated with high coefficients of correlation of 0.761 and 0.765, respectively (Table 3).

Table 3.
Correlation analysis between gene expression and AsA content in transgenic tomato leaves.
4. Discussion
4.1. Overexpressing RrGGP2 and RrDHAR in Tomato Indicates That RrGGP2 Is the Key Control Point of AsA Biosynthesis and Metabolism
Over the years, genes relating to AsA biosynthesis and metabolism in R. roxburghii have been identified and cloned, and their functions have been verified by heterologous overexpression. For instance, the overexpression of RrGDH and RrGGP2 in tobacco increased the leaf AsA content by an average of 1.1-fold and 12-fold [16,39], while increasing the leaf AsA content by 3.02-fold and 2.11-fold was achieved by overexpressing RrDHAR and RrGME in Arabidopsis [37]. Indeed, RrGGP2 and RrDHAR are particularly good candidates for increasing AsA biosynthesis in R. roxburghii [49]; however, further validation of these two genes was required in a model plant that has edible fruit, such as tomato, to assess their effectiveness in a plant with commercial applications. Our results reveal that overexpressing RrGGP2 increases AsA content in tomato fruits to a greater extent than RrDHAR, which suggests that RrGGP2 may have greater potential to enhance fruit AsA content in other fruit crops via molecular breeding.
GGP catalyzes the conversion of GDP-L-galactose into L-galactose-1-phosphate, making it the first specific enzyme in the L-galactose pathway. Numerous previous studies on a variety of crops have demonstrated that overexpressing GGP can significantly augment AsA content; these crops include Arabidopsis [15,50,51,52], tobacco [23,39,53,54,55], tomato [31,36,56,57], rice [2,58,59], kiwifruit [60], strawberry, and potato [31]. Although other genes in this pathway may also amplify AsA content, such as PMM in Acerola and GME in alfalfa [50,61], not all genes have the same effect on recipient plants. For instance, GMP and GME in peach [62] were found to not increase AsA content. After summarizing experimental data from previous studies and conducting a comparative analysis, we have determined that overexpressing native GGP has a greater impact on fruit AsA content compared to overexpressing other genes in the AsA pathway. For instance, overexpressing SlGGP in tomatoes resulted in a 3-fold increase in fruit AsA content [36], which was higher than the increases observed with SlGMP (1.22 to 1.60-fold) [63], SlGME (1.22 to 1.42-fold) [35], SlDHAR (1.4 to 1.5-fold), and SlMDHAR [64]. Furthermore, overexpressing Arabidopsis AsA biosynthesis-related GMP, GME, GGP, GPP, GDH, and GLDH in Arabidopsis leaves resulted in 1.3, 1.4, 2.9, 1.5, 1.2, and 1.8-fold increases in the leaves’ AsA content, respectively [51]. Although not all the differences were significant, it offers initial evidence that indicates that the single transformation of GGP is more effective than other genes in increasing AsA content, while other genes have additive effects compared to GGP alone. While other genes may not be as efficient as GGP, studies show that co-transforming genes such as GME, GPP, and GLDH can significantly increase the AsA content, ultimately leading to an increase in AsA accumulation in plants. Metabolic control analysis of known kinetic parameters in Arabidopsis can explain why GGP is the key gene in this conclusion, while manipulating other genes can have minimal impact on AsA content. This is due to feedback inhibition of the GGP catalytic step that provides high flow control coefficients [52,65]. Apart from physiological and biochemical evidence, bioinformatics analysis also identified tomato GGP as a key gene for AsA biosynthesis [33,36]. AsA accumulation in 11 wild and cultivated tomatoes and QTL analysis for ascorbic acid content in strawberry fruit reveals a complex genetic architecture and association with GDP-L-galactose phosphorylase [66,67]. As we have speculated, GGP was recently identified once again as a key gene in two kiwifruits with distinct AsA content based on transcriptomic data [60], further solidifying its role in AsA accumulation.
Why does GGP play such a crucial and distinctive role in the regulation of AsA biosynthesis and metabolism? With the rapid advancement of genome sequencing technology, we now have some answers from the perspective of gene evolution. GGP genes are present in all plants, and due to their unique whole-gene replication mode, GGP is significantly amplified in angiosperms. The majority of GGP proteins have similar catalytic functions, which can be attributed to their conserved motif arrangement and composition. This may explain why angiosperms have a higher AsA content and can adapt more readily to environmental changes [68]. Previous evolutionary analysis has shown that R. roxburghii and strawberries are closely related [39], and strawberries are known for their high AsA content, which may be due to their relatively conservative motif arrangement and composition. Furthermore, research has revealed that the expression of GGP in various plants is significantly regulated by light and undergoes drastic changes under stress [19,69]. Analysis of the GGP promoter sequence has shown that it contains numerous cis-acting elements associated with light response and stress response [70]. Additionally, the light and photosynthesis-dependent rate-limiting enzyme GGP is activated and plays a critical role in the regulation of the ascorbate pool size [71]. Recent studies on the mechanism of GGP translation regulation propose a model that allows for a feedback response to regulate AsA synthesis under adverse conditions. Under rapidly changing conditions, uORF directly regulates GGP translation without gene transcriptional modification, and this points to a more dependable way to regulate AsA concentration [72]. All the evidence mentioned above once again confirms that, from the perspective of genetic structure composition, GGP plays a key role in AsA biosynthesis.
4.2. Similarities and Differences for the Mechanism of AsA Accumulation in Tomato Fruit and Leaf
The accumulation of AsA depends on the interplay between biosynthesis, catabolism, and cycling [73]. In this study, we comprehensively analyzed these three pathways to investigate AsA accumulation in the fruits and leaves of transgenic tomatoes. Our findings revealed both similarities and differences.
GMP, GME, and GGP have been identified as good candidates for promoting AsA accumulation in tomato [63]. The overexpression of SlGMP, SlGME, or SlGGP has been found to increase AsA content in tomato [35,36,63]. In our experiment, at least two of the three key genes were simultaneously upregulated in the fruits and leaves of transgenic tomatoes, which is likely why the AsA content was higher in transgenic tomatoes than in the control. Furthermore, through comparison and summarization, we suggest that there may be an additive model centered on GGP. From the perspective of increasing AsA content, GMP-GME-GGP co-expression yields higher results than GGP-GMP/GGP-GME, which is higher than GMP-GME and also higher than GMP/GME/GGP [15,56,74,75]. This may explain why the AsA content in fruits of tomatoes overexpressing RrGGP2 is higher than that of RrDHAR and why the AsA content in leaves of tomatoes overexpressing RrDHAR is higher than that of RrGGP2. In transgenic RrGGP2 tomato fruits, the expression of SlGME and SlGGP was upregulated simultaneously, while in transgenic RrDHAR tomato fruits, the expression of SlGMP and SlGME was upregulated and SlGGP expression was downregulated. The expression of SlGMP, SlGME, and SlGGP was simultaneously upregulated in transgenic RrDHAR tomato leaves, but only SlGMP and SlGGP were upregulated in transgenic RrGGP2 tomato leaves, and SlGME was downregulated. Additionally, the expression of SlGGP in transgenic RrDHAR tomato leaves was much higher than that in RrGGP2, and the correlation results further confirmed that the upregulation of key genes in the biosynthetic pathway, especially SlGGP, may be the main factor contributing to the increase in AsA. Recent studies have shown that GMP, GME, and GGP proteins are located in the cytoplasm [65] and that these enzyme complexes likely interact, demonstrating channelization [76]. This finding confirms the complex regulation of AsA pool size in tomato.
In terms of catabolism and recycling pathways, SlAPX was found to be significantly upregulated in the leaves of both transgenic tomatoes. It was observed that the high expression of SlAPX could be detrimental to AsA accumulation, while almost no expression of SlAO was very beneficial to AsA accumulation [77,78]. Although the expression levels of the two genes were opposite, the final phenotypic results of tomatoes showed that AsA was still elevated. The correlation analysis results confirmed the positive effect of SlAO on AsA accumulation. However, it was not immediately clear why the significant downregulation of SlAPX expression did not result in AsA content remaining constant or decreasing. This may be related to the multiple functions of APX in plants; in addition to its oxidative function in AsA metabolism, APX also acts as an important antioxidant enzyme to scavenge ROS under various stress conditions. The overexpression of APX can improve tolerance to various stresses in tobacco [79], tomato [80], and Arabidopsis [81,82]. Therefore, the upregulation of SlAPX expression may not only affect the catabolism of AsA but also perform tasks related to stress resistance. Interestingly, the expression of SlAO or SlAPX in fruits was not significantly inhibited; this may be because fruits contain more abundant secondary metabolites and more hierarchical biological structures than leaves, thus having a potentially higher stress resistance effect. The expression of SlDHAR was significantly upregulated in the fruits and leaves of the two transgenic tomatoes; although the correlation coefficient was not statistically significant, it was still at a relatively high level with all biosynthetic and metabolic genes. We believe that the upregulation of SlDHAR expression in the recycling pathway is also an important factor for the accumulation of AsA in tomatoes. In addition to the function of DHA recovery, the AsA-GSH system involved in DHAR plays an important role in managing H2O2 and indirectly participates in ROS scavenging to protect plants from environmental stress and better accumulate AsA [83,84,85,86,87,88]. The expression of SlMDHAR has no obvious regularity in transgenic lines. Some studies have shown that MDHAR is not a key gene regulated by AsA [64].
5. Conclusions
In summary, this is the first functional validation of RrGGP2 and RrDHAR in tomato, a valuable agricultural crop. The results indicate that the overexpression of RrGGP2 and RrDHAR can increase AsA content in tomato leaves and fruits, with the effect of RrGGP2 being more significant in fruits. Expression measurements of genes relating to AsA biosynthesis and catabolism in transgenic lines revealed both similarities and differences in the mechanisms of AsA accumulation in fruits and leaves, in which SlGGP may play a key role. Additionally, this study provides evidence that can lead to a better understanding of the crucial role that RrGGP2 plays in R. roxburghii AsA biosynthesis.
Author Contributions
Conceptualization, resources, wring—review and editing, H.A., M.L. and R.A.L.; investigation, methodology and formal analysis, Z.L, T.R. and Y.Y.; validation, data curation, writing–original draft preparation, Z.L.; Project administration, M.L. and H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32060657, 32260730), the Basic Research Program of Guizhou Province (20201Y113), and the Innovation and Entrepreneurship Training Program of Guizhou University (2021016).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets presented in this study are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Broad, R.C.; Bonneau, J.P.; Hellens, R.P.; Johnson, A.A.T. Manipulation of Ascorbate Biosynthetic, Recycling, and Regulatory Pathways for Improved Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 1790. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2012, 64, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Pastori, G.M.; Kiddle, G.; Antoniw, J.; Bernard, S.; Veljovic-Jovanovic, S.; Verrier, P.J.; Noctor, G.; Foyer, C.H. Leaf Vitamin C Contents Modulate Plant Defense Transcripts and Regulate Genes That Control Development through Hormone Signaling. Plant Cell 2003, 15, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Viviani, A.; Fambrini, M.; Giordani, T.; Pugliesi, C. L-Ascorbic acid in plants: From biosynthesis to its role in plant development and stress response. Agrochimica 2021, 65, 151–171. [Google Scholar] [CrossRef]
- Barth, C.; Moeder, W.; Klessig, D.F.; Conklin, P.L. The Timing of Senescence and Response to Pathogens Is Altered in the Ascorbate-Deficient Arabidopsis Mutant Vitamin c-1. Plant Physiol. 2004, 134, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Lachapelle, M.Y.; Drouin, G. Inactivation dates of the human and guinea pig vitamin C genes. Genetica 2011, 139, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Shi, Q.; Yu, X. Ascorbic acid contents in transgenic potato plants overexpressing two dehydroascorbate reductase genes. Mol. Biol. Rep. 2011, 38, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 2012, 108, 692–698. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 5. [Google Scholar] [CrossRef]
- Agius, F.; González-Lamothe, R.; Caballero, J.; Munoz-Blanco, J.; Botella, M.A. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 2003, 21, 177–181. [Google Scholar] [CrossRef]
- Lorence, A.; Chevone, B.I.; Mendes, P.; Nessler, C.L. myo -Inositol Oxygenase Offers a Possible Entry Point into Plant Ascorbate Biosynthesis. Plant Physiol. 2004, 134, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Wolucka, B.A.; Van Montagu, M. GDP-Mannose 3′,5′-Epimerase Forms GDP-L-gulose, a Putative Intermediate for the de Novo Biosynthesis of Vitamin C in Plants. J. Biol. Chem. 2003, 278, 47483–47490. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Wang, P.; Ma, F. Ascorbic Acid Accumulation and Expression of Genes Involved in Its Biosynthesis and Recycling in Developing Apple Fruit. J. Am. Soc. Hortic. Sci. 2011, 136, 231–238. [Google Scholar] [CrossRef]
- Bulley, S.M.; Rassam, M.; Hoser, D.; Otto, W.; Schunemann, N.; Wright, M.; MacRae, E.; Gleave, A.; Laing, W. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J. Exp. Bot. 2009, 60, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; An, H.M.; Yang, M. Overexpression of Rosa roxburghii l-galactono-1,4-lactone dehydrogenase in tobacco plant enhances ascorbate accumulation and abiotic stress tolerance. Acta Physiol. Plant. 2013, 35, 1617–1624. [Google Scholar] [CrossRef]
- Liu, F.H.; Wang, L.; Gu, L.; Zhao, W.; Su, H.Y.; Cheng, X.H. Higher transcription levels in ascorbic acid biosynthetic and recycling genes were associated with higher ascorbic acid accumulation in blueberry. Food Chem. 2015, 188, 399–405. [Google Scholar] [CrossRef]
- Mellidou, I.; Kanellis, A.K. Genetic Control of Ascorbic Acid Biosynthesis and Recycling in Horticultural Crops. Front. Chem. 2017, 5, 50. [Google Scholar] [CrossRef]
- Dowdle, J.; Ishikawa, T.; Gatzek, S.; Rolinski, S.; Smirnoff, N. Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability: Role of GDP-l-Gal phosphorylase in ascorbate biosynthesis. Plant J. 2007, 52, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Maruta, T.; Yonemitsu, M.; Yabuta, Y.; Tamoi, M.; Ishikawa, T.; Shigeoka, S. Arabidopsis Phosphomannose Isomerase 1, but Not Phosphomannose Isomerase 2, Is Essential for Ascorbic Acid Biosynthesis. J. Biol. Chem. 2008, 43, 28842–28851. [Google Scholar] [CrossRef]
- Qian, W.Q.; Yu, C.M.; Qin, M.; Liu, X.; Zhang, A.M.; Johansen, I.E.; Wang, D.W. Molecular and functional analysis of phosphomannomutase (PMM) from higher plants and genetic evidence for the invoIvement of PMM in ascorbic acid biosynthesis in Arabidopsis and Nicotiana benthamiana. Plant J. 2007, 49, 399–413. [Google Scholar] [CrossRef]
- Conklin, P.L.; Norris, S.R.; Wheeler, G.L.; Williams, E.H.; Smimoff, N.; Last, R.L. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 4198–4203. [Google Scholar] [CrossRef]
- Laing, W.A.; Wright, M.A.; Cooney, J.; Bulley, S.M. The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an l -galactose guanyltransferase, increases leaf ascorbate content. Proc. Natl. Acad. Sci. USA 2007, 104, 9534–9539. [Google Scholar] [CrossRef] [PubMed]
- Conklin, P.L.; Gatzek, S.; Wheeler, G.L.; Dowdle, J.; Raymond, M.J.; Rolinski, S.; Isupov, M.; Littlechild, J.A.; Smirnoff, N. Arabidopsis thaliana VTC4 encodes L-galactose-1-phosphatase, a plant ascorbic acid biosynthetic enzyme. J. Biol. Chem. 2006, 281, 15662–15670. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, J.; Donahue, J.L.; Gunesekera, B.N.; Allen-Daniels, M.J.; Gilkpy, G.E. VTC4 is a bifunctional enzyme that affects myoinositol and ascorbate biosynthesis in plants. Plant Physiol. 2009, 150, 951–961. [Google Scholar] [CrossRef]
- Gatek, S.; Wheeler, G.L.; Smirnoff, N. Antisense suppression of L-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated L-galactose synthesis. Plant J. 2002, 30, 541–553. [Google Scholar] [CrossRef]
- Imai, T.; Karita, S.; Shiratori, G.; Hattori, M.; Nunome, T.; Oba, K.; Himi, M. L- galactono-gamma-1actone dehydrogenase from sweet potato: Purification and cDNA sequence analysis. Plant Cell Physiol. 1998, 39, 1350–1358. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Eltayeb, A.E.; Kawanob, N.; Badawic, G.H.; Kaminakaa, H.; Sanekatad, T.; Inanaga STanaka, K. 0verexpression of monodehydroascorbate reductase in tmnsgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 2007, 225, 1255–1264. [Google Scholar] [CrossRef]
- Yoshida, S.; Tamaoki, M.; Shikano, T.; Nakajima, N.; Ogawa, D.; Ioki, M.; Aono, M.; Kubo, A.; Kamada, H.; Inoue, Y.; et al. Cytosolic dehydroascorbate reducmse is impoaam for ozone tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 304–308. [Google Scholar] [CrossRef]
- Bulley, S.; Wright, M.; Rommens, C.; Yan, H.; Rassam, M.; Lin-Wang, K.; Andre, C.; Brewster, D.; Karunairetnam, S.; Allan, A.C.; et al. Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene GDP-l-galactose phosphorylase: Enhanced ascorbate in fruits and tubers. Plant Biotechnol. J. 2012, 10, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, F.; Liang, D.; Li, J.; Wang, Y. Ascorbate Biosynthesis during Early Fruit Development Is the Main Reason for Its Accumulation in Kiwi. PLoS ONE 2010, 5, e14281. [Google Scholar] [CrossRef]
- Mellidou, I.; Keulemans, J.; Kanellis, A.K.; Davey, M.W. Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant Biol. 2012, 12, 239. [Google Scholar] [CrossRef]
- Gilbert, L.; Alhagdow, M.; Nunes-Nesi, A.; Quemener, B.; Guillon, F.; Bouchet, B.; Faurobert, K.; Gouble, B.; Page, D.; Garcia, V.; et al. GDP-d-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. Plant J. 2009, 60, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Zhang, Y.; Cai, X.; Gong, P.; Zhang, J.; Wang, T.; Li, H.; Ye, Z. Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 2011, 30, 389–398. [Google Scholar] [CrossRef]
- Koukounaras, A.; Mellidou, I.; Patelou, E.; Kostas, S.; Shukla, V.; Engineer, C.; Papaefthimiou, D.; Amari, F.; Chatzopoulos, D.; Mattoo, A.K.; et al. Over-expression of GGP1 and GPP genes enhances ascorbate content and nutritional quality of tomato. Plant Physiol. Biochem. 2022, 193, 124–138. [Google Scholar] [CrossRef]
- Huang, M.; Xu, Q.; Deng, X.X. L-Ascorbic acid metabolism during fruit development in an ascorbate-rich fruit crop chestnut rose (Rosa roxburghii Tratt). J. Plant Physiol. 2014, 171, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Yang, M.; An, H.M. Expression of GDP-L-galactose pyrophosphatase and its relationship with Ascorbate accumulation in Rosa roxburghii. Acta Hortic. Sin. 2014, 41, 1175–1182. [Google Scholar]
- Yan, Y.; Liu, Y.; Lu, M.; Lu, C.; Ludlow, R.A.; Yang, M.; Huang, W.; Liu, Z.; An, H. Gene expression profiling in Rosa roxburghii fruit and overexpressing RrGGP2 in tobacco and tomato indicates the key control point of AsA biosynthesis. Front. Plant Sci. 2023, 13, 1096493. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lu, M.; Rao, T.; Liu, Z.; Wu, X.; An, H. Comparative analysis of fruit metabolome using widely targeted metabolomics reveals nutritional characteristics of different Rosa roxburghii genotypes. Foods 2022, 11, 850. [Google Scholar] [CrossRef]
- Li, N.; Jiang, L.; Liu, Y.; Zou, S.; Lu, M.; An, H. Metabolomics Combined with Transcriptomics Analysis Revealed the Amino Acids, Phenolic Acids, and Flavonol Derivatives Biosynthesis Network in Developing Rosa roxburghii Fruit. Foods 2022, 11, 1639. [Google Scholar] [CrossRef]
- Wang, L.; Wei, T.; Zheng, L.; Jiang, F.; Ma, W.; Lu, M.; Wu, X.; An, H. Recent Advances on Main Active Ingredients, Pharmacological Activities of Rosa roxbughii and Its Development and Utilization. Foods 2023, 12, 1051. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, H.S.; An, H.M. Chloroplast DNA-based genetic variation of Rosa roxburghii in southwest China: Phylogeography and conservation implications. Hortic. Plant J. 2021, 7, 286–294. [Google Scholar] [CrossRef]
- Gong, L.S.; Lu, M.; An, H.M. Generation of Composite Rosa roxburghii Plants with Transgenic Roots by Agrobacterium-Mediated Transformation. Horticulturae 2022, 8, 1079. [Google Scholar] [CrossRef]
- Lu, M.; An, H.M.; Li, L.L. Genome survey sequencing for the characterization of the genetic background of Rosa roxburghii tratt and leaf ascorbate metabolism genes. PLoS ONE 2016, 11, e0147530. [Google Scholar] [CrossRef]
- Chen, W.F.; Hu, T.X.; Ye, J.; Wang, B.; Liu, G.Z.; Wang, Y.; Yuan, L.; Li, J.M.; Li, F.M.; Ye, Z.B.; et al. CCAAT-binding factor, SlNFYA10, negatively regulates ascorbate accumulation by modulating the d-mannose/l-galactose pathway in tomato. Hortic. Res. 2020, 7, 200. [Google Scholar] [CrossRef]
- Munir, S.; Mumtaz, M.A.; Ahiakpa, J.K.; Liu, G.Z.; Chen, W.F.; Zhou, G.L.; Zheng, W.; Ye, Z.B.; Zhang, Y.Y. Genome-wide analysis of Myo-inositol oxygenase gene family in tomato reveals their involvement in ascorbic acid accumulation. BMC Genom. 2020, 21, 284. [Google Scholar] [CrossRef]
- Takahama, U.; Oniki, T. Regulations of peroxidase-dependent oxidation of phenolics in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol. 1992, 33, 379–387. [Google Scholar]
- Li, G.; Lin, G.; Hu, Y.; Zhang, H.; Bai, J.; An, H.; Lu, M. Sequence polymorphisms of GGP and DHAR genes and their association with vitamin C content in R. roxburghii. Jiangsu Agric. Sci. 2020, 48, 63–69. [Google Scholar]
- Badejo, A.A.; Eltelib, H.A.; Fujikawa, Y.; Esaka, M. Genetic Manipulation for Enhancing Vitamin C Content in Tobacco Expressing Acerola (Malpighia glabra) GDP-L-galactose phosphorylase Gene. Hort. Environ. Biotechnol. 2009, 50, 329–333. [Google Scholar]
- Zhou, Y.; Tao, Q.C.; Wang, Z.N.; Fan, R.; Li, Y.; Sun, X.F.; Tang, K.X. Engineering ascorbic acid biosynthetic pathway in Arabidopsis leaves by single and double gene transformation. Biol. Plant. 2012, 56, 451–457. [Google Scholar] [CrossRef]
- Fenech, M.; Amorim-Silva, V.; del Valle, A.E.; Arnaud, D.; Ruiz-Lopez, N.; Castillo, A.G.; Smirnoff, N.; Botella, M.A. The role of GDP- l -galactose phosphorylase in the control of ascorbate biosynthesis. Plant Physiol. 2021, 185, 1574–1594. [Google Scholar] [CrossRef]
- Wang, L.Y.; Meng, X.; Yang, D.Y.; Ma, N.N.; Wang, G.D.; Meng, Q.W. Overexpression of tomato GDP-l-galactose phosphorylase gene in tobacco improves tolerance to chilling stress. Plant Cell Rep. 2014, 33, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Laing, W.A.; Martinez-Sanchez, M.; Wright, M.A.; Bulley, S.M.; Brewster, D.; Dare, A.P.; Rassam, M.; Wang, D.; Storey, R.; Macknight, R.C. An upstream open reading frame is essential for feedback regulation of ascorbate biosynthesis in Arabidopsis. Plant Cell 2015, 27, 772–786. [Google Scholar] [CrossRef]
- Broad, R.C.; Bonneau, J.P.; Beasley, J.T.; Roden, S.; Philips, J.G.; Baumann, U.; Hellens, R.P.; Johnson, A.A.T. Genome-wide identification and characterization of the GDP-L-galactose phosphorylase gene family in bread wheat. BMC Plant Biol. 2019, 19, 515. [Google Scholar] [CrossRef]
- Li, X.; Ye, J.; Munir, S.; Yang, T.; Chen, W.; Liu, G.; Zheng, W.; Zhang, Y. Biosynthetic Gene Pyramiding Leads to Ascorbate Accumulation with Enhanced Oxidative Stress Tolerance in Tomato. Int. J. Mol. Sci. 2019, 20, 1558. [Google Scholar] [CrossRef]
- Yang, D.Y.; Zhuang, K.Y.; Ma, N.N. Overexpression of SlGGP-LIKE gene enhanced the resistance of tomato to salt stress. Protoplasma 2023, 260, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Liu, R.R.; Zhang, C.Q.; Tang, K.X.; Sun, M.F.; Yan, G.H.; Liu, Q.Q. Manipulation of the Rice L-Galactose Pathway: Evaluation of the Effects of Transgene Overexpression on Ascorbate Accumulation and Abiotic Stress Tolerance. PLoS ONE 2015, 10, e0125870. [Google Scholar] [CrossRef]
- Ali, B.; Pantha, S.; Acharya, R.; Ueda, Y.; Wu, L.B.; Ashrafuzzaman, M.; Ishizaki, T.; Wissuwa, M.; Bulley, S.; Frei, M. Enhanced ascorbate level improves multi-stress tolerance in a widely grown indica rice variety without compromising its agronomic characteristics. J. Plant Physiol. 2019, 240, 152998. [Google Scholar] [CrossRef]
- Liu, X.; Xie, X.; Zhong, C.; Li, D. Comparative Transcriptome Analysis Revealed the Key Genes Regulating Ascorbic Acid Synthesis in Actinidia. Int. J. Mol. Sci. 2021, 22, 12894. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Liu, W.; Liu, Z. Overexpression of an alfalfa GDP-mannose 3, 5-epimerase gene enhances acid, drought and salt tolerance in transgenic Arabidopsis by increasing ascorbate accumulation. Biotechnol. Lett. 2014, 36, 2331–2341. [Google Scholar] [CrossRef]
- Imai, T.; Ban, Y.; Yamamoto, T.; Moriguchi, T. Ectopic overexpression of peach GDP-d-mannose pyrophosphorylase and GDP-d-mannose-3′,5′-epimerase in transgenic tobacco. Plant Cell Tissue Organ Cult. 2012, 111, 1–13. [Google Scholar] [CrossRef]
- Zhang, C.J.; Ouyang, B.; Yang, C.X.; Zhang, X.H.; Liu, H.; Zhang, Y.Y.; Zhang, J.H.; Li, H.X.; Ye, Z.B. Reducing AsA Leads to Leaf Lesion and Defence Response in Knock-Down of the AsA Biosynthetic Enzyme GDP-D-Mannose Pyrophosphorylase Gene in Tomato Plant. PLoS ONE 2013, 8, e61987. [Google Scholar] [CrossRef] [PubMed]
- Haroldsen, V.M.; Chi-Ham, C.L.; Kulkarni, S.; Lorence, A.; Bennett, A.B. Constitutively expressed DHAR and MDHAR influence fruit, but not foliar ascorbate levels in tomato. Plant Physiol. Biochem. 2011, 49, 1244–1249. [Google Scholar] [CrossRef]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C Content in Fruits: Biosynthesis and Regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef] [PubMed]
- Tyapkina, D.U.; Kochieva, E.Z.; Slugina, M.A. Identification and Analysis of VTC2 Homologs Encoding the Key Enzyme of L-Ascorbic Acid Biosynthesis in Tomato Species (Solanum Section of Lycopersicon). Dokl. Biochem. Biophys. 2018, 483, 374–378. [Google Scholar] [CrossRef]
- Muñoz, P.; Castillejo, C.; Gómez, J.A.; Miranda, L.; Lesemann, S.; Olbricht, K.; Petit, A.; Chartier, P.; Haugeneder, A.; Trinkl, J.; et al. QTL analysis for ascorbic acid content in strawberry fruit reveals a complex genetic architecture and association with GDP-L-galactose phosphorylase. Hortic. Res. 2023, 10, uhad006. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Hao, Z.; Huang, C. Molecular evolution of GDP-L-galactose phosphorylase, a key regulatory gene in plant ascorbate biosynthesis. AoB Plants 2020, 12, plaa055. [Google Scholar] [CrossRef]
- Li, J.; Liang, N.; Li, M.; Ma, F. Light and abiotic stresses regulate the expression of GDP-l-galactose phosphorylase and levels of ascorbic acid in two kiwifruit genotypes via light-responsive and stress-inducible cis-elements in their promoters. Planta 2013, 238, 535–547. [Google Scholar] [CrossRef]
- Zhang, S.X. Cloning, Analysis and Functional Verification of GGP Gene Promoter in Rosa roxburghii Tratt. Master’s Thesis, Guizhou University, Guizhou, China, 2018. [Google Scholar]
- Maruta, T. How does light facilitate vitamin C biosynthesis in leaves? Biosci. Biotechnol. Biochem. 2022, 86, 1173–1182. [Google Scholar] [CrossRef]
- Deslous, P.; Bournonville, C.; Decros, G.; Okabe, Y.; Mauxion, J.-P.; Jorly, J.; Gadin, S.; Brès, C.; Mori, K.; Ferrand, C.; et al. Overproduction of ascorbic acid impairs pollen fertility in tomato. J. Exp. Bot. 2021, 72, 3091–3107. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Li, C.; Yu, X. Enhanced ascorbic acid accumulation through overexpression of dehydroascorbate reductase confers tolerance to methyl viologen and salt stresses in tomato. Czech J. Genet. Plant Breed. 2012, 48, 74–86. [Google Scholar] [CrossRef]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef]
- Suekawa, M.; Fujikawa, Y.; Inoue, A.; Kondo, T.; Uchida, E.; Koizumi, T.; Esaka, M. High levels of expression of multiple enzymes in the Smirnoff-Wheeler pathway are important for high accumulation of ascorbic acid in acerola fruits. Biosci. Biotechnol. Biochem. 2019, 83, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free. Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, H.X.; Shu, W.B.; Zhang, C.J.; Zhang, W.; Ye, Z.B. Suppressed Expression of Ascorbate Oxidase Gene Promotes Ascorbic Acid Accumulation in Tomato Fruit. Plant Mol. Biol. Report. 2011, 29, 638–645. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Shu, W.; Zhang, C.; Ye, Z. RNA interference of a mitochondrial APX gene improves vitamin C accumulation in tomato fruit. Sci. Hortic. 2011, 129, 220–226. [Google Scholar] [CrossRef]
- Lee, Y.P.; Baek, K.H.; Lee, H.S.; Kwak, S.S.; Bang, J.W.; Kwon, S.Y. Tobacco seeds simultaneously over-expressing Cu/Zn-superoxide dismutase and ascorbate peroxidase display enhanced seed longevity and germination rates under stress conditions. J. Exp. Bot. 2010, 61, 2499–2506. [Google Scholar] [CrossRef]
- Wang, Y.; Wisniewski, M.; Meilan, R.; Cui, M.; Fuchigami, L. Transgenic tomato (Lycopersicon esculentum) overexpressing cAPX exhibits enhanced tolerance to UV-B and heat stress. J. Appl. Hortic. 2006, 8, 87–90. [Google Scholar] [CrossRef]
- Murgia, I.; Tarantino, D.; Vannini, C.; Bracale, M.; Carravieri, S.; Soave, C. Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J. 2004, 38, 940–953. [Google Scholar] [CrossRef]
- Xu, W.; Shi, W.; Ueda, A.; Takabe, T. Mechanisms of salt tolerance in transgenic Arabidopsis thaliana carrying a peroxisomal ascorbate peroxidase gene from barley. Pedosphere 2008, 18, 486–495. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agr. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Potters, G.; Horemans, N.; Caubergs, R.J.; Asard, H. Ascorbate and dehydroascorbate influence cell cycle progression in a tobacco cell suspension. Plant Physiol. 2000, 124, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Completing a pathway to plant vitamin C synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 9109–9110. [Google Scholar] [CrossRef] [PubMed]
- Harb, J.; Khraiwesh, B.; Streif, J.; Reski, R.; Frank, W. Characterization of blueberry monodehydroascorbate reductase gene and changes in levels of ascorbic acid and the antioxidative capacity of water soluble antioxidants upon storage of fruits under various conditions. Sci. Hortic. 2010, 125, 390–395. [Google Scholar] [CrossRef]
- Melino, V.J.; Soole, K.L.; Ford, C.M. Ascorbate metabolism and the developmental demand for tartaric and oxalic acids in ripening grape berries. BMC Plant Biol. 2009, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, Y.; Chen, W.; Tang, K.; Zhang, L. Increased Vitamin C Content Accompanied by an Enhanced Recycling Pathway Confers Oxidative Stress Tolerance inArabidopsis. J. Integr. Plant Biol. 2010, 52, 400–409. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).