If Only You Could Catch Me—Catch Me If You Can: Monitoring Aphids in Protected Cucumber Cultivations by Means of Sticky Traps
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Material
2.2. Plant Material
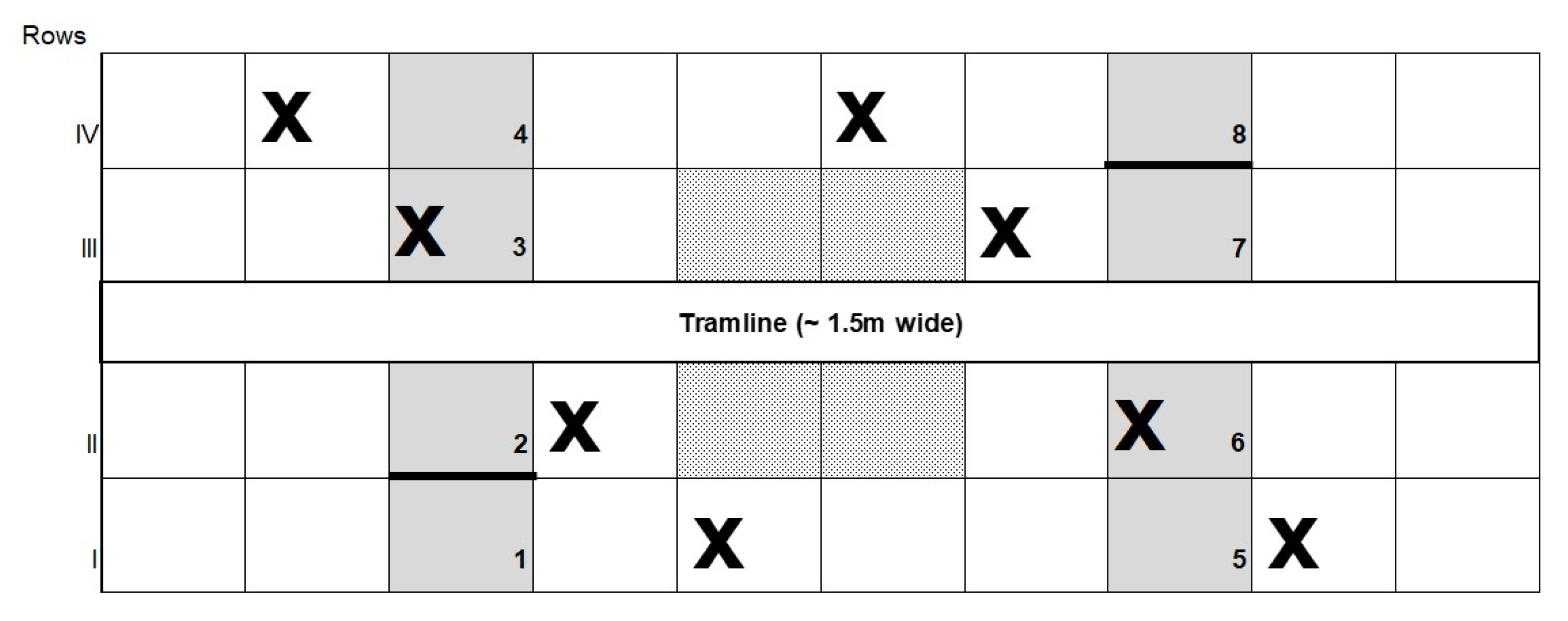
2.3. Experimental Setup
2.4. Statistical Analyses
3. Results
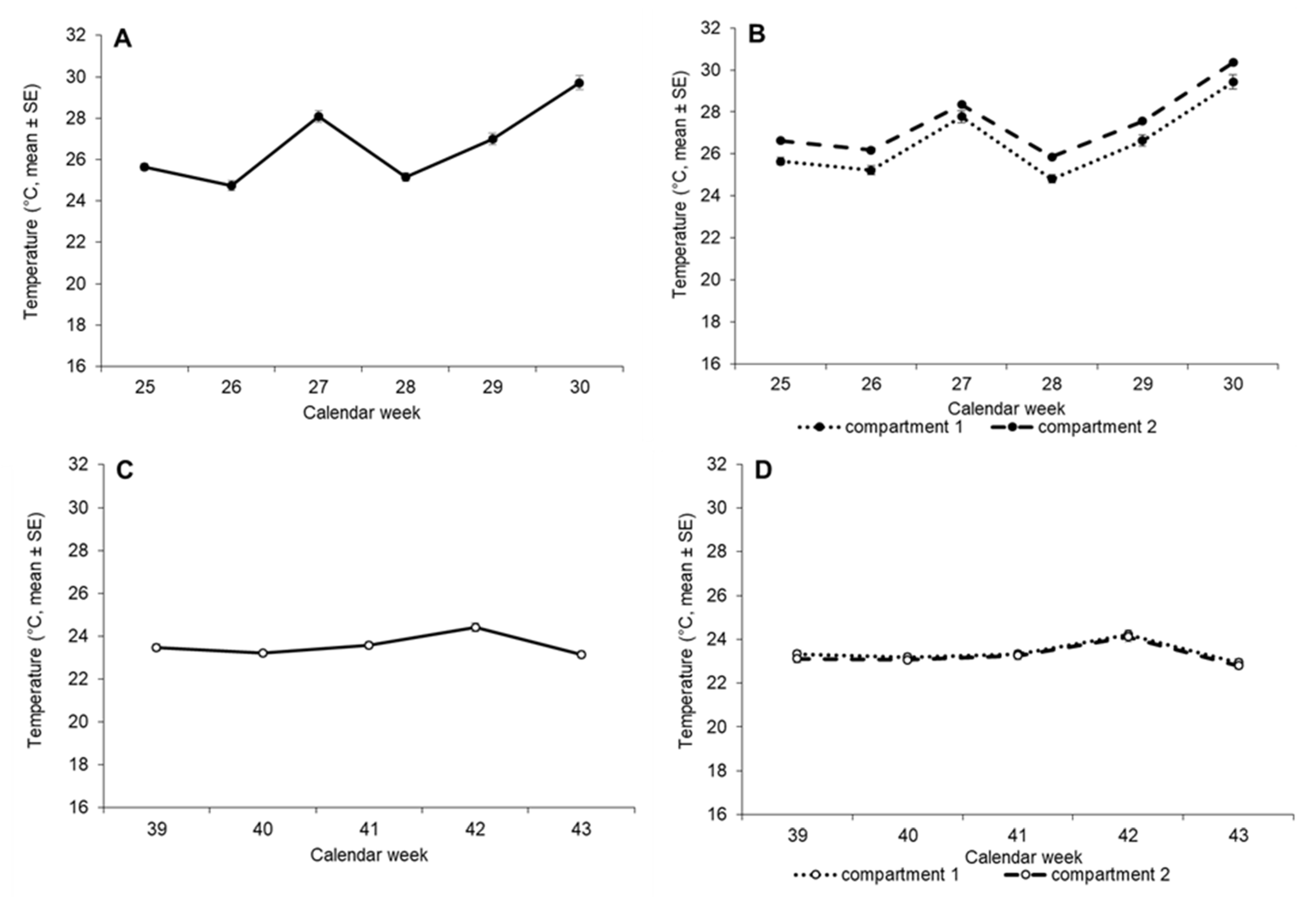
3.1. Temperatures over Time
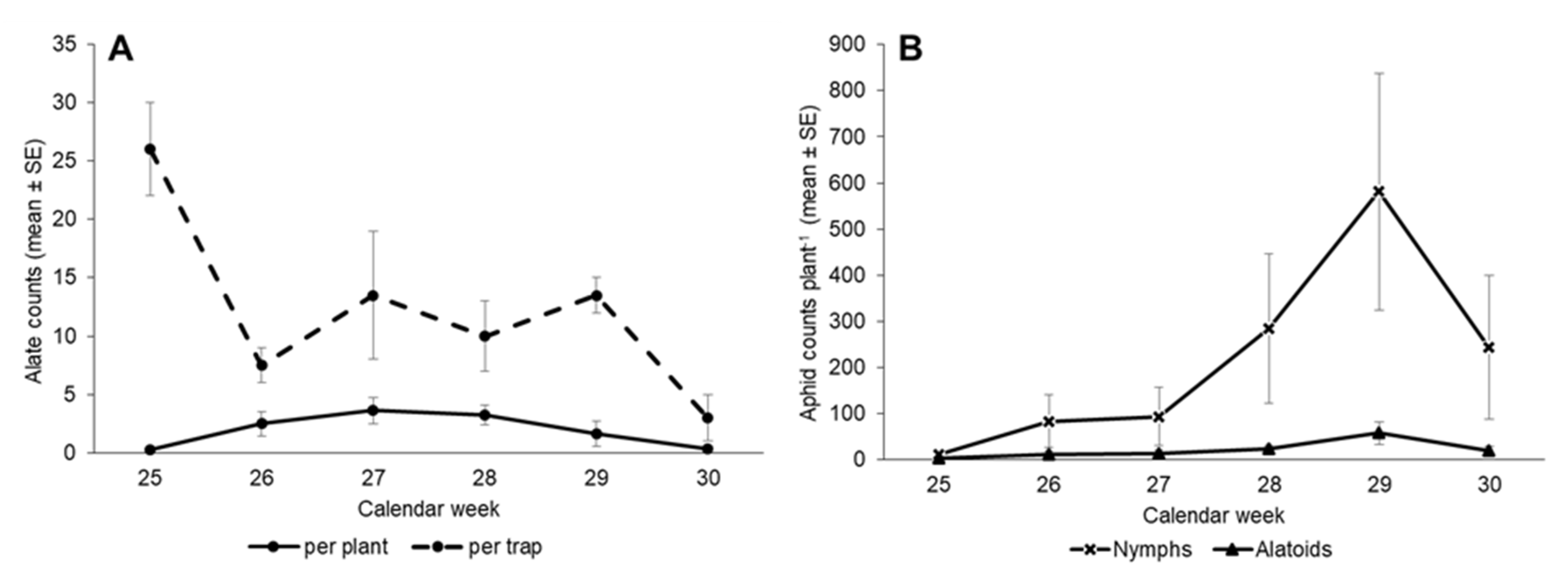
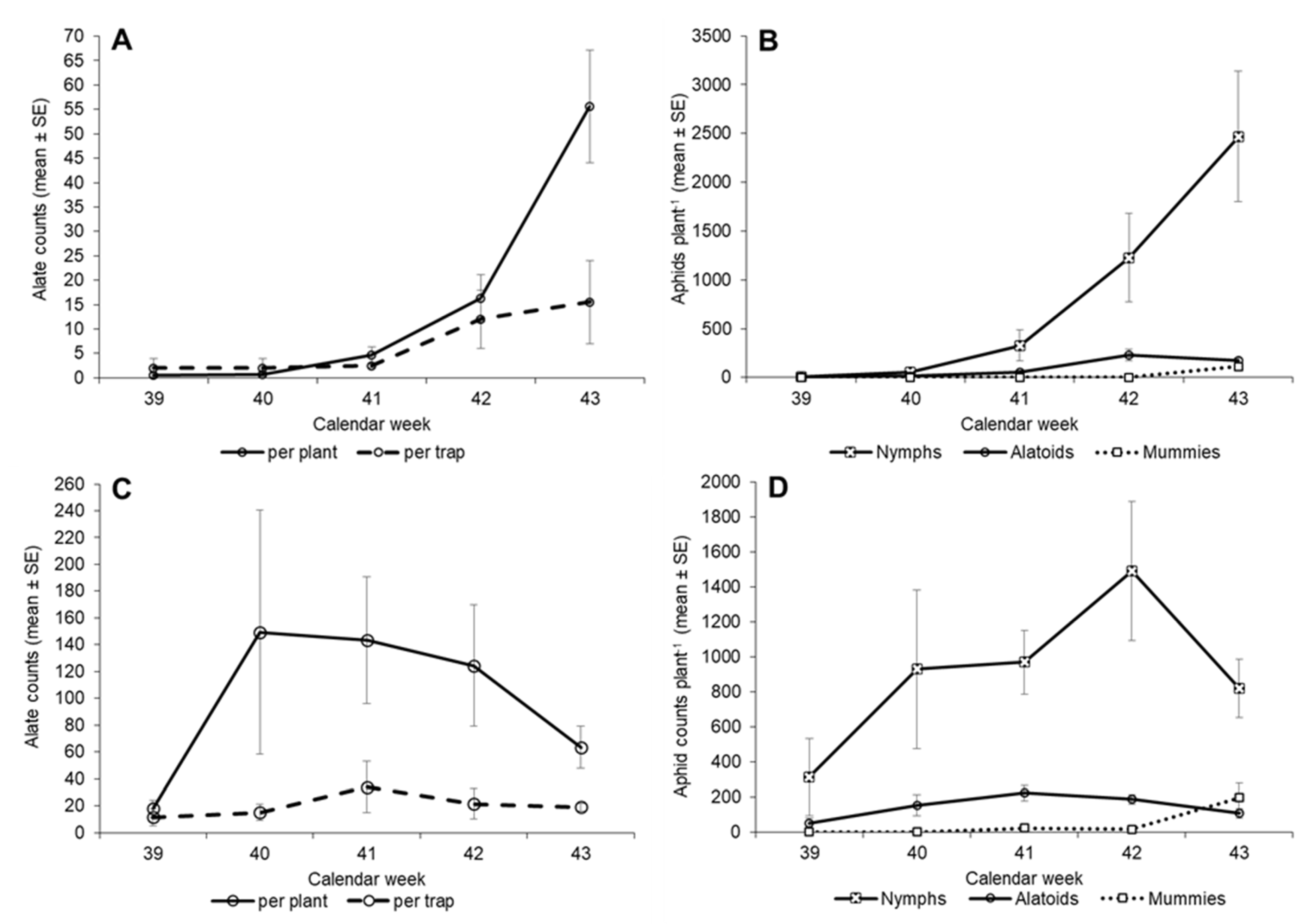
3.2. First Planting—Insect Counts over Time
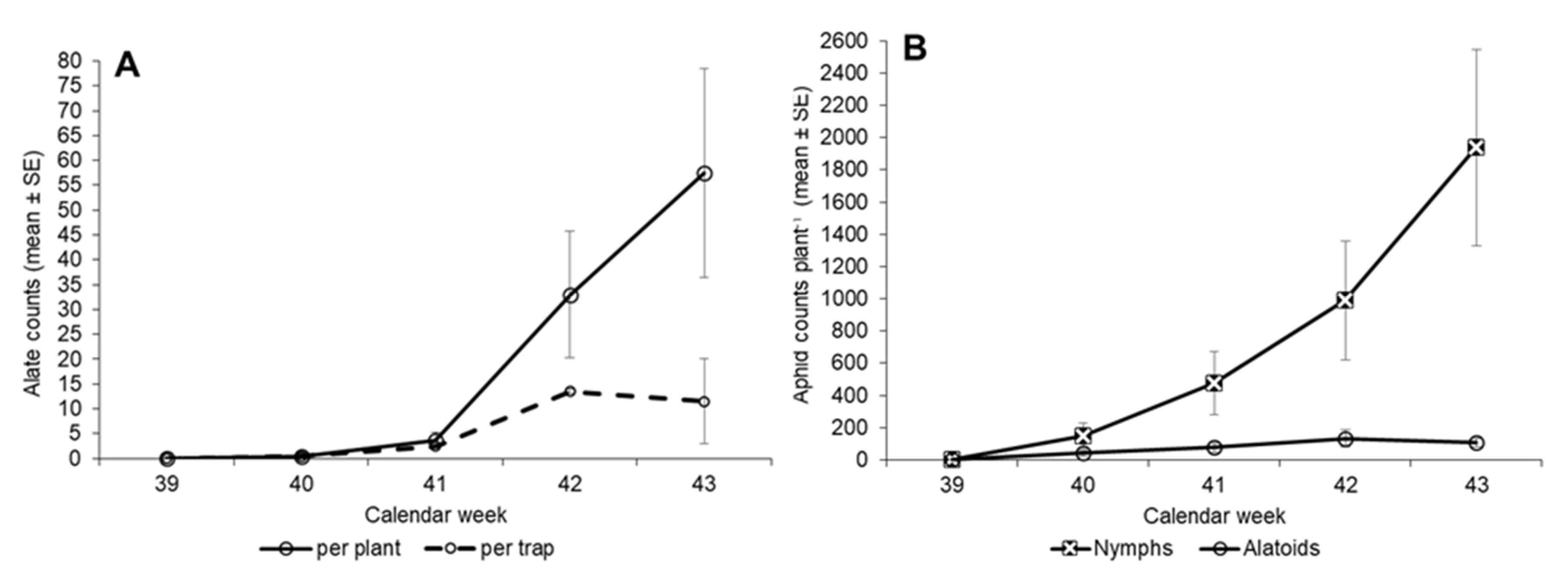
3.3. Second Planting—Insect Counts over Time
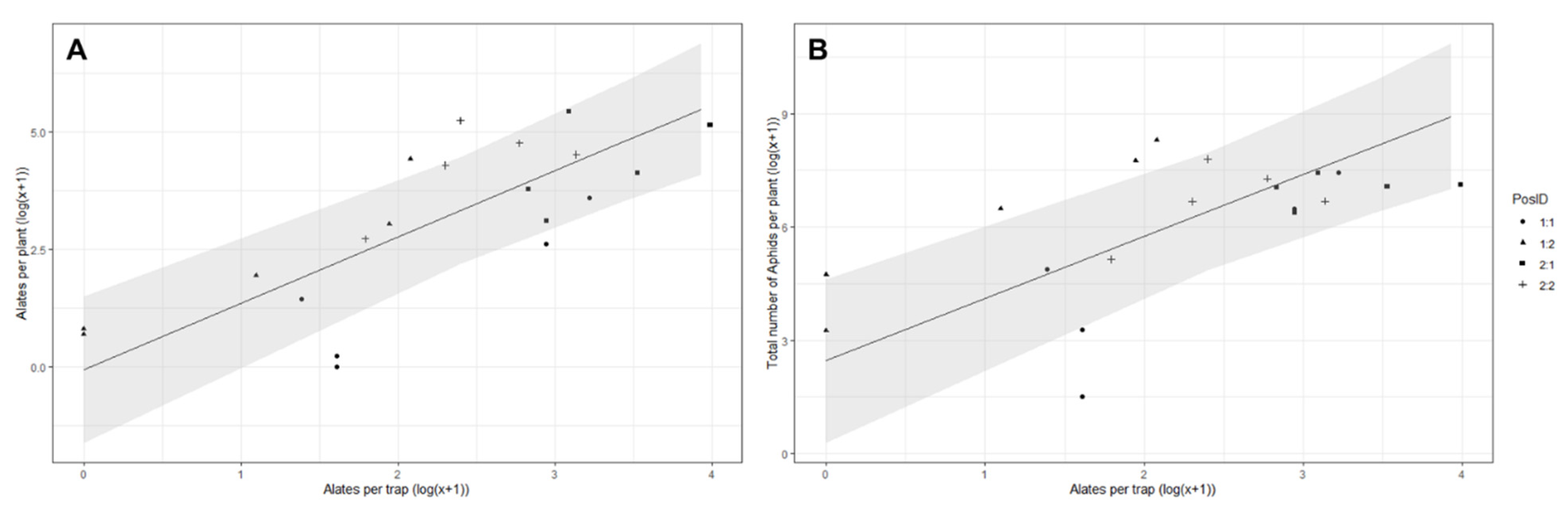
3.4. First and Second Planting—Correlation between Plant and Trap Counts in the Control Treatments
3.5. First and Second Planting—Correlation between Plant and Trap Counts in the Two Treatment Compartments
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radcliffe, E.B.; Hutchison, W.D.; Cancelado, R.E. Radcliffe’s IPM World Textbook; University of Minesota: St. Paul, MN, USA, 2020. [Google Scholar]
- Pedigo, L.P.; Rice, M.E. Entomology and Pest Management, 6th ed.; Waveland Press Inc.: Long Grove, IL, USA, 2015; ISBN 1478622857. [Google Scholar]
- Hajek, A.E.; Eilenberg, J. Natural Enemies: An Introduction to Biological Control, 2nd ed.; Cambridge University Press: Cambridge, UK, 2018; ISBN 9781107280267. [Google Scholar]
- Koppert. Horiver. Available online: www.koppertbio.de/horiver/ (accessed on 5 March 2023).
- Bae, S.D.; Kim, H.J.; Yoon, Y.N.; Lee, Y.H.; Park, I.H.; Kang, H.W.; Mainali, B.P. Yellow sticky card offers composite attractiveness to western flower thrips and greenhouse whitefly. J. Entomol. Zool. Stud. 2015, 3, 110–113. [Google Scholar]
- Vernon, R.S.; Gillespie, D.R. Spectral responsiveness of Frankliniella occidentalis (Thysanoptera, Thripidae) determined by trap catches in greenhouses. Environ. Entomol. 1990, 19, 1229–1241. [Google Scholar] [CrossRef]
- Moericke, V. Farben als Landereize für geflügelte Blattläuse (Aphidoidea). Z. Naturforsch. B 1952, 7, 304–309. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Owens, E.D. Visual detection of plants by herbivorous insects. Annu. Rev. Entomol. 1983, 28, 337–364. [Google Scholar] [CrossRef]
- Gerling, D.; Horowitz, A.R. Yellow traps for evaluating the population-levels and dispersal patterns of Bemisia tabaci (Gennadius) (Homoptera, Aleyrodidae). Ann. Entomol. Soc. Am. 1984, 77, 753–759. [Google Scholar] [CrossRef]
- Doering, T.F.; Chittka, L. Visual ecology of aphids-a critical review on the role of colours in host finding. Arthropod Plant Interact. 2007, 1, 3–16. [Google Scholar] [CrossRef]
- Roach, S.H.; Agee, H.R. Trap colors: Preference of alate aphids. Environ. Entomol. 1972, 1, 797–798. [Google Scholar] [CrossRef]
- Aubert, B.; Hua, X.Y. Monitoring flight activity of Diaphorina citri on citrus and Murraya canopies. In Rehabilitation of Citrus Industry in the Asia Pacific Region, Proceedings of the 4th International Asia Pacific Conference on Citrus Rehabilitation, Chiang Mai, Thailand, 4–10 February 1990; Food and Agriculture Organization of the United Nations: Rome, Italy, 1990; pp. 4–10. [Google Scholar]
- Hall, D.G. An assessment of yellow sticky card traps as indicators of the abundance of adult Diaphorina citri (Hemiptera: Psyllidae) in Citrus. J. Econ. Entomol. 2009, 102, 446–452. [Google Scholar] [CrossRef]
- Döring, T.F.; Röhrig, K. Behavioural response of winged aphids to visual contrasts in the field. Ann. Appl. Biol. 2016, 168, 421–434. [Google Scholar] [CrossRef]
- Van Tol, R.W.H.M.; Tom, J.; Roher, M.; Schreurs, A.; van Dooremalen, C. Haze of glue determines preference of western flower thrips (Frankliniella occidentalis) for yellow or blue traps. Sci. Rep. 2021, 11, 6557. [Google Scholar] [CrossRef]
- Straw, N.A.; Williams, D.T.; Green, G. Influence of sticky trap color and height above ground on capture of alate Elatobium abietinum (Hemiptera: Aphididae) in Sitka spruce plantations. Environ. Entomol. 2011, 40, 120–125. [Google Scholar] [CrossRef]
- Byrne, D.N.; von Bretzel, P.K.; Hoffman, C.J. Impact of trap design and placement when monitoring for the Bandedwinged Whitefly and the Sweetpotato Whitefly (Homoptera: Aleyrodidae). Environ. Entomol. 1986, 15, 300–304. [Google Scholar] [CrossRef]
- A’Brook, J. Observations on different methods of aphid trapping. Ann. Appl. Biol. 1973, 74, 263–277. [Google Scholar] [CrossRef]
- Moericke, V. Über die Lebensgewohnheiten der geflügelten Blattläuse (Aphidina) unter besonderer Berücksichtigung des Verhaltens beim Landen. J. Appl. Entomol. 1955, 37, 29–91. [Google Scholar]
- Boeckmann, E.; Hommes, M.; Meyhoefer, R. Yellow traps reloaded: What is the benefit for decision making in practice? J. Pest. Sci. 2015, 88, 439–449. [Google Scholar] [CrossRef]
- Pizzol, J.; Nammour, D.; Hervouet, P.; Bout, A.; Desneux, N.; Mailleret, L. Comparison of two methods of monitoring thrips populations in a greenhouse rose crop. J. Pest. Sci. 2010, 83, 191–196. [Google Scholar] [CrossRef]
- Plant, R.E. An integrated expert decision support system for agricultural management. Agric. Syst. 1989, 29, 49–66. [Google Scholar] [CrossRef]
- Clarke, N.D.; Shipp, J.L.; Papadopoulos, A.P.; Jarvis, W.R.; Khosla, S.; Jewett, T.J.; Ferguson, G. Development of the Harrow Greenhouse Manager: A decision-support system for greenhouse cucumber and tomato. Comput. Electron. Agric. 1999, 24, 195–204. [Google Scholar] [CrossRef]
- Von Kröcher, C.; Röhrig, M. Monitoring of plant pests and diseases as a base of the Germany-wide online decision support system ISIP. J. Verbr. Lebensm. 2007, 2, 50–51. [Google Scholar] [CrossRef]
- Jones, V.P.; Brunner, J.F.; Grove, G.G.; Petit, B.; Tangren, G.V.; Jones, W.E. A Web-Based decision support system to enhance IPM programs in Washington tree fruit. Pest. Manag. Sci. 2010, 66, 587–595. [Google Scholar] [CrossRef]
- Perini, A.; Susi, A. Developing a decision support system for integrated production in agriculture. Environ. Model. Softw. 2004, 19, 821–829. [Google Scholar] [CrossRef]
- Samietz, J.; Graf, B.; Höhn, H.; Schaub, L.; Höpli, H.U. Phenology modelling of major insect pests in fruit orchards from biological basics to decision support: The forecasting tool SOPRA. Bull. OEPP 2007, 37, 255–260. [Google Scholar] [CrossRef]
- Wubs, M.; Böckmann, E.; Meyhöfer, R.; Hemerik, L. Modelling development of the whitefly Trialeurodes vaporariorum for a decision-support system. Neth. Entomol. Soc. Meet. 2014, 25, 9–23. [Google Scholar]
- Ebert, T.A. Melon Aphid, Aphis gossypii (Hemiptera: Aphididae). In Encyclopedia of Entomology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 1375–1378. ISBN 0792386701. [Google Scholar]
- Ebert, T.A.; Cartwright, B. Biology and ecology of Aphis gossypii Glover (Homoptera: Aphididae). Southwest. Entomol. 1997, 22, 116–153. [Google Scholar]
- Gadhave, K.R.; Gautam, S.; Rasmussen, D.A.; Srinivasan, R. Aphid transmission of Potyvirus: The largest Plant-Infecting RNA Virus Genus. Viruses 2020, 12, 773. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Trees: An Identification and Information Guide; CAB International: Wallingford, UK, 1994.
- Lampel, G.; Meier, W. Aphididae; Centre Suisse de Cartographie de la Faune: Neuchâtel, Switzerland, 2007; ISBN 9782884140287. [Google Scholar]
- Ramakers, P.M.J.; O’Neill, T.M. Cucurbits. In Integrated Pest and Disease Management in Greenhouse Crops; Albajes, R., Lodovica Gullino, M., van Lenteren, J.C., Elad, Y., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 435–453. ISBN 9780792356318. [Google Scholar]
- Rahsepar, A.; Haghani, M.; Sedaratian-Jahromi, A. Different cucumber (Cucumis sativus) varieties could affects biological performance of cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae), a case study at laboratory condition. Entomofauna 2016, 37, 353–364. [Google Scholar]
- Destatis Statistisches Bundesamt. Available online: https://www.destatis.de (accessed on 25 April 2023).
- Muller, C.B.; Williams, I.S.; Hardie, J. The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecol. Entomol. 2001, 26, 330–340. [Google Scholar] [CrossRef]
- Hatano, E.; Baverstock, J.; Kunert, G.; Pell, J.K.; Weisser, W.W. Entomopathogenic fungi stimulate transgenerational wing induction in pea aphids, Acyrthosiphon pisum (Hemiptera: Aphididae). Ecol. Entomol. 2012, 37, 75–82. [Google Scholar] [CrossRef]
- Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M.; Hardie, J. The chemical ecology of aphids. Annu. Rev. Entomol. 1992, 37, 67–90. [Google Scholar] [CrossRef]
- Van Tol, R.; Helsen, H.H.M.; Griepink, F.C.; de Kogel, W.J. Female-Induced Increase of Host-Plant Volatiles Enhance Specific Attraction of Aphid Male Dysaphis plantaginea (Homoptera: Aphididae) to the Sex Pheromone. Bull. Entomol. Res. 2009, 99, 593–602. [Google Scholar] [CrossRef]
- Holland, J.M.; McHugh, N.M.; Salinari, F. Field specific monitoring of cereal yellow dwarf virus aphid vectors and factors influencing their immigration within fields. Pest. Manag. Sci. 2021, 77, 4100–4108. [Google Scholar] [CrossRef]
- Bonneau, P.; Brisson, J.D.; Tellier, S.; Fournier, V. Flight phenology and trap selection for monitoring potential viral vector Aphididae and Aleyrodidae (Hemiptera) in strawberry (Rosaceae) fields of Québec, Canada. Can. Entomol. 2019, 151, 378–390. [Google Scholar] [CrossRef]
- Kafeshani, F.A.; Rajabpour, A.; Aghajanzadeh, S.; Gholamian, E.; Farkhari, M. Comparison of different sampling procedures for population monitoring of important Citrus aphids on two orange species. J. Entomol. Res. Soc. 2019, 21, 57–63. [Google Scholar]
- Bărăscu, N.; Donescu, D.; Donescu, V.; Urdă, C. Monitoring of aphids species in the potato crop from “Tara Barsei”. Rom. Agric. Res. 2022, 39, 489–496. [Google Scholar]
- Vučetić, A.; Vukov, T.; Jovičić, I.; Petrović-Obradović, O. Monitoring of aphid flight activities in seed potato crops in Serbia. Zookeys 2013, 319, 333–346. [Google Scholar] [CrossRef]
- Döring, T.F.; Kirchner, S.M. A model for colour preference behaviour of spring migrant aphids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20210283. [Google Scholar] [CrossRef]
- Hardie, J.; Storer, J.R.; Nottingham, S.F.; Peace, L.; Harrington, R.; Merritt, L.A.; Wadhams, L.J.; Wood, D.K. The Interactino of Sex Pheromone and Plant Volatiles for Field Attraction of Male Bird-Cherry Aphid, Rhopalosiphum padi. In Proceedings of the Brighton Crop Protection Conference—Pests and Diseases, Brighton, UK, 21–24 November 1994; Volume 1–3, pp. 1223–1230, ISBN 0948404809. [Google Scholar]
- Hardie, J.; Storer, J.R.; Cook, F.J.; Campbell, C.A.M.; Wadhams, L.J.; Lilley, R.; Peace, L. Sex pheromone and visual trap interactions in mate location strategies and aggregation by host-alternating aphids in the field. Physiol. Entomol. 1996, 21, 97–106. [Google Scholar] [CrossRef]
- Gabrys, B.J.; Gadomski, H.J.; Klukowski, Z.; Pickett, J.A.; Sobota, G.T.; Wadhams, L.J.; Woodcock, C.M. Sex pheromone of cabbage aphid Brevicoryne brassicae: Identification and field trapping of male aphids and parasitoids. J. Chem. Ecol. 1997, 23, 1881–1890. [Google Scholar] [CrossRef]
- Capinera, J.L. Relationships between insect pests and weeds: An evolutionary perspective. Weed Sci. 2005, 53, 892–901. [Google Scholar] [CrossRef]
- Krug, H.; Liebig, H.-P.; Stützel, H. Gemüseproduktion: Ein Lehr-und Nachschlagewerk für Studium und Praxis; mit 113 Tabellen; Ulmer: Stuttgart, Germany, 2003; ISBN 9783800135844. [Google Scholar]
- Lattauschke, G. Anbauverfahren unter Glas; Landesamt für Umwelt, Landwirtschaft und Geologie: Dresden, Germany, 2006. [Google Scholar]
- Mondor, E.B.; Rosenheim, J.A.; Addicott, J.F. Predator-induced transgenerational phenotypic plasticity in the cotton aphid. Oecologia 2005, 142, 104–108. [Google Scholar] [CrossRef]
- Weisser, W.W.; Braendle, C.; Minoretti, N. Predator-induced morphological shift in the pea aphid. Proc. R. Soc. Lond. B 1999, 266, 1175–1181. [Google Scholar] [CrossRef]
- van Steenis, M.J.; El-Khawass, K. Life-history of Aphis gossypii on cucumber—Influence of temperature, host-plant and parasitism. Entomol. Exp. Appl. 1995, 76, 121–131. [Google Scholar] [CrossRef]
- Satar, S.; Kersting, U.; Uygun, N. Effect of temperature on development and fecundity of Aphis gossypii Glover (Homoptera: Aphididae) on cucumber. J. Pest. Sci. 2005, 78, 133–137. [Google Scholar] [CrossRef]
- Kocourek, F.; Havelka, J.; Berankova, J.; Jarosik, V. Effect of temperature on development rate and intrinsic rate of increase of Aphis gossypii reared on greenhouse cucumbers. Entomol. Exp. Appl. 1994, 71, 59–64. [Google Scholar] [CrossRef]
- Xia, J.Y.; van der Werf, W.; Rabbinge, R. Influence of temperature on bionomics of cotton aphid, Aphis gossypii, on cotton. Entomol. Exp. Appl. 1999, 90, 25–35. [Google Scholar] [CrossRef]
- Hoelmer, K.A.; Simmons, A.M. Yellow Sticky Trap Catches of Parasitoids of Bemisia tabaci (Hemiptera: Aleyrodidae) in Vegetable Crops and Their Relationship to In-Field Populations. Environ. Entomol. 2008, 37, 391–399. [Google Scholar] [CrossRef]
- Grupe, B.; Dieckhoff, C.; Meyhöfer, R. Keep an eye on natural enemies: What Aphidius on sticky traps tells us about aphid pest population dynamics. Entomol. Exp. Appl. 2023; accepted. [Google Scholar]
- Dieckhoff, C.; Meyhöfer, R. Dataset: Monitoring Aphids in Protected Cultivations by Sticky Traps; Leibniz University Hannover: Hannover, Germany, 2023. [Google Scholar] [CrossRef]


| Response Variables | Regression Equation | Slope SEM | r2 * | df | F | Pf > F | |
|---|---|---|---|---|---|---|---|
| First planting | Number of nymphs previous week | Y = 7.0237 − 1.3063X | 0.7461 | 0.24 | 10 | 3.07 | 0.1105 |
| Total number of aphids previous week | Y = 6.9135 − 1.1794X | 0.7162 | 0.22 | 10 | 2.71 | 0.1306 | |
| Number of alates same week | Y = 0.72537 + 0.07919X | 0.26859 | 0.01 | 10 | 0.09 | 0.7742 | |
| Total number of aphids same week | Y = 4.8175 − 0.08067X | 0.65671 | 0.08 | 10 | 0.02 | 0.9047 | |
| Second planting | Number of nymphs previous week | Y = 1.7254 + 1.8992X | 0.3482 | 0.79 | 7 | 29.75 | <0.001 |
| Total number of aphids previous week | Y = 2.1119 + 1.8081X | 0.3257 | 0.79 | 7 | 30.83 | <0.0001 | |
| Number of alates same week | Y = 0.06445 + 1.34920X | 0.18160 | 0.86 | 8 | 55.20 | <0.0001 | |
| Total number of aphids same week | Y = 3.6553 + 1.4888X | 0.3635 | 0.65 | 8 | 16.77 | <0.01 |
| Response Variables | Regression Equation | Slope SEM | r2 * | df | F | Pf > F | |
|---|---|---|---|---|---|---|---|
| First planting | Number of nymphs previous week | Y = 3.5339 + 0.4374X | 0.5857 | 0.06 | 22 | 0.5577 | 0.4631 |
| Total number of aphids previous week | Y = 3.8805 + 0.3831X | 0.5657 | 0.12 | 20 | 0.4585 | 0.5059 | |
| Number of alates same week | Y = 0.6319 + 0.2568X | 0.2508 | 0.04 | 22 | 1.0484 | 0.317 | |
| Total number of aphids same week | Y = 6.2326 − 0.3971X | 0.4592 | 0.29 | 20 | 0.7478 | 0.3975 | |
| Second planting | Number of nymphs previous week | Y = 4.5543 + 0.6371X | 0.3872 | 0.24 | 14 | 2.7078 | 0.1228 |
| Total number of aphids previous week | Y = 4.7228 + 0.6557X | 0.3834 | 0.27 | 14 | 2.9259 | 0.1086 | |
| Number of alates same week | Y = −0.05012 + 1.40864X | 0.2271 | 0.87 | 18 | 38.465 | <0.0001 | |
| Total number of aphids same week | Y = 2.4525 + 1.6471X | 0.3208 | 0.83 | 18 | 26.359 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieckhoff, C.; Meyhöfer, R. If Only You Could Catch Me—Catch Me If You Can: Monitoring Aphids in Protected Cucumber Cultivations by Means of Sticky Traps. Horticulturae 2023, 9, 571. https://doi.org/10.3390/horticulturae9050571
Dieckhoff C, Meyhöfer R. If Only You Could Catch Me—Catch Me If You Can: Monitoring Aphids in Protected Cucumber Cultivations by Means of Sticky Traps. Horticulturae. 2023; 9(5):571. https://doi.org/10.3390/horticulturae9050571
Chicago/Turabian StyleDieckhoff, Christine, and Rainer Meyhöfer. 2023. "If Only You Could Catch Me—Catch Me If You Can: Monitoring Aphids in Protected Cucumber Cultivations by Means of Sticky Traps" Horticulturae 9, no. 5: 571. https://doi.org/10.3390/horticulturae9050571
APA StyleDieckhoff, C., & Meyhöfer, R. (2023). If Only You Could Catch Me—Catch Me If You Can: Monitoring Aphids in Protected Cucumber Cultivations by Means of Sticky Traps. Horticulturae, 9(5), 571. https://doi.org/10.3390/horticulturae9050571







