Temperature-Driven Selection of Predatory Mirid Bugs for Improving Aphid Control in Sweet Pepper Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Insects
2.2. Predation Capacity
2.3. Developmental Time and Juvenile Survivorship
2.4. Reproductive Parameters
2.5. Demographic Growth Indexes
2.6. Statistical Analysis
3. Results
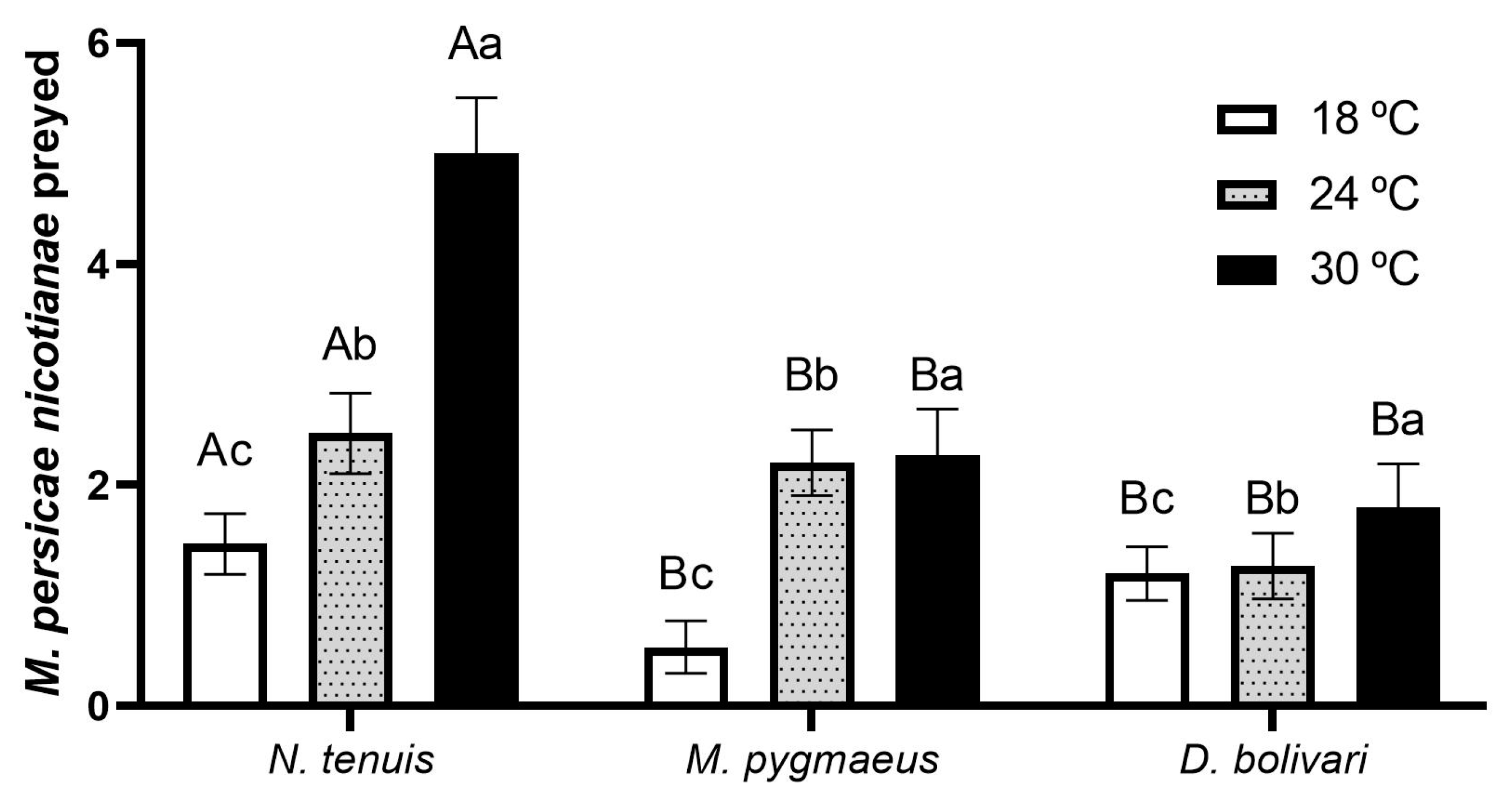
3.1. Predation Capacity
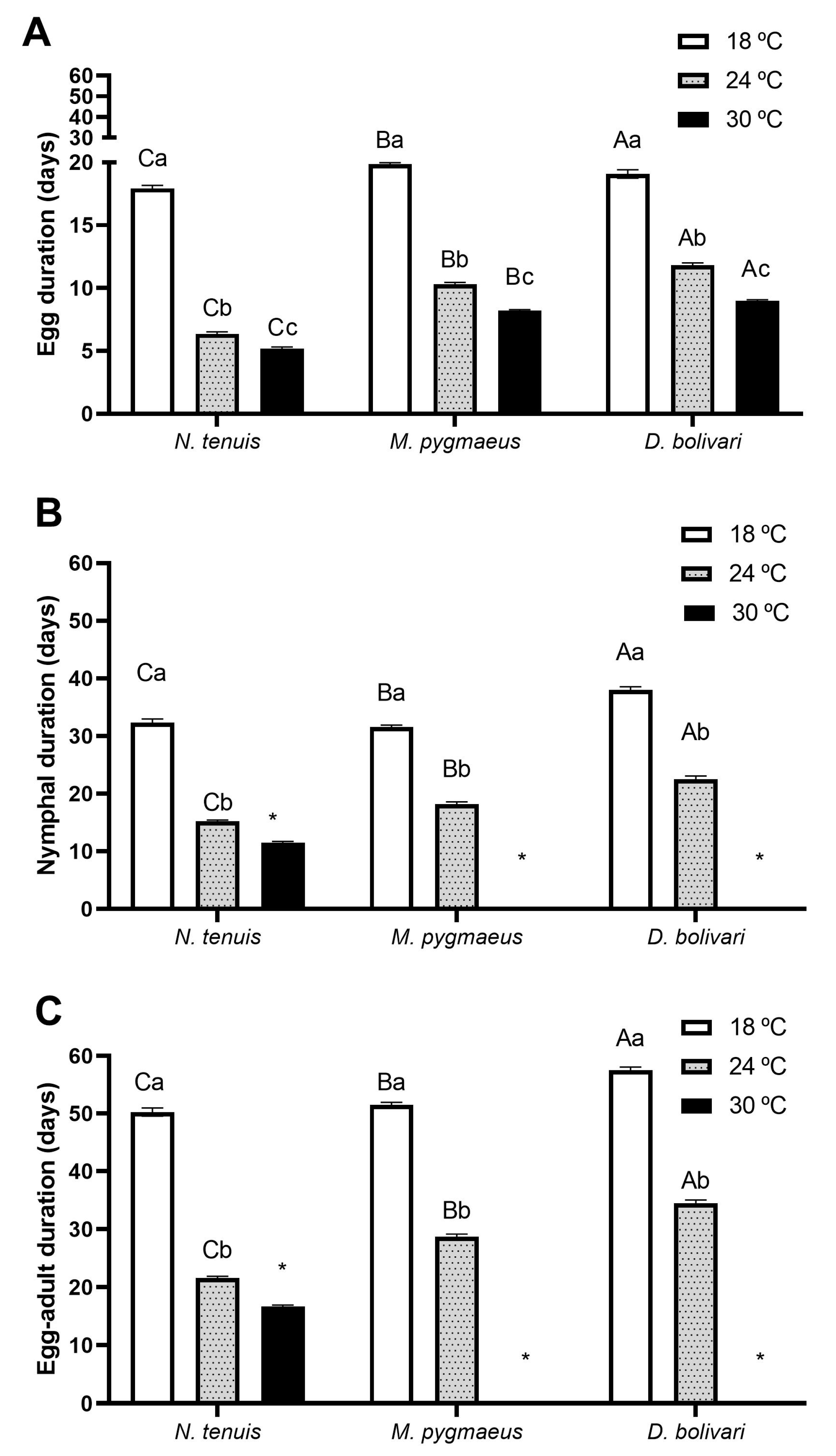
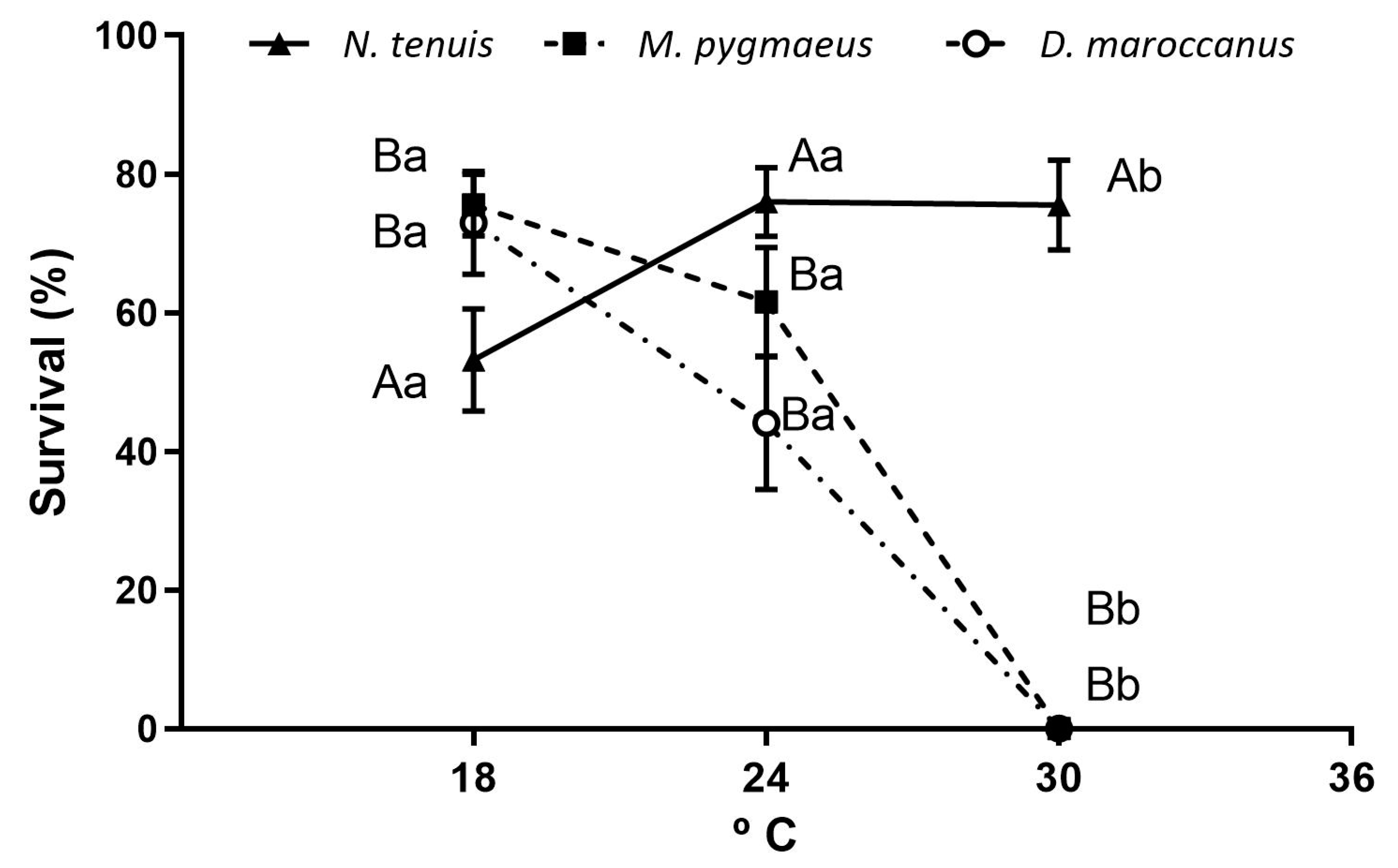
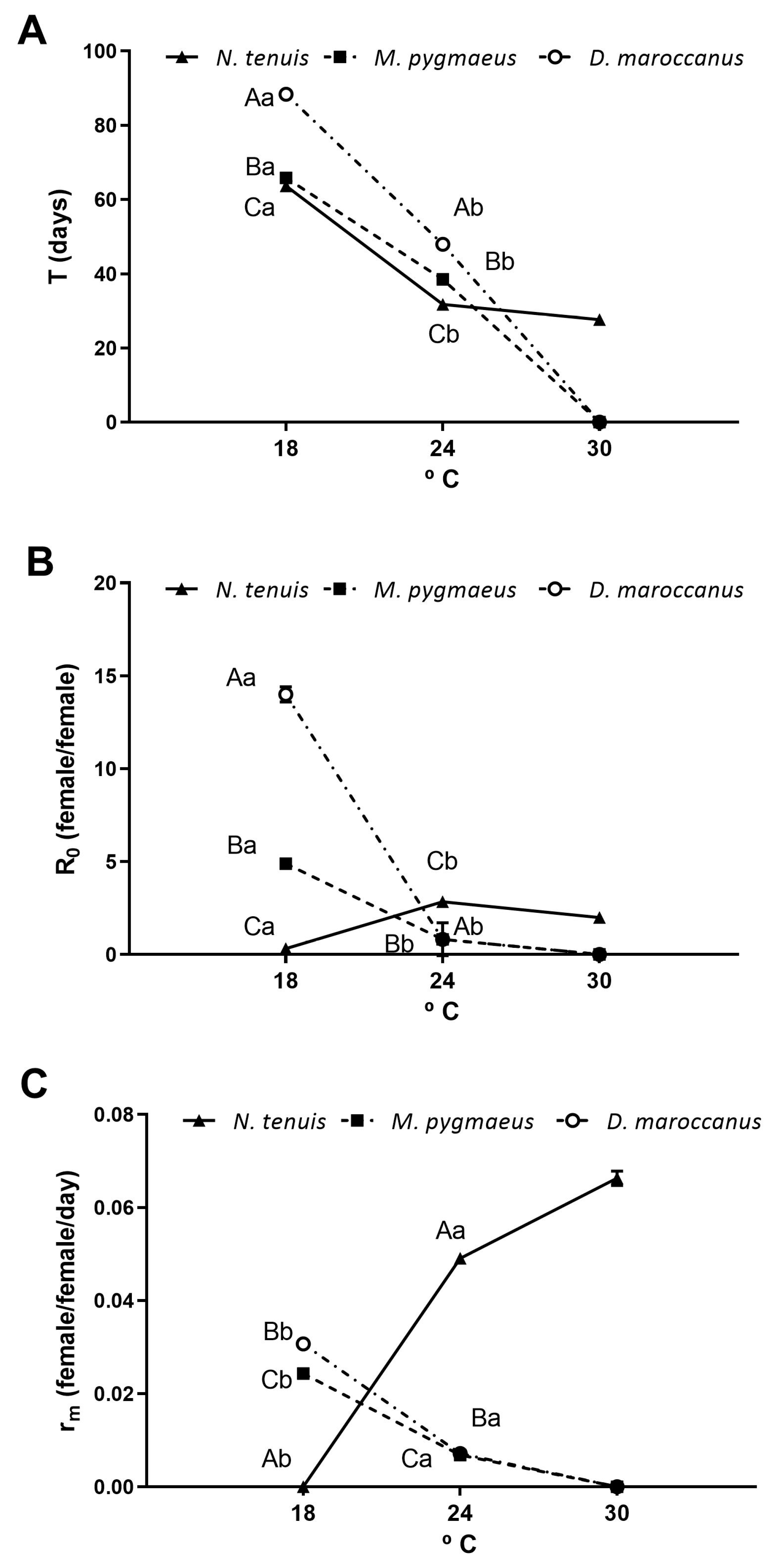
3.2. Developmental Time and Survivorship of Immatures
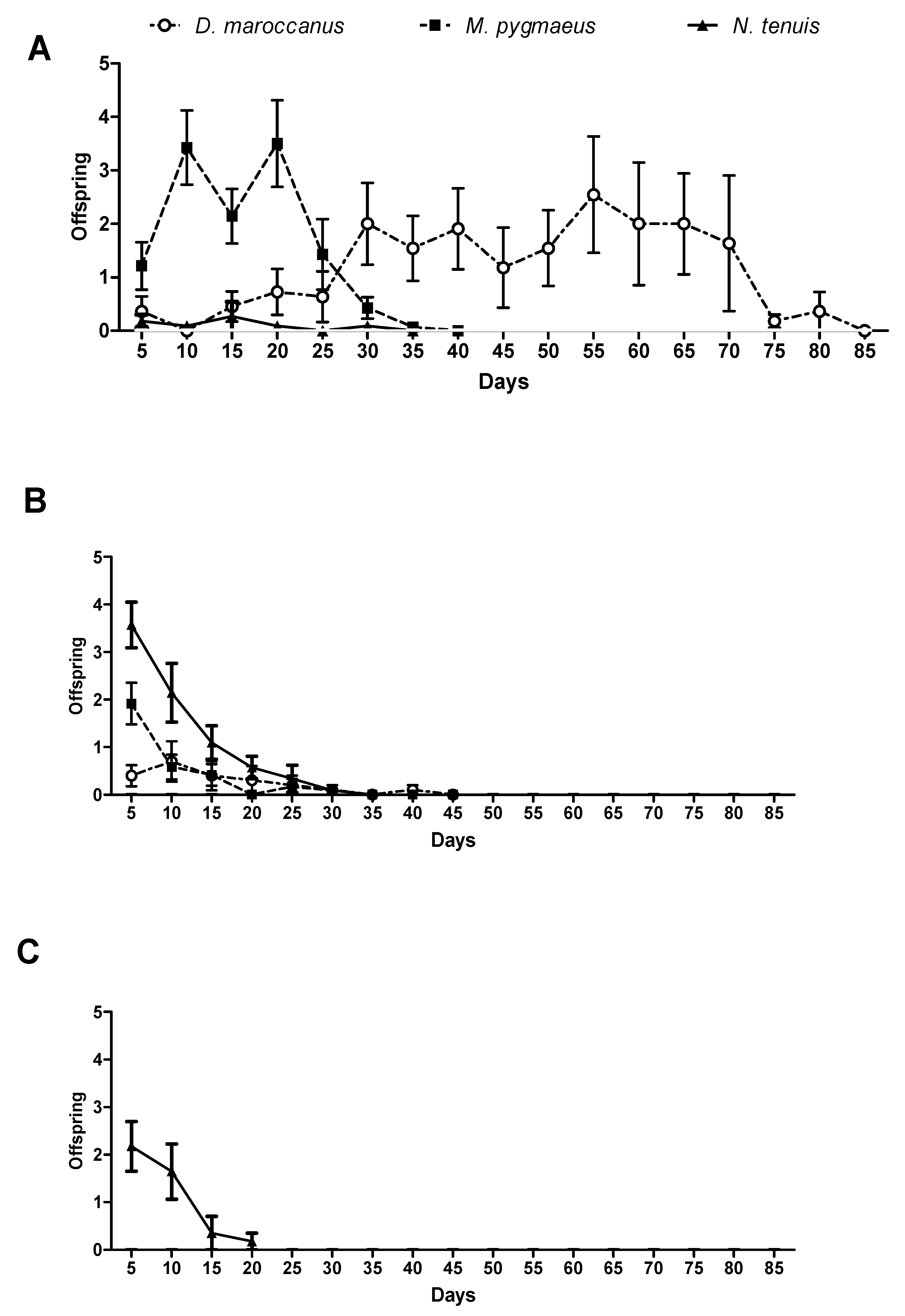
3.3. Reproductive Parameters
3.4. Demographic Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Lenteren, J.C.; Alomar, O.; Ravensberg, W.J.; Urbaneja, A. Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-22303-8. [Google Scholar]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Roca, E.; Aramburu, J.; Moriones, E. Comparative host reactions and Frankliniella occidentalis transmission of different isolates of tomato spotted wilt tospovirus from Spain. Plant Pathol. 1997, 46, 407–415. [Google Scholar] [CrossRef]
- Bouagga, S.; Urbaneja, A.; Depalo, L.; Rubio, L.; Pérez-Hedo, M. Zoophytophagous predator-induced defences restrict accumulation of the tomato spotted wilt virus. Pest Manag. Sci. 2020, 76, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Stansly, P.A.; Natwick, E.T. Integrated Systems for Managing Bemisia tabaci in Protected and Open Field Agriculture. In Bemisia: Bionomics and Management of a Global Pest; Stansly, P.A., Naranjo, S.E., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2010; pp. 467–502. [Google Scholar]
- Urbaneja, A.; Stansly, P.A. Host suitability of different instars of the whitefly Bemisia tabaci “biotype Q” for Eretmocerus mundus. BioControl 2004, 49, 153–161. [Google Scholar] [CrossRef]
- Blom, J. Pimiento bajo abrigo. In Control Biológico de Plagas Agrícolas; Jacas, J.A., Urbaneja, A., Eds.; Phytoma: Valencia, Spain, 2008; pp. 399–409. [Google Scholar]
- Van der Blom, J.; Ramos, M.; Ravensberg, W. Biological pest control in sweet pepper in Spain: Introduction rates of predators of Frankiniella occidentalis. IOBC Bull. 1997, 20, 196–202. [Google Scholar]
- Stansly, P.A.; Calvo, F.J.; Urbaneja, A. Augmentative biological control of Bemisia tabaci biotype “Q” in Spanish greenhouse pepper production using Eretmocerus spp. Crop Prot. 2005, 24, 829–835. [Google Scholar] [CrossRef]
- Urbaneja, A.; Sanchez, E.; Stansly, P.A. Life history of Eretmocerus mundus, a parasitoid of Bemisia tabaci, on tomato and sweet pepper. BioControl 2007, 52, 25–39. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Lacasa, A. Modelling population dynamics of Orius laevigatus and O. albidipennis (Hemiptera: Anthocoridae) to optimise their use as biological control agents of Frankliniella occidentalis (Thysanoptera: Thripidae). Bull. Entomol. Res. 2002, 92, 77–88. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Alcazar, A.; Lacasa, A.; Llamas, A.; Bielza, P. Integrated pest management strategies in sweet pepper plastic houses in the Southeast of Spain. IOBC Bull. 2000, 23, 21–27. [Google Scholar]
- Urbaneja, A.; Arán, E.; León, P.; Gallego, A. Efecto combinado de altas temperaturas y de humedades relativas en la supervivencia, fecundidad y fertilidad de Orius laevigatus y Orius albidipennis (Hem.: Anthocoridae). Bol. San. Veg. Plagas. 2002, 29, 27–35. [Google Scholar]
- Van der Blom, J.; Urbaneja García, A.; Lara, L. Instalación, distribución y eficacia de Orius laevigatus (Fieber) y O. albidipennis (Reuter), (Hemiptera: Anthocoridae) en invernaderos de pimiento en Almería. Boletín Sanid. Veg. 2002, 28, 251–262. [Google Scholar]
- Calvo, F.J.; Bolckmans, K.; Belda, J.E. Biological control-based IPM in sweet pepper greenhouses using Amblyseius swirskii (Acari: Phytoseiidae). Biocontrol Sci. Technol. 2012, 22, 1398–1416. [Google Scholar] [CrossRef]
- Calvo, J.; Bolckmans, K.; Stansly, P.A.; Urbaneja, A. Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 2009, 54, 237–246. [Google Scholar] [CrossRef]
- Koppert, B.S. Swirski Ulti-Mite. Available online: https://www.koppert.com/swirski-ulti-mite (accessed on 19 April 2023).
- Nomikou, M. Combating Whiteflies: Predatory Mites as a Novel Weapon. Ph.D. Thesis, Faculteit der Natuurwetenschappen, University of Amsterdam, Amsterdam, The Netherlands, 2003. [Google Scholar]
- Nomikou, M.; Janssen, A.; Schraag, R.; Sabelis, M.W. Phytoseiid predators as potential biological control agents for Bemisia tabaci. Exp. Appl. Acarol. 2001, 25, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.J.; Bolckmans, K.; Belda, J.E. Control of Bemisia tabaci and Frankliniella occidentalis in cucumber by Amblyseius swirskii. BioControl 2011, 56, 185–192. [Google Scholar] [CrossRef]
- Acebedo, M.M.; Diánez, F.; Santos, M. Almeria’s Green Pest Management Revolution: An Opportunity That Arose from a Food Safety Alert. Agronomy 2022, 12, 619. [Google Scholar] [CrossRef]
- Bouagga, S.; Urbaneja, A.; Rambla, J.L.; Granell, A.; Pérez-Hedo, M. Orius laevigatus strengthens its role as a biological control agent by inducing plant defenses. J. Pest Sci. 2018, 91, 55–64. [Google Scholar] [CrossRef]
- Belliure, B.; Pérez, P.; Marcos, M.A.; Michelena, J.M.; Hermoso de Mendoza, A. Control biológico de pulgones. In Control Biológico de Plagas Agrícolas; Jacas, J.A., Urbaneja, A., Eds.; Phytoma España: Valencia, Spain, 2008. [Google Scholar]
- Sanchez, J.A.; La-Spina, M.; Michelena, J.M.; Lacasa, A.; de Mendoza, A.H. Ecology of the aphid pests of protected pepper crops and their parasitoids. Biocontrol Sci. Technol. 2011, 21, 171–188. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bloemhard, C.M.J.; Kok, L.; Janssen, A. Generalist predatory bugs control aphids in sweet pepper. IOBC/WPRS Bull. 2011, 68, 115–118. [Google Scholar]
- Pekas, A.; De Craecker, I.; Boonen, S.; Wäckers, F.L.; Moerkens, R. One stone; two birds: Concurrent pest control and pollination services provided by aphidophagous hoverflies. Biol. Control 2020, 149, 104328. [Google Scholar] [CrossRef]
- Moerkens, R.; Boonen, S.; Wäckers, F.L.; Pekas, A. Aphidophagous hoverflies reduce foxglove aphid infestations and improve seed set and fruit yield in sweet pepper. Pest Manag. Sci. 2021, 77, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hedo, M.; Urbaneja, A. Prospects for predatory mirid bugs as biocontrol agents of aphids in sweet peppers. J. Pest Sci. 2015, 88, 65–73. [Google Scholar] [CrossRef]
- Bouagga, S.; Urbaneja, A.; Pérez-Hedo, M. Comparative biocontrol potential of three predatory mirids when preying on sweet pepper key pests. Biol. Control 2018, 121, 168–174. [Google Scholar] [CrossRef]
- Bouagga, S.; Urbaneja, A.; Pérez-Hedo, M. Combined use of predatory mirids with Amblyseius swirskii (Acari: Phytoseiidae) to enhance pest management in sweet pepper. J. Econ. Entomol. 2018, 111, 1112–1120. [Google Scholar] [CrossRef]
- Perdikis, D.C.; Lykouressis, D.P. Thermal Requirements for Development of the Polyphagous Predator Macrolophus pygmaeus (Hemiptera: Miridae). Environ. Entomol. 2002, 31, 661–667. [Google Scholar] [CrossRef]
- Messelink, G.J.; Janssen, A. Increased control of thrips and aphids in greenhouses with two species of generalist predatory bugs involved in intraguild predation. Biol. Control 2014, 79, 1–7. [Google Scholar] [CrossRef]
- Horsfall, J.L. Life history studies of Myzus persicae Sulzer. Pa. Agric. Exp. Stn. Bull. 1924, 185, 16. [Google Scholar]
- Abbas, S.; Pérez-Hedo, M.; Colazza, S.; Urbaneja, A. The predatory mirid Dicyphus maroccanus as a new potential biological control agent in tomato crops. BioControl 2014, 59, 565–574. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Lacasa, A.; Arnó, J.; Castañé, C.; Alomar, O. Life history parameters for Nesidiocoris tenuis (Reuter) (Het., Miridae) under different temperature regimes. J. Appl. Entomol. 2009, 133, 125–132. [Google Scholar] [CrossRef]
- Birch, L.C. The Intrinsic Rate of Natural Increase of An Insect Population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Mackauer, M. Quantitative assessment of Aphidius smithi (Hymenoptera: Aphidiidae): Fecundity, intrinsic rate of increase, and functional response. Can. Entomol 1983, 115, 399–415. [Google Scholar] [CrossRef]
- Maia, A.D.H.N.; Luiz, A.J.B.; Campanhola, C. Statistical inference on associated fertility life table parameters using jackknife technique: Computational aspects. J. Econ. Entomol. 2000, 93, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.E.; Bale, J.S.; Sterk, G. Thermal biology and establishment potential in temperate climates of the predatory mirid Nesidiocoris tenuis. BioControl 2009, 54, 785–795. [Google Scholar] [CrossRef]
- Hughes, G.E.; Alford, L.; Sterk, G.; Bale, J.S. Thermal activity thresholds of the predatory mirid Nesidiocoris tenuis: Implications for its efficacy as a biological control agent. BioControl 2010, 55, 493–501. [Google Scholar] [CrossRef]
- Ziaei-Madbouni, M.A.; Samih, M.A.; Namvar, P.; Biondi, A. Temperature-dependent functional response of Nesidiocoris tenuis (Hemiptera: Miridae) to different densities of pupae of cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Eur. J. Entomol. 2017, 114, 325–331. [Google Scholar] [CrossRef]
- Gavkare, O.; Sharma, P.L. Influence of temperature on development of Nesidiocoris tenuis (Reuter) preying on Trialeurodes vaporariorum (Westwood) on tomato. Entomol. News 2017, 127, 230–241. [Google Scholar] [CrossRef]
- Ingegno, B.L.; Messelink, G.J.; Leman, A.; Sacco, D.; Tavella, L. Development and thermal activity thresholds of European mirid predatory bugs. Biol. Control 2021, 152, 104423. [Google Scholar] [CrossRef]
- Martínez-García, H.; Sáenz-Romo, M.G.; Aragón-Sánchez, M.; Román-Fernández, L.R.; Sáenz-de-Cabezón, E.; Marco-Mancebón, V.S.; Pérez-Moreno, I. Temperature-dependent development of Macrolophus pygmaeus and its applicability to biological control. BioControl 2017, 62, 481–493. [Google Scholar] [CrossRef]
- Perdikis, D.C.; Fantinou, A.A.; Lykouressis, D.P. Constant rate allocation in nymphal development in species of Hemiptera. Physiol. Entomol. 2003, 28, 331–339. [Google Scholar] [CrossRef]
- Lykouressis, D.; Perdikis, D.; Michalaki, M. Nymphal development and survival of Macrolophus pygmaeus Rambur (Hemiptera: Miridae) on two eggplant varieties as affected by temperature and presence/absence of prey. Biol. Control 2001, 20, 222–227. [Google Scholar] [CrossRef]
- Perdikis, D.C.; Lykouressis, D.P. Life table and biological characteristics of Macrolophus pygmaeus when feeding on Myzus persicae and Trialeurodes vaporariorum. Entomol. Exp. Appl. 2002, 102, 261–272. [Google Scholar] [CrossRef]
- Perdikis, D.C.; Lykouressis, D.P.; Economou, L.P. The influence of temperature, photoperiod and plant type on the predation rate of Macrolophus pygmaeus on Myzus persicae. BioControl 1999, 44, 281–289. [Google Scholar] [CrossRef]
- Ricupero, M.; Abbes, K.; Haddi, K.; Kurtulus, A.; Desneux, N.; Russo, A.; Siscaro, G.; Biondi, A.; Zappalà, L. Combined thermal and insecticidal stresses on the generalist predator Macrolophus pygmaeus. Sci. Total Environ. 2020, 729, 138922. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, H.; Román-Fernández, L.R.; Sáenz-Romo, M.G.; Pérez-Moreno, I.; Marco-Mancebón, V.S. Optimizing Nesidiocoris tenuis (Hemiptera: Miridae) as a biological control agent: Mathematical models for predicting its development as a function of temperature. Bull. Entomol. Res. 2016, 106, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Siscaro, G.; Lo Pumo, C.; Tropea Garzia, G.; Tortorici, S.; Gugliuzzo, A.; Ricupero, M.; Biondi, A.; Zappalà, L. Temperature and tomato variety influence the development and the plant damage induced by the zoophytophagous mirid bug Nesidiocoris tenuis. J. Pest Sci. 2019, 92, 1049–1056. [Google Scholar] [CrossRef]
- Pazyuk, I.M.; Musolin, D.L.; Reznik, S.Y. Geographic variation in thermal and photoperiodic effects on development of zoophytophagous plant bug Nesidiocoris tenuis. J. Appl. Entomol. 2014, 138, 36–44. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Riahi, C.; Urbaneja, A. Use of zoophytophagous mirid bugs in horticultural crops: Current challenges and future perspectives. Pest Manag. Sci. 2021, 77, 33–42. [Google Scholar] [CrossRef]
- Perdikis, D.; Lucas, E.; Garantonakis, N.; Giatropoulos, A.; Kitsis, P.; Maselou, D.; Panagakis, S.; Lampropoulos, P.; Paraskevopoulos, A.; Lykouressis, D.; et al. Intraguild predation and sublethal interactions between two zoophytophagous mirids, Macrolophus pygmaeus and Nesidiocoris tenuis. Biol. Control 2014, 70, 35–41. [Google Scholar] [CrossRef]
- Salas Gervassio, N.G.; Pérez-Hedo, M.; Luna, M.G.; Urbaneja, A. Intraguild predation and competitive displacement between Nesidiocoris tenuis and Dicyphus maroccanus, two biological control agents in tomato pests. Insect Sci. 2017, 24, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Lucas, É.; Alomar, O. Macrolophus caliginosus (Wagner) as an Intraguild Prey for the Zoophytophagous Dicyphus tamaninii Wagner (Heteroptera: Miridae). Biol. Control 2001, 20, 147–152. [Google Scholar] [CrossRef]
- Moreno-Ripoll, R.; Gabarra, R.; Symondson, W.O.C.; King, R.A.; Agusti, N. Do the interactions among natural enemies compromise the biological control of the whitefly Bemisia tabaci? J. Pest Sci. 2014, 87, 133–141. [Google Scholar] [CrossRef]
- Moreno-Ripoll, R.; Agusti, N.; Berruezo, R.; Gabarra, R. Conspecific and heterospecific interactions between two omnivorous predators on tomato. Biol. Control 2012, 62, 189–196. [Google Scholar] [CrossRef]
- Lucas, É.; Alomar, O. Impact of the presence of Dicyphus tamaninii Wagner (Heteroptera: Miridae) on whitefly (Homoptera: Aleyrodidae) predation by Macrolophus caliginosus (Wagner) (Heteroptera: Miridae). Biol. Control 2002, 25, 123–128. [Google Scholar] [CrossRef]
- Mouratidis, A.; Leman, A.; Poelman, E.H.; Messelink, G. Dicyphus predatory bugs pre-established on tomato plants reduce Nesidiocoris tenuis population growth. J. Pest Sci. 2022, 95, 1659–1670. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Urbaneja, A. The zoophytophagous predator Nesidiocoris tenuis: A successful but controversial biocontrol agent in tomato crops. In Advances in Insect Control and Resistance Management; Horowitz, A.R., Ishaaya, I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 121–138. ISBN 978-3-319-31798-4. [Google Scholar]
- Abraços-Duarte, G.; Ramos, S.; Valente, F.; Borges da Silva, E.; Figueiredo, E. Functional Response and Predation Rate of Dicyphus cerastii Wagner (Hemiptera: Miridae). Insects 2021, 12, 530. [Google Scholar] [CrossRef] [PubMed]
- Perdikis, D.C.; Lykouressis, D.P. Myzus persicae (Homoptera: Aphididae) as suitable prey for Macrolophus pygmaeus (Hemiptera: Miridae) population increase on pepper plants. Environ. Entomol 2004, 33, 499–505. [Google Scholar] [CrossRef]
- Perdikis, D.; Lykouressis, D. Effects of various items, host plants, and temperatures on the development and survival of Macrolophus pygmaeus Rambur (Hemiptera: Miridae). Biol. Control 2000, 17, 55–60. [Google Scholar] [CrossRef]
- Bass, C.; Zimmer, C.T.; Riveron, J.M.; Wilding, C.S.; Wondji, C.S.; Kaussmann, M.; Field, L.M.; Williamson, M.S.; Nauen, R. Gene amplification and microsatellite polymorphism underlie a recent insect host shift. Proc. Natl. Acad. Sci. USA 2013, 110, 19460–19465. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R. Neonicotinoids—From zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- Jalali, M.A.; Michaud, J.P. Aphid-plant interactions affect the suitability of Myzus spp. as prey for the two spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae). Eur. J. Entomol. 2012, 109, 345–352. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Lanzoni, A.; Hemerik, L.; Bueno, V.H.P.; Bajonero Cuervo, J.G.; Biondi, A.; Burgio, G.; Calvo, F.J.; de Jong, P.W.; López, S.N.; et al. The pest kill rate of thirteen natural enemies as aggregate evaluation criterion of their biological control potential of Tuta absoluta. Sci. Rep. 2021, 11, 10756. [Google Scholar] [CrossRef] [PubMed]
- Messelink, G.J.; Bloemhard, C.M.J.; Hoogerbrugge, H.; van Schelt, J.; Ingegno, B.L.; Tavella, L. Evaluation of mirid predatory bugs and release strategy for aphid control in sweet pepper. J. Appl. Entomol. 2015, 139, 333–341. [Google Scholar] [CrossRef]
| Temp. | Instar | N. tenuis | M. pygmaeus | D. bolivari |
|---|---|---|---|---|
| 18 °C | Lifetime fertility | 0.9 ± 0.5 Ba (n = 10) | 12.2 ± 1.8 Aa (n = 13) | 20.6 ± 5.3 Aa (n = 10) |
| Female longevity | 29.4 ± 4.2 Ba (n = 11) | 44.1 ± 2.6 Ba (n = 13) | 75.5 ± 13.4 Aa (n = 10) | |
| Progeny sex-ratio | 75.0 ± 25.0 Aa (n = 4) | 52.8 ± 2.1 Ba (n = 12) | 93.1 ± 3.6 Aa (n = 7) | |
| 24 °C | Lifetime fertility | 7.3 ± 1.0 Bb (n = 21) | 3.2 ± 0.7 Ab (n = 12) | 2.7 ± 0.7 Ab (n = 10) |
| Female longevity | 17. 1± 2.0 Bb (n = 21) | 17.8 ± 3.6 Bb (n = 12) | 29.3 ± 3.8 Ab (n = 10) | |
| Progeny sex-ratio | 50.9 ± 7.0 Ab (n = 16) | 42.0 ± 6.2 Bb (n = 5) | 75.8 ± 9.2 Ab (n = 6) | |
| 30 °C | Lifetime fertility | 6.4 ± 1.6 (n = 11) | * | * |
| Female longevity | 7. 2± 1.3 (n = 11) | * | * | |
| Progeny sex-ratio | 62.0 ± 0.1 (n = 6) | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Hedo, M.; Pedroche, V.; Urbaneja, A. Temperature-Driven Selection of Predatory Mirid Bugs for Improving Aphid Control in Sweet Pepper Crops. Horticulturae 2023, 9, 572. https://doi.org/10.3390/horticulturae9050572
Pérez-Hedo M, Pedroche V, Urbaneja A. Temperature-Driven Selection of Predatory Mirid Bugs for Improving Aphid Control in Sweet Pepper Crops. Horticulturae. 2023; 9(5):572. https://doi.org/10.3390/horticulturae9050572
Chicago/Turabian StylePérez-Hedo, Meritxell, Virginia Pedroche, and Alberto Urbaneja. 2023. "Temperature-Driven Selection of Predatory Mirid Bugs for Improving Aphid Control in Sweet Pepper Crops" Horticulturae 9, no. 5: 572. https://doi.org/10.3390/horticulturae9050572
APA StylePérez-Hedo, M., Pedroche, V., & Urbaneja, A. (2023). Temperature-Driven Selection of Predatory Mirid Bugs for Improving Aphid Control in Sweet Pepper Crops. Horticulturae, 9(5), 572. https://doi.org/10.3390/horticulturae9050572






