The Dormancy Types and Germination Characteristics of the Seeds of Berberis koreana Palibin, an Endemic Species of Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Investigation of Internal and External Characteristics of Seeds
2.3. Seed Disinfection and Setting
2.4. Water Imbibition Test
2.5. Effect of Temperature on Germination: A Move-Along Experiment
2.6. Effect of Cold Stratification Experiment on Germination
2.7. Experiment to Determine the Effect of GA3 on Germination
2.8. Effect of Light Conditions on Seed Germination
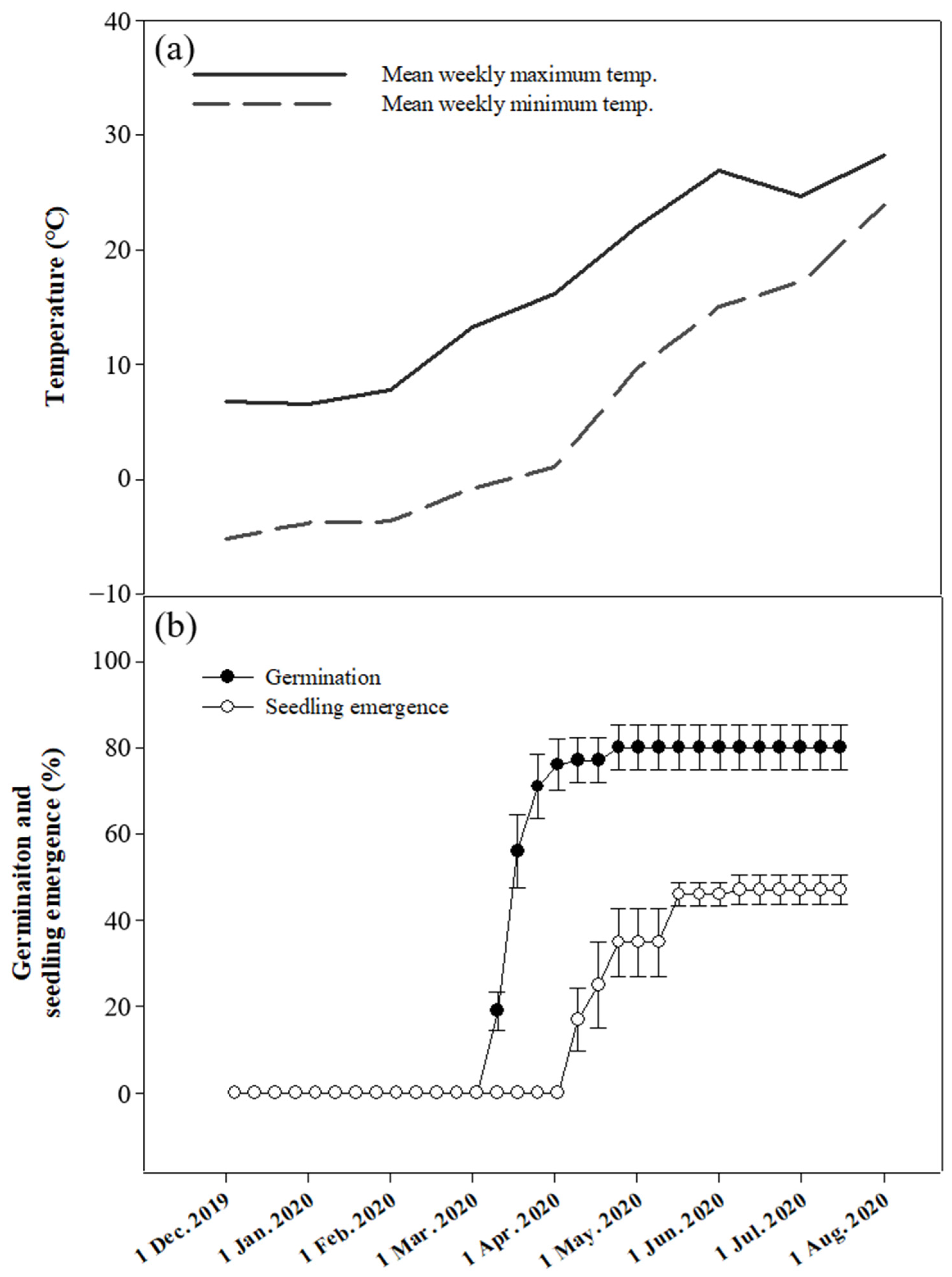
2.9. Phenology of Embryo Growth, Germination, and Seedling Emergence under Natural Environmental Conditions
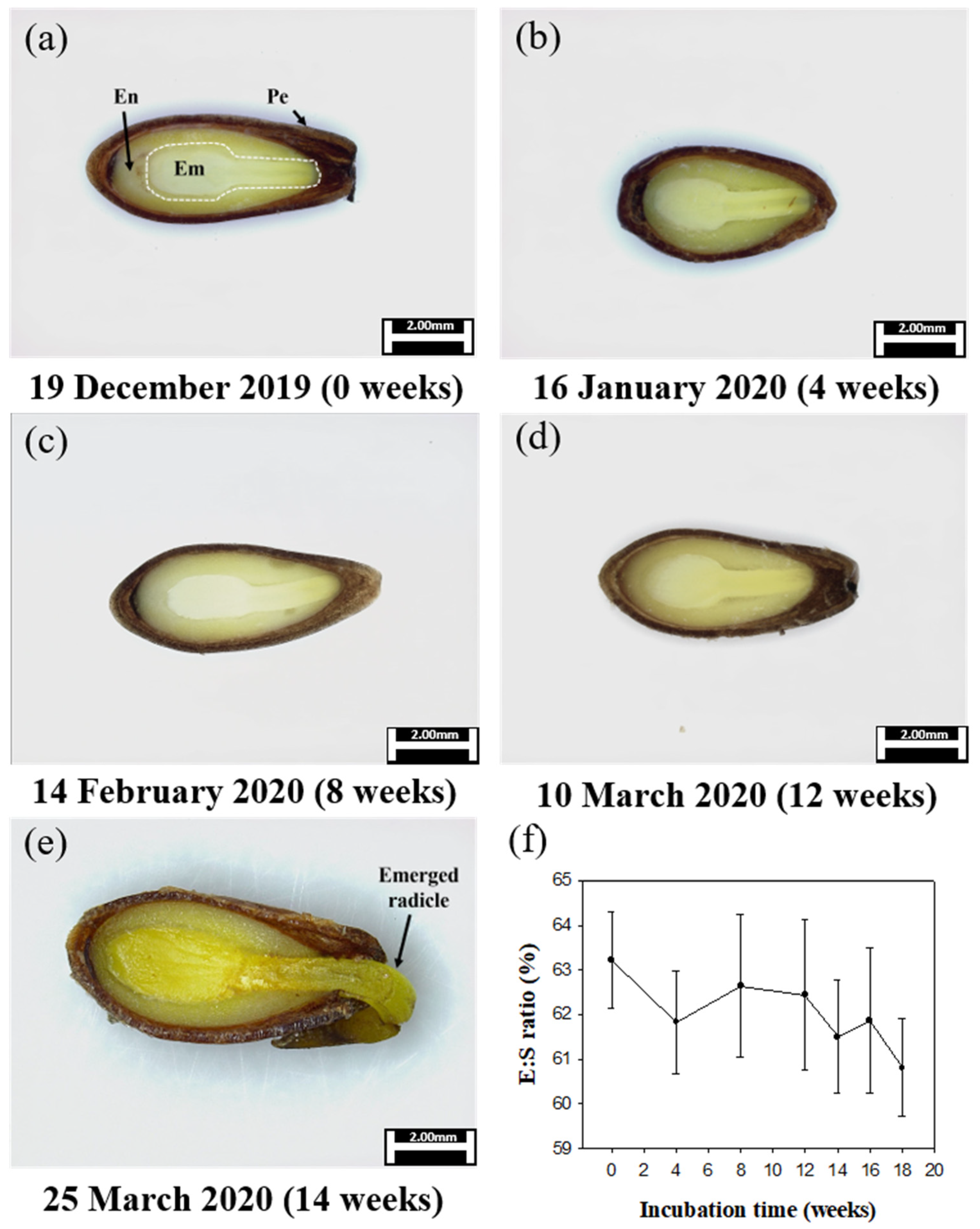
2.9.1. Embryo Growth
2.9.2. Germination
2.9.3. Seedling Emergence
2.10. Statistical Analyses
3. Results
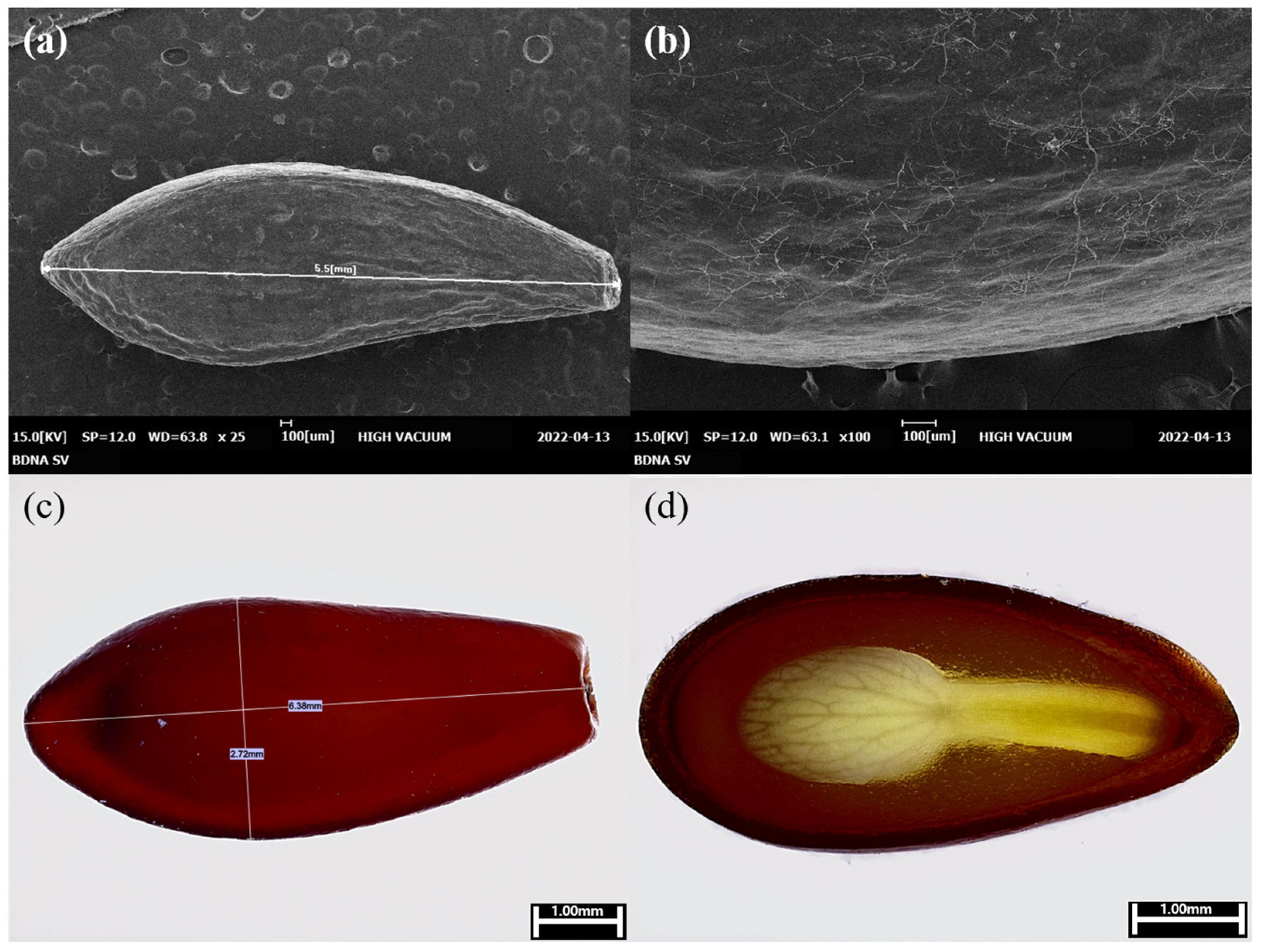
3.1. Investigation of Internal and External Characteristics of Seeds
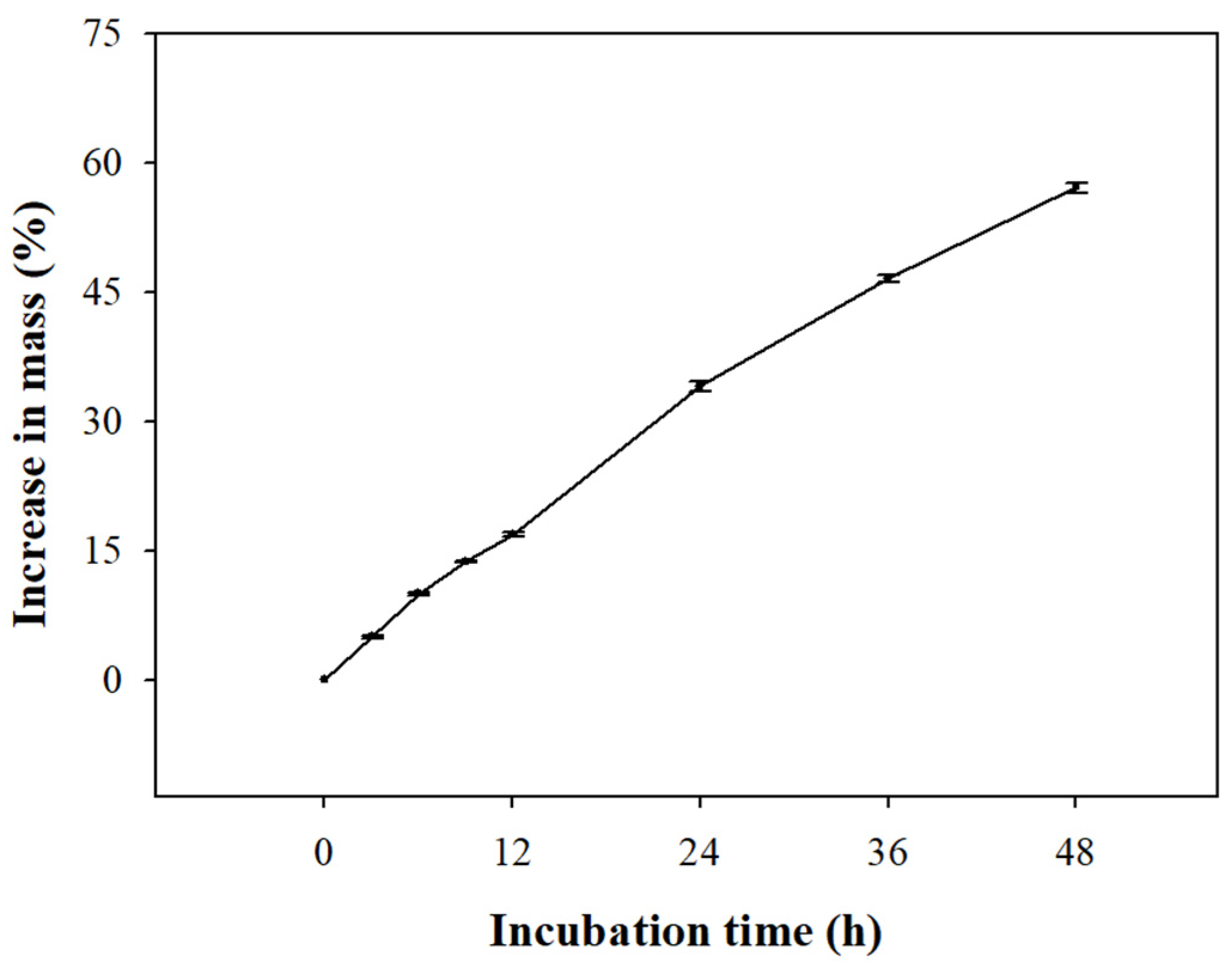
3.2. Water Imbibition Test
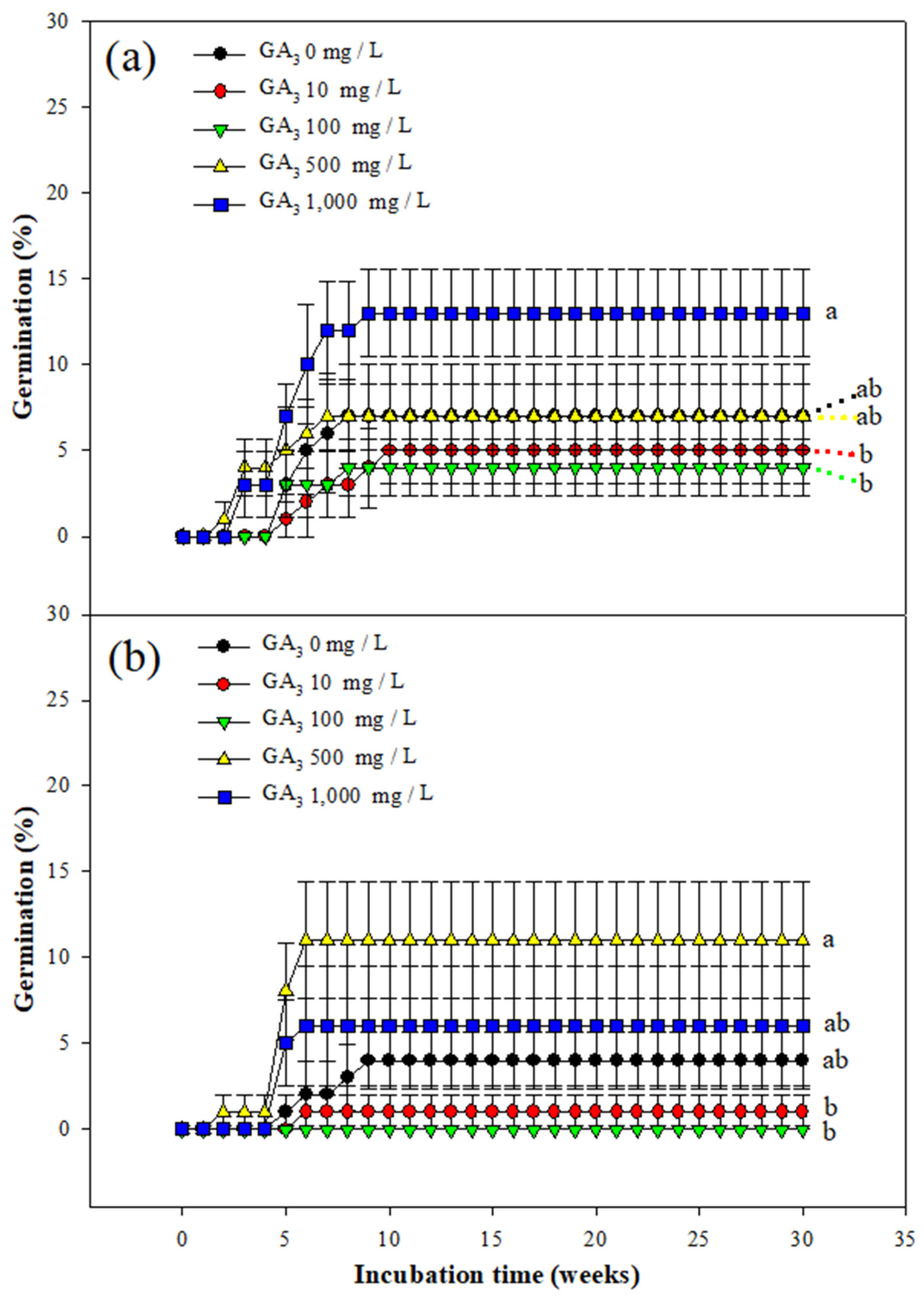
3.3. Effect of GA3 Treatment on Seed Germination
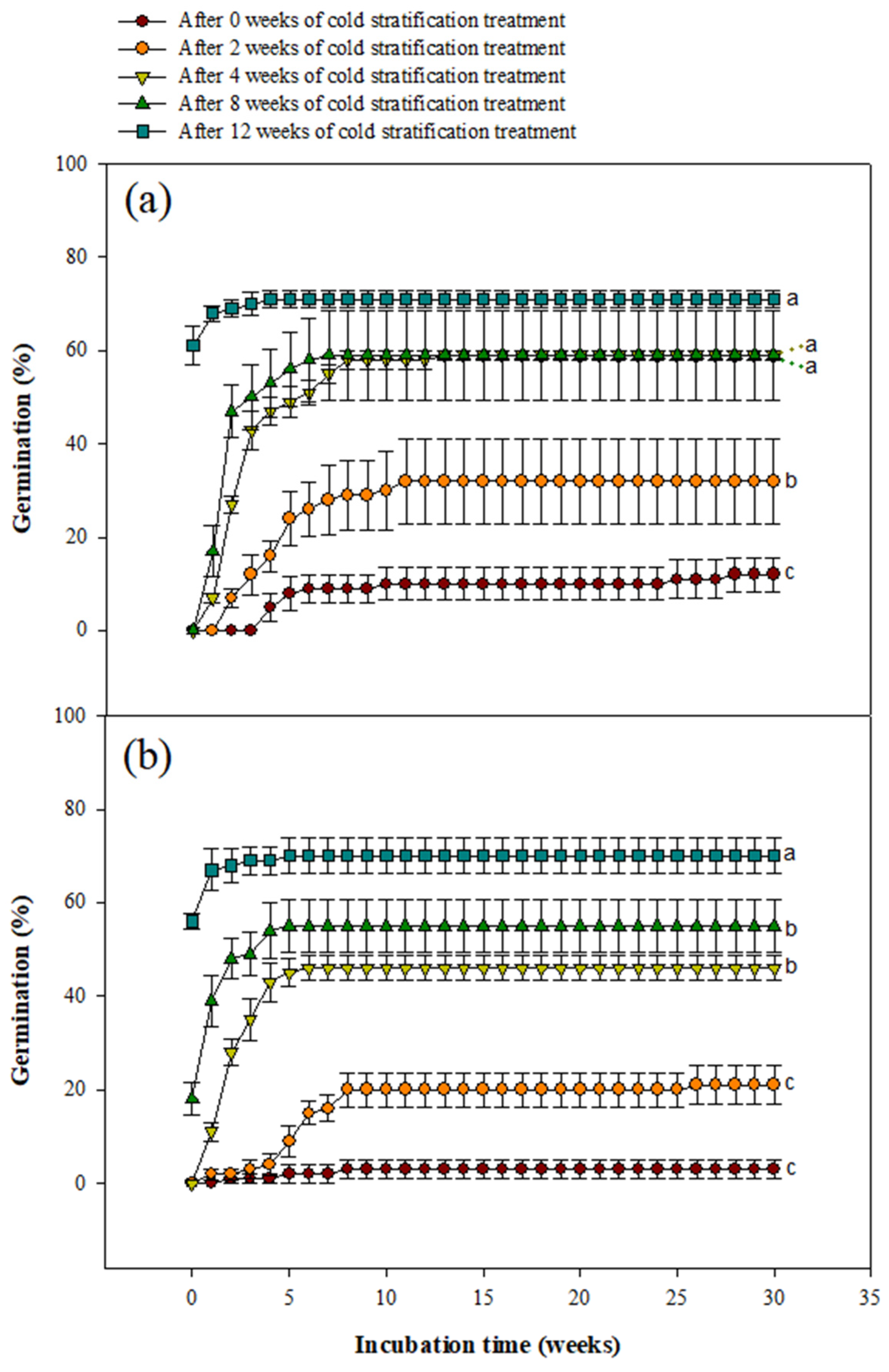
3.4. Effect of Cold Stratification Experiment on Seed Germination
3.5. Seed Germination Based on Temperature Conditions: A Move-along Experiment
3.6. Phenology of Embryo Growth, Germination, and Seedling Emergence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, S.Y.; Rhie, Y.H.; Kim, Y.J. Morphological and morphophysiological dormancy in seeds of several spring ephemerals native to Korea. Flower Res. J. 2012, 20, 193–199. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Elsevier Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Lang, G.A. Dormancy: A new universal terminology. HortScience 1987, 22, 817–820. [Google Scholar] [CrossRef]
- Yoo, S.J.; Lee, K.B.; Kwak, J.H. Studies on the seasonal variation of berberine contents in Berberis koreana. Korean J. Pharmacogn. 1986, 17, 123–128. [Google Scholar]
- Hyun, M.S.; Woo, W.H.; Hur, J.M.; Kim, D.; Mun, Y.J. The role of ROS and p38 MAP kinase in berberine-induced apoptosis on human hepatoma Hep G2 cells. J. Korean Soc. Appl. Biol. Chem. 2008, 51, 129–135. [Google Scholar]
- Ling, J.; Ha, J.; Choi, Y.; Seo, Y.; Kim, J.; Kim, Y.; Cha, S.; Kim, J.; Lee, H. Enhancement of cosmeceutical activities of Berberis koreana bark by high pressure and ultrasonification extraction processes. Korean J. Med. Crop Sci. 2011, 19, 54–65. [Google Scholar] [CrossRef]
- Verma, N.; Singh, J.P.; Mishra, D.; Nath, A.; Kashyap, P.; Dutta, D. Indian barberry (Berberis aristata D. C). In Underutilized Fruit Crops: Importance and Cultivation; Jaya Publishing House: New Delhi, India, 2017; pp. 143–154. [Google Scholar]
- Belwal, T.; Bisht, A.; Bhatt, I.D.; Rawal, R.S. Influence of seed priming and storage time on germination and enzymatic activity of selected Berberis species. Plant Growth Regul. 2015, 77, 189–199. [Google Scholar] [CrossRef]
- Thakur, A.; Thakur, P.S.; Mehta, R. Studies on germination, viability and vigour in Indian barberry (Berberis aristata DC.)—An endangered medicinal plant species of western Himalayas. Indian For. 2006, 132, 485–492. [Google Scholar]
- Wang, J.H.; Du, G.Z.; Cui, X.L.; Zheng, X.F.; Qi, W. Germination characteristics of 61 common woody species from the eastern Qinghai-Tibet Plateau of China and their life history correlates. J. Plant Ecol. 2009, 33, 171–179. [Google Scholar]
- Wang, J.H.; Baskin, C.C.; Chen, W.; Du, G.Z. Variation in seed germination between populations of five sub-alpine woody species from eastern Qinghai-Tibet Plateau following dry storage at low temperatures. Ecol. Res. 2010, 25, 195–203. [Google Scholar] [CrossRef]
- Deb, C.R.; Sangtam, T.L.; Jamir, N.S. Seed Biology of Berberis manipurana Ahrendt: A Threatened Natural Dye Yielding Plant. Am. J. Plant Sci. 2017, 8, 1285–1295. [Google Scholar] [CrossRef]
- Bahuguna, V.K.; Rawart, M.M.S.; Joshi, S.R. Preliminary studies on seed germination behaviour of Berberis lycium Royle—An important shrub for reclamation of wastelands in the Himalaya. Indian For. 1988, 114, 181–183. [Google Scholar]
- Sathyakumar, S.; Viswanath, S. Observations on food habits of Asiatic black bear in Kedarnath Wildlife Sanctuary, India: Preliminary evidence on their role in seed germination and dispersal. Ursus 2003, 14, 99–103. [Google Scholar]
- Rudolf, P.O.; Buckeye, A.L. Horse chestnut. Seeds of Woody Plants in the United States. Agric. Handb. 1974, 450, 195–199. [Google Scholar]
- Taylor, J. Propagation successes, failures and lessons learned. In Proceedings of the Conference: Native Plant Propagation and Restoration Strategies; Nursery Technology Cooperative and Western Forestry and Conservation Association: Eugene, Oregon, 2002; pp. 45–54. [Google Scholar]
- Jannatizadeh, A.; Khadivi-Khub, A. Morphological variability of Berberis integerrima from Iran. Erwerbs-Obstbau 2016, 58, 247–252. [Google Scholar] [CrossRef]
- Karlović, K.; Kremer, D.; Liber, Z.; Šatović, Z.; Vršek, I. Intra- and interpopulation variability and taxonomic status of Berberis croatica Horvat. Plant Biosyst. 2009, 143, 40–46. [Google Scholar] [CrossRef]
- Akbulut, M.; Çalişir, S.; Marakoğlu, T.; Çoklar, H. Some physicomechanical and nutritional properties of barberry (Berberis vulgaris L.) fruits. J. Food Process Eng. 2009, 32, 497–511. [Google Scholar] [CrossRef]
- Allen, R.B.; Wilson, J.B. Fruit and seed production in Berberis darwinii Hook., a shrub recently naturalised in New Zealand. N. Z. J. Bot. 1992, 30, 45–55. [Google Scholar] [CrossRef]
- Kremer, D.; Jurišić Grubješić, R.; Popović, Z.; Karlović, K. Fruit and seed traits of Berberis croatica Horvat and Berberis vulgaris L. Acta Bot. Croat. 2012, 71, 115–123. [Google Scholar] [CrossRef]
- ISTA. International Seed Testing Association. International rules for seed testing. Seed Sci. Technol. 1999, 27, 333. [Google Scholar]
- Lee, S.Y.; Rhie, Y.H.; Kim, K.S. Non-deep simple morphophysiological dormancy in seeds of Thalictrum rochebrunianum, an endemic perennial herb in the Korean Peninsula. Hortic. Environ. Biotechnol. 2015, 56, 366–375. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. When breaking seed dormancy is a problem: Try a move-along experiment. Nat. Plants J. 2003, 4, 17–21. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Zheng, R.; Ma, Z.; Jiang, L.; Zhao, Z.; Shi, X.; Wang, L. Germination characteristics of plump and shriveled seeds of Tamarix ramosissima matured in different seasons. Seed Sci. Technol. 2022, 50, 21–25. [Google Scholar] [CrossRef]
- Bravo, C.; Chamorro, D.; Hiraldo, F.; Speziale, K.; Lambertucci, S.A.; Tella, J.L.; Blanco, G. Physiological dormancy broken by endozoochory: Austral parakeets (Enicognathus ferrugineus) as legitimate dispersers of calafate (Berberis microphylla) in the Patagonian Andes. J. Plant Ecol. 2020, 13, 538–544. [Google Scholar] [CrossRef]
- Khudonogova, E.; Zatsepina, O.; Polovinkina, S.; Rachenko, M.; Tyapaeva, M. Seed germination of woody and shrubby introduced species. IOP Conf. Ser. Earth Environ. Sci. 2019, 316, 12–21. [Google Scholar] [CrossRef]
- Larsen, S.U.; Eriksen, E.N. Delayed release of primary dormancy and induction of secondary dormancy in seeds of woody taxa caused by temperature alternations. Acta Hortic. 2004, 630, 91–100. [Google Scholar] [CrossRef]
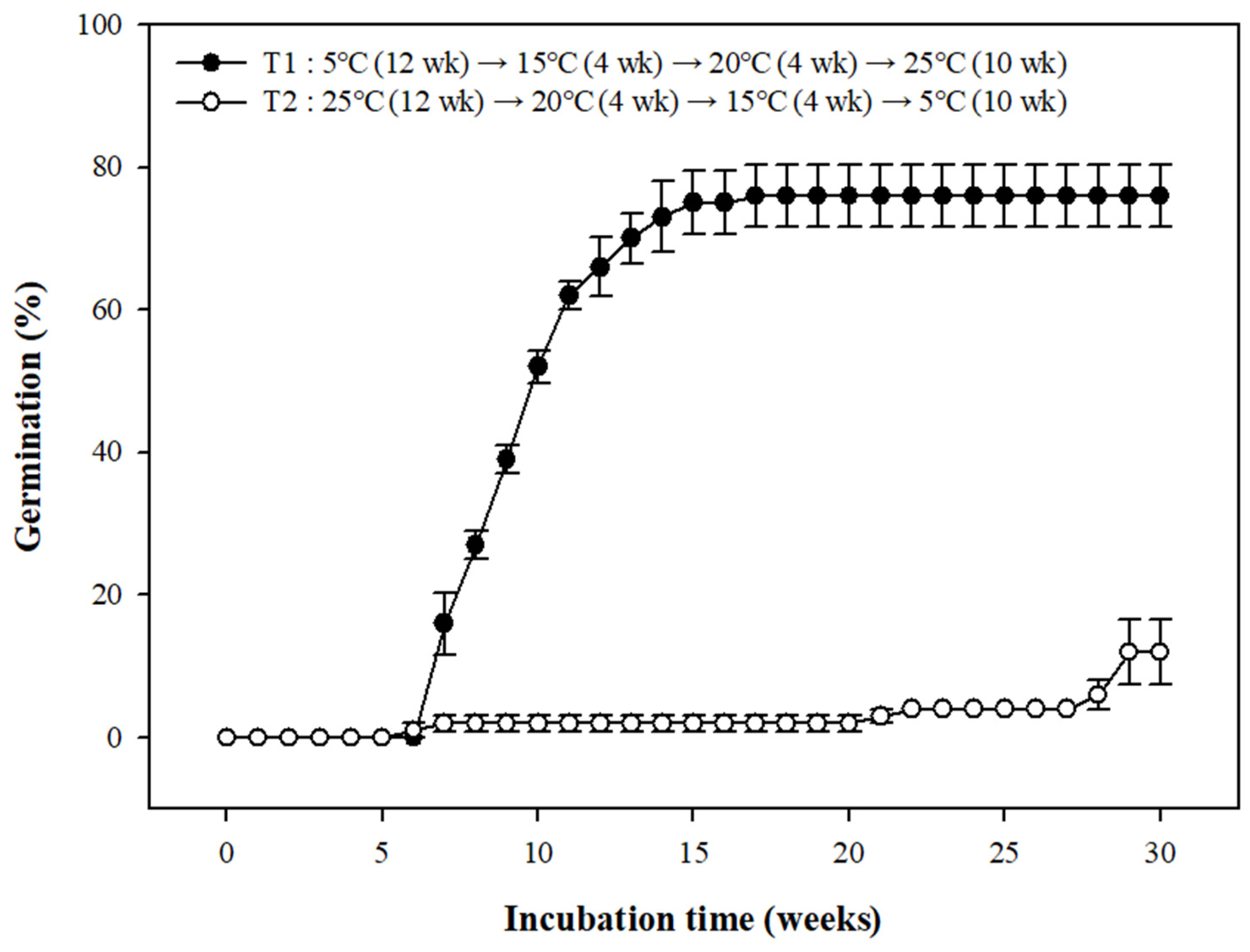
| No. of Weeks at Treatment Temperatures | 4 | 4 | 4 | 4 | 4 | 4 | |
|---|---|---|---|---|---|---|---|
| Move along | T1 | 5 °C winter | 5 °C winter | 5 °C winter | 15 °C early spring | 20 °C late spring | 25 °C summer |
| T2 | 25 °C summer | 25 °C summer | 25 °C summer | 20 °C early autumn | 15 °C early autumn | 5 °C winter | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Kim, S.-G.; Lee, H.; Na, C.-S.; Lee, D.-H. The Dormancy Types and Germination Characteristics of the Seeds of Berberis koreana Palibin, an Endemic Species of Korea. Horticulturae 2023, 9, 547. https://doi.org/10.3390/horticulturae9050547
Kim D-H, Kim S-G, Lee H, Na C-S, Lee D-H. The Dormancy Types and Germination Characteristics of the Seeds of Berberis koreana Palibin, an Endemic Species of Korea. Horticulturae. 2023; 9(5):547. https://doi.org/10.3390/horticulturae9050547
Chicago/Turabian StyleKim, Do-Hyun, Sang-Geun Kim, Hayan Lee, Chae-Sun Na, and Do-Hyung Lee. 2023. "The Dormancy Types and Germination Characteristics of the Seeds of Berberis koreana Palibin, an Endemic Species of Korea" Horticulturae 9, no. 5: 547. https://doi.org/10.3390/horticulturae9050547
APA StyleKim, D.-H., Kim, S.-G., Lee, H., Na, C.-S., & Lee, D.-H. (2023). The Dormancy Types and Germination Characteristics of the Seeds of Berberis koreana Palibin, an Endemic Species of Korea. Horticulturae, 9(5), 547. https://doi.org/10.3390/horticulturae9050547








