Abstract
Fresh-cut pumpkins refer to fresh pumpkin that has been graded, cleaned, peeled, sliced, preserved, and packaged. It has the qualities of freshness, nutrition, convenience, and being 100% edible. However, mechanical damages during the cutting processing can accelerate the quality deterioration, aging, and loss of nutritional values of fresh-cut pumpkins. Nisin, a natural preservative, has been widely used in fruits and vegetables with good preservation effects. To investigate the effect of different concentrations (0, 0.2, 0.4, and 0.6 g/L) of nisin on the quality of fresh-cut pumpkins, the critical indexes involved in weight loss, firmness, color, respiration intensity, reactive oxygen species (ROS) metabolism, ascorbate (AsA)—glutathione (GSH) cycle, and antioxidant capacity were monitored for fresh-cut pumpkins during storage at 4 °C for 10 days. The results showed that 0.4 g/L nisin was the best preservation concentration. Compared with 0 g/L nisin, 0.4 g/L nisin reduced the weight loss rate and whitening rate of fresh-cut pumpkins by 13.53% and 13.61%, inhibited respiration rate by 45.83%, and maintained hardness by 1.18 times. Meanwhile, 0.4 g/L nisin increased the activities of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) and maintained higher contents of GSH and AsA. It prevented the rapid increase in ROS levels by improving antioxidant capacity, including DPPH, ABTS free radical scavenging rate, and T-AOC (total antioxidant capacity). The collected results showed that nisin has an obvious influence on the quality by regulating physiological and antioxidant activity metabolism. It is envisaged that the combination of nisin and physical and chemical preservation technology will further enhance the quality of fresh-cut pumpkins during storage in the future.
1. Introduction
Pumpkin is one of the most widely grown crops on the planet [1]. It is favored by consumers because of its sweet and mild flesh, as well as its high nutritional content [2]. Pumpkin polysaccharides [3], phenols (including flavonoids and phenolic acids), carotenoids [4], minerals (including potassium, phosphorus, magnesium, iron, and selenium), and vitamins (including B, C, E, and K) [5] are all found in pumpkin. These nutrients have antioxidant activity, which is useful for maintaining the body’s immunological function and lowering the risk of some types of cancer, and they are especially vital for diabetics and those with vascular damage [6,7]. Thus, it is also regarded as a vegetable in many countries’ healthy diets and traditional medicine. China is the world’s largest producer of pumpkins. According to the Food and Agriculture Organization of the United Nations, China accounted for about one-third of the world’s pumpkin production in 2021 [8]. Despite the fact that pumpkin is a store-tolerant crop, it is still wasted owing to quality degradation during the production, manufacture, transportation, retail, and home storage stages. Furthermore, the sheer size of pumpkins might make them difficult to transport and handle for the typical customer.
Fresh-cut pumpkins refer to fresh pumpkins that have been graded, cleaned, peeled, sliced, preserved, and packaged [9]. It has the qualities of freshness, nutrition, convenience, and being 100% edible. In line with consumer demands in the restaurants and retail markets for fresh-cut vegetables. However, mechanical cutting resulted in a number of quality changes that reduced the shelf life and marketability of fresh-cut pumpkins, including microbial infection, faster nutrient loss, yellowing and whitening of the cut surface, tissue softening, and flavor alterations [9]. For these reasons, food scientists have developed three different techniques. Firstly, the physical preservation method is the most widely employed for fresh-cut pumpkins because of its advantages of easy control of processing conditions and little influence on the structure and nutritional tastes, but it requires a relatively large investment in equipment [10]. Secondly, while the chemical preservation method has an excellent preservation effect, it uses chemicals that are easy to leave residue, which causes safety issues [11]. Finally, due to its safety, non-toxicity, and great efficiency, biological preservation technology has attracted special attention from scholars all over the world in recent decades, and it is utilized in a variety of foods [10].
Nisin, a bacteriocin produced by the Lactococcus lactis subspecies, is a safe and non-toxic natural biological preservative. Nisin has been widely used as a food preservative for more than 60 years. The U.S. Food and Drug Administration (FDA) classifies Nisin as Gras and other countries allow its use as a food preservative [12,13]. In addition, nisin can be rapidly digested and decomposed into various amino acids by protease, and it does not cause toxic side effects on the human body. Therefore, nisin has been widely used in the preservation of fruits and vegetables with good preservation effects [14,15]. Studies have shown that nisin combined with green tea (GTE), modified atmosphere packaging (MAP), and modified initial atmosphere (MIA) treatment on fresh-cut beet leaves greatly increased total polyphenol contents and antioxidant capacity [16]. Nisin and ε-polylysine with chitosan coating significantly (p < 0.05) inhibited respiration rate, decline of AsA, and white blush in fresh-cut carrots [17].
At present, there is no information available on the treatment of nisin on fresh-cut pumpkin to improve its quality and antioxidant activity during storage. Therefore, the purpose of this study is to investigate the effects of different nisin concentrations (0, 0.2, 0.4, and 0.6 g/L) on the weight loss, firmness, color, and respiratory intensity of fresh-cut pumpkins stored at 4 °C for 10 days. Additionally, a suitable concentration was chosen to measure the ROS level, antioxidant enzyme activity, antioxidant content, and antioxidant capacity of fresh-cut pumpkins in order to investigate how nisin affects their antioxidant activity. Simultaneously, the study provides a biological preservation strategy for maintaining the quality and extending the shelf life of fresh-cut pumpkins.
2. Materials and Methods
2.1. Processing and Treatments of Fresh-Cut Pumpkins
Pumpkins (Cucurbita moschata, Duch) of the cultivar “Luli” were obtained from a commercial farm near Wuchang City, Heilongjiang Province. The harvest area of “Luli” pumpkins was located in a high-cold and high-latitude area, with sufficient light in the growing season, long sunshine times, and a large temperature difference between day and night. The harvest area is fertile black soil, characterized by high organic content, fertile soil, and loose soil. The mature tropical pumpkins were harvested at the mature green 86 stage (65 days after flowering).
Pumpkins with uniform melon shapes and similar maturities, without diseases, pests, or mechanical damages, were selected and stored at 4 °C for later use. The pumpkins were soaked in 0.1% sodium hypochlorite water for 2 min, rinsed with distilled water, and then the skin and pulp were removed. The pumpkins were cut into 1/4 circular arc slices with a thickness of 0.5 cm and a width of 2 cm. Soak them in nisin (nisin A, purchased from Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China) solution at a concentration of 0, 0.2, 0.4, and 0.6 g/L for 10 min, and then make fresh-cut pumpkins after being drained. Then, fresh-cut pumpkins were packed inside a polypropylene container with polyethylene films and stored at 4 °C for 10 days. Samples were taken every 2 days to determine the optimal concentration of nisin for weight loss, firmness, color, and respiration. Then the samples of the control group and the optimal concentration treatment group were ground into a powder after being frozen with liquid nitrogen, then stored at −80 °C for antioxidant index measurements.
2.2. Weight Loss
The weight of samples was weighed using an electronic balance, and three sets were set up in parallel; thus, the weight loss was expressed as the ratio of the mass difference value to the initial fresh mass (%) [18].
2.3. Firmness
The firmness of fresh-cut pumpkin slices was measured by carrying out texture profile analysis (TPA) using a TA-XT texture analyzer. The cylindrical P5 probe with a diameter of 5 mm was pressed down the slice at a rate of 1 mm/s, the trigger force was 0.5 N, and the depth of penetration was 4 mm [19]. Different parts of fresh-cut pumpkins in each treatment group were measured, and the maximum force (N) was measured as the firmness.
2.4. Color
The color (L*, a*, and b*) of fresh-cut pumpkin slices was measured by a CR-400 colorimeter, where the L* value defines brightness, the a* value defines red-green, and the b* value defines yellow-blue. The whitening index (WI) indicates the degree of whitening on the cut surface of fresh-cut pumpkins [20]. The formula is as follows:
2.5. Respiration Intensity
The respiration intensity of fresh-cut pumpkins was measured by a gas analyzer (F-940 STORE II, Felix, Camas, WA, USA) [21]. Place 50 g of fresh-cut pumpkins in a 550-mL sealed crisper to store the sample at 4 °C for 1 h. Then the CO2 content in the crisper was measured for 25 s, and repeated three times. The respiratory intensity was calculated according to the following formula:
where C1 represents the CO2 content (%) in the sealed crimp box, C2 represents the atmospheric CO2 content (%), V represents the container volume (mL), m represents the sample mass (kg), and t represents the placement time (h).
Respiration Intensity (mg/(kg·h)) = ((C1 − C2) ÷ 100 × V) ÷ (m × t) × 1.96
2.6. Superoxide Anion (O2−·) Production Rate
Approximately 0.2 g of the frozen sample was used for the O2−· rate of production assay according to the protocol of the OFR measurement kit (SA-1-G, Suzhou Keming Biotechnology Co., Ltd., Suzhou, China) [22]. After adding reagents, the light absorption for each sample was determined by measuring the UV absorbances (A1) at 530 nm against a blank solution (A2) (ΔA = A1 − A2). A standard curve was generated for O2−· rate of production measurement (y = 0.0121x − 0.0027, R2 = 0.9980). Finally, the O2−· rate of production is measured by the following formula: (nmol/g·min) = (ΔA + 0.0027) ÷ 0.0121 × V1 ÷ (V2 ÷ V3 × W) × 2 ÷ T (V1: total response volume; V2: reaction sample volume; V3: extraction liquid volume; W: sample mass; 2: 2 molecules of O2− participating in the reaction to form 1 molecule of NO2−; T: reaction time.) Three independent replicates were used for each treatment.
2.7. H2O2 Content
Approximately 0.2 g of the frozen sample was used for H2O2 content assay according to the protocol of the H2O2 content measurement kit (H2O2-1-Y, Suzhou Keming Biotechnology Co., Ltd., Suzhou, China) [22]. After adding reagents, the light absorption for each sample was determined by measuring the UV absorbances (A1) at 415 nm against a blank solution (A2) (ΔA = A1 − A2). Regression curves were generated for the determination of H2O2 content under standard conditions (y = 0.3744x + 0.0006). Finally, the H2O2 content is measured by the following formula: (μmol/g) = [(ΔA − 0.0006) ÷ 0.3744 × V1] ÷ (W × V1 ÷ V2) (V1: sample volume in the reaction system; V2: extraction liquid volume; W: sample quality). Three independent replicates were used for each treatment.
2.8. Superoxide Dismutase Activity (SOD)
Approximately 0.2 g of the frozen sample was used for the SOD activity assay according to the protocol of the SOD measurement kit (SOD-1-Y, Suzhou Keming Biotechnology Co., Ltd., Suzhou, China) [22]. After adding reagents, the percentage inhibition was determined by measuring the UV absorbance at 560 nm for each sample (A1) and the light absorption of the control solution (A2) (inhibition rate = (A2 − A1) ÷ A2 × 100%). One unit of SOD activity was defined as SOD enzyme activity in the reaction system when the percentage inhibition was 50%. Three independent replicates were used for each treatment.
2.9. Catalase Activity (CAT)
Approximately 0.2 g of the frozen sample was used for the CAT activity assay according to the protocol of the CAT measurement kit (CAT-1-Y, Suzhou Keming Biotechnology Co., Ltd., Suzhou, China) [22]. After adding reagents, the light absorption for each sample was determined by measuring the initial UV absorbances (A1) at 240 nm against the absorbance value after 1 min (A2) (ΔA = A1 − A2). One unit of CAT activity was defined as catalyzing 1 nmol of H2O2 degradation per minute per g of tissue at 240 nm. Three independent replicates were used for each treatment.
2.10. Ascorbate Peroxidase Activity (APX)
The APX activity determination method refers to Yan et al. [23,24]. The frozen, fresh-cut pumpkin tissues (5 g) were added to 20 mL of 0.1 mol/L potassium phosphate buffer (pH 7.5), and the supernatant was centrifuged at 12,000× g for 30 min at 4 °C as the enzyme extraction solution, which was stored at a low temperature for later use. APX was determined by mixing a 2.6 mL reaction buffer (containing 0.1 mmol/L EDTA and 0.5 mmol/L ascorbic acid) with 0.1 mL of enzyme extract, then adding 0.3 mL of a 2 mmol/L H2O2 solution to start the reaction. The absorbance value at 290 nm was determined within 15 s. One unit of enzyme activity was defined as U/g when the absorbance value at 290 nm of the APX enzymatic reaction system decreased by 0.01 per gram of the fresh-cut pumpkin sample.
2.11. Glutathione Reductase Activity (GR)
Approximately 0.2 g of the frozen sample was used for the GR activity assay according to the protocol of the GR measurement kit (GR-1-W, Suzhou Keming Biotechnology Co., Ltd., Suzhou, China) [22]. After adding reagents, the light absorption for each sample was determined by measuring the initial UV absorbance (A1) at 240 nm against the absorbance value after 180 s (A2) (ΔA = A1 − A2). One unit of GR enzyme activity was defined as catalyzing the oxidation of 1 nmol of NADPH per minute per g of tissue at 340 nm at pH 8.0 at a specified temperature. Three independent replicates were used for each treatment.
2.12. Reduced Glutathione Content (GSH)
Approximately 0.2 g of the frozen sample was used for the GSH content assay according to the protocol of the GSH measurement kit (GSH-1-W, Suzhou Keming Biotechnology Co., Ltd., Suzhou, China) [25]. After adding reagents, the light absorption for each sample was determined by measuring the UV absorbances (A1) at 412 nm against the absorbance of the blank solution (A2) (ΔA = A1 − A2). A standard curve was generated for GSH content measurement (y = 0.75x). Finally, the GSH content is measured by the following formula: (μmol/g) = ΔA ÷ 0.75 × V1 ÷ (V1 ÷ V2 × W) (V1: accumulation of supernatant liquid in the reaction system; V2: total volume of supernatant; W: sample quality). Three replicates were used for each treatment.
2.13. Ascorbic Acid Content (AsA)
Approximately 0.2 g of the frozen sample was used for the AsA content assay according to the protocol of the AsA measurement kit (AsA-1-W, Suzhou Keming Biotechnology Co., Ltd., Suzhou, China) [22]. After adding reagents, the light absorption for each sample was determined by measuring the UV absorbances (A1) at 420 nm against the absorbance of the blank solution (A2) (ΔA = A1 − A2). A standard curve was generated for AsA content measurement (y = 0.0044x − 0.018, R2 = 0.9978). Finally, the AsA content is measured by the following formula: (μg/g) = (ΔA + 0.018) ÷ 0.0044 × V1 ÷ (W × V1 ÷ V2) (V1: reaction sample volume; V2: extraction liquid volume; W: sample quality). Three replicates were used for each treatment.
2.14. DPPH, ABTS Free Radical Scavenging Rate
Approximately 0.2 g of the frozen sample was used for the DPPH and ABTS clearance rate assays according to the protocol of the DPPH and ABTS measurement kits (DPPH-1-D and ABTS-1-D, Suzhou Keming Biotechnology Co., Ltd., Suzhou, China), respectively [22]. After adding reagents, the light absorption of DPPH and ABTS for each sample was determined by measuring the UV absorbances (A1) at 515 nm against the absorbance of the blank solution (A2) (ΔA = A2 − A1), respectively. DPPH/ABTS free radical scavenging rate (%) = (A2 − A1) ÷ A2 × 100%.
2.15. Total Antioxidant Capacity (T-AOC)
Approximately 0.2 g of the frozen sample was used for the total antioxidant capacity assay according to the protocol of the FRAP measurement kit (FRAP-1-G, Suzhou Keming Biotechnology Co., Ltd., Suzhou, China) [22]. After adding reagents, the light absorption for each sample was determined by measuring the UV absorbances (A1) at 593 nm against the absorbance of the blank solution (A2) (ΔA = A1 − A2). A standard curve was generated for FRAP content measurement (y = 1.2416x + 0.0134, R2 = 0.9996). Finally, the FRAP content is measured by the following formula: (μmol/g) = (ΔA − 0.0134) ÷ 1.2416 × V1 ÷ (V1 ÷ V2 × W) (V1: reaction sample volume; V2: extraction liquid volume; W: sample quality). Three replicates were used for each treatment.
2.16. Statistical Analysis
The data were presented as the mean ± SD (standard deviation). Originpro 2021 software (OriginLab., Northampton, MA, USA) was used for the drawing of figures. Using SPSS Version 26 (IBM Corp., Armonk, NY, USA), one-way analysis of variance (ANOVA) and minimal significance difference (LSD) were used to differentiate mean values at the p < 0.05 level and the correlation between parameters, using Heml Version 2.0 (The CUCKOO Workgroup, Wuhan, China) software to draw a heat map.
3. Results
3.1. Weight Loss and Firmness
Due to direct contact with air, the transpiration of fresh-cut pumpkins is rapidly accelerated, resulting in increased weight loss [26]. As shown in Figure 1a, the weight loss of fresh-cut pumpkins in all treatment groups showed an increasing trend. The values in the nisin groups were significantly lower than those in the control group at 0–6 days, and the weight loss in the nisin group with 0.2 g/L, which was almost the same as that of the control group, was higher than that in the other two nisin groups from 8 to 10 days. However, the 0.4 g/L nisin had the lowest weight loss and the most obvious inhibition of water loss during the whole storage period. Compared with the control group, the weight loss rate at the end of the storage period in the 0.4 g/L treatment group was reduced by 13.53%.
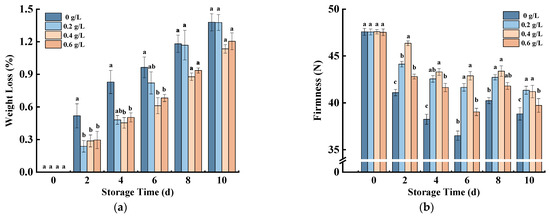
Figure 1.
Effects of nisin treatment on weight loss (a) and firmness (b) in fresh-cut pumpkins. Vertical bars represent the standard deviation of the mean (n = 3). Different letters indicate significant differences between the groups (p < 0.05).
The firmness of fresh-cut pumpkins is easily affected by moisture content, lignin formation, and oxidation [27]. Figure 1b shows that the firmness of the four groups of fresh-cut pumpkins decreased rapidly and then increased slowly. During the whole storage period, the firmness of the 0.4 g/L nisin was higher than that of the other groups, and the value was the lowest in the control group. The 0.4 g/L Nisin most clearly preserved the hardness of fresh-cut pumpkin at 6 days, which was 1.18 times higher than the control group. The firmness increased slowly from 6 to 8 days, which may be due to the callus response of the pumpkin slices under the injury stress, which generated callus at the cut site, leading to the firmness increase of the fresh-cut pumpkins. Furthermore, applying a 1% nisin nano-coating to a white button similarly showed minimal weight loss and firmness [28].
3.2. Whitening Index (WI)
The surface of fresh-cut pumpkins easily produces a white substance when it loses water, and WI is used to indicate the degree of whitening [17]. Figure 2 shows an upward trend in the whitening index of fresh-cut pumpkins during storage, but nisin could effectively inhibit this change. It is possible that nisin reduced whitening on fresh-cut pumpkins by inhibiting surface weight loss. During the whole storage period, 0.4 g/L nisin-treated slowed the rise of the whitening index, and the values in all nisin-treated samples were lower than those in the control group. At the end of the storage period, the whitening index was reduced by 13.61% compared with the control group. Similar results were found in fresh-cut carrots [17] and white buttons [28], which indicated that nisin was extremely well maintained in the color of fruit and vegetables.
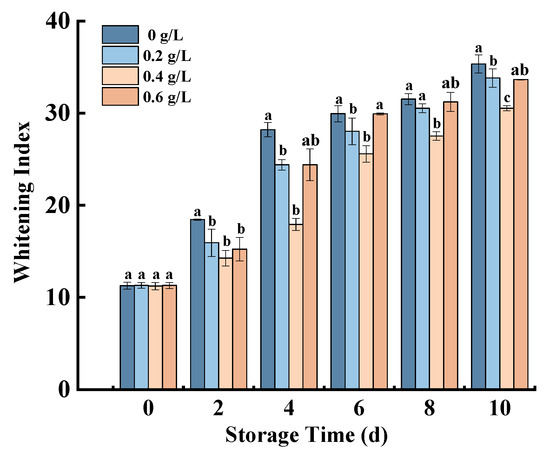
Figure 2.
Effects of nisin treatment on WI in fresh-cut pumpkins. Vertical bars represent the standard deviation of the mean (n = 3). Different letters indicate significant differences between the groups (p < 0.05).
3.3. Respiration Intensity
Increased respiration rates can cause fruit and vegetable aging [29]. Figure 3 showed that the respiratory intensity of fresh-cut pumpkins in each treatment group decreased first and then increased during storage. The values reached their lowest intensity at 6 d and then rose. The respiratory intensity of all nisin-treated groups was lower than that of the control group, and the 0.4 g/L nisin group had a significant effect on the respiratory intensity of fresh-cut pumpkins. Respiratory rates of fresh-cut pumpkins were greatly reduced by 0.4 g/L Nisin at 8 d and inhibited by 45.83% compared with the control group. Thus, it can effectively delay the aging process of fresh-cut pumpkins. In addition, similar results were found in fresh-cut carrots [17] and button mushrooms [30]. Those results indicated that nisin was extremely efficient in decreasing the respiration rates of fruits and vegetables.
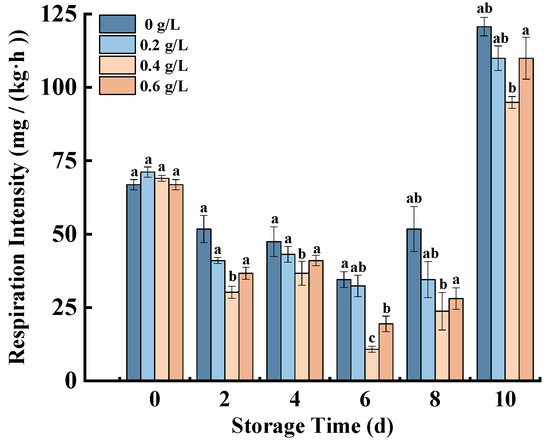
Figure 3.
Effects of nisin treatment on respiration intensity in fresh-cut pumpkins. Vertical bars represent the standard deviation of the mean (n = 3). Different letters indicate significant differences between the groups (p < 0.05).
In conclusion, 0.4 g/L nisin treatment could effectively reduce the weight loss, hardness loss, whitening, and respiration intensity of fresh-cut pumpkins and better maintain the storage quality. Therefore, we selected the nisin concentration of 0.4 g/L for subsequent analysis of antioxidant activity indexes to explore the effect of nisin on the antioxidant capacity of fresh-cut pumpkins.
3.4. O2−· Production Rate and H2O2 Contents
Fresh-cut fruits and vegetables under cutting stress will trigger the production of a large number of ROS, such as O2−·and H2O2, in cells. However, excessive production and accumulation of ROS will seriously accelerate the aging of fruits and vegetables [31]. The O2−· production rate of fresh-cut pumpkins was at its highest rate at 0 d (Figure 4a). The control group showed a downward trend from 0 to 4 days and then slowly increased to 6 days and then decreased. After treatment with 0.4 g/L nisin, the O2−· production rate of the nisin group was lower than that of the control group, except for the 4th day. Nisin reduced the O2−· production rate of fresh-cut pumpkins by 65.11% compared with the control group. Figure 4b showed that H2O2 content increased during the storage time, but the 0.4 g/L nisin effectively inhibited the accumulation of H2O2, compared with the control group. At day 8, 0.4 g/L nisin reduced the H2O2 content of fresh-cut pumpkins by 40% compared with the control. Similar results were found in mushrooms [30], indicating that nisin reduced O2−· production rate and H2O2 content.
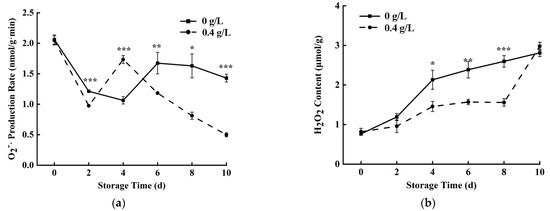
Figure 4.
Effects of 0.4 g/L nisin treatment on O2−· (a) and H2O2 content production rate (b) in fresh-cut pumpkins (*, ** and *** indicate the significant difference between the 0.4 g/L nisin treatment and the control at p < 0.05, p < 0.01, and p < 0.001, respectively. Vertical bars represent the standard deviation of the mean (n = 3)).
3.5. Antioxidant Metabolism-Related Enzyme Activities
SOD, CAT, APX, GR, and other enzymes work together to defend against the damage caused by reactive oxygen species or other peroxide free radicals to the cell membrane system, as well as maintain the balance of reactive oxygen metabolism and play an antioxidant role [32,33].
As can be seen from Figure 5a,c, the SOD and APX activities of fresh-cut pumpkins decreased first and then increased. Compared with the control group, 0.4 g/L nisin treatment could increase SOD activity at 2–4 days, while the trend in the later storage period was opposite to the early storage period. APX activity in the 0.4 g/L nisin group decreased from 0 to 4 days and then increased slowly from 4 to 10 days, whereas in the control group, it decreased to a nadir at 6 d and then increased, but it was always higher in the 0.4 g/L nisin group than that in the control group. Figure 5b showed that the CAT activity of fresh-cut pumpkins increased first and then decreased. CAT activity in the 0.4 g/L nisin group peaked at 6 d and in the control group at 4 d, but CAT activity was significantly higher in the 0.4 g/L nisin group at 0–6 days. Figure 5d showed that GR activity had been on the rise, and the activity of the 0.4 g/L nisin group was always higher than that of the control group during the storage. The activity of the 0.4 g/L nisin group was 759.51 U/g at 4 d, which was significantly higher than that of the control group.
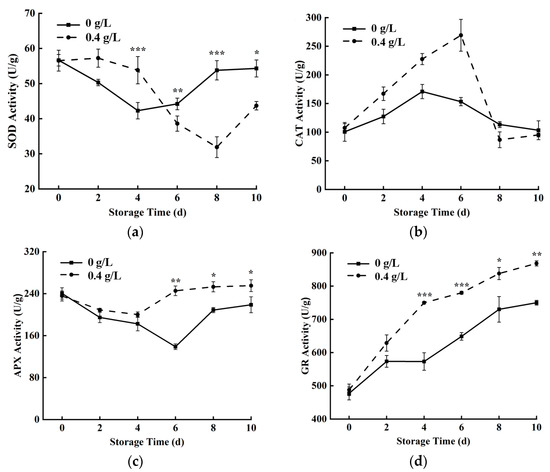
Figure 5.
Effects of 0.4 g/L nisin treatment on SOD activity (a), CAT activity (b), APX activity (c), and GR activity (d) in fresh-cut pumpkins (*, **, and *** indicate the significant difference between the 0.4 g/L nisin treatment and the control at p < 0.05, p < 0.01, and p < 0.001, respectively. Vertical bars represent the standard deviation of the mean (n = 3)).
3.6. Antioxidant Metabolism-Related Substances Contents
AsA and GSH are important antioxidants in the AsA-GSH cycle, and they complement each other in the process of scavenging reactive oxygen free radicals [34,35]. Figure 6a,b showed an increasing trend of both AsA and GSH in fresh-cut pumpkins. After 0.4 g/L nisin treatment, the AsA content of fresh-cut pumpkins accumulated rapidly from 4 to 10 days, which was significantly higher than that of the control group. In addition, the change trends of AsA content and APX enzyme activity were consistent, indicating that AsA can reduce the accumulation of H2O2 catalyzed by APX. Moreover, 0.4 g/L nisin treatment could promote the accumulation of GSH content, which was higher than that of the control group during 4–10 days, which was conducive to the accumulation of antioxidants.
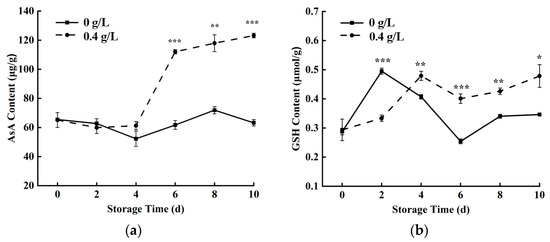
Figure 6.
Effects of 0.4 g/L nisin treatment on AsA content (a) and GSH content (b) in fresh-cut pumpkins (*, **, and *** indicate the significant difference between the 0.4 g/L nisin treatment and the control at p < 0.05, p < 0.01, and p < 0.001, respectively. Vertical bars represent the standard deviation of the mean (n = 3)).
3.7. Antioxidant Capacity
DPPH, ABTS, and T-AOC were used to evaluate the antioxidant capacity of fresh-cut pumpkins [32,36]. In the control group, DPPH, ABTS, and T-AOC of fresh-cut pumpkins showed an overall decreasing trend during storage (Figure 7a–c), and the scavenging ability of ROS decreased as a whole, but the antioxidant ability was improved after 0.4 g/L nisin treatment. The free radical scavenging rates of DPPH and ABTS reached their peaks on the second day, and the T-AOC was in an upward state, which was always higher than that of the control group, indicating that the 0.4 g/L nisin group had a higher antioxidant capacity.
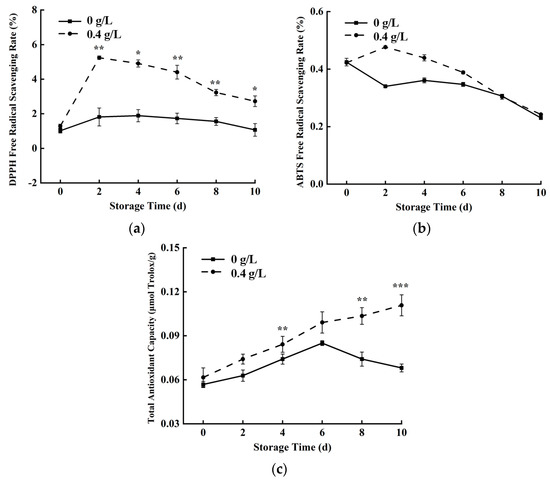
Figure 7.
Effects of 0.4 g/L nisin treatment on DPPH (a), ABTS (b) free radical scavenging rate, and T−AOC (c) in fresh−cut pumpkins (*, **, and *** indicate the significant difference between the 0.4 g/L nisin treatment and the control at p < 0.05, p < 0.01, and p < 0.001, respectively. Vertical bars represent the standard deviation of the mean (n = 3)).
3.8. Correlation Analysis
The Pearson correlation coefficient between different parameters of fresh-cut pumpkins after 0.4 g/L nisin treatment is shown in Figure 8. Firmness was significantly positively correlated with APX and ABTS (p < 0.05) and significantly negatively correlated with H2O2 (p < 0.05). H2O2 was extremely significantly positively correlated with weight loss rate and WI (p < 0.01), while ABTS was extremely significantly negatively correlated (p < 0.01). ABTS was extremely significantly negatively correlated with weight loss and WI (p < 0.01). These results indicated that the antioxidant system of fresh-cut pumpkins was closely related to their storage quality. In addition, O2−· was extremely significantly negatively correlated with GR, GSH, and FRAP (p < 0.01), as well as significantly negatively correlated with AsA (p < 0.05), indicating that the AsA−GSH cycle was conducive to O2−· clearance.
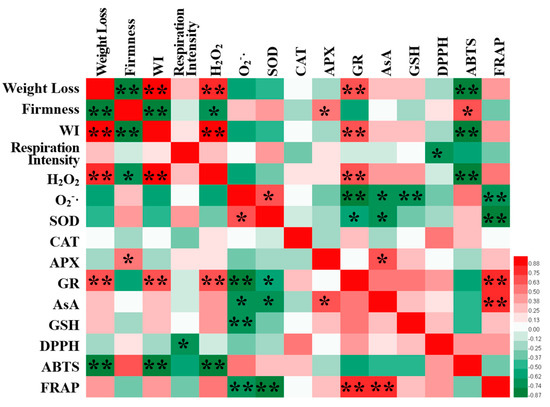
Figure 8.
Pearson correlation matrix of each indicator. * and ** indicate that correlation between values reach the level of p < 0.05 and p < 0.01, respectively.
4. Discussion
Mechanically damaged pumpkin tissue is prone to a series of quality degradation problems such as cell rupture, water loss, and nutrient outflow, resulting in the weight loss rate decreasing, the tissue softening, the section turning white, and the respiration rate increasing [37,38]. Therefore, it is of great significance to explore safe and natural preservation technology to maintain the storage quality of fresh-cut pumpkins. Nisin, a safe food additive, is a safe and natural biological preservative agent, which has been widely concerned by researchers at home and abroad [39,40]. Many scholars have applied it to various foods and achieved good fresh-keeping effects, such as cucumber juice drinks [41], fresh-cut carrots [17], button mushrooms [30], fresh apple-kale blend juice [42], grape juice [43], fresh-cut beet leaves [16], fresh-cut onions [44], and fruit- and vegetable-based beverages [45]. In this study, it was found that nisin treatment could effectively inhibit the weight loss rate, the whiteness of the cut surface, the decrease in hardness, and the increase in respiration rate of fresh-cut pumpkins, as well as activate the antioxidant system and preserve the better quality of fresh-cut pumpkins.
Cutting stress leads to the accumulation of ROS and the aggravation of membrane lipid peroxidation, resulting in tissue oxidative damage and the acceleration of the aging process of fresh-cut pumpkins [46,47]. After broccoli was cut, high-intensity injury increased H2O2 content, low-intensity injury increased O2−· content, and SOD, POD, and CAT enzymes induced by the trauma accelerated the decomposition of ROS [48]. The antioxidant defense system is vital in the preservation of fruits and vegetables due to the fact that it can eliminate ROS and delay the aging process. SOD, CAT, APX, and GR are antioxidant enzymes that work together to maintain the metabolic equilibrium of ROS and function in an antioxidant role. SOD is a first-line defense system against ROS, capable of mutating O2−· into H2O2 and O2. [49]. APX and GR serve as key enzymes in the AsA-GSH cycle [50]. APX and CAT enzymes catalyze the decomposition of H2O2 into O2 and H2O. GSH and AsA are important antioxidant substances in the ASA-GSH cycle, which are beneficial to maintain the activity of APX and provide ROS scavenging and reducing power [50,51].
In this study, ROS content was increased in the control group, while the production rate of O2−· and H2O2 content of fresh-cut pumpkins was decreased by nisin treatment almost during the storage period, which significantly inhibited ROS content. Additionally, nisin elevated SOD and CAT activities early in the storage period and APX activity later in the storage period. It is possible that SOD enzymes diminish O2−· production early in the storage period, whereas CAT and APX enzymes break down H2O2 later in the storage period, inhibiting ROS accumulation throughout the storage period. In the study of Agaricus bisporus using nisin in combination with chitosan and nano-silica, it was also found that the contents of O2−· and H2O2 could be maintained at the lowest levels, and the activities of SOD and CAT enzymes were significantly increased due to the addition of nisin [30]. Nisin treatment significantly raised the GR activity, GSH, and AsA contents of fresh-cut pumpkins, promoting the transport rate of the AsA-GSH cycle and increasing antioxidant capacity. Similar research has demonstrated that combining nisin and chitosan treatments could increase GR activity, GSH, and AsA contents in feijoa, improve fruit resistance, and so postpone plant senescence and fruit deterioration [52].
Furthermore, the correlation analysis results (Figure 8) show that O2−· was negatively correlated with GR, AsA, GSH, and FRAP, and H2O2 had a positive correlation with weight loss and the whitening index and was negatively correlated with hardness and ABTS. These findings revealed that ROS accumulation can lower the quality of fresh-cut pumpkins, but the antioxidant enzymes and substances are advantageous to ROS elimination.
In conclusion, fresh-cut pumpkins treated with nisin could increase the activity of the SOD enzyme in the early stage of storage to reduce the production rate of O2−· and activate the CAT and APX enzymes to remove H2O2, which results in reduced ROS levels. Meanwhile, GR activity and contents of GSH and AsA were also significantly increased, which promoted the transport rate of the AsA-GSH cycle and enhanced antioxidant capacity. Previous studies have also shown that nisin could enhance these antioxidant capacities in fresh-cut beet leaves [16], grape juice [43], fresh apple-kale blend juice [42], and Agaricus bisporus [30].
5. Conclusions
Nisin has a positive effect on the preservation of fresh-cut pumpkins. This study showed that 0.4 g/L nisin, compared with the control group, effectively reduced water loss, maintained firmness, inhibited whitening, and decreased the respiration rate for fresh-cut pumpkins to maintain a good appearance quality. Nisin could also reduce the accumulation of ROS (O2−· and H2O2) by enhancing the activities of SOD and CAT and regulating the conversion of antioxidant enzymes (GR and APX) and antioxidant substances (GSH and AsA) in the AsA-GSH cycle. In addition, combined with DPPH, the ABTS free radical scavenging rate, and T-AOC, nisin could effectively enhance the antioxidant activity of fresh-cut pumpkins. In conclusion, nisin treatment has the potential to maintain the preservation quality and delay the aging process during the storage period, which is a natural biological preservative that can effectively improve the quality of fresh-cut pumpkins.
Author Contributions
Conceptualization, W.H., C.C. and N.Y.; Data curation, W.H., C.C., N.Y., Y.G. and Y.W.; Formal analysis, W.H., C.C., Y.G. and N.Y.; Funding acquisition, W.H.; Investigation, W.H. and N.Y.; Methodology, W.H., C.C. and N.Y.; Project administration, W.H.; Resources, W.H.; Software, W.H. and N.Y.; Supervision, Y.G. and Y.W.; Visualization, N.Y., Y.G. and Y.W.; Writing—original draft, W.H., C.C. and N.Y.; Writing—review and editing, N.Y., Y.G. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the “Thirteenth Five-Year Plan” for the National Key Research and Development Program (No. 2016YFD0400903), National Natural Science Foundation of China (No. 31471923).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ji, X.; Peng, B.; Ding, H.; Cui, B.; Nie, H.; Yan, Y. Purification, Structure and Biological Activity of Pumpkin Polysaccharides: A Review. Food Rev. Int. 2021, 39, 307–319. [Google Scholar] [CrossRef]
- Sharma, S.; Ramana Rao, T.V. Nutritional quality characteristics of pumpkin fruit as revealed by its biochemical analysis. Int. Food Res. J. 2013, 20, 2309–2316. [Google Scholar]
- Oloyede, F.M.; Adebooye, O.C.; Obuotor, E.M. Planting date and fertilizer affect antioxidants in pumpkin fruit. Sci. Hortic. 2014, 168, 46–50. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. The Profile of Carotenoids and Other Bioactive Molecules in Various Pumpkin Fruits (Cucurbita maxima Duchesne) Cultivars. Molecules 2019, 24, 3212. [Google Scholar] [CrossRef] [PubMed]
- Zdunic, G.M.; Menkovic, N.R.; Jadranin, M.B.; Novakovic, M.M.; Savikin, K.P.; Zivkovic, J.C. Phenolic compounds and carotenoids in pumpkin fruit and related traditional products. Hem. Ind. 2016, 70, 429–433. [Google Scholar] [CrossRef]
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.B.K.S.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data (accessed on 1 March 2023).
- Yüksel, Ç.; Atalay, D.; Erge, H.S. The effects of chitosan coating and vacuum packaging on quality of fresh-cut pumpkin slices during storage. J. Food Process. Preserv. 2022, 46, e16365. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef]
- Rashid, M.H.; Khan, M.R.; Roobab, U.; Rajoka, M.S.R.; Inam-ur-Raheem, M.; Anwar, R.; Ahmed, W.; Jahan, M.; Ijaz, M.R.A.; Asghar, M.M.; et al. Enhancing the shelf stability of fresh-cut potatoes via chemical and nonthermal treatments. J. Food Process. Preserv. 2021, 45, e15582. [Google Scholar] [CrossRef]
- Khan, I.; Oh, D.-H. Integration of nisin into nanoparticles for application in foods. Innov. Food Sci. Emerg. 2016, 34, 376–384. [Google Scholar] [CrossRef]
- Ali, A.H.; Hale, O.W.; Khasawneh, F.A.; Urban, R.S.; Werner, H.V.; Smalligan, R.D. NISIN and Clostridium difficile: A Potentially Effective Treatment for an Increasingly Problematic Disease. Am. J. Gastroenterol. 2013, 108, 625. [Google Scholar] [CrossRef] [PubMed]
- Fusieger, A.; Perin, L.M.; Teixeira, C.G.; de Carvalho, A.F.; Nero, L.A. The ability of Lactococcus lactis subsp. lactis bv. diacetylactis strains in producing nisin. Antonie Van Leeuwenhoek 2020, 113, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.H.A.; Cutter, C.N. Development and evaluation of pullulan-based composite antimicrobial films (CAF) incorporated with nisin, thymol and lauric arginate to reduce foodborne pathogens associated with muscle foods. Int. J. Food Microbiol. 2020, 320, 108519. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.V.; Jagus, R.J.; Aguero, M.V. Application of a combined treatment using natural antimicrobials and modified atmosphere packaging to enhance safety, quality, and shelf-life of fresh-cut beet leaves. J. Food Saf. 2018, 38, e12556. [Google Scholar] [CrossRef]
- Song, Z.Y.; Li, F.; Guan, H.; Xu, Y.F.; Fu, Q.J.; Li, D.P. Combination of nisin and epsilon-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control 2017, 74, 34–44. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Wang, J.Y.; Wu, Z.H.; Xu, Y.P.; Wu, Z.X. Ozone treatment promotes physicochemical properties and antioxidant capacity of fresh-cut red pitaya based on phenolic metabolism. Front. Nutr. 2022, 9, 1–14. [Google Scholar] [CrossRef]
- Sarengaowa; Hu, W.Z.; Jiang, A.L.; Xiu, Z.L.; Feng, K. Effect of thyme oil-alginate-based coating on quality and microbial safety of fresh-cut apples. J. Sci. Food Agric. 2018, 98, 2302–2311. [Google Scholar] [CrossRef]
- BOLIN, H.R.; HUXSOLL, C.C. Control of minimally processed carrot (Daucus carotova) surface discoloration caused by abrasion peeling. J. Food Sci. 1991, 56, 416–418. [Google Scholar]
- Zhou, F.H.; Xu, D.Y.; Liu, C.H.; Chen, C.; Tian, M.X.; Jiang, A.L. Ascorbic acid treatment inhibits wound healing of fresh-cut potato strips by controlling phenylpropanoid metabolism. Postharvest Biol. Technol. 2021, 181, 111644. [Google Scholar] [CrossRef]
- Coming Biotechnology Company Home Page of SOD Activity Kit. Available online: http://www.cominbio.com/index.html (accessed on 2 November 2022).
- Yan, J.; Song, Y.; Li, J.; Jiang, W. Forced-air precooling treatment enhanced antioxidant capacities of apricots. J. Food Process. Pres. 2018, 42, e13320. [Google Scholar] [CrossRef]
- Zhou, F.; Zuo, J.; Xu, D.; Gao, L.; Wang, Q.; Jiang, A. Low intensity white light-emitting diodes (LED) application to delay senescence and maintain quality of postharvest pakchoi (Brassica campestris L. ssp. chinensis (L.) Makino var. communis Tsen et Lee). Sci. Hortic. 2020, 262, 109060. [Google Scholar]
- Guan, Y.; Ji, Y.; Yang, X.; Pang, L.; Cheng, J.; Lu, X.; Zheng, J.; Yin, L.; Hu, W. Antioxidant activity and microbial safety of fresh-cut red cabbage stored in different packaging films. LWT-Food Sci. Technol. 2023, 175, 114478. [Google Scholar] [CrossRef]
- Piagentini, A.M.; Güemes, D.R. Shelf life of fresh-cut spinach as affected by chemical treatment and type of packaging film. Braz. J. Chem. Eng. 2002, 19, 383–389. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, B.; Chen, X.; Su, L.; Jiao, C.; Chen, S.; Fan, J.; Liu, H. Short peptides secreted by Bacillus subtilis inhibit the growth of mold on fresh-cut pumpkin (Cucurbita pepo). J. Sci. Food Agric. 2020, 100, 936–944. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Almushhin, A.; Alharbi, M.; Almatrafi, M.; Benajiba, N.; Fikry, M.; Helal, M. Enhancement in physicochemical parameters and microbial populations of mushrooms as influenced by nano-coating treatments. Sci. Rep. 2021, 11, 7915. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, H.; Huang, M.; Zhou, D.; Cao, Q.; Ma, K. Effects of high pressure nitrogen treatments on the quality of fresh-cut pears at cold storage. Innov. Food Sci. Emerg. Technol. 2015, 32, 56–63. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Fikry, M.; Helal, M. The combined effect of coating treatments to nisin, nano-silica, and chitosan on oxidation processes of stored button mushrooms at 4 °C. Sci. Rep. 2021, 11, 6031. [Google Scholar] [CrossRef]
- Li, C.; Tao, J.; Wu, Z. Gaseous ozone regulates reactive oxygen species metabolism and ascorbate-glutathione cycle to delay the senescence of fresh-cut red pitaya (Selenicereus undatus) fruit. Sci. Hortic. 2023, 312, 111839. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Wu, X.; Liu, W.; Jing, X.; Liu, Y.; Yan, J.; Liu, S.; Qin, W. Combination of calcium lactate impregnation with UV-C irradiation maintains quality and improves antioxidant capacity of fresh-cut kiwifruit slices. Food Chem. X 2022, 14, 100329. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Liu, J.; Sun, W.; Min, D.; Li, F.; Li, X. Methyl salicylate pretreatment maintains quality and antioxidant capacity of fresh-cut pitaya fruit by modulating phenylpropanoid metabolism and antioxidant system. Sci. Hortic. 2023, 309, 111705. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Li, M.; Fu, X.; Zhao, X.; Min, D.; Li, F.; Li, X.; Zhang, X. Hot air pretreatment alleviates browning of fresh-cut pitaya fruit by regulating phenylpropanoid pathway and ascorbate-glutathione cycle. Postharvest Biol. Technol. 2022, 190, 111954. [Google Scholar] [CrossRef]
- Chen, C.; Hu, W.Z.; Zhang, R.D.; Jiang, A.L.; Liu, C.H. Effects of hydrogen sulfide on the surface whitening and physiological responses of fresh-cut carrots. J. Sci. Food Agric. 2018, 98, 4726–4732. [Google Scholar] [CrossRef]
- Zhou, C.-L.; Liu, W.; Zhao, J.; Yuan, C.; Song, Y.; Chen, D.; Ni, Y.-Y.; Li, Q.-H. The effect of high hydrostatic pressure on the microbiological quality and physical–chemical characteristics of Pumpkin (Cucurbita maxima Duch.) during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2014, 21, 24–34. [Google Scholar] [CrossRef]
- Nicola, S.; Tibaldi, G.; Gaino, W.; Pignata, G. Cutting shape, film and storage temperature affect the shelf-life of fresh-cut pumpkin. Acta Hortic. 2018, 1209, 399–408. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- McManamon, O.; Kaupper, T.; Scollard, J.; Schmalenberger, A. Nisin application delays growth of Listeria monocytogenes on fresh-cut iceberg lettuce in modified atmosphere packaging, while the bacterial community structure changes within one week of storage. Postharvest Biol. Technol. 2019, 147, 185–195. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Liu, F.; Dong, P.; Huang, W.; Xiong, L.; Liao, X. Comparing the effects of high hydrostatic pressure and thermal pasteurization combined with nisin on the quality of cucumber juice drinks. Innov. Food Sci. Emerg. Technol. 2013, 17, 27–36. [Google Scholar] [CrossRef]
- Mok, J.H.; Pyatkovskyy, T.; Yousef, A.; Sastry, S.K. Effects of combination shear stress, moderate electric field (MEF), and nisin on kinetics and mechanisms of inactivation of Escherichia coli K12 and Listeria innocua in fresh apple-kale blend juice. J. Food Eng. 2021, 292, 110262. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Wang, L.; Yang, Y.; Yang, W.; Wang, H.; Lan, T.; Zhang, Q.; Sun, X. Ultrasound-Combined Sterilization Technology: An Effective Sterilization Technique Ensuring the Microbial Safety of Grape Juice and Significantly Improving Its Quality. Foods 2020, 9, 1512. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, W.; Zhang, R.; Jiang, A.; Zou, Y. Levels of phenolic compounds, antioxidant capacity, and microbial counts of fresh-cut onions after treatment with a combination of nisin and citric acid. Hortic. Environ. Biotechnol. 2016, 57, 266–273. [Google Scholar] [CrossRef]
- Nieva, S.G.; Jagus, R.J.; Agüero, M.V.; Fernandez, M.V. Fruit and vegetable smoothies preservation with natural antimicrobials for the assurance of safety and quality. LWT-Food Sci. Technol. 2022, 154, 112663. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Xu, Y.; Yang, X.; Ji, Y.; Feng, K.; Sarengaowa. Metabolomics and physiological analyses validates previous findings on the mechanism of response to wounding stress of different intensities in broccoli. Food Res. Int. 2021, 140, 110058. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Jian, W. Effect of Chitosan and Nisin on the Antioxidant Quality of Feijoa during Storage. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).