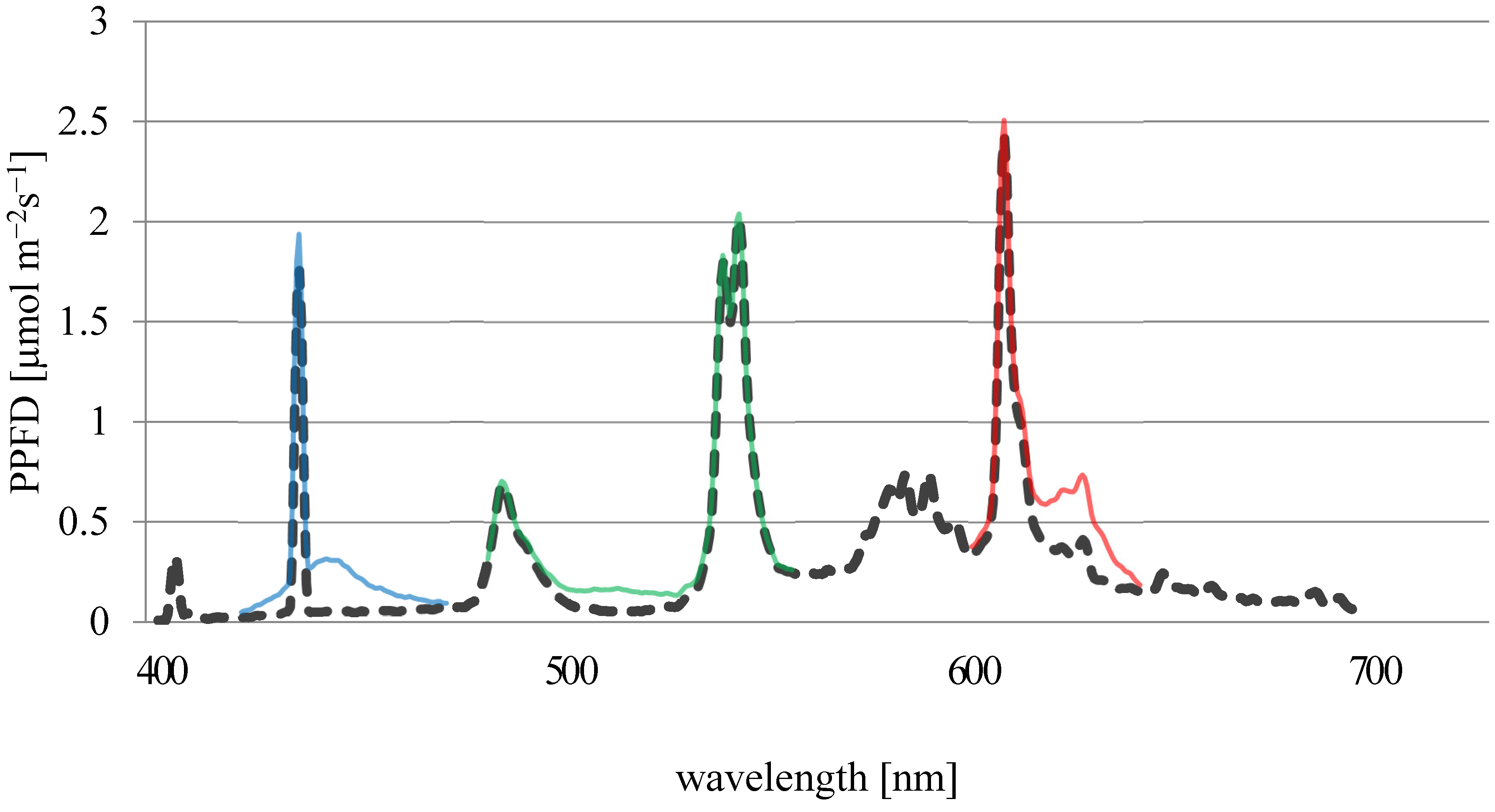
Figure 1.
Light spectrum of fluorescent tubes (FTs) (dashed black line), modified using LEDs (blue line: bLED; green line: gLED; red line: rLED) in the climate chamber, measured using an AvantesAvaSpec NIR 256 spectrometer.
Figure 1.
Light spectrum of fluorescent tubes (FTs) (dashed black line), modified using LEDs (blue line: bLED; green line: gLED; red line: rLED) in the climate chamber, measured using an AvantesAvaSpec NIR 256 spectrometer.
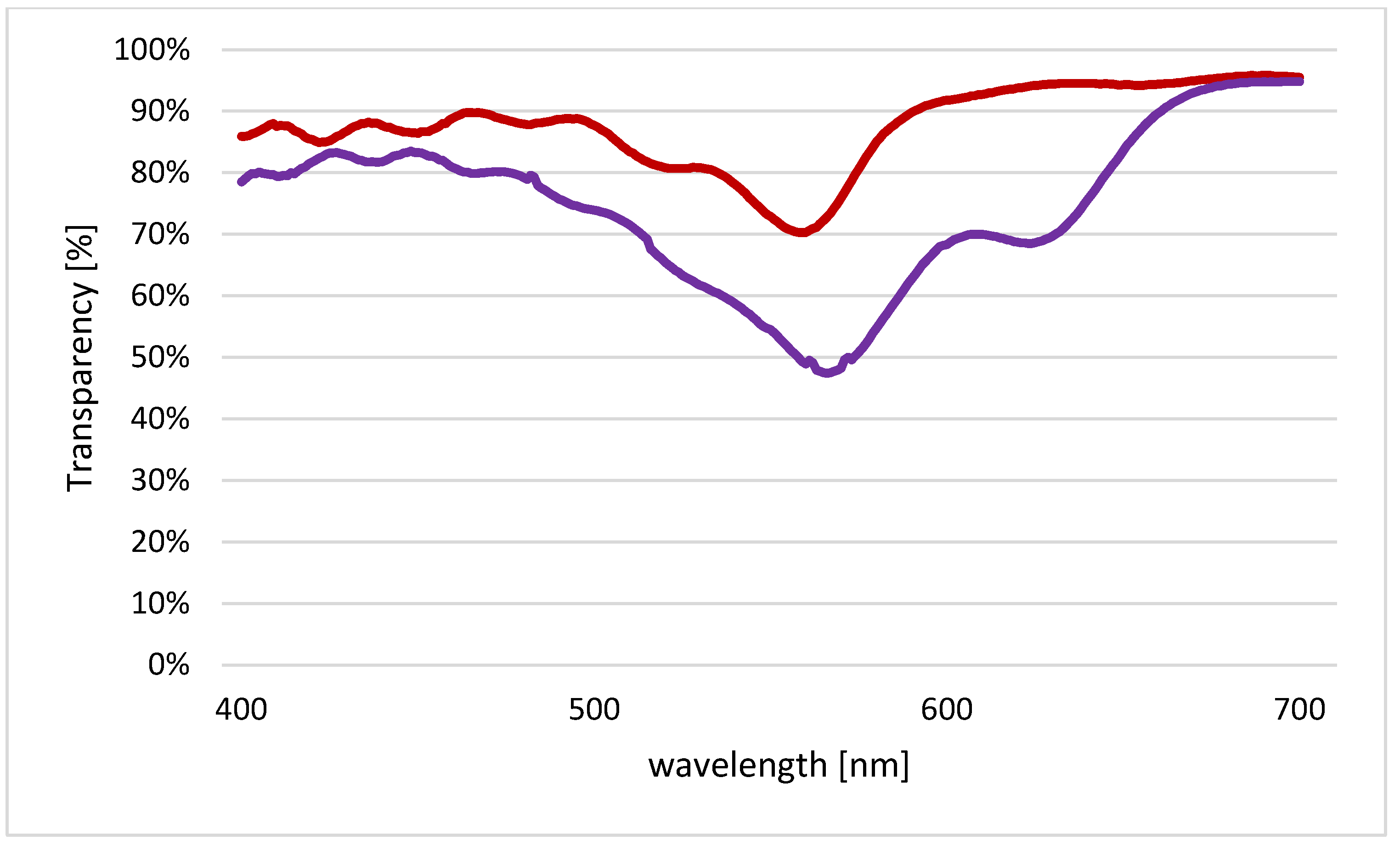
Figure 2.
Transparency of photoselective plastic films (red: Half Minus Green (“H”), purple: Pale Lavender (“P”)), measured using Unicam UV/Vis Spectrometer UV2.
Figure 2.
Transparency of photoselective plastic films (red: Half Minus Green (“H”), purple: Pale Lavender (“P”)), measured using Unicam UV/Vis Spectrometer UV2.
Table 1.
Daylight integral and spectral light composition of fluorescent tubes (FTb, FTg, FTr = control), modified by LEDs (FT + B: emission maximum at 443 nm, irradiance: 11 µmol·m−2·s−1; FT + G: emission maximum at 515 nm, irradiance: 7 µmol·m−2·s−1; FT + R: emission maximum at 629 nm, irradiance: 12 µmol·m−2·s−1) in a climate chamber, measured using an Avantes-AvaSpec NIR 256 spectrometer.
Table 1.
Daylight integral and spectral light composition of fluorescent tubes (FTb, FTg, FTr = control), modified by LEDs (FT + B: emission maximum at 443 nm, irradiance: 11 µmol·m−2·s−1; FT + G: emission maximum at 515 nm, irradiance: 7 µmol·m−2·s−1; FT + R: emission maximum at 629 nm, irradiance: 12 µmol·m−2·s−1) in a climate chamber, measured using an Avantes-AvaSpec NIR 256 spectrometer.
| Treatment | Daylight Integral [mol·m−2·day−1] | Share of Spectral Ranges on Total Radiation (%) |
|---|
| 401–450 nm | 451–500 nm | 501–550 nm | 551–600 nm | 601–650 nm | 651–700 nm |
|---|
| FTb | 4.68 | 7.8 | 10.6 | 22.8 | 23.9 | 27.7 | 6.8 |
| FT + B | 5.07 | 15.3 | 13.0 | 20.1 | 21.1 | 24.4 | 6.1 |
| FTg | 6.51 | 7.8 | 10.6 | 22.8 | 23.9 | 27.7 | 6.8 |
| FT + G | 6.30 | 7.4 | 11.0 | 26.1 | 22.7 | 26.2 | 6.6 |
| FTr | 9.06 | 7.8 | 10.6 | 22.8 | 23.9 | 27.7 | 6.8 |
| FT + R | 8.61 | 7.3 | 9.9 | 21.2 | 22.3 | 32.8 | 6.5 |
Table 2.
Daylight integral and spectral light composition of fluorescent tubes (FTh, FTp1, FTp2 = control), modified by photoselective plastic films (FT + H: covering with “Half Minus Green”; FT + P1: covering with “Pale Lavender”, decrease in light intensity by 43%; FT + P2: covering with “Pale Lavender”, decrease in light intensity by 60%) in climate chamber, measured using an Avantes-AvaSpec NIR 256 spectrometer.
Table 2.
Daylight integral and spectral light composition of fluorescent tubes (FTh, FTp1, FTp2 = control), modified by photoselective plastic films (FT + H: covering with “Half Minus Green”; FT + P1: covering with “Pale Lavender”, decrease in light intensity by 43%; FT + P2: covering with “Pale Lavender”, decrease in light intensity by 60%) in climate chamber, measured using an Avantes-AvaSpec NIR 256 spectrometer.
| Treatment | Daylight Integral [mol·m−2·day−1] | Share of Spectral Ranges on Total Radiation (%) |
|---|
| 401–450 nm | 451–500 nm | 501–550 nm | 551–600 nm | 601–650 nm | 651–700 nm |
|---|
| FTh | 6.51 | 7.8 | 10.6 | 22.8 | 23.9 | 27.7 | 6.8 |
| FT + H | 4.69 | 8.1 | 11.1 | 20.7 | 23.6 | 30.5 | 7.7 |
| FTp1 | 4.68 | 7.8 | 10.6 | 22.8 | 23.9 | 27.7 | 6.8 |
| FT + P1 | 2.67 | 9.7 | 12.4 | 20.1 | 20.7 | 29.5 | 9.5 |
| FTp2 | 9.06 | 7.8 | 10.6 | 22.8 | 23.9 | 27.7 | 6.8 |
| FT + P2 | 3.62 | 9.7 | 12.4 | 20.1 | 20.7 | 29.5 | 9.5 |
Table 3.
Spectral light composition of natural light (NL = control), modified by photoselective plastic films (NL + H: covering with “Half Minus Green”; NL + P: covering with “Pale Lavender”, in greenhouse, measured using an Avantes-AvaSpec NIR 256 spectrometer.
Table 3.
Spectral light composition of natural light (NL = control), modified by photoselective plastic films (NL + H: covering with “Half Minus Green”; NL + P: covering with “Pale Lavender”, in greenhouse, measured using an Avantes-AvaSpec NIR 256 spectrometer.
| Treatment | Transparency (%) | Share of Spectral Ranges on Total Radiation (%) |
|---|
| 401–450 nm | 451–500 nm | 501–550 nm | 551–600 nm | 601–650 nm | 651–700 nm |
|---|
| NL | 100 | 1.8 | 15.6 | 17.1 | 18.9 | 19.0 | 18.8 |
| NL + H | 77.9 | 11.0 | 16.3 | 14.1 | 15.2 | 21.6 | 21.9 |
| NL + P | 53.9 | 13.4 | 18.2 | 13.2 | 10.8 | 16.7 | 28.1 |
Table 4.
Influence of light conditions on inorganic constituents and growth parameters (fresh and dry matter, water content) in P. odorata, cultivated in greenhouse under natural light (NL), modified by additional LEDs (NL + B: emission maximum at 443 nm, irradiance: 11 µmol·m−2·s−1; NL + G: emission maximum at 515 nm, irradiance: 7 µmol·m−2·s−1; NL + R: emission maximum at 629 nm, irradiance: 12 µmol·m−2·s−1).
Table 4.
Influence of light conditions on inorganic constituents and growth parameters (fresh and dry matter, water content) in P. odorata, cultivated in greenhouse under natural light (NL), modified by additional LEDs (NL + B: emission maximum at 443 nm, irradiance: 11 µmol·m−2·s−1; NL + G: emission maximum at 515 nm, irradiance: 7 µmol·m−2·s−1; NL + R: emission maximum at 629 nm, irradiance: 12 µmol·m−2·s−1).
| | NL | NL + B | NL + G | NL + R |
|---|
| Nitrate [mg·g−1 DM] | 16.72 b | 24.16 a | 11.70 c | 13.30 bc |
| P [mg·g−1 DM] | 7.86 ab | 7.84 ab | 7.56 b | 7.94 a |
| K [mg·g−1 DM] | 38.90 b | 41.66 a | 40.53 ab | 42.55 a |
| Mg [mg·g−1 DM]) | 7.84 a | 6.73 ab | 6.50 b | 6.60 b |
| Ca [mg·g−1 DM] | 26.26 | 23.73 | 24.63 | 24.58 |
| Fe [mg·g−1 DM] | 0.12 | 0.13 | 0.14 | 0.16 |
| Fresh matter [g/plant] | 32.18 ab | 33.97 a | 28.79 c | 30.62 bc |
| Dry matter [g/plant] | 4.38 a | 4.26 a | 3.50 b | 3.48 b |
| Water content [%] | 86.57 c | 87.96 ab | 87.39 bc | 88.74 a |
Table 5.
Influence of light conditions on inorganic constituents and growth parameters (fresh and dry matter, water content) in P. odorata, cultivated in greenhouse under natural light (NL), modifiedby covering plants with photoselective plastic films (NL + H: covering with “Half Minus Green”; NL + P: covering with “Pale Lavender”).
Table 5.
Influence of light conditions on inorganic constituents and growth parameters (fresh and dry matter, water content) in P. odorata, cultivated in greenhouse under natural light (NL), modifiedby covering plants with photoselective plastic films (NL + H: covering with “Half Minus Green”; NL + P: covering with “Pale Lavender”).
| | NL | NL + H | NL + P |
|---|
| Nitrate [mg·g−1 DM] | 16.72 a | 10.97 b | 10.14 b |
| P [mg·g−1 DM] | 7.86 | 7.79 | 7.41 |
| K [mg·g−1 DM] | 38.9 | 39.85 | 40.1 |
| Mg [mg·g−1 DM]) | 7.48 a | 6.65 b | 6.31 b |
| Ca [mg·g−1 DM] | 26.26 | 27.54 | 25.76 |
| Fe [mg·g−1 DM] | 0.12 b | 0.14 a | 0.15 a |
| Fresh matter [g/plant] | 32.18 a | 21.68 b | 18.19 c |
| Dry matter [g/plant] | 4.38 a | 2.83 b | 2.46 b |
| Water content [%] | 86.57 ab | 87.19 a | 86.43 b |
Table 6.
Influence of light conditions on inorganic constituents and growth parameters (fresh and dry matter, water content) in P. odorata, cultivated in the climate chamber under fluorescent tubes (FTb, FTg, FTr = control), modified by additional LEDs (FT + B: emission maximum at 443 nm, irradiance: 11 µmol·m−2·s−1; FT + G: emission maximum at 515 nm, irradiance: 7 µmol·m−2·s−1; FT + R: emission maximum at 629 nm, irradiance: 12 µmol·m−2·s−1).
Table 6.
Influence of light conditions on inorganic constituents and growth parameters (fresh and dry matter, water content) in P. odorata, cultivated in the climate chamber under fluorescent tubes (FTb, FTg, FTr = control), modified by additional LEDs (FT + B: emission maximum at 443 nm, irradiance: 11 µmol·m−2·s−1; FT + G: emission maximum at 515 nm, irradiance: 7 µmol·m−2·s−1; FT + R: emission maximum at 629 nm, irradiance: 12 µmol·m−2·s−1).
| | FTb | FT + B | FTg | FT + G | FTr | FT + R |
|---|
| Daylight integral [mol·m−2·day−1] | 4.68 | 5.07 | 6.51 | 6.30 | 9.06 | 8.61 |
| Nitrate [mg·g−1 DM] | 21.07 | 18.52 | 21.80 | 18.53 | 27.10 a | 15.66 b |
| P [mg·g−1 DM] | 7.93 | 7.90 | 8.08 | 7.69 | 7.41 a | 5.87 b |
| K [mg·g−1 DM] | 37.56 | 37.59 | 38.67 | 37.66 | 40.90 a | 35.41 b |
| Mg [mg·g−1 DM]) | 6.99 b | 7.70 a | 8.46 b | 9.42 a | 7.77 | 8.47 |
| Ca [mg·g−1 DM] | 25.09 | 26.21 | 25.13 b | 27.48 a | 22.46 | 24.43 |
| Fe [mg·g−1 DM] | 0.14 | 0.15 | 0.13 | 0.13 | 0.13 | 0.14 |
| Fresh matter [g/plant] | 21.29 b | 28.83 a | 32.57 | 34.51 | 43.15 a | 37.80 b |
| Dry matter [g/plant] | 2.78 b | 3.86 a | 4.16 | 4.87 | 5.03 | 5.28 |
| Water content [%] | 86.95 | 86.59 | 87.24 a | 85.86 b | 88.35 a | 85.95 b |
Table 7.
Influence of light conditions on inorganic constituents and growth parameters (fresh and dry matter, water content) in P. odorata, cultivated in the climate chamber under fluorescent tubes (FTh, FTp1, FTp2 = control), modified by covering plants with photoselective plastic films (FT + H: covering with “Half Minus Green”; FT + P1: covering with “Pale Lavender”, decrease in light intensity by 43%; FT + P2: covering with “Pale Lavender”, decrease in light intensity by 60%).
Table 7.
Influence of light conditions on inorganic constituents and growth parameters (fresh and dry matter, water content) in P. odorata, cultivated in the climate chamber under fluorescent tubes (FTh, FTp1, FTp2 = control), modified by covering plants with photoselective plastic films (FT + H: covering with “Half Minus Green”; FT + P1: covering with “Pale Lavender”, decrease in light intensity by 43%; FT + P2: covering with “Pale Lavender”, decrease in light intensity by 60%).
| | FTh | FT + H | FTp1 | FT + P1 | FTp2 | FT + P2 |
|---|
| Daylight integral [mol·m−2·day−1] | 6.51 | 4.69 | 4.68 | 2.67 | 9.06 | 3.62 |
| Nitrate [mg·g−1 DM] | 21.80 | 20.13 | 21.07 | 20.40 | 27.10 | 30.28 |
| P [mg·g−1 DM] | 8.08 | 7.98 | 7.93 | 7.99 | 7.41 | 7.70 |
| K [mg·g−1 DM] | 38.67 | 37.65 | 37.56 | 37.38 | 40.90 | 41.23 |
| Mg [mg·g−1 DM]) | 8.46 | 8.44 | 6.99 | 7.35 | 7.77 a | 7.00 b |
| Ca [mg·g−1 DM] | 25.13 | 25.60 | 25.09 | 26.98 | 22.46 b | 27.84 a |
| Fe [mg·g−1 DM] | 0.13 | 0.12 | 0.14 | 0.15 | 0.13 b | 0.15 a |
| Fresh matter [g/plant] | 32.57 | 30.47 | 21.29 a | 18.45 b | 43.15 a | 19.40 b |
| Dry matter [g/plant] | 4.16 | 3.86 | 2.78 a | 2.47 b | 5.03 a | 2.10 b |
| Water content [%] | 87.24 | 87.29 | 86.95 | 86.50 | 88.35 | 89.17 |
Table 8.
Interdependencies (average partial correlation coefficients) between light parameters and contents of inorganic constituents or fresh and dry matter, as well as water content, of P. odorata, cultivated in the greenhouse or climate chamber under natural light or fluorescent tubes, modified by additional LEDs or photoselective plastic films.
Table 8.
Interdependencies (average partial correlation coefficients) between light parameters and contents of inorganic constituents or fresh and dry matter, as well as water content, of P. odorata, cultivated in the greenhouse or climate chamber under natural light or fluorescent tubes, modified by additional LEDs or photoselective plastic films.
| | Partial Correlation Coefficients |
|---|
| DLI | Share of Spectral Ranges | Ratio between Spectral Ranges |
|---|
| 401–450 | 451–500 | 501–550 | 551–600 | 601–650 | 651–700 | B:R | BR:G | B:G | R:G | R:FR |
|---|
| Nitrate | 0.30 | 0.18 | 0.01 | −0.20 | 0.02 | 0.03 | 0.39 | 0.30 | 0.45 | 0.45 | −0.03 | −0.68 |
| P | −0.03 | −0.02 | 0.01 | 0.08 | 0.28 | −0.10 | −0.08 | −0.15 | −0.48 | 0.00 | 0.05 | 0.34 |
| K | 0.21 | 0.20 | 0.15 | −0.05 | 0.02 | −0.27 | 0.26 | 0.16 | −0.15 | 0.08 | −0.08 | −0.25 |
| Mg | 0.36 | 0.00 | −0.02 | −0.13 | −0.05 | 0.17 | −0.24 | −0.14 | 0.37 | 0.12 | −0.04 | −0.07 |
| Ca | −0.39 | 0.20 | −0.09 | −0.29 | −0.16 | −0.10 | −0.04 | −0.12 | 0.03 | −0.03 | 0.22 | 0.16 |
| Fe | −0.31 | 0.29 | 0.46 | 0.14 | −0.51 | −0.06 | −0.30 | 0.27 | −0.13 | 0.10 | 0.15 | −0.04 |
| FM | 0.75 | 0.37 | 0.32 | 0.08 | −0.14 | −0.34 | 0.16 | 0.22 | 0.45 | 0.41 | −0.27 | −0.32 |
| DM | 0.64 | −0.31 | −0.22 | 0.02 | −0.06 | 0.04 | −0.20 | −0.15 | −0.14 | −0.22 | −0.12 | 0.13 |
| Water content | 0.14 | −0.40 | −0.35 | −0.09 | 0.53 | 0.15 | −0.41 | −0.31 | −0.21 | −0.42 | −0.22 | 0.73 |
Table 9.
Interdependencies (Pearson correlation coefficients) between contents of inorganic constituents and dry matter or water content of plants.
Table 9.
Interdependencies (Pearson correlation coefficients) between contents of inorganic constituents and dry matter or water content of plants.
| | Nitrate | P | K | Mg | Ca | Fe |
|---|
| Dry matter [g/plant] | 0.02 | −0.51 | −0.26 | 0.65 | −0.37 | −0.37 |
| Water content [%] | 0.23 | 0.09 | 0.49 | 0.16 | 0.04 | 0.02 |