Applicability of Variable-Rate Nitrogen Top Dressing Based on Measurement of the Within-Field Variability of Soil Nutrients for Cabbage Production
Abstract
1. Introduction
2. Materials and Methods
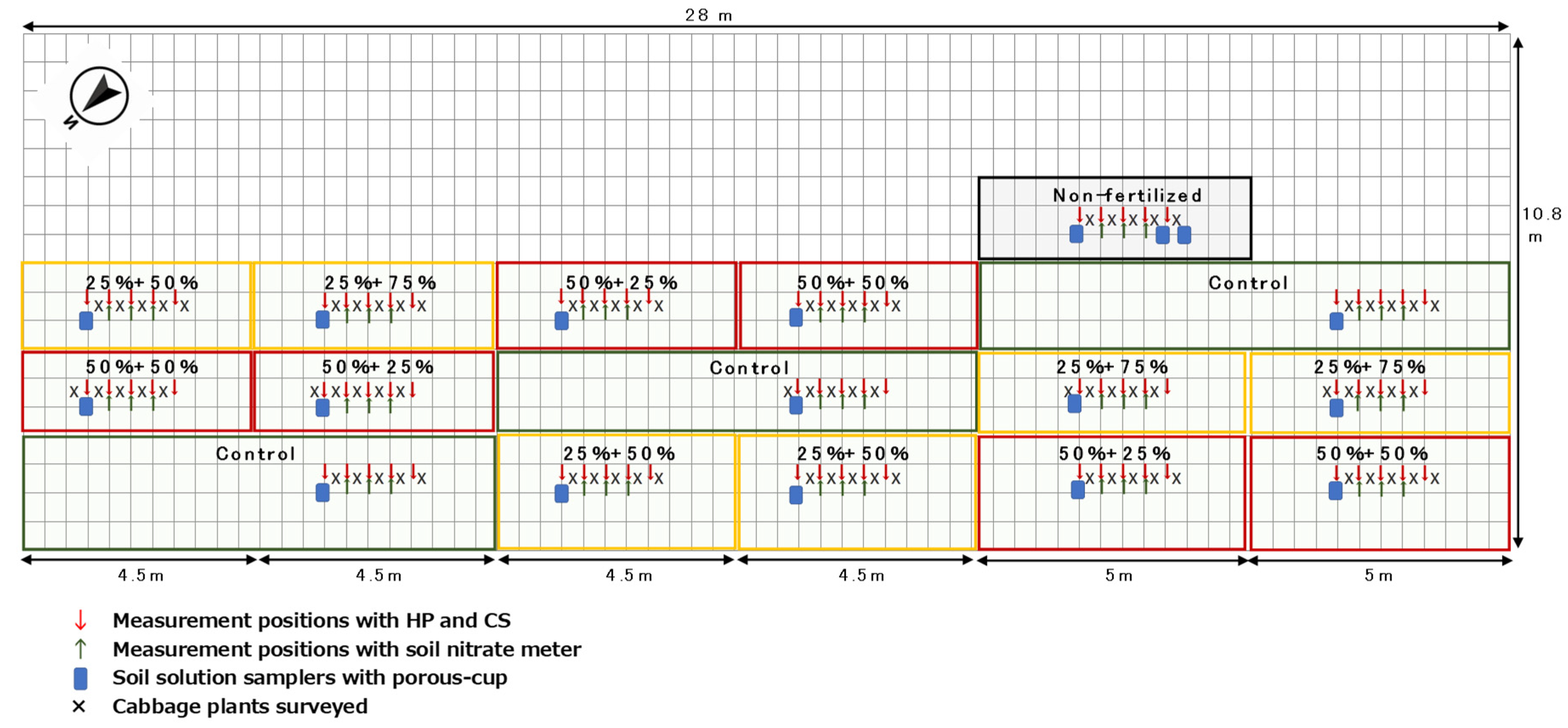
2.1. Trial and Sampling Details
2.2. Selection of Suitable Crop and Soil Measurements for Diagnosis (Trials 1 and 2)
2.3. Verification of the Effect of Variable-Rate N Top Dressing Based on Soil Diagnosis (Trial 3)
2.4. Statistical Analyses
3. Results
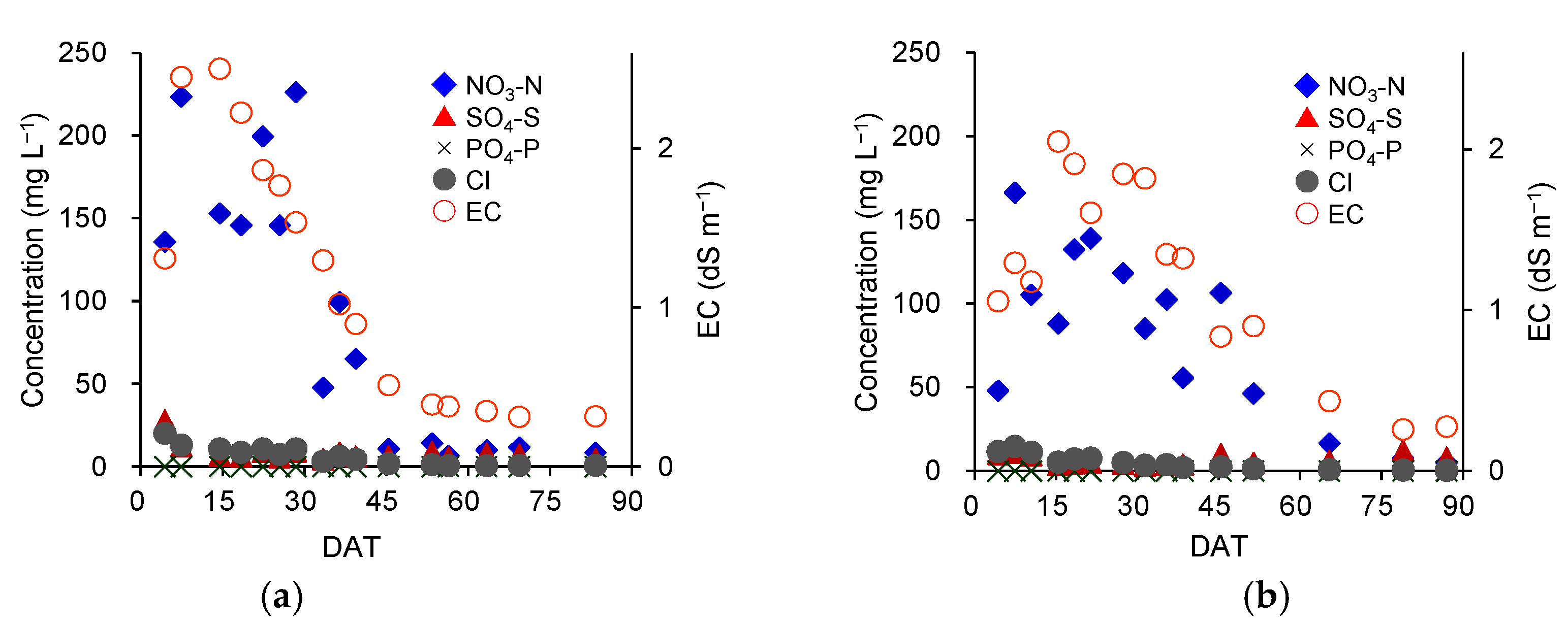
3.1. Climate and Soil Environment
3.2. Cabbage Yield and N Absorption
3.3. Correlation of Crop Growth Indicators with Yield at Early Stages (Trials 1 and 2)
3.4. Comparison of Measurement Sensors for Variable-Rate N Fertilization (Trials 1 and 2)
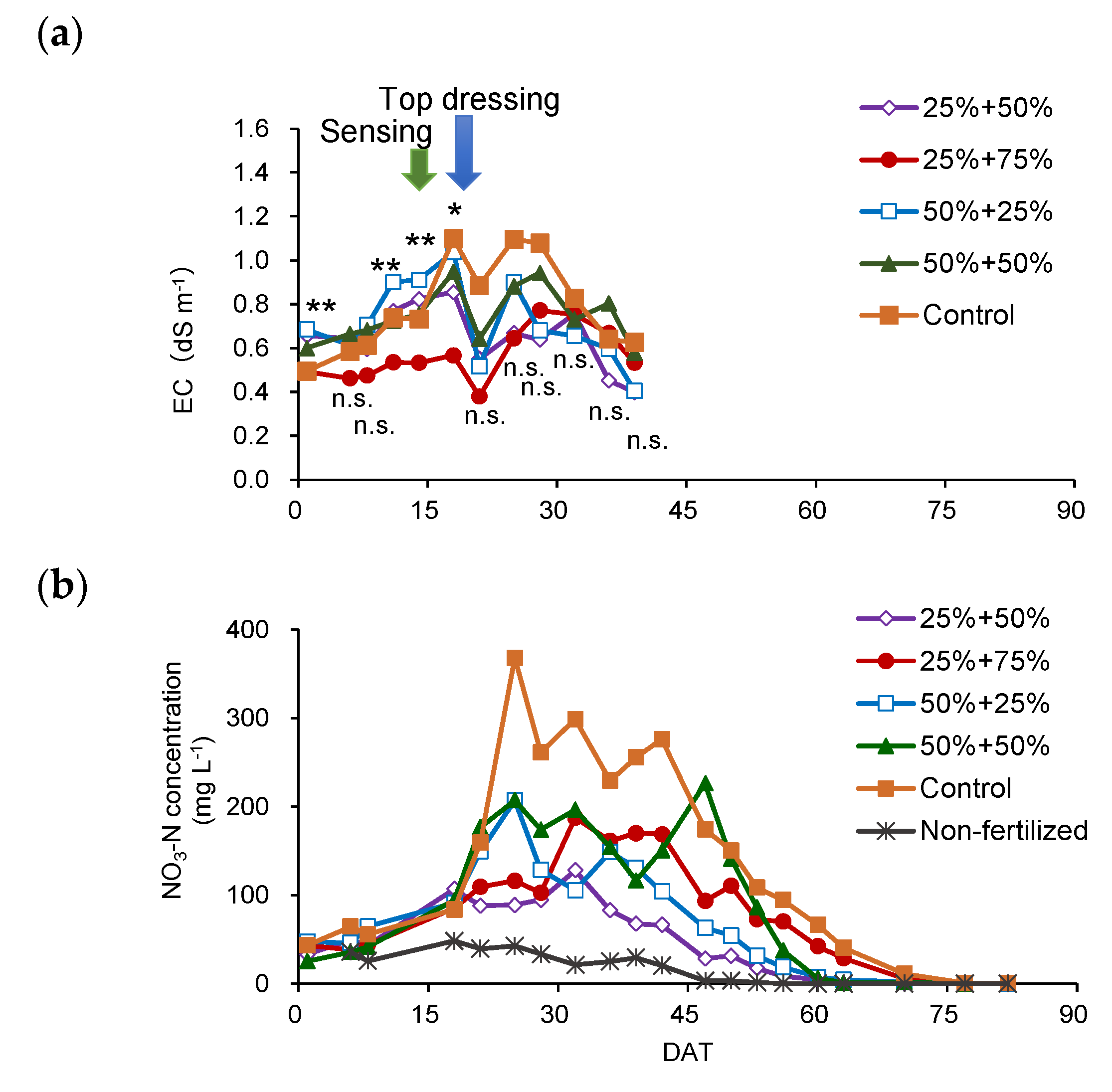
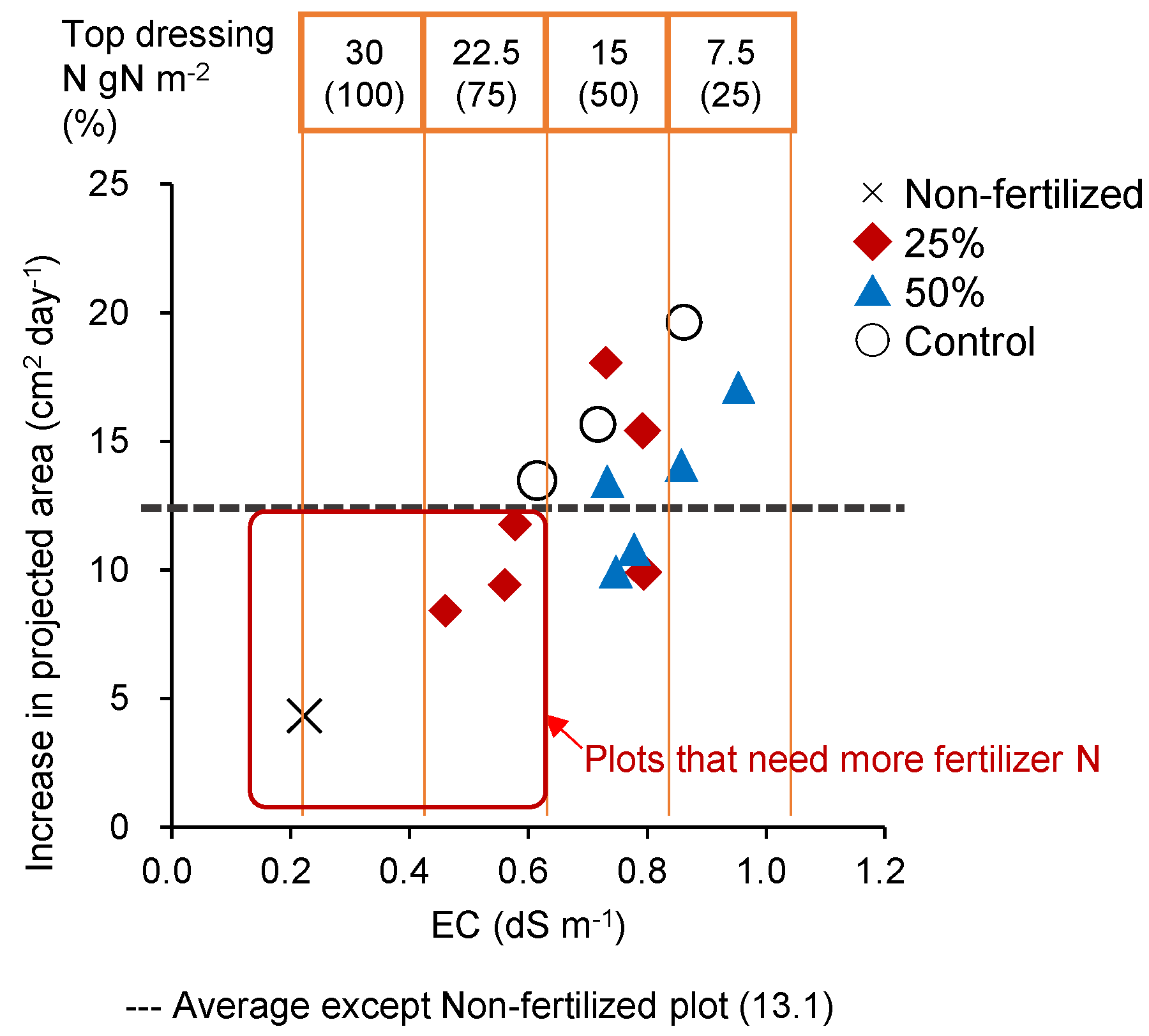
3.5. Verification of the Effect of Variable-Rate N Top Dressing Based on Soil Diagnosis (Trial 3)
4. Discussion
4.1. Indicators and Timing of Crop Diagnosis
4.2. Indicators and Timing of Soil Diagnosis
4.3. Effect of Variable N Top Dressing on Cabbage Yield and N Fertilizer Utilization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agriculture & Livestock Industries Corporation. Report on the Survey of the Actual Demand Structure of Vegetables for Processing and Commercial Use in the COVID-19 Crisis; Agriculture & Livestock Industries Corporation: Tokyo, Japan, 2021; Available online: https://www.alic.go.jp/content/001194698.pdf (accessed on 20 February 2023). (In Japanese)
- Ministry of Agriculture, Forestry and Fisheries (Japan). Strategy for Sustainable Food Systems, MeaDRI; Ministry of Agriculture, Forestry and Fisheries (Japan): Tokyo, Japan, 2021. Available online: https://www.maff.go.jp/e/policies/env/env_policy/meadri.html (accessed on 20 February 2023).
- Salo, T. Effects of band placement and nitrogen rate on dry matter accumulation, yield and nitrogen uptake of cabbage, carrot and onion. Agric. Food Sci. 1999, 8, 157–232. [Google Scholar] [CrossRef]
- Aichi Prefectural Government. Fertilizer Standards for Agricultural Crops; Aichi Prefectural Government: Nagoya, Japan, 2021. Available online: https://www.pref.aichi.jp/soshiki/nogyo-keiei/sehikijun.html (accessed on 20 February 2023). (In Japanese)
- Gunma Prefectural Government. Fertilizer Standards for Agricultural Crops; Gunma Prefectural Government: Tokyo, Japan, 2004. Available online: https://aic.pref.gunma.jp/agriculturals/management_support/soil01 (accessed on 20 February 2023). (In Japanese)
- Fink, M.; Feller, C. An empirical model for describing growth and nitrogen uptake of white cabbage (Brassica oleracea var. capitata). Sci. Hortic. 1998, 73, 75–88. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.G.; Xu, W.H.; Chi, S.L.; Zhao, W.Y.; Li, Y.H.; Zhang, C.L.; Feng, D.Y.; He, Z.M.; Wang, Z.Y. Effects of coated slow-release fertilizer with urease and nitrification inhibitors on nitrogen release characteristic and uptake and utilization of nitrogen, phosphorus and potassium in cabbage. Int. J. Agric. Biol. 2018, 20, 422–430. [Google Scholar] [CrossRef]
- Chohura, P.; Kołota, E. Suitability of some nitrogen fertilizers for the cultivation of early cabbage. J. Elem. 2014, 19, 661–672. [Google Scholar] [CrossRef]
- Onodera, M.; Miki, N.; Akashi, K. Effect of stripe dressing on cabbage for reducing 30% of nitrogen fertilizer. Jpn. J. Soil Sci. Plant Nutr. 2000, 71, 714–717. (In Japanese) [Google Scholar] [CrossRef]
- Shindo, H.; Sato, F.; Kaneta, Y. Cultivation of cabbage for summer harvest by single basal application of controlled release fertilizer localized deep placement in cool region. Jpn. J. Soil Sci. Plant Nutr. 2001, 72, 803–806. (In Japanese) [Google Scholar] [CrossRef]
- Müller, T.; von Fragstein und Niemsdorff, P. Organic fertilizers derived from plant materials Part II: Turnover in field trials. J. Plant. Nutr. Soil Sci. 2006, 169, 265–273. [Google Scholar] [CrossRef]
- Sato, T.; Sato, T.; Onodera, Y.; Takakai, F.; Yasuda, K.; Ishida, T.; Shibuya, M.; Nakagawa, S.; Watanabe, K.; Kaneta, Y. Effect of hairy-vetch treatment on nitrogen absorption and yield of cabbage in a heavy clay soil field converted from a paddy field. Jpn. J. Soil Sci. Plant Nutr. 2019, 90, 249–256, (In Japanese with English abstract). [Google Scholar] [CrossRef]
- Hokkaido Government Opendata CC-BY4. Hokkaido Fertilizer Recommendations. 2020. Available online: https://www.harp.lg.jp/opendata/dataset/1056/resource/4481/source-url (accessed on 20 February 2023). (In Japanese)
- Magdoff, F.R. Understanding the Magdoff pre-sidedress nitrate test for corn. J. Prod. Agric. 1991, 4, 297–305. [Google Scholar] [CrossRef]
- Heckman, J.R.; Morris, T.; Sims, J.T.; Sieczka, J.B.; Krogmann, U.; Nitzsche, P.; Ashley, R. Pre-sidedress soil nitrate test is effective for fall cabbage. HortScience 2002, 37, 113–117. [Google Scholar] [CrossRef]
- Ito, S.; Baba, K.; Asano, Y.; Takesako, H.; Wada, H. Development of a nitrate ion-selective electrode based on an urushi matrix membrane and its application to the direct measurement of nitrate-nitrogen in upland soils. Talanta 1996, 43, 1869–1881. [Google Scholar] [CrossRef]
- Kim, D.W.; Yun, H.; Jeong, S.J.; Kwon, Y.S.; Kim, S.G.; Lee, W.S.; Kim, H.J. Modeling and testing of growth status for Chinese cabbage and white radish with UAV-based RGB imagery. Remote Sens. 2018, 10, 563. [Google Scholar] [CrossRef]
- Kang, Y.S.; Ryu, C.S.; Kim, S.H.; Jun, S.R.; Jang, S.H.; Park, J.W.; Sarkar, T.K.; Song, H.Y. Yield prediction of chinese cabbage (Brassicaceae) using broadband multispectral imagery mounted unmanned aerial system in the air and narrowband hyperspectral imagery on the ground. Biosyst. Eng. 2018, 43, 138–147. [Google Scholar] [CrossRef]
- Oki, K.; Mitsuishi, S.; Ito, T.; Mizoguchi, M. An agricultural monitoring system based on the use of remotely sensed imagery and field server web camera data. GISci. Remote Sens. 2009, 46, 305–314. [Google Scholar] [CrossRef]
- Tanaka, M.; Hama, A.; Tsurusaki, Y.; Shibato, Y. Methods of aerial photography using drone and image analyses for evaluation of cabbage growth at individual level. J. Remote Sens. Soc. Jpn. 2021, 41, 375–385, (In Japanese with English abstract). [Google Scholar] [CrossRef]
- SkymatiX, Inc. System and Method for Supporting Work through Use of Drone. Japan Patent JP2018-169995, 1 November 2018. [Google Scholar]
- Ubelhor, A.; Munz, S.; Graeff-Honninger, S.; Claupein, W. Evaluation of the CROPGRO model for white cabbage production under temperate European climate conditions. Sci. Hortic. 2015, 182, 110–118. [Google Scholar] [CrossRef]
- Go, S.H.; Lee, D.H.; Na, S.I.; Park, J.H. Analysis of growth characteristics of kimchi cabbage using drone-based cabbage surface model image. Agriculture 2022, 12, 216. [Google Scholar] [CrossRef]
- Sim, H.S.; Jo, W.J.; Lee, H.J.; Moon, Y.H.; Wool, U.J.; Jung, S.B.; Ahn, S.R.; Kim, S.K. Determination of optimal growing degree days and cultivars of kimchi cabbage for growth and yield during spring cultivation under shading conditions. Hortic. Sci. Technol. 2021, 39, 714–725. [Google Scholar] [CrossRef]
- Okada, K.; Sasaki, H. Development of a growth model for prediction of top dry weight, head dry weight and head fresh weight of cabbage grown in winter season. Bull. Natl. Inst. Veg. Tea Sci. 2016, 15, 67–76, (In Japanese with English abstract). [Google Scholar]
- Sugahara, K.; Okada, K.; Sato, F. Leaf Area Calculating Method and Yield Predicting Method. Japan Patent JP2021-151228, 30 September 2021. [Google Scholar]
- Purba, J.; Sharma, R.K.; Jat, M.L.; Thind, H.S.; Gupta, R.K.; Chaudhary, O.P.; Chandna, P.; Khurana, H.S.; Kumar, A.; Uppal, H.S.; et al. Site-specific fertilizer nitrogen management in irrigated transplanted rice (Oryza sativa) using an optical sensor. Precis. Agric. 2015, 16, 455–475. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, X.; Pei, D.; Shao, X. Digital image processing technology under backpropagation neural network and K-Means Clustering algorithm on nitrogen utilization rate of Chinese cabbages. PLoS ONE 2021, 16, e0248923. [Google Scholar] [CrossRef]
- Shaw, R.; Lark, R.M.; Williams, A.P.; Chadwick, D.R.; Jones, D.L. Characterising the within-field scale spatial variation of nitrogen in a grassland soil to inform the efficient design of in-situ nitrogen sensor networks for precision agriculture. Agric. Ecosyst. Environ. 2016, 230, 294–306. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.A.; Chiou, C.P.; Bond, L.J. Development of field mobile soil nitrate sensor technology to facilitate precision fertilizer management. Precis. Agric. 2019, 20, 40–55. [Google Scholar] [CrossRef]
- Hilhorst, M.A. A pore water conductivity sensor. Soil Sci. Soc. Am. J. 2000, 64, 1922–1925. [Google Scholar] [CrossRef]
- National Astronomical Observatory of Japan. Dielectric material. Liquid. In Chronological Scientific Tables, 2020; Maruzen Publishing Co., Ltd.: Tokyo, Japan, 2020; p. 438. ISBN 978-4621304259. [Google Scholar]
- Oishi, Y.; Habaragamuwa, H.; Zhang, Y.; Sugiura, R.; Asano, K.; Akai, K.; Shibata, H.; Fujimoto, T. Automated abnormal potato plant detection system using deep learning models and portable video cameras. Int. J. Appl. Earth Obs. Geoinf. 2021, 104, 102509. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Hokkaido Prefectural Government. Nitrogen Nutrient Diagnosis and Fertilization Methods to Improve Cabbage Quality. In Annual Report of Results; 1996. Available online: https://www.hro.or.jp/list/agricultural/center/kenkyuseika/gaiyosho/h08gaiyo/1995130.htm (accessed on 20 February 2023). (In Japanese)
- Rongting, J.; Shi, W.; Wang, Y.; Zhang, H.; Min, J. Nondestructive estimation of bok choy nitrogen status with an active canopy sensor in comparison to a chlorophyll meter. Pedosphere 2020, 30, 769–777. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef]
- Westerveld, S.M.; McKeown, A.W.; Scott-Dupree, C.D.; Mcdonald, M.R. How well do critical nitrogen concentrations work for cabbage, carrot, and onion crops? HortScience 2003, 38, 1122–1128. [Google Scholar] [CrossRef]
- Jamil, N.; Kootstra, G.; Kooistra, L. Evaluation of individual plant growth estimation in an intercropping field with UAV imagery. Agriculture 2022, 12, 102. [Google Scholar] [CrossRef]
- Fujiwara, T.; Yoshioka, H.; Shikata, H.; Sato, F. Effects of transplanting depth of plug seedlings on the establishment and the growth of cabbage. J. Jpn. Soc. Hortic. 1998, 67, 767–772. [Google Scholar] [CrossRef]
- Padilla, F.M.; Farneselli, M.; Gianquintoc, G.; Tei, F.; Thompson, R.B. Monitoring nitrogen status of vegetable crops and soils for optimal nitrogen management. Agric. Water Manag. 2020, 241, 106356. [Google Scholar] [CrossRef]
- Yamamoto, T.; Matsuo, K.; Yamasaki, A. Effect of seedling transplant depth on the yield, lodging, and root distribution of cabbages grown for processing under an integrated mechanical cultivation system. Root Res. 2015, 24, 3–10. [Google Scholar] [CrossRef]
- Kelleners, T.J.; Paige, G.B.; Gray, S.T. Measurement of the dielectric properties of Wyoming soils using electromagnetic sensors. Soil Sci. Soc. Am. J. 2009, 73, 1626–1637. [Google Scholar] [CrossRef]
- Lo, T.H.; Rudnick, D.R.; Singh, J.; Nakabuye, H.N.; Katimbo, A.; Heeren, D.M.; Ge, Y. Field assessment of interreplicate variability from eight electromagnetic soil moisture sensors. Agric. Water Manag. 2020, 231, 105984. [Google Scholar] [CrossRef]
- Noda, S.; Miyake, Y.; Nakano, Y.; Kogoshi, M.; Iijima, W.; Nakagawa, J. A mobile laboratory robot for various and precise measurements of crops and soil in agricultural fields: Development and pilot study (optional). Adv. Robot. 2022; submitted. [Google Scholar]
- Everaarts, A.P.; Booi, R. The effect of nitrogen application on nitrogen utilization by white cabbage (Brassica oleracea var. capitata) and on nitrogen in the soil at harvest. J. Hortic. Sci. Biotechnol. 2015, 75, 705–712. [Google Scholar] [CrossRef]



| Trial | Treatment | Fresh Weight (g plant−1) | Nitrogen Content (% on a Dry Weight Basis) | Plant Nitrogen (g plant−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Head | Total Shoots | Head | Outer Leaves | Stem | Total Shoots | ||||||||
| Trial 1 | Fertilized | 1374 | (1196–1492) | 2176 | (1888–2270) | 3.0 | (2.7–3.1) | 3.5 | (3.4–3.6) | 3.9 | (3.6–4.3) | 4.7 | (4.2–5.0) |
| Non-fertilized | 343 | (278–421) | 817 | (756–983) | 2.5 | (2.3–2.6) | 2.1 | (2.0–2.1) | 2.4 | (2.1–2.4) | 1.7 | (1.6–2.0) | |
| p-values | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Trial 2 | Fertilized | 1277 | (1162–1420) | 2146 | (1914–2284) | 2.9 | (2.8–3.2) | 3.5 | (3.4–3.5) | 3.3 | (3.2–3.6) | 4.9 | (4.5–5.2) |
| Non-fertilized | 651 | (564–823) | 1318 | (1096–1511) | 2.2 | (2.0–2.3) | 2.0 | (1.9–2.4) | 2.0 | (1.9–2.1) | 2.4 | (2.1–2.7) | |
| p-values | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Growth Stage | Indicator | Trial 1 | Trial 2 | ||
|---|---|---|---|---|---|
| Head FW | Total Shoot FW | Head FW | Total Shoot FW | ||
| Seedling a | SPAD | 0.402 * | 0.416 * | 0.510 ** | 0.440 * |
| Height | −0.014 | 0.018 | 0.246 | 0.094 | |
| Increase in projected leaf area | 0.658 *** | 0.591 *** | 0.595 *** | 0.577 *** | |
| Projected leaf area | 0.672 *** | 0.592 *** | 0.599 *** | 0.571 *** | |
| Soil NO3-N | 0.41 * | 0.431 * | 0.574 *** | 0.485 ** | |
| Soil EC with HP sensor | 0.396 * | 0.417 * | 0.456 ** | 0.342 | |
| Soil EC with CS sensor | 0.519 ** | 0.554 ** | 0.364 * | 0.277 | |
| Rosette b | SPAD | −0.386 | 0.100 | −0.002 | −0.109 |
| Height | 0.478 ** | 0.467 ** | 0.476 ** | 0.351 * | |
| Increase in projected leaf area | 0.621 *** | 0.593 *** | 0.629 *** | 0.619 *** | |
| Projected leaf area | 0.644 *** | 0.587 *** | 0.649 *** | 0.647 *** | |
| Soil NO3-N | 0.458 *** | 0.429 * | 0.255 | 0.140 | |
| Soil EC with HP sensor | 0.470 * | 0.458 * | 0.402 * | 0.284 | |
| Soil EC with CS sensor | 0.510 ** | 0.565 ** | 0.424 * | 0.323 | |
| Treatment Basal + Top Dressing | Fresh Weight a (g Plant−1) | Nitrogen Content b (% on a Dry Weight Basis) | Plant Nitrogen b (g Plant−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Head | Total Shoot | Head | Outer Leaves | Stem | Total Shoot | ||||||||
| 25 + 50% | 3018 | (2821–3219) | 4041 | (3865–4152) | 3.0 | (2.7–3.1) | 2.7 | ab | (2.0–2.8) | 3.8 | (3.5–4.2) | 6.2 | (6.2–7.0) |
| 25 + 75% | 2749 | (2583–3010) | 3544 | (3437–3967) | 2.9 | (2.7–3.1) | 2.7 | ab | (2.2–2.9) | 3.9 | (3.6–4.0) | 5.8 | (5.5–6.4) |
| 50 + 25% | 2903 | (2740–3036) | 3738 | (3617–4057) | 3.1 | (3.0–3.2) | 2.6 | ab | (2.3–2.7) | 4.0 | (3.8–4.3) | 6.4 | (6.1–6.8) |
| 50 + 50% | 2959 | (2841–3138) | 3891 | (3724–4178) | 2.8 | (2.6–2.9) | 2.5 | b | (2.2–2.6) | 4.0 | (3.7–4.1) | 6.6 | (5.8–6.8) |
| Control | 2917 | (2819–3130) | 3933 | (3680–4143) | 3.3 | (3.0–3.4) | 3.0 | a | (2.2–3.0) | 3.9 | (3.6–4.2) | 6.6 | (6.2–7.2) |
| p-values | n.s. | n.s. | n.s. | <0.01 | n.s. | n.s. | |||||||
| Treatment Basal + Top Dressing | NO3-N | NH4-N | ||
|---|---|---|---|---|
| (g 100 g−1 Dry Soil) | (g 100 g−1 Dry Soil) | |||
| 25 + 50% | 0.82 | (0.19) | 1.23 | (0.08) |
| 25 + 75% | 1.30 | (1.23) | 1.10 | (0.09) |
| 50 + 25% | 0.59 | (0.03) | 1.17 | (0.10) |
| 50 + 50% | 0.46 | (0.04) | 1.30 | (0.19) |
| Control | 1.77 | (1.50) | 1.12 | (0.04) |
| p-value | n.s. | n.s. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, Y.; Noda, S.; Miyake, Y.; Kogoshi, M.; Sato, F.; Iijima, W. Applicability of Variable-Rate Nitrogen Top Dressing Based on Measurement of the Within-Field Variability of Soil Nutrients for Cabbage Production. Horticulturae 2023, 9, 506. https://doi.org/10.3390/horticulturae9040506
Nakano Y, Noda S, Miyake Y, Kogoshi M, Sato F, Iijima W. Applicability of Variable-Rate Nitrogen Top Dressing Based on Measurement of the Within-Field Variability of Soil Nutrients for Cabbage Production. Horticulturae. 2023; 9(4):506. https://doi.org/10.3390/horticulturae9040506
Chicago/Turabian StyleNakano, Yuka, Shintaro Noda, Yasunari Miyake, Masayuki Kogoshi, Fumio Sato, and Wataru Iijima. 2023. "Applicability of Variable-Rate Nitrogen Top Dressing Based on Measurement of the Within-Field Variability of Soil Nutrients for Cabbage Production" Horticulturae 9, no. 4: 506. https://doi.org/10.3390/horticulturae9040506
APA StyleNakano, Y., Noda, S., Miyake, Y., Kogoshi, M., Sato, F., & Iijima, W. (2023). Applicability of Variable-Rate Nitrogen Top Dressing Based on Measurement of the Within-Field Variability of Soil Nutrients for Cabbage Production. Horticulturae, 9(4), 506. https://doi.org/10.3390/horticulturae9040506






