Breakthrough Analysis of Chemical Composition and Applied Chemometrics of European Plum Cultivars Grown in Norway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Plant Materials
2.2.1. Locations of Plant Material
2.2.2. Weather Conditions
2.2.3. Cultivation Conditions
2.3. Sample Preparation
2.4. Methods and Instrumentations
2.5. Chemometric Analysis
3. Results and Discussion
3.1. ICP-OES Results of Element Content
3.2. IC Results of Sugar Content
3.3. IC Results of Organic Acid Content
3.4. Phenolic Quantification
3.5. Antioxidant Activity
3.6. Chemometric Analyses
3.6.1. Chemometric Analysis of the Results of Element Content
3.6.2. Chemometric Analysis of the Results of Sugar Content
3.6.3. Chemometric Analysis of the Results of Organic Acid Content
3.6.4. Chemometric Analysis of the Results of Phenolic Content, TPC, and RSA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Additional Figures

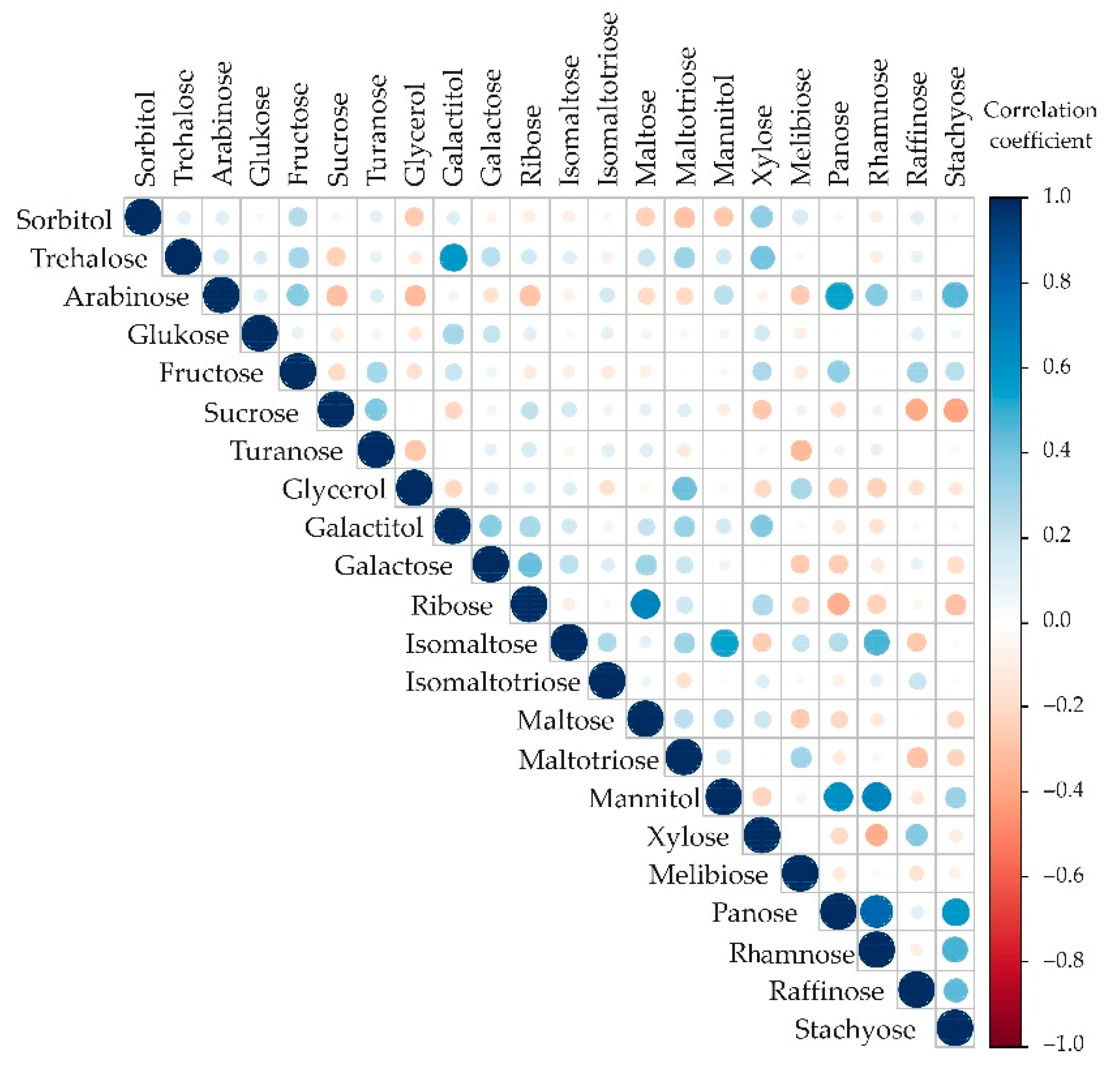
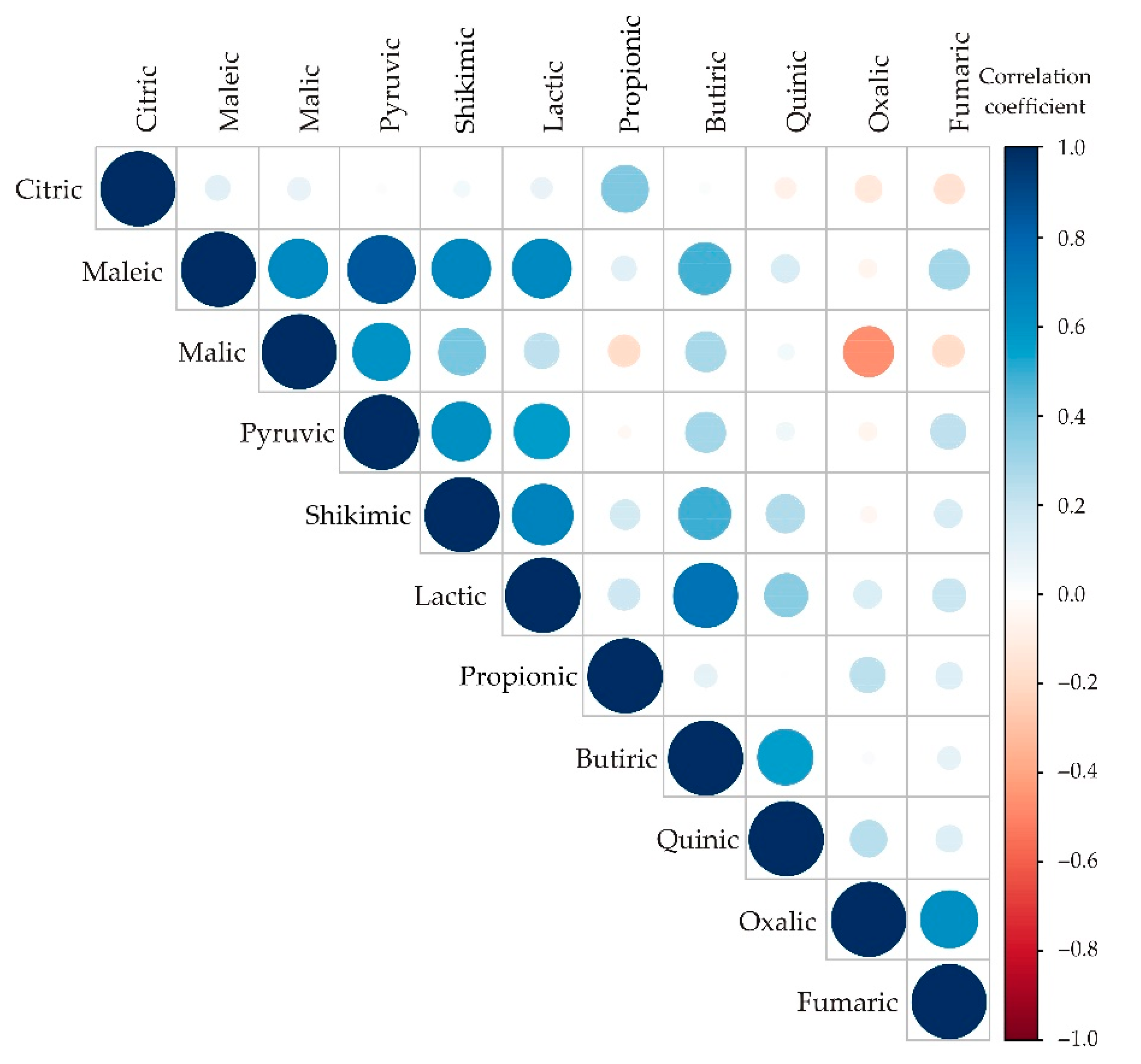
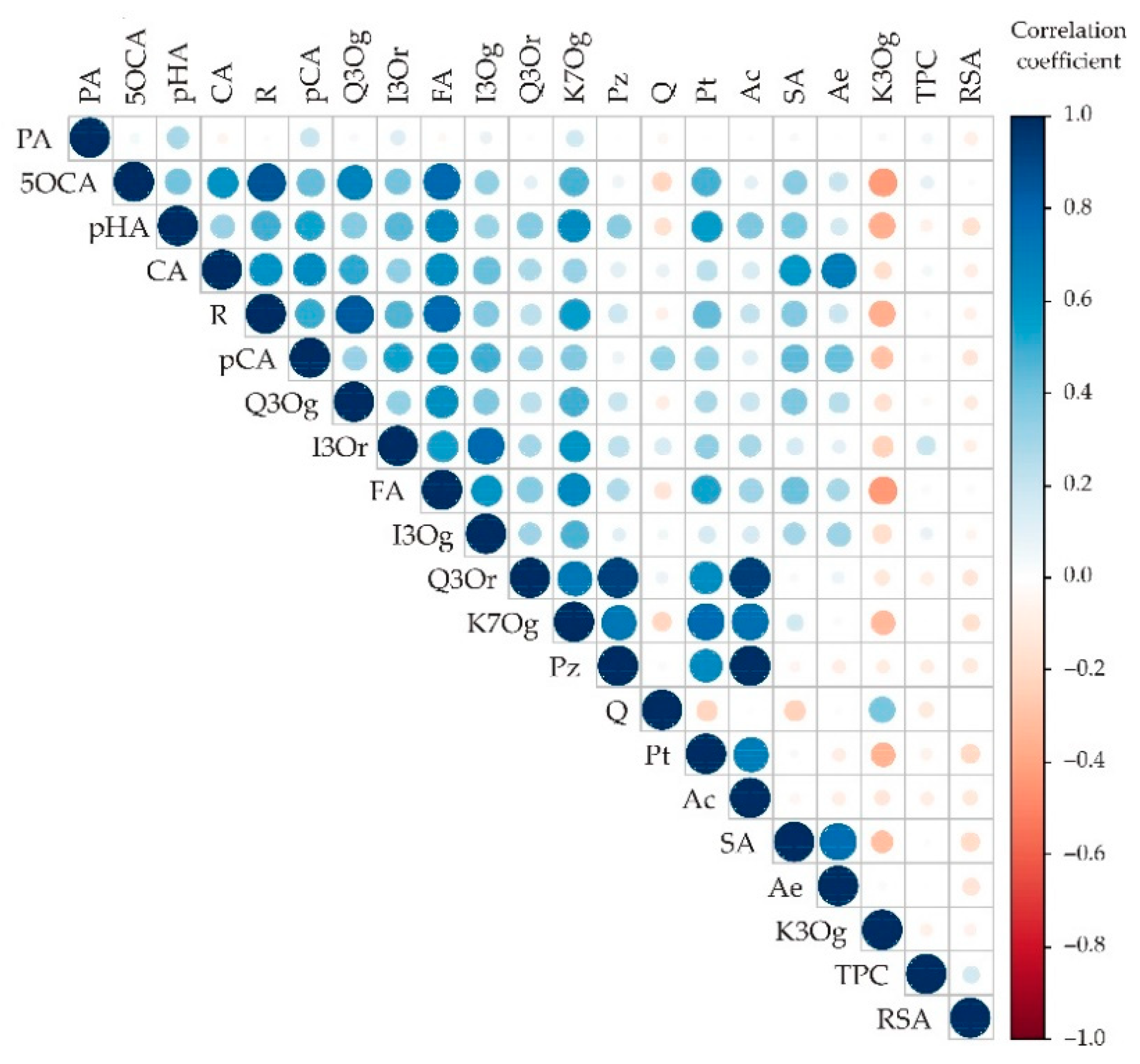
Appendix B. Additional Tables
| No | Plum Cultivar | Al | B | Ca | Cu | Fe | K | Mg | Mn | Na | P | S | Zn | N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Admiral Rigny | 11.27 | 2.05 | 312.65 | 2.54 | 8.65 | 5123.33 | 158.92 | 4.03 | 45.70 | 8523.65 | 345.70 | 4.90 | 3.09 |
| 2 | Reine Claude d’Althanns | 5.09 | 12.19 | 49.84 | 2.74 | 3.43 | 1503.75 | 94.99 | 0.98 | 14.54 | 9766.32 | 209.15 | 8.59 | 3.03 |
| 3 | Anita | 28.65 | 1.55 | 63.33 | 2.86 | 2.89 | 2114.12 | 105.65 | 1.00 | 38.99 | 5421.26 | 187.54 | 5.65 | 2.87 |
| 4 | Avalon | 6.59 | 1.65 | 198.26 | 2.99 | 5.62 | 3147.66 | 92.65 | 2.15 | 15.65 | 8523.62 | 189.65 | 8.66 | 3.06 |
| 5 | Bleue de Belgique | 8.13 | 6.79 | 142.70 | 3.67 | 5.88 | 2568.04 | 154.21 | 1.38 | 31.89 | 7930.17 | 246.96 | 24.60 | 3.12 |
| 6 | Czar | 7.14 | 7.38 | 159.01 | 2.98 | 6.41 | 2496.32 | 126.12 | 0.98 | 9.75 | 7493.81 | 227.78 | 6.89 | 3.03 |
| 7 | Diana | 7.22 | 3.66 | 63.59 | 4.22 | 7.05 | 3874.27 | 123.54 | 2.52 | 52.65 | 6536.66 | 195.36 | 6.86 | 3.22 |
| 8 | Edda | 4.51 | 13.24 | 116.84 | 2.56 | 5.71 | 1902.43 | 165.90 | 1.48 | 13.15 | 6333.46 | 187.57 | 6.04 | 2.96 |
| 9 | Edwards | 8.90 | 5.53 | 61.08 | 2.24 | 5.29 | 3291.22 | 76.76 | 0.99 | 31.19 | 6294.38 | 171.64 | 5.32 | 3.11 |
| 10 | Excalibur | 7.09 | 9.13 | 82.71 | 2.01 | 6.97 | 2598.22 | 96.95 | 1.56 | 18.80 | 5253.15 | 201.62 | 6.74 | 3.26 |
| 11 | Frostaplomme | 7.65 | 3.26 | 108.98 | 3.26 | 8.96 | 2562.69 | 110.53 | 1.32 | 9.65 | 8112.65 | 256.33 | 8.65 | 3.42 |
| 12 | Grand Duke | 4.65 | 1.02 | 263.13 | 1.89 | 7.89 | 4256.33 | 135.66 | 3.16 | 41.37 | 7856.63 | 325.27 | 4.66 | 3.02 |
| 13 | Haganta | 4.88 | 9.76 | 102.63 | 2.82 | 3.53 | 1830.21 | 108.32 | 0.86 | 46.12 | 5972.07 | 192.67 | 8.79 | 2.90 |
| 14 | Helgøyplomme | 2.65 | 1.66 | 283.33 | 2.66 | 41.26 | 8536.99 | 278.65 | 8.96 | 101.24 | 5985.62 | 365.33 | 10.25 | 2.98 |
| 15 | Herman | 10.29 | 5.65 | 115.44 | 2.70 | 5.70 | 2578.84 | 118.91 | 1.30 | 15.88 | 7550.90 | 367.56 | 11.26 | 3.17 |
| 16 | Jefferson | 11.26 | 2.65 | 285.37 | 4.66 | 29.62 | 8523.62 | 245.62 | 6.33 | 85.33 | 4965.32 | 301.27 | 8.26 | 3.24 |
| 17 | Jubileum | 9.92 | 4.15 | 277.09 | 2.62 | 16.71 | 5915.36 | 300.74 | 4.01 | 69.08 | 5275.34 | 194.33 | 4.52 | 3.20 |
| 18 | Kirkes | 8.01 | 4.26 | 71.99 | 5.21 | 8.24 | 4125.65 | 153.65 | 3.05 | 64.56 | 8321.51 | 232.57 | 7.13 | 3.12 |
| 19 | Mallard | 14.80 | 0.70 | 482.97 | 3.93 | 23.19 | 11,545.75 | 513.84 | 9.42 | 84.71 | 7852.33 | 355.62 | 9.28 | 3.11 |
| 20 | Mount Royal | 7.99 | 6.62 | 127.52 | 2.85 | 8.27 | 3340.60 | 106.08 | 1.03 | 40.96 | 8844.27 | 258.83 | 8.00 | 3.38 |
| 21 | Njøs II | 6.64 | 8.55 | 280.36 | 2.11 | 3.35 | 2261.36 | 111.49 | 0.75 | 29.53 | 7079.04 | 124.00 | 12.07 | 3.00 |
| 22 | Ontario | 18.65 | 3.65 | 257.99 | 3.65 | 7.99 | 4889.32 | 201.51 | 6.66 | 85.33 | 9001.65 | 365.26 | 5.93 | 2.89 |
| 23 | Opal | 9.46 | 14.98 | 146.15 | 4.56 | 14.03 | 1700.97 | 199.10 | 1.20 | 17.57 | 8084.77 | 272.83 | 2.71 | 2.90 |
| 24 | Reine Claude d’Oullins ‘Henjum | 6.99 | 3.26 | 326.33 | 3.89 | 36.66 | 9652.32 | 268.95 | 7.22 | 95.33 | 5213.32 | 352.65 | 9.13 | 3.05 |
| 25 | Prosser 84 | 5.38 | 5.72 | 101.29 | 1.70 | 2.58 | 1884.16 | 110.74 | 0.69 | 0.50 | 8230.53 | 163.45 | 5.55 | 2.68 |
| 26 | R5 | 6.11 | 3.85 | 103.33 | 3.12 | 6.85 | 2456.62 | 99.65 | 1.00 | 7.65 | 7412.36 | 301.24 | 6.25 | 3.14 |
| 27 | Raud Eplevik | 12.66 | 2.65 | 185.66 | 2.65 | 8.96 | 3658.66 | 105.65 | 2.66 | 32.65 | 6985.65 | 305.67 | 7.41 | 3.08 |
| 28 | Reeves | 16.05 | 0.78 | 262.89 | 4.70 | 40.29 | 9155.97 | 353.11 | 6.75 | 78.38 | 6196.64 | 227.10 | 9.54 | 3.03 |
| 29 | Reine Claude Althanns | 5.24 | 12.68 | 52.10 | 2.78 | 3.50 | 1574.34 | 99.37 | 0.94 | 15.14 | 10,225.25 | 218.89 | 8.91 | 2.98 |
| 30 | Reine Claude Noire | 7.15 | 7.34 | 58.18 | 3.27 | 3.56 | 2438.93 | 124.44 | 0.69 | 36.46 | 7227.61 | 309.69 | 10.94 | 3.07 |
| 31 | Reine Claude Souffriau | 5.37 | 6.98 | 88.97 | 2.13 | 7.21 | 2030.02 | 112.23 | 1.40 | 24.72 | 5582.01 | 201.02 | 7.34 | 3.23 |
| 32 | Rives Early Prolific | 16.92 | 6.30 | 180.60 | 4.14 | 10.06 | 2865.71 | 142.26 | 2.43 | 38.48 | 8190.41 | 327.09 | 8.53 | 2.94 |
| 33 | Rød Victoria | 5.43 | 15.56 | 106.26 | 3.57 | 32.29 | 1972.94 | 148.09 | 1.42 | 34.77 | 6235.81 | 252.27 | 22.60 | 3.25 |
| 34 | Ruth Gerstetter | 10.26 | 3.26 | 209.66 | 6.23 | 21.66 | 9259.33 | 326.33 | 8.65 | 91.26 | 4563.32 | 257.65 | 9.99 | 3.42 |
| 35 | Sanctus Hubertus | 14.72 | 5.04 | 147.83 | 4.64 | 19.56 | 5111.60 | 248.67 | 4.60 | 49.60 | 6098.21 | 256.24 | 7.69 | 3.17 |
| 36 | Sviske frå Tveit | 35.52 | 4.87 | 42.70 | 1.50 | 5.19 | 2779.32 | 78.27 | 0.72 | 75.42 | 6850.72 | 229.06 | 7.78 | 3.24 |
| 37 | Thames Cross | 5.02 | 12.66 | 97.27 | 2.84 | 4.05 | 2432.24 | 144.49 | 1.15 | 17.71 | 6345.55 | 225.97 | 11.38 | 3.08 |
| 38 | Valor | 9.43 | 0.68 | 343.41 | 3.99 | 28.06 | 6571.50 | 325.85 | 4.70 | 57.15 | 4788.37 | 288.74 | 7.26 | 3.18 |
| 39 | Victoria | 11.87 | 7.30 | 404.86 | 4.31 | 17.59 | 7044.10 | 303.06 | 4.30 | 54.31 | 9120.75 | 254.36 | 6.26 | 3.08 |
| 40 | Vinterplomme | 8.26 | 4.67 | 125.65 | 4.02 | 7.65 | 2896.33 | 121.65 | 1.04 | 10.26 | 8014.55 | 288.65 | 9.65 | 3.65 |
| 41 | Washington | 9.82 | 3.42 | 58.62 | 3.89 | 6.02 | 4128.66 | 92.33 | 1.82 | 45.62 | 7124.26 | 185.26 | 6.33 | 3.01 |
| 42 | Yakima | 7.85 | 2.33 | 296.33 | 1.99 | 18.65 | 6853.21 | 301.26 | 2.86 | 71.26 | 4896.33 | 198.65 | 4.96 | 3.04 |
| 43 | Edda | 19.85 | 0.60 | 540.67 | 5.21 | 15.20 | 11,575.99 | 547.76 | 8.46 | 90.65 | 7706.97 | 404.39 | 9.33 | 3.10 |
| 44 | Jubileum | 9.13 | 3.87 | 263.83 | 2.37 | 14.64 | 5723.61 | 292.51 | 3.79 | 66.45 | 4928.48 | 186.23 | 4.32 | 3.15 |
| 45 | Čačanska lepotica | 11.22 | 3.43 | 365.27 | 3.73 | 23.74 | 8180.19 | 367.17 | 5.04 | 67.77 | 5848.60 | 93.18 | 4.38 | 3.16 |
| 46 | Mallard | 24.38 | 0.91 | 500.89 | 3.97 | 27.53 | 11,696.62 | 529.53 | 9.48 | 88.75 | 7864.60 | 333.32 | 8.90 | 2.95 |
| 47 | Opal | 4.73 | 0.56 | 120.27 | 1.44 | 3.17 | 2745.22 | 121.49 | 1.79 | 37.23 | 3312.55 | 74.26 | 2.56 | 3.15 |
| 48 | Reeves | 20.45 | 1.00 | 348.76 | 5.81 | 54.72 | 11,456.59 | 470.71 | 8.61 | 101.33 | 7965.93 | 300.57 | 11.70 | 3.21 |
| 49 | Valor | 9.49 | 0.69 | 369.20 | 4.02 | 29.88 | 6827.79 | 343.46 | 4.88 | 62.85 | 4985.72 | 316.59 | 7.64 | 2.99 |
| 50 | Victoria | 14.53 | 6.58 | 339.60 | 3.82 | 14.88 | 6240.41 | 265.89 | 4.10 | 61.50 | 8540.89 | 227.98 | 6.49 | 3.16 |
| 51 | Blue Rock | 7.28 | 7.14 | 132.40 | 2.31 | 5.27 | 1860.78 | 110.13 | 1.07 | 23.45 | 8416.16 | 170.04 | 23.99 | 3.15 |
| 52 | Czar | 10.68 | 9.75 | 280.22 | 2.78 | 9.16 | 3118.25 | 129.14 | 1.52 | 19.97 | 11,895.27 | 247.72 | 9.99 | 3.04 |
| 53 | Diamond | 31.98 | 6.50 | 172.91 | 1.28 | 4.64 | 2489.74 | 122.37 | 1.36 | 29.02 | 6398.91 | 190.01 | 6.99 | 3.29 |
| 54 | Edwards | 11.18 | 4.70 | 72.82 | 2.07 | 9.47 | 2677.96 | 63.57 | 0.84 | 26.24 | 6878.40 | 137.65 | 6.60 | 3.01 |
| 55 | Emil | 11.91 | 12.25 | 117.08 | 2.33 | 8.00 | 2680.04 | 100.89 | 0.87 | 36.14 | 12,198.01 | 187.64 | 8.33 | 2.98 |
| 56 | Excalibur | 10.23 | 12.70 | 80.60 | 1.92 | 8.78 | 1904.59 | 78.71 | 1.11 | 31.31 | 10,005.00 | 161.84 | 6.76 | 3.05 |
| 57 | Experimentalfältets sviskon | 17.67 | 6.34 | 164.64 | 2.36 | 2.98 | 2206.17 | 90.77 | 1.43 | 37.55 | 6808.62 | 187.87 | 10.89 | 3.01 |
| 58 | Herman | 9.58 | 6.61 | 149.58 | 2.83 | 5.98 | 2369.52 | 137.99 | 1.34 | 16.83 | 7215.14 | 385.43 | 10.43 | 3.13 |
| 59 | Ive | 7.22 | 10.50 | 76.96 | 2.61 | 8.31 | 1840.81 | 76.34 | 0.92 | 26.30 | 10,397.93 | 202.13 | 8.74 | 3.03 |
| 60 | Mount Royal | 8.73 | 6.05 | 96.26 | 2.40 | 7.59 | 2317.29 | 83.97 | 0.86 | 24.73 | 6922.51 | 185.52 | 6.16 | 3.10 |
| 61 | Reine Claude Noir | 12.56 | 7.58 | 56.71 | 2.96 | 8.14 | 2085.00 | 106.75 | 0.79 | 31.64 | 7414.57 | 269.62 | 9.76 | 3.04 |
| 62 | Reine Claude d’Oullins | 42.77 | 9.09 | 93.32 | 2.26 | 9.80 | 1562.42 | 72.26 | 0.64 | 21.00 | 7893.83 | 130.50 | 4.90 | 2.96 |
| 63 | Reine Claude Souffriau | 5.38 | 6.99 | 88.96 | 2.14 | 7.22 | 2031.29 | 112.49 | 1.41 | 24.69 | 5581.36 | 201.47 | 7.37 | 3.05 |
| 64 | Rivers Early Prolific | 6.14 | 5.85 | 112.69 | 2.59 | 6.13 | 1778.97 | 109.44 | 1.06 | 21.78 | 5861.86 | 216.47 | 9.23 | 3.13 |
| 65 | Sinikka | 17.58 | 4.15 | 81.06 | 1.49 | 9.53 | 2359.34 | 93.99 | 1.33 | 15.35 | 6060.25 | 177.30 | 8.26 | 3.09 |
| 66 | Søgne | 8.79 | 4.88 | 76.27 | 1.70 | 5.48 | 1612.86 | 89.90 | 0.94 | 21.89 | 6355.70 | 148.00 | 5.82 | 2.97 |
| 67 | Sviske frå Tveit | 40.28 | 8.75 | 75.20 | 1.90 | 13.78 | 2557.94 | 88.84 | 0.99 | 47.56 | 8956.24 | 189.50 | 7.17 | 3.15 |
| 68 | Traneplommer | 14.20 | 6.33 | 73.67 | 2.85 | 6.58 | 2052.23 | 126.99 | 1.37 | 50.10 | 6559.98 | 282.97 | 9.49 | 3.13 |
| No | Sorbitol | Trehalose | Arabinose | Glukose | Fructose | Sucrose | Turanose | Glycerol | Galactitol | Galactose | Ribose | Isomaltose | Isomaltotriose | Maltose | Maltotriose | Mannitol | Xylose | Melibiose | Panose | Rhamnose | Raffinose | Stachyose |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 101.27 | 1.02 | 1.57 | 245.65 | 211.27 | 219.33 | 17.96 | 0.63 | 1.79 | 21.40 | 2.01 | 1.65 | 1.99 | 4.13 | 0.74 | 1.88 | 2.86 | 8.11 | 4.33 | 5.89 | 1.03 | 1.41 |
| 2 | 34.11 | 0.81 | 0.05 | 245.62 | 181.11 | 211.49 | 16.97 | 1.11 | 3.54 | 31.69 | 11.82 | 0.58 | 0.47 | 19.94 | 0.68 | 2.61 | 8.31 | 5.11 | 1.21 | 1.98 | 2.09 | 0.74 |
| 3 | 4.16 | 0.63 | 0.32 | 183.62 | 179.73 | 155.03 | 2.31 | 7.20 | 0.75 | 31.22 | 3.94 | 2.34 | 0.68 | 1.62 | 1.59 | 3.34 | 0.24 | 8.48 | 3.06 | 0.93 | 0.72 | 0.96 |
| 4 | 84.26 | 1.00 | 1.57 | 226.33 | 178.65 | 199.65 | 10.66 | 1.00 | 1.99 | 35.66 | 3.26 | 4.66 | 2.14 | 2.89 | 0.86 | 1.90 | 2.65 | 5.21 | 6.33 | 8.57 | 0.89 | 0.98 |
| 5 | 119.15 | 2.84 | 1.65 | 245.12 | 213.56 | 140.97 | 14.43 | 0.89 | 2.63 | 16.17 | 1.98 | 0.57 | 4.48 | 1.56 | 0.30 | 0.34 | 12.23 | 7.14 | 6.00 | 2.27 | 2.80 | 3.01 |
| 6 | 92.34 | 1.84 | 0.66 | 256.28 | 193.86 | 156.97 | 15.57 | 0.96 | 3.93 | 29.35 | 7.27 | 1.88 | 1.48 | 11.95 | 0.61 | 3.26 | 5.22 | 4.82 | 4.27 | 6.38 | 1.47 | 0.77 |
| 7 | 100.45 | 4.11 | 1.94 | 244.94 | 183.54 | 102.50 | 6.81 | 0.77 | 6.85 | 28.57 | 7.21 | 0.75 | 2.61 | 2.25 | 0.38 | 0.41 | 7.01 | 5.58 | 4.14 | 1.47 | 0.96 | 1.61 |
| 8 | 69.06 | 0.17 | 0.24 | 326.42 | 158.68 | 170.06 | 10.50 | 0.87 | 4.32 | 34.78 | 8.69 | 2.02 | 1.76 | 24.40 | 0.68 | 2.18 | 7.08 | 4.90 | 0.88 | 2.57 | 2.40 | 0.86 |
| 9 | 85.19 | 2.36 | 1.02 | 241.02 | 171.01 | 83.11 | 8.66 | 1.06 | 5.14 | 33.67 | 1.59 | 4.51 | 3.03 | 13.74 | 0.19 | 7.68 | 3.39 | 7.60 | 1.88 | 1.71 | 1.37 | 1.76 |
| 10 | 114.53 | 0.82 | 0.89 | 211.44 | 178.17 | 227.29 | 15.38 | 0.45 | 1.36 | 16.49 | 1.78 | 1.29 | 1.35 | 4.08 | 0.28 | 2.10 | 5.41 | 9.48 | 4.47 | 6.63 | 0.93 | 1.36 |
| 11 | 133.85 | 0.14 | 0.36 | 205.75 | 141.50 | 114.10 | 7.38 | 2.29 | 2.39 | 21.61 | 4.63 | 1.92 | 1.41 | 4.07 | 0.57 | 2.17 | 6.00 | 10.46 | 5.27 | 8.40 | 2.75 | 4.55 |
| 12 | 91.65 | 0.99 | 1.15 | 235.69 | 201.33 | 225.33 | 16.99 | 0.59 | 1.33 | 19.89 | 1.94 | 1.55 | 1.66 | 3.79 | 0.66 | 1.53 | 2.33 | 7.86 | 4.13 | 6.13 | 0.99 | 1.89 |
| 13 | 103.54 | 0.52 | 0.47 | 308.74 | 151.66 | 106.14 | 4.88 | 1.01 | 4.00 | 32.03 | 6.66 | 2.30 | 2.41 | 17.08 | 0.59 | 1.78 | 5.95 | 4.93 | 0.56 | 3.53 | 2.73 | 1.07 |
| 14 | 71.65 | 0.91 | 2.33 | 175.33 | 145.67 | 112.26 | 8.32 | 0.57 | 1.88 | 21.33 | 3.26 | 0.87 | 3.26 | 14.26 | 0.72 | 2.00 | 4.65 | 3.53 | 1.99 | 4.33 | 1.10 | 1.96 |
| 15 | 164.54 | 1.35 | 1.00 | 210.19 | 275.64 | 127.16 | 20.57 | 1.03 | 3.21 | 33.29 | 2.74 | 0.26 | 0.23 | 5.86 | 0.17 | 0.07 | 6.48 | 3.05 | 4.04 | 1.48 | 2.99 | 2.52 |
| 16 | 84.65 | 1.26 | 0.90 | 194.65 | 175.65 | 135.65 | 18.65 | 0.36 | 2.45 | 24.65 | 4.65 | 0.95 | 3.60 | 14.67 | 0.48 | 1.99 | 6.65 | 4.33 | 1.02 | 4.65 | 3.14 | 2.65 |
| 17 | 144.19 | 0.10 | 0.47 | 231.01 | 126.85 | 199.17 | 18.44 | 2.26 | 1.77 | 35.73 | 5.46 | 2.52 | 3.07 | 7.67 | 0.27 | 1.49 | 5.48 | 6.24 | 0.04 | 6.14 | 2.73 | 1.61 |
| 18 | 82.33 | 5.32 | 1.65 | 258.66 | 171.56 | 152.33 | 5.23 | 1.00 | 2.33 | 32.36 | 5.63 | 0.92 | 1.99 | 4.33 | 0.33 | 0.59 | 4.33 | 4.37 | 2.15 | 5.67 | 1.25 | 2.14 |
| 19 | 85.88 | 9.70 | 0.30 | 241.60 | 176.85 | 129.00 | 6.20 | 1.49 | 2.57 | 37.92 | 11.07 | 1.25 | 0.50 | 29.88 | 0.61 | 2.60 | 8.18 | 5.07 | 1.18 | 2.00 | 2.07 | 0.75 |
| 20 | 118.28 | 2.84 | 1.89 | 221.39 | 196.93 | 180.54 | 15.95 | 0.30 | 1.88 | 21.68 | 7.81 | 4.21 | 2.72 | 23.08 | 0.55 | 7.81 | 4.63 | 6.38 | 20.56 | 18.51 | 0.53 | 1.31 |
| 21 | 61.54 | 0.51 | 0.42 | 294.95 | 160.89 | 127.51 | 4.37 | 1.54 | 1.91 | 34.80 | 5.76 | 2.24 | 2.95 | 16.76 | 0.35 | 1.02 | 3.58 | 4.09 | 0.10 | 4.00 | 2.32 | 1.10 |
| 22 | 88.65 | 1.25 | 1.79 | 204.67 | 198.37 | 201.33 | 13.66 | 0.73 | 1.70 | 22.33 | 2.26 | 1.77 | 2.01 | 4.33 | 0.25 | 1.63 | 2.57 | 9.00 | 5.32 | 5.66 | 1.15 | 1.65 |
| 23 | 52.04 | 0.78 | 0.12 | 304.57 | 94.49 | 193.01 | 5.78 | 1.29 | 2.30 | 36.51 | 5.19 | 2.00 | 2.90 | 7.54 | 0.35 | 1.19 | 4.17 | 4.72 | 0.14 | 4.61 | 2.14 | 1.28 |
| 24 | 91.66 | 0.86 | 1.05 | 201.67 | 155.66 | 145.62 | 9.66 | 0.37 | 3.53 | 25.62 | 5.63 | 1.00 | 4.13 | 19.82 | 0.65 | 2.65 | 7.89 | 5.62 | 1.66 | 5.62 | 4.33 | 3.00 |
| 25 | 102.71 | 0.32 | 0.30 | 166.51 | 60.95 | 135.74 | 3.84 | 0.78 | 2.18 | 32.12 | 4.25 | 1.67 | 2.45 | 6.70 | 0.30 | 1.34 | 4.25 | 5.77 | 1.34 | 4.88 | 1.97 | 1.76 |
| 26 | 61.03 | 0.42 | 0.32 | 167.38 | 182.10 | 115.31 | 11.26 | 1.09 | 3.58 | 27.78 | 1.64 | 3.23 | 2.08 | 3.64 | 0.79 | 2.48 | 5.10 | 7.80 | 7.52 | 9.93 | 2.66 | 1.92 |
| 27 | 85.33 | 0.95 | 0.93 | 223.26 | 192.36 | 214.66 | 15.27 | 0.53 | 1.54 | 18.33 | 1.85 | 1.36 | 1.48 | 3.26 | 0.52 | 1.14 | 1.26 | 6.76 | 3.85 | 6.33 | 0.96 | 1.63 |
| 28 | 57.35 | 0.82 | 0.55 | 202.63 | 173.21 | 203.85 | 26.01 | 1.21 | 1.92 | 34.38 | 11.43 | 1.72 | 3.24 | 32.12 | 0.40 | 0.95 | 3.37 | 3.87 | 0.08 | 3.75 | 2.84 | 1.03 |
| 29 | 42.05 | 1.01 | 0.08 | 222.74 | 173.23 | 169.68 | 20.92 | 1.38 | 4.37 | 39.07 | 4.58 | 0.73 | 0.59 | 24.59 | 0.85 | 3.23 | 5.25 | 6.30 | 1.50 | 2.45 | 2.59 | 0.92 |
| 30 | 73.35 | 2.00 | 0.65 | 220.33 | 188.63 | 207.80 | 29.45 | 0.60 | 1.28 | 20.09 | 6.16 | 0.33 | 1.26 | 5.16 | 0.30 | 0.19 | 6.81 | 2.90 | 3.88 | 1.42 | 2.83 | 0.33 |
| 31 | 97.54 | 0.75 | 1.00 | 238.79 | 193.77 | 226.27 | 15.17 | 0.70 | 4.02 | 32.08 | 0.60 | 3.83 | 2.47 | 3.38 | 1.02 | 2.37 | 5.63 | 7.00 | 5.99 | 10.00 | 0.67 | 0.70 |
| 32 | 77.52 | 1.02 | 0.68 | 200.83 | 172.88 | 151.28 | 16.19 | 0.75 | 1.88 | 26.93 | 4.60 | 0.78 | 2.14 | 13.20 | 0.61 | 0.54 | 5.77 | 6.29 | 2.54 | 2.36 | 1.96 | 1.01 |
| 33 | 28.33 | 0.22 | 0.48 | 349.79 | 136.61 | 139.12 | 6.75 | 0.97 | 1.16 | 16.43 | 2.42 | 1.01 | 1.23 | 3.11 | 0.33 | 1.68 | 4.63 | 8.03 | 3.67 | 5.66 | 2.32 | 2.71 |
| 34 | 48.96 | 3.26 | 0.84 | 205.65 | 163.66 | 131.33 | 11.26 | 1.65 | 1.96 | 31.26 | 5.62 | 1.00 | 2.33 | 9.65 | 0.37 | 2.02 | 5.03 | 3.21 | 1.53 | 4.65 | 2.05 | 0.56 |
| 35 | 47.74 | 1.00 | 0.66 | 156.69 | 206.32 | 131.11 | 14.35 | 1.10 | 2.90 | 35.52 | 7.20 | 2.72 | 2.77 | 19.75 | 0.71 | 2.25 | 5.04 | 7.22 | 4.49 | 6.73 | 2.42 | 4.02 |
| 36 | 54.64 | 5.29 | 3.34 | 257.68 | 225.38 | 76.78 | 17.71 | 0.32 | 2.82 | 30.67 | 2.57 | 1.01 | 1.49 | 8.36 | 0.31 | 6.16 | 3.62 | 1.96 | 16.53 | 14.63 | 4.34 | 10.37 |
| 37 | 89.66 | 1.12 | 1.87 | 215.33 | 155.37 | 102.36 | 11.02 | 0.43 | 2.33 | 18.66 | 4.70 | 1.02 | 2.15 | 25.33 | 0.33 | 1.86 | 5.90 | 4.22 | 1.57 | 3.25 | 2.02 | 1.74 |
| 38 | 121.06 | 0.93 | 0.61 | 225.13 | 184.48 | 191.22 | 21.86 | 0.25 | 2.97 | 39.04 | 9.28 | 0.80 | 2.90 | 2.29 | 0.25 | 1.34 | 5.03 | 5.64 | 0.84 | 5.47 | 2.08 | 1.46 |
| 39 | 77.28 | 1.94 | 1.45 | 261.52 | 149.43 | 131.19 | 31.55 | 1.21 | 2.42 | 24.38 | 3.46 | 2.41 | 2.96 | 10.43 | 0.36 | 1.39 | 4.40 | 5.99 | 1.33 | 5.24 | 2.02 | 1.81 |
| 40 | 54.96 | 0.21 | 0.42 | 186.60 | 178.15 | 112.51 | 2.80 | 2.17 | 1.67 | 20.86 | 4.27 | 1.37 | 1.37 | 3.09 | 0.59 | 1.76 | 5.03 | 8.85 | 3.82 | 6.08 | 2.30 | 2.86 |
| 41 | 68.95 | 2.36 | 0.79 | 235.63 | 165.66 | 178.65 | 7.32 | 1.12 | 4.33 | 21.66 | 4.33 | 0.89 | 1.24 | 12.66 | 1.09 | 0.69 | 2.22 | 6.54 | 3.37 | 2.27 | 2.04 | 1.12 |
| 42 | 75.65 | 0.83 | 0.57 | 204.33 | 168.95 | 175.66 | 11.74 | 2.99 | 1.43 | 24.52 | 4.11 | 2.98 | 3.01 | 6.52 | 0.37 | 1.70 | 5.69 | 6.35 | 0.59 | 4.22 | 1.89 | 1.64 |
| 43 | 89.04 | 2.54 | 0.46 | 232.49 | 200.33 | 238.20 | 12.60 | 1.30 | 4.82 | 20.48 | 11.59 | 2.18 | 2.82 | 28.19 | 0.90 | 2.92 | 9.29 | 6.33 | 1.27 | 3.18 | 1.54 | 1.16 |
| 44 | 104.84 | 0.29 | 0.61 | 231.39 | 150.04 | 269.02 | 16.24 | 2.15 | 1.70 | 31.13 | 4.91 | 2.40 | 2.87 | 6.87 | 0.42 | 1.49 | 5.00 | 5.67 | 3.97 | 5.59 | 1.94 | 1.58 |
| 45 | 114.32 | 34.66 | 1.10 | 248.94 | 244.51 | 97.03 | 22.67 | 0.22 | 9.80 | 40.28 | 5.67 | 3.68 | 2.08 | 16.94 | 1.33 | 3.70 | 11.56 | 7.11 | 1.71 | 2.71 | 2.97 | 1.13 |
| 46 | 91.11 | 10.49 | 0.39 | 247.11 | 186.28 | 168.41 | 6.75 | 1.67 | 2.85 | 34.57 | 11.84 | 1.44 | 0.62 | 32.29 | 0.74 | 2.92 | 8.88 | 5.59 | 1.33 | 2.24 | 2.33 | 0.87 |
| 47 | 76.03 | 4.18 | 0.37 | 230.89 | 168.50 | 198.71 | 10.06 | 1.75 | 4.38 | 26.58 | 9.45 | 2.51 | 3.55 | 13.41 | 0.74 | 2.17 | 7.22 | 6.98 | 0.59 | 5.93 | 3.08 | 1.82 |
| 48 | 67.79 | 1.00 | 0.73 | 223.43 | 182.09 | 279.02 | 29.76 | 1.47 | 2.28 | 39.61 | 13.12 | 2.04 | 3.87 | 37.54 | 0.54 | 1.21 | 4.01 | 4.58 | 0.17 | 4.39 | 3.38 | 1.29 |
| 49 | 109.45 | 1.28 | 0.95 | 278.16 | 205.84 | 219.73 | 25.75 | 0.53 | 3.68 | 45.58 | 10.80 | 1.17 | 3.58 | 2.86 | 0.52 | 1.79 | 6.07 | 6.80 | 0.31 | 6.60 | 2.64 | 1.89 |
| 50 | 87.65 | 2.24 | 1.51 | 263.24 | 206.28 | 195.91 | 29.90 | 1.08 | 1.54 | 32.16 | 2.44 | 2.73 | 4.65 | 7.95 | 0.33 | 2.74 | 2.50 | 4.75 | 6.66 | 9.51 | 2.42 | 3.98 |
| 51 | 94.47 | 2.17 | 1.24 | 211.27 | 195.99 | 67.43 | 11.38 | 0.62 | 2.02 | 12.70 | 1.48 | 0.36 | 3.38 | 1.12 | 0.14 | 0.17 | 9.71 | 5.60 | 4.70 | 1.70 | 5.32 | 5.71 |
| 52 | 92.03 | 1.65 | 0.56 | 196.36 | 190.54 | 175.61 | 15.47 | 0.89 | 4.12 | 30.13 | 7.53 | 1.84 | 1.38 | 12.87 | 0.59 | 2.90 | 5.56 | 4.94 | 3.71 | 5.82 | 3.61 | 3.41 |
| 53 | 112.46 | 4.37 | 1.94 | 319.13 | 204.78 | 96.51 | 7.14 | 0.71 | 7.62 | 31.86 | 7.98 | 0.70 | 2.81 | 2.42 | 0.30 | 0.29 | 7.35 | 5.79 | 4.32 | 1.44 | 4.08 | 4.34 |
| 54 | 62.10 | 1.85 | 0.86 | 257.24 | 209.89 | 86.71 | 6.54 | 0.88 | 3.97 | 25.20 | 1.29 | 3.50 | 2.35 | 10.31 | 0.23 | 4.95 | 2.25 | 5.76 | 12.60 | 12.10 | 3.90 | 11.17 |
| 55 | 120.33 | 0.61 | 0.96 | 170.31 | 142.18 | 227.09 | 12.33 | 1.64 | 2.24 | 14.95 | 3.31 | 1.74 | 0.96 | 3.61 | 0.33 | 1.69 | 4.56 | 8.17 | 4.09 | 5.74 | 2.17 | 3.11 |
| 56 | 108.59 | 1.13 | 0.71 | 207.93 | 148.36 | 198.15 | 14.44 | 0.24 | 0.98 | 11.65 | 1.26 | 0.88 | 0.96 | 2.92 | 0.12 | 1.49 | 3.99 | 7.17 | 3.28 | 5.55 | 2.12 | 2.44 |
| 57 | 109.06 | 2.03 | 1.23 | 286.11 | 270.94 | 78.37 | 9.28 | 0.25 | 1.43 | 27.74 | 0.97 | 1.28 | 3.42 | 6.39 | 0.25 | 0.19 | 13.36 | 6.75 | 5.12 | 2.11 | 6.55 | 1.07 |
| 58 | 144.01 | 0.98 | 0.80 | 211.16 | 285.38 | 118.70 | 17.94 | 0.93 | 2.83 | 29.37 | 2.45 | 0.24 | 0.21 | 5.16 | 0.15 | 0.06 | 5.85 | 2.72 | 3.64 | 1.31 | 2.66 | 2.30 |
| 59 | 64.64 | 0.74 | 1.04 | 221.99 | 189.58 | 185.07 | 19.54 | 0.34 | 1.31 | 24.93 | 2.21 | 3.04 | 2.55 | 3.63 | 0.24 | 6.28 | 2.83 | 2.33 | 15.57 | 14.68 | 2.46 | 1.18 |
| 60 | 75.96 | 1.35 | 0.80 | 220.85 | 167.64 | 136.24 | 9.94 | 0.90 | 1.33 | 18.52 | 4.99 | 2.18 | 1.74 | 10.88 | 0.43 | 4.01 | 4.36 | 6.86 | 10.25 | 10.81 | 3.39 | 6.93 |
| 61 | 78.13 | 2.00 | 0.56 | 138.26 | 175.92 | 195.01 | 29.30 | 0.51 | 1.17 | 20.11 | 5.83 | 0.22 | 1.16 | 5.16 | 0.18 | 0.07 | 5.66 | 2.85 | 3.96 | 1.35 | 2.78 | 1.95 |
| 62 | 102.10 | 0.32 | 0.30 | 165.52 | 160.59 | 134.93 | 3.82 | 0.78 | 2.16 | 31.92 | 4.23 | 1.66 | 2.44 | 6.66 | 0.30 | 1.34 | 4.23 | 5.74 | 1.33 | 4.85 | 1.96 | 1.75 |
| 63 | 85.62 | 0.58 | 0.83 | 240.05 | 198.05 | 233.16 | 14.03 | 0.54 | 3.53 | 29.08 | 0.42 | 3.33 | 2.21 | 2.91 | 0.81 | 2.30 | 4.17 | 6.22 | 7.16 | 9.12 | 2.35 | 6.36 |
| 64 | 124.95 | 0.68 | 0.57 | 228.59 | 202.83 | 87.66 | 9.28 | 1.33 | 2.13 | 22.96 | 2.48 | 1.00 | 1.53 | 3.01 | 0.66 | 1.16 | 7.12 | 9.46 | 4.78 | 4.87 | 3.93 | 5.62 |
| 65 | 104.63 | 2.29 | 1.25 | 225.62 | 180.75 | 75.83 | 4.72 | 2.01 | 1.12 | 20.01 | 4.01 | 0.97 | 1.30 | 2.31 | 0.61 | 1.53 | 4.50 | 7.84 | 3.27 | 6.02 | 1.98 | 2.74 |
| 66 | 98.54 | 1.03 | 0.83 | 194.78 | 189.89 | 191.67 | 1.47 | 1.22 | 0.48 | 37.62 | 1.03 | 1.46 | 3.11 | 4.39 | 0.28 | 0.13 | 2.11 | 7.67 | 3.06 | 1.07 | 6.16 | 1.00 |
| 67 | 52.47 | 5.15 | 3.28 | 256.80 | 227.74 | 95.79 | 17.32 | 0.31 | 2.72 | 29.79 | 2.47 | 1.00 | 1.48 | 8.12 | 0.30 | 6.02 | 3.31 | 2.06 | 15.76 | 14.16 | 4.24 | 10.08 |
| 68 | 134.36 | 4.82 | 1.68 | 253.67 | 221.58 | 73.33 | 17.93 | 2.18 | 2.31 | 28.98 | 7.14 | 1.04 | 1.71 | 8.53 | 0.53 | 0.35 | 7.33 | 6.19 | 4.37 | 1.36 | 4.22 | 4.21 |
| No | Plum Cultivar | Citric | Maleic | Malic | Pyruvic | Shikimic | Lactic | Propionic | Butiric | Quinic | Oxalic | Fumaric |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Admiral Rigny | 0.87 | 1.33 | 27.30 | 0.90 | 0.18 | 0.14 | 0.002 | 0.36 | 8.74 | 0.13 | 0.24 |
| 2 | Reine Claude d’Althanns | 0.20 | 0.78 | 19.92 | 0.78 | 0.08 | 0.08 | 0.002 | 0.07 | 3.27 | 0.18 | 0.01 |
| 3 | Anita | 2.06 | 1.06 | 15.40 | 0.67 | 0.14 | 0.09 | 0.009 | 0.07 | 2.80 | 1.18 | 0.40 |
| 4 | Avalon | 1.13 | 1.52 | 26.90 | 1.53 | 0.12 | 0.13 | 0.003 | 0.10 | 2.90 | 1.11 | 0.65 |
| 5 | Bleue de Belgique | 1.78 | 1.16 | 29.63 | 1.16 | 0.15 | 0.10 | 0.001 | 0.09 | 4.51 | 0.05 | 0.38 |
| 6 | Czar | 0.40 | 0.86 | 21.93 | 0.86 | 0.09 | 0.08 | 0.001 | 0.08 | 3.81 | 0.25 | 0.05 |
| 7 | Diana | 1.12 | 1.09 | 28.00 | 1.09 | 0.16 | 0.10 | 0.001 | 0.10 | 3.74 | 0.83 | 0.41 |
| 8 | Edda | 0.76 | 0.68 | 17.45 | 0.68 | 0.08 | 0.08 | 0.001 | 0.07 | 3.13 | 0.18 | 0.06 |
| 9 | Edwards | 1.30 | 1.14 | 29.15 | 1.14 | 0.13 | 0.09 | 0.002 | 0.09 | 4.72 | 0.81 | 0.26 |
| 10 | Excalibur | 0.70 | 0.92 | 23.52 | 0.92 | 0.16 | 0.12 | 0.002 | 0.11 | 7.77 | 0.06 | 0.27 |
| 11 | Frostaplomme | 0.78 | 0.56 | 7.26 | 0.56 | 0.13 | 0.13 | 0.003 | 0.12 | 8.55 | 9.69 | 1.10 |
| 12 | Grand Duke | 0.95 | 1.03 | 26.63 | 0.99 | 0.18 | 0.13 | 0.004 | 0.24 | 9.06 | 0.11 | 0.30 |
| 13 | Haganta | 1.04 | 0.65 | 16.68 | 0.65 | 0.09 | 0.08 | 0.002 | 0.07 | 3.15 | 0.16 | 0.11 |
| 14 | Helgøyplomme | 1.03 | 2.33 | 31.59 | 1.99 | 0.37 | 0.24 | 0.004 | 0.26 | 5.33 | 1.65 | 0.25 |
| 15 | Herman | 0.13 | 1.64 | 14.06 | 1.64 | 0.14 | 0.10 | 0.001 | 0.10 | 2.35 | 5.25 | 2.25 |
| 16 | Jefferson | 0.79 | 1.13 | 23.65 | 1.86 | 0.24 | 0.19 | 0.003 | 0.15 | 4.26 | 1.15 | 0.27 |
| 17 | Jubileum | 1.30 | 0.68 | 17.36 | 0.68 | 0.11 | 0.10 | 0.003 | 0.09 | 3.85 | 0.99 | 0.13 |
| 18 | Kirkes | 1.36 | 1.63 | 31.26 | 1.11 | 0.20 | 0.14 | 0.002 | 0.14 | 4.22 | 1.11 | 0.65 |
| 19 | Mallard | 0.25 | 1.06 | 27.05 | 1.06 | 0.11 | 0.10 | 0.003 | 0.10 | 3.85 | 1.91 | 0.82 |
| 20 | Mount Royal | 1.19 | 0.94 | 24.17 | 0.94 | 0.17 | 0.10 | 0.001 | 0.10 | 4.31 | 0.12 | 0.44 |
| 21 | Njøs II | 1.27 | 0.69 | 17.70 | 0.69 | 0.09 | 0.08 | 0.002 | 0.08 | 2.62 | 0.09 | 0.10 |
| 22 | Ontario | 1.07 | 1.65 | 28.96 | 1.03 | 0.19 | 0.22 | 0.001 | 0.42 | 8.96 | 0.14 | 0.27 |
| 23 | Opal | 1.25 | 0.41 | 10.39 | 0.41 | 0.08 | 0.08 | 0.002 | 0.08 | 3.02 | 0.09 | 0.03 |
| 24 | Reine Claude d’Oullins ‘Henjum | 1.00 | 1.56 | 26.90 | 2.63 | 0.26 | 0.22 | 0.002 | 0.17 | 5.00 | 1.13 | 0.13 |
| 25 | Prosser 84 | 1.05 | 0.26 | 6.70 | 0.26 | 0.09 | 0.08 | 0.001 | 0.07 | 3.69 | 0.08 | 0.07 |
| 26 | R5 | 0.71 | 0.41 | 10.41 | 0.41 | 0.11 | 0.10 | 0.003 | 0.10 | 6.85 | 10.19 | 1.16 |
| 27 | Raud Eplevik | 0.73 | 0.96 | 25.63 | 0.94 | 0.17 | 0.13 | 0.003 | 0.22 | 7.21 | 0.07 | 0.29 |
| 28 | Reeves | 1.47 | 0.70 | 17.84 | 0.70 | 0.11 | 0.09 | 0.002 | 0.09 | 2.61 | 1.13 | 0.14 |
| 29 | Reine Claude Althanns | 0.27 | 1.05 | 16.90 | 1.05 | 0.10 | 0.11 | 0.003 | 0.10 | 4.41 | 4.25 | 1.75 |
| 30 | Reine Claude Noire | 0.62 | 1.06 | 27.23 | 1.06 | 0.12 | 0.10 | 0.001 | 0.10 | 2.28 | 1.06 | 0.17 |
| 31 | Reine Claude Souffriau | 2.56 | 1.32 | 19.18 | 0.83 | 0.17 | 0.12 | 0.011 | 0.08 | 3.48 | 1.47 | 0.50 |
| 32 | Rives Early Prolific | 1.25 | 1.16 | 24.22 | 1.21 | 0.13 | 0.11 | 0.001 | 0.09 | 5.36 | 0.21 | 0.20 |
| 33 | Rød Victoria | 0.53 | 0.59 | 15.03 | 0.59 | 0.09 | 0.08 | 0.002 | 0.07 | 5.14 | 0.09 | 0.11 |
| 34 | Ruth Gerstetter | 0.97 | 1.36 | 29.65 | 1.36 | 0.25 | 0.09 | 0.002 | 0.10 | 3.65 | 1.36 | 0.45 |
| 35 | Sanctus Hubertus | 0.86 | 1.01 | 27.11 | 1.06 | 0.16 | 0.10 | 0.001 | 0.09 | 4.09 | 0.88 | 0.34 |
| 36 | Sviske frå Tveit | 0.89 | 1.44 | 18.95 | 1.44 | 0.27 | 0.10 | 0.001 | 0.10 | 6.93 | 0.11 | 1.07 |
| 37 | Thames Cross | 1.33 | 1.79 | 23.85 | 1.45 | 0.24 | 0.27 | 0.002 | 0.31 | 4.22 | 2.26 | 1.09 |
| 38 | Valor | 1.40 | 0.95 | 24.25 | 0.95 | 0.11 | 0.09 | 0.001 | 0.08 | 3.98 | 1.09 | 0.19 |
| 39 | Victoria | 1.69 | 0.91 | 23.38 | 0.91 | 0.14 | 0.10 | 0.001 | 0.09 | 4.22 | 0.56 | 0.29 |
| 40 | Vinterplomme | 0.73 | 0.73 | 9.67 | 0.73 | 0.10 | 0.10 | 0.003 | 0.09 | 6.77 | 10.17 | 0.63 |
| 41 | Washington | 0.85 | 1.59 | 19.54 | 1.24 | 0.24 | 0.22 | 0.001 | 0.10 | 5.24 | 1.00 | 0.54 |
| 42 | Yakima | 0.84 | 0.80 | 21.33 | 0.85 | 0.23 | 0.12 | 0.003 | 0.13 | 4.52 | 0.13 | 0.16 |
| 43 | Edda | 0.30 | 0.88 | 22.79 | 0.90 | 0.14 | 0.10 | 0.002 | 0.09 | 3.80 | 0.18 | 0.12 |
| 44 | Jubileum | 1.37 | 0.74 | 18.55 | 0.73 | 0.12 | 0.11 | 0.003 | 0.10 | 4.11 | 0.11 | 0.14 |
| 45 | Čačanska lepotica | 1.05 | 2.23 | 32.52 | 2.18 | 0.16 | 0.11 | 0.001 | 0.10 | 5.50 | 0.41 | 0.30 |
| 46 | Mallard | 0.24 | 0.89 | 23.69 | 0.92 | 0.30 | 0.08 | 0.002 | 0.08 | 3.38 | 0.17 | 0.07 |
| 47 | Opal | 0.16 | 0.83 | 23.78 | 0.85 | 0.11 | 0.09 | 0.001 | 0.08 | 4.00 | 0.28 | 0.12 |
| 48 | Reeves | 1.46 | 0.69 | 17.68 | 0.69 | 0.10 | 0.09 | 0.002 | 0.08 | 2.59 | 0.11 | 0.13 |
| 49 | Valor | 1.54 | 0.98 | 25.38 | 0.98 | 0.12 | 0.09 | 0.001 | 0.09 | 4.14 | 0.71 | 0.20 |
| 50 | Victoria | 1.78 | 1.03 | 24.90 | 1.10 | 0.14 | 0.11 | 0.002 | 0.10 | 4.60 | 1.92 | 0.32 |
| 51 | Blue Rock | 1.62 | 1.03 | 26.44 | 1.03 | 0.13 | 0.08 | 0.001 | 0.08 | 3.98 | 0.04 | 0.33 |
| 52 | Czar | 0.61 | 0.92 | 23.71 | 0.92 | 0.10 | 0.09 | 0.001 | 0.08 | 3.35 | 0.17 | 0.14 |
| 53 | Diamond | 1.21 | 0.88 | 22.53 | 0.88 | 0.15 | 0.08 | 0.001 | 0.07 | 3.70 | 0.08 | 0.46 |
| 54 | Edwards | 0.97 | 1.06 | 27.12 | 1.06 | 0.14 | 0.09 | 0.001 | 0.09 | 5.98 | 0.16 | 0.34 |
| 55 | Emil | 0.50 | 0.68 | 17.40 | 0.68 | 0.13 | 0.09 | 0.001 | 0.08 | 5.89 | 0.12 | 0.27 |
| 56 | Excalibur | 0.50 | 0.66 | 17.05 | 0.66 | 0.11 | 0.09 | 0.0005 | 0.08 | 5.52 | 0.04 | 0.20 |
| 57 | Experimentalfältets sviskon | 1.47 | 1.16 | 29.80 | 1.16 | 0.12 | 0.08 | 0.001 | 0.07 | 4.32 | 0.07 | 0.29 |
| 58 | Herman | 0.09 | 1.22 | 31.39 | 1.22 | 0.10 | 0.08 | 0.001 | 0.07 | 1.74 | 0.04 | 0.19 |
| 59 | Ive | 1.21 | 1.00 | 25.81 | 1.00 | 0.13 | 0.08 | 0.001 | 0.08 | 1.64 | 0.07 | 0.27 |
| 60 | Mount Royal | 0.99 | 0.79 | 20.08 | 0.79 | 0.14 | 0.08 | 0.0004 | 0.08 | 3.55 | 0.10 | 0.37 |
| 61 | Reine Claude Noir | 0.52 | 0.61 | 15.61 | 0.61 | 0.10 | 0.08 | 0.001 | 0.08 | 4.01 | 0.10 | 0.10 |
| 62 | Reine Claude d’Oullins | 0.98 | 0.24 | 19.67 | 0.24 | 0.08 | 0.07 | 0.0003 | 0.07 | 3.45 | 0.08 | 0.07 |
| 63 | Reine Claude Souffriau | 0.95 | 0.85 | 21.78 | 0.85 | 0.11 | 0.08 | 0.001 | 0.07 | 3.98 | 0.22 | 0.20 |
| 64 | Rivers Early Prolific | 0.79 | 1.19 | 30.36 | 1.19 | 0.11 | 0.09 | 0.001 | 0.08 | 5.97 | 0.22 | 0.20 |
| 65 | Sinikka | 0.68 | 0.94 | 24.16 | 0.94 | 0.14 | 0.09 | 0.001 | 0.08 | 6.17 | 0.20 | 0.37 |
| 66 | Søgne | 1.64 | 1.00 | 25.55 | 1.00 | 0.12 | 0.09 | 0.001 | 0.08 | 6.02 | 0.09 | 0.25 |
| 67 | Sviske frå Tveit | 0.80 | 1.18 | 30.21 | 1.18 | 0.20 | 0.10 | 0.001 | 0.09 | 5.04 | 0.18 | 0.66 |
| 68 | Traneplommer | 0.74 | 0.95 | 24.37 | 0.95 | 0.14 | 0.08 | 0.002 | 0.08 | 3.96 | 0.14 | 0.40 |
| No | PA | 5COA | pHBA | CA | R | pCA | Q3Og | I3Or | FA | I3Og | Q3Or | K7Og | Pz | Q | Pt | Ac | SA | Ae | K3Og | SUM | TPC | RSA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NF | 8.87 | NF | 2.98 | 18.15 | NF | 7.66 | NF | NF | NF | NF | NF | NF | 6.50 | NF | NF | NF | 1.38 | 0.84 | 46.38 | 12.79 | 75.29 |
| 2 | 1.08 | 161.84 | 4.40 | 3.36 | 107.73 | 1.46 | 11.50 | 5.15 | 3.99 | 0.19 | NF | 0.99 | 0.95 | 1.99 | 0.18 | 0.17 | NF | NF | NF | 305.00 | 10.37 | 80.68 |
| 3 | NF | 2.22 | NF | 5.79 | 2.40 | NF | 3.09 | NF | NF | NF | NF | NF | NF | 6.67 | NF | NF | NF | 0.45 | 0.22 | 20.84 | 8.25 | 78.48 |
| 4 | NF | 0.57 | NF | 4.90 | 1.52 | NF | 0.86 | NF | NF | NF | NF | NF | NF | 7.22 | NF | NF | NF | 1.52 | 0.02 | 16.61 | 11.82 | 48.15 |
| 5 | NF | 15.08 | NF | 7.67 | 19.51 | NF | 7.61 | NF | NF | NF | NF | NF | NF | 8.64 | NF | NF | NF | 5.28 | 0.79 | 64.58 | 5.76 | 34.97 |
| 6 | 2.28 | 43.28 | 3.51 | 3.92 | 35.21 | 1.43 | 7.76 | 35.92 | 3.30 | 4.93 | 1.20 | 1.14 | 0.54 | 6.82 | NF | 0.25 | NF | 1.12 | 0.46 | 153.08 | 10.79 | 40.18 |
| 7 | NF | 0.55 | NF | NF | 1.54 | NF | 0.91 | NF | NF | NF | NF | NF | NF | 7.66 | NF | NF | NF | 0.76 | 0.14 | 11.56 | 8.81 | 32.78 |
| 8 | 2.07 | 118.94 | 2.85 | 3.52 | 122.38 | 0.24 | 20.02 | 3.75 | 2.72 | 0.13 | 0 | 1.04 | 0.96 | 3.10 | 0.30 | 0.15 | NF | NF | NF | 282.19 | 8.70 | 40.61 |
| 9 | NF | 2.42 | NF | 1.65 | 4.76 | NF | 2.57 | NF | NF | NF | NF | NF | NF | 8.04 | NF | NF | NF | 0.55 | 0.45 | 20.44 | 3.47 | 12.14 |
| 10 | NF | 1.11 | NF | 1.19 | 3.31 | NF | 4.77 | NF | NF | NF | NF | NF | NF | 5.70 | NF | NF | NF | 0.88 | 0.30 | 17.26 | 5.83 | 71.87 |
| 11 | NF | 0.19 | NF | 0.30 | 0.62 | NF | 2.52 | NF | NF | NF | NF | NF | NF | 7.15 | NF | NF | NF | 0.21 | 0.18 | 11.17 | 6.91 | 82.06 |
| 12 | NF | NF | NF | 0.69 | 0.66 | NF | 0.94 | NF | NF | NF | NF | NF | NF | 6.62 | NF | NF | NF | 0.48 | 0.21 | 9.60 | 6.16 | 21.15 |
| 13 | 1.67 | 90.94 | 4.18 | 6.62 | 60.48 | 2.18 | 13.81 | 3.83 | 2.67 | 0.31 | 0.21 | 0.91 | 0.90 | 2.66 | 0.23 | NF | NF | NF | NF | 191.60 | 7.92 | 30.53 |
| 14 | NF | 3.48 | NF | 2.45 | 9.76 | NF | 5.41 | NF | NF | NF | NF | NF | NF | 9.30 | NF | NF | NF | 1.68 | 1.21 | 33.29 | 6.06 | 16.27 |
| 15 | NF | 0.56 | NF | 0.81 | 0.83 | NF | 1.49 | NF | NF | NF | NF | NF | NF | 9.32 | NF | NF | NF | 0.20 | 0.33 | 13.54 | 6.77 | 27.13 |
| 16 | NF | NF | NF | 1.24 | 2.41 | NF | 0.62 | NF | NF | NF | NF | NF | NF | 7.36 | NF | NF | NF | 0.32 | 0.09 | 12.04 | 10.36 | 72.97 |
| 17 | NF | 2.67 | NF | 1.67 | 2.03 | NF | 0.97 | NF | NF | NF | NF | NF | NF | 7.41 | NF | NF | NF | 1.15 | 0.19 | 16.09 | 9.83 | 62.98 |
| 18 | NF | NF | NF | 1.10 | 2.91 | NF | 1.13 | NF | NF | NF | NF | NF | NF | 8.11 | NF | NF | NF | 0.35 | 0.07 | 13.67 | 8.60 | 30.52 |
| 19 | NF | 0.72 | NF | 2.28 | 2.59 | NF | 1.30 | NF | NF | NF | NF | NF | NF | 8.34 | NF | NF | NF | 1.51 | 0.11 | 16.85 | 6.71 | 37.41 |
| 20 | NF | 1.89 | NF | 2.40 | 4.15 | NF | 5.22 | NF | NF | NF | NF | NF | NF | 5.83 | NF | NF | NF | 1.29 | 0.35 | 21.13 | 11.75 | 23.88 |
| 21 | 1.27 | 129.12 | 2.67 | 3.73 | 42.95 | 1.21 | 3.89 | 2.04 | 1.60 | 0.16 | 0.16 | 0.43 | 0.59 | 2.12 | 0.47 | 0.91 | NF | NF | NF | 193.31 | 7.65 | 24.02 |
| 22 | NF | 0.18 | NF | 0.77 | 0.80 | NF | 0.54 | NF | NF | NF | NF | NF | NF | 5.90 | NF | NF | NF | 0.74 | 0.21 | 9.14 | 9.16 | 35.98 |
| 23 | 2.42 | 93.07 | 8.48 | 3.86 | 21.32 | 1.40 | 2.42 | 3.99 | 3.27 | 0.13 | NF | 0.52 | 0.52 | 1.89 | 0.25 | 0.28 | NF | NF | NF | 143.81 | 12.35 | 46.34 |
| 24 | NF | 0.74 | NF | 2.04 | 3.02 | NF | 1.45 | NF | NF | NF | NF | NF | NF | 6.66 | NF | NF | NF | 0.67 | 0.29 | 14.87 | 16.08 | 32.80 |
| 25 | 1.83 | 95.60 | 5.30 | 5.43 | 99.42 | 1.57 | 9.55 | 10.37 | 3.05 | 0.38 | NF | 0.58 | 1.13 | 2.65 | NF | NF | NF | NF | NF | 236.86 | 14.35 | 45.31 |
| 26 | NF | 0.18 | NF | 1.12 | 1.94 | NF | 1.98 | NF | NF | NF | NF | NF | NF | 7.98 | NF | NF | NF | 0.32 | 0.15 | 13.67 | 8.81 | 27.01 |
| 27 | NF | 2.61 | NF | 0.46 | 6.50 | NF | 2.45 | NF | NF | NF | NF | NF | NF | 6.71 | NF | NF | NF | 0.60 | 0.26 | 19.59 | 8.48 | 44.44 |
| 28 | NF | 1.37 | NF | 1.85 | 8.20 | NF | 1.49 | NF | NF | NF | NF | NF | NF | 8.37 | NF | NF | NF | 1.13 | 0.34 | 22.75 | 5.78 | 103.52 |
| 29 | NF | 1.59 | NF | 1.13 | 0.96 | NF | 0.73 | NF | NF | NF | NF | NF | NF | 6.08 | NF | NF | NF | 0.80 | 0.17 | 11.46 | 3.90 | 58.18 |
| 30 | NF | 0.81 | NF | 1.38 | 10.50 | NF | 7.12 | NF | NF | NF | NF | NF | NF | 7.83 | NF | NF | NF | 0.77 | 0.50 | 28.91 | 10.55 | 35.39 |
| 31 | NF | 0.78 | NF | 2.29 | 1.90 | NF | 2.17 | NF | NF | NF | NF | NF | NF | 7.31 | NF | NF | NF | NF | 0.09 | 14.54 | 7.96 | 47.36 |
| 32 | 12.82 | 44.16 | NF | 4.14 | 77.65 | 1.50 | 22.35 | NF | NF | NF | NF | NF | NF | 5.86 | NF | NF | 9.46 | 2.06 | 0.34 | 180.33 | 10.28 | 25.66 |
| 33 | 2.63 | 69.76 | 3.55 | 4.46 | 95.17 | 0.83 | 9.40 | 2.30 | 2.19 | 0.12 | NF | 0.48 | 0.38 | 2.10 | 0.21 | 0.24 | NF | NF | NF | 193.84 | 7.10 | 21.93 |
| 34 | NF | NF | NF | 0.48 | 0.57 | NF | 1.08 | NF | NF | NF | NF | NF | NF | 6.84 | NF | NF | NF | 0.22 | 0.28 | 9.47 | 6.99 | 57.39 |
| 35 | 7.15 | 82.85 | NF | 4.45 | 59.73 | 0.54 | 8.93 | NF | NF | NF | NF | NF | NF | 6.09 | NF | NF | 6.23 | 2.16 | 0.26 | 178.38 | 5.81 | 31.93 |
| 36 | NF | 1.16 | NF | 1.09 | 2.92 | NF | 3.90 | NF | NF | NF | NF | NF | NF | 6.88 | NF | NF | NF | 1.34 | 0.33 | 17.62 | 6.36 | 30.11 |
| 37 | NF | NF | NF | 1.03 | 2.05 | NF | 0.73 | NF | NF | NF | NF | NF | NF | 7.14 | NF | NF | NF | 0.89 | 0.13 | 11.97 | 9.55 | 41.57 |
| 38 | NF | 1.91 | NF | 1.59 | 5.81 | NF | 3.81 | NF | NF | NF | NF | NF | NF | 9.69 | NF | NF | NF | 1.57 | 0.30 | 24.68 | 7.50 | 24.29 |
| 39 | 0.79 | 57.14 | 7.46 | 4.13 | 42.47 | 1.70 | 3.17 | 4.64 | 2.32 | 0.02 | NF | 0.61 | 0.53 | 6.00 | 0.21 | 0.18 | NF | 2.38 | 0.59 | 134.34 | 7.80 | 27.86 |
| 40 | NF | 7.98 | NF | 3.68 | 7.17 | NF | 5.44 | NF | NF | NF | NF | NF | NF | 16.47 | NF | NF | NF | 1.99 | 1.05 | 43.78 | 7.84 | 24.01 |
| 41 | NF | 1.00 | NF | 1.50 | 0.88 | NF | 0.24 | NF | NF | NF | NF | NF | NF | 5.76 | NF | NF | NF | 0.47 | 0.14 | 9.99 | 17.09 | 45.98 |
| 42 | NF | NF | NF | 0.64 | 0.78 | NF | 0.33 | NF | NF | NF | NF | NF | NF | 6.00 | NF | NF | NF | 0.37 | 0.12 | 8.24 | 6.76 | 43.09 |
| 43 | 4.63 | 16.19 | 1.63 | 1.25 | 17.98 | 0.48 | 5.78 | 1.73 | NF | NF | 0.18 | 0.48 | 1.40 | 6.81 | 0.25 | 0.21 | NF | 0.53 | 0.58 | 60.11 | 6.82 | 30.01 |
| 44 | 2.75 | 22.49 | 3.05 | 1.65 | 14.89 | 4.72 | 1.13 | 27.68 | NF | 1.13 | 0.75 | 0.98 | 1.05 | 5.30 | 0.25 | 0.71 | NF | 0.65 | 0.03 | 89.22 | 20.59 | 36.66 |
| 45 | 26.69 | 16.35 | 3.17 | 1.24 | 18.77 | 4.03 | 3.23 | 11.84 | NF | 0.47 | 0.28 | 1.73 | 1.03 | 5.51 | 0.22 | 0.14 | NF | 0.41 | 0.14 | 95.26 | 6.49 | 18.11 |
| 46 | 8.46 | 13.50 | 5.47 | 1.07 | 7.37 | 1.10 | 0.58 | 1.08 | NF | NF | 0.14 | 0.16 | 0.75 | 4.59 | 0.25 | 0.29 | NF | 0.58 | 0.04 | 45.43 | 10.89 | 39.13 |
| 47 | 173.61 | 16.08 | 11.49 | 1.04 | 9.03 | 4.09 | 3.33 | 6.24 | NF | 0.35 | 0.26 | 0.71 | 0.74 | 6.50 | NF | NF | NF | 0.50 | 0.43 | 234.42 | 10.39 | 36.40 |
| 48 | 36.62 | 54.89 | 2.96 | 0.93 | 27.86 | 1.50 | 3.32 | 9.79 | NF | 0.32 | 0.47 | 0.64 | 1.25 | 4.54 | 0.24 | NF | NF | 0.30 | 0.06 | 145.69 | 6.49 | 25.49 |
| 49 | 1.40 | 14.15 | 2.13 | 1.36 | 9.25 | 1.13 | 2.02 | 1.97 | NF | NF | 0.18 | 0.36 | 0.72 | 5.98 | 0.22 | 0.61 | NF | 0.61 | 0.05 | 42.14 | 4.77 | 36.39 |
| 50 | 7.57 | 31.25 | 8.74 | 3.58 | 33.75 | 3.43 | 2.02 | 7.14 | 2.41 | 0.24 | 0.35 | 0.69 | 0.57 | 5.14 | 0.22 | 0.31 | 11.03 | 1.72 | 0.13 | 120.29 | 8.65 | 26.65 |
| 51 | 6.67 | 84.75 | 6.81 | 7.68 | 82.73 | 2.99 | 15.26 | 11.02 | 2.74 | 0.74 | NF | 0.70 | 0.46 | 3.56 | NF | NF | 9.40 | 6.16 | NF | 241.67 | 7.89 | 39.08 |
| 52 | 10.71 | 67.19 | 6.74 | 7.98 | 74.73 | 3.69 | 13.06 | 18.60 | 2.70 | 2.59 | 0.56 | 0.90 | 0.55 | 4.70 | NF | 0.23 | 14.81 | 5.88 | NF | 235.61 | 11.17 | 41.32 |
| 53 | 3.92 | 137.53 | 10.47 | 8.01 | 132.82 | 12.35 | 15.31 | 28.94 | 4.06 | 1.29 | 0.19 | 0.62 | 0.87 | 26.01 | 0.20 | 0.53 | NF | NF | NF | 383.11 | 6.46 | 47.61 |
| 54 | 8.61 | 42.88 | 20.77 | 5.76 | 80.88 | 6.50 | 7.05 | 3.65 | 2.92 | NF | NF | 1.13 | 0.59 | 4.01 | 0.19 | 0.28 | 12.62 | 3.67 | NF | 201.52 | 7.84 | 30.51 |
| 55 | 6.61 | 74.23 | 5.09 | 3.93 | 27.18 | 2.96 | 2.89 | 0.80 | 3.23 | NF | 0.23 | 0.33 | 2.42 | 3.11 | 0.23 | 0.27 | 6.32 | 2.89 | NF | 142.70 | 13.84 | 58.48 |
| 56 | 4.43 | 74.43 | 4.23 | 3.38 | 59.47 | 2.44 | 14.93 | 3.42 | 2.13 | 0.48 | NF | 0.78 | 0.52 | 2.72 | NF | NF | 6.25 | 0.94 | NF | 180.57 | 10.86 | 40.26 |
| 57 | 2.06 | 18.33 | 14.29 | 1.21 | 34.62 | 0.22 | 12.08 | 4.30 | 1.82 | 0.51 | NF | 0.46 | 0.29 | 3.38 | 0.19 | 0.25 | NF | NF | NF | 94.02 | 4.62 | 28.99 |
| 58 | 24.16 | 106.11 | 5.96 | 3.46 | 89.38 | 1.54 | 18.03 | 10.01 | 2.78 | 2.93 | 1.45 | 1.51 | 0.82 | 3.96 | 0.19 | 0.37 | NF | NF | NF | 272.65 | 10.73 | 54.49 |
| 59 | 2.80 | 56.65 | 4.60 | 5.37 | 28.98 | 2.34 | 6.34 | 11.03 | 4.30 | 2.43 | NF | 1.02 | 1.06 | 3.17 | 0.19 | 0.45 | 5.97 | 2.73 | NF | 139.43 | 4.86 | 42.70 |
| 60 | 5.79 | 106.21 | 6.98 | 4.20 | 74.01 | 2.73 | 20.99 | 3.44 | 3.71 | 0.50 | 0.15 | 1.11 | 0.93 | 2.75 | 0.19 | NF | 7.54 | 2.20 | NF | 243.43 | 7.15 | 35.19 |
| 61 | 5.35 | 32.75 | 1.66 | 2.29 | 48.32 | 0.86 | 7.82 | 3.72 | 0.79 | 0.28 | NF | 0.33 | 0.31 | 2.99 | 0.10 | NF | 7.13 | 0.89 | NF | 115.61 | 5.44 | 35.07 |
| 62 | 7.99 | 46.83 | NF | 6.84 | 19.20 | 2.34 | 2.11 | NF | NF | NF | NF | NF | NF | 1.97 | NF | NF | 8.07 | 2.54 | NF | 97.89 | 4.30 | 22.33 |
| 63 | 2.77 | 34.67 | 5.47 | 1.36 | 41.15 | 0.86 | 7.75 | 6.37 | 1.69 | 0.48 | 0.16 | 0.44 | 1.19 | 3.01 | 0.18 | 0.12 | NF | NF | NF | 107.69 | 7.59 | 32.50 |
| 64 | 11.95 | 51.32 | 9.38 | 2.51 | 36.06 | 1.69 | 11.61 | 6.65 | 2.39 | 1.63 | NF | 0.47 | 0.48 | 3.18 | NF | NF | 11.62 | 3.60 | NF | 154.54 | 6.35 | 25.84 |
| 65 | 7.72 | 77.38 | 6.75 | 13.13 | 79.10 | 17.56 | 11.89 | 9.71 | 4.89 | 3.27 | 7.62 | 0.71 | 1.53 | 13.71 | 0.19 | 0.55 | 11.20 | 7.48 | NF | 274.39 | 8.26 | 25.54 |
| 66 | 7.28 | 112.14 | 3.78 | 10.06 | 44.72 | 1.80 | 8.36 | 4.67 | 1.71 | 0.38 | NF | 0.61 | 0.52 | 2.92 | 0.21 | NF | 14.00 | 7.34 | NF | 220.50 | 12.36 | 33.28 |
| 67 | 4.30 | 21.08 | 17.38 | 2.24 | 15.39 | 4.27 | 2.41 | 8.88 | 1.77 | 0.64 | NF | 0.42 | 0.98 | 2.93 | 0.20 | NF | 5.55 | 1.38 | NF | 89.83 | 4.58 | 39.63 |
| 68 | 4.61 | 49.17 | 16.27 | 5.33 | 76.34 | 2.63 | 14.25 | 16.32 | 4.04 | 1.11 | 19.06 | 3.98 | 102.16 | 5.53 | 0.83 | 10.02 | NF | NF | NF | 331.65 | 5.52 | 23.81 |
References
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.E.E.; Morgan, D.R.; Kerr, M.; Robertson, K.R.; Arsenault, M.; Dickinson, T.A.; et al. Phylogeny and classification of Rosaceae. Plant Syst. Evol. 2007, 266, 5–43. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N. Chapter 5: Plum (Prunus spp.) Breeding. In Advances in Plant Breeding Strategies: Fruits; Al-Khayri, J.M., Jain, S., Johanson, D., Eds.; Springer: Edinburgh, UK, 2018; pp. 165–215. [Google Scholar] [CrossRef]
- Hartmann, W.; Neumüller, M. Plum breeding. In Breeding Plantation Tree Crops: Temperate Species, 1st ed.; Jain, S.M., Priyadarshan, P.M., Eds.; Springer Science Business Publishing: New York, NJ, USA, 2009; pp. 161–231. [Google Scholar]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World, 4th ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- FAOStat. 2020. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 26 November 2020).
- Abanoz, Y.Y.; Okcu, Z. Biochemical content of cherry laurel (Prunus laurocerasus L.) fruits with edible coatings based on caseinat, Semperfresh and lecithin. Turk. J. Agric. For. 2022, 46, 908–918. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 144, 529–534. [Google Scholar] [CrossRef]
- Gill, S.K.; Lever, E.; Emery, P.W.; Whelan, K. Nutrient, fibre, sorbitol and chlorogenic acid content of prunes (Prunus domestica): An updated analysis and comparison of different countries of origin and database values. Int. J. Food Sci. Nutr. 2019, 70, 924–931. [Google Scholar] [CrossRef]
- Farcuh, M.; Bosheng, L.; Rivero, R.M.; Shlizerman, L.; Sadka, A.; Blumwald, E. Sugar metabolism reprogramming in a non-climacteric bud mutant of a climacteric plum fruit during development on the tree. J. Exp. Bot. 2017, 68, 5813–5828. [Google Scholar] [CrossRef]
- Kim, H.Y.; Farcuh, M.; Cohen, Y.; Crisosto, C.; Sadka, A.; Blumwald, E. Non-climacteric ripening and sorbitol homeostasis in plum fruits. Plant Sci. 2015, 231, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Drkenda, P.; Music, O.; Oras, A.; Haracic, S.; Haseljic, S.; Blanke, M.; Hudina, M. Sugar, Acid and Phenols in Fruit of the Sharka-Tolerant Autochthonous Plum Genotype ‘Mrkosljiva’. Erwerbs-Obstbau 2022, 64, 569–580. [Google Scholar] [CrossRef]
- Walkowiak-Tomczak, J.; Reguła, J.; Łysiak, G. Physico-chemical properties and antioxidant activity of selected plum cultivars fruit. Acta Sci. Pol. Technol. Aliment. 2008, 7, 15–22. [Google Scholar]
- Rampáčková, E.; Göttingerová, M.; Kiss, T.; Ondrášek, I.; Venuta, R.; Wolf, J.; Nečas, T.; Ercisli, S. CIELAB analysis and quantitative correlation of total anthocyanin content in European and Asian plums. Eur. J. Hortic. Sci. 2021, 86, 453–460. [Google Scholar] [CrossRef]
- Sahamishirazi, S.; Moehring, J.; Claupein, W.; Graeff-Hoenninger, S. Quality assessment of 178 cultivars of plum regarding phenolic, anthocyanin and sugar content. Food Chem. 2017, 214, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.M.; Williams, A.A. The flavour of plums (Prunus domestica L.). An examination of the aroma components of plum juice from the cultivar victoria. J. Agric. Food Chem. 1981, 32, 613–619. [Google Scholar] [CrossRef]
- Lenchyk, L.V. Determination of content of flavonoids, hydroxycinnamic acids and volatile compounds in plum leaves. Int. J. Adv. Pharm. Biol. Chem. 2016, 5, 131–136. [Google Scholar]
- Radulović, N.S.; Đorđević, A.S.; Zlatković, B.K.; Palić, R.M. GC–MS analyses of flower ether extracts of Prunus domestica L. and Prunus padus L. (Rosaceae). Chem. Pap. 2009, 63, 377–384. [Google Scholar] [CrossRef]
- Groh, B.; Bauer, H.; Treutter, D. Chemotaxonomical investigations of Prunus domestica by isoenzyme markers and phenolic compounds. Sci. Hort. 1994, 58, 41–55. [Google Scholar] [CrossRef]
- Parmar, V.S.; Vardhan, A.; Nagarajan, G.R.; Jain, R. Dihydroflavonols from Prunus domestica. Phytochemistry 1992, 31, 2185–2186. [Google Scholar] [CrossRef]
- Ortega-Vidal, J.; Cobo, A.; Ortega-Morente, E.; Gálvez, A.; Martínez-Bailén, M.; Salido, S.; Altarejos, J. Antimicrobial activity of phenolics isolated from the pruning wood residue of European plum (Prunus domestica L.). Ind. Crops Prod. 2022, 176, 114296. [Google Scholar] [CrossRef]
- Islam, N.U.; Amin, R.; Shahid, M.; Amin, M.; Zaib, S.; Iqbal, J. A multi-target therapeutic potential of Prunus domestica gum stabilized nanoparticles exhibited prospective anticancer, antibacterial, urease-inhibition, anti-inflammatory and analgesic properties. BMC Complement. Altern. Med. 2017, 17, 276. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Mesarović, J.; Gašić, U.; Trifković, J.; Milatović, D.; Meland, M. Determination of phenolic profile in kernels of different plum cultivars. Acta Hortic. 2019, 1260, 229–234. [Google Scholar] [CrossRef]
- Slimestad, R.; Vangal, E.; Brede, C. Analysis of Phenolic Compounds in Six Norwegian Plum Cultivars (Prunus domestica L.). J. Agric. Food Chem. 2009, 57, 11370–11375. [Google Scholar] [CrossRef] [PubMed]
- Fanning, K.J.; Topp, B.; Russell, D.; Stanley, R.; Netzel, M. Japanese plums (Prunus salicina Lindl.) and phytochemi.cals–breeding, horticultural practice, postharvest storage, processing and bioactivity. J. Sci. Food Agric. 2014, 94, 2137–2147. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Yilmaz, B.; Pateiro, M.; Kumar, M.; Domínguez, M.A.S.; Hano, C.; Lorenzo, J.M. Valorization of by-products from Prunus genus fruit processing: Opportunities and applications. Crit. Rev. Food Sci. Nutr. 2022; 1–16, Online ahead of print. [Google Scholar] [CrossRef]
- Birwal, P.; Deshmukh, G.; Saurabh, S.P.; Pragati, S. Plums: A Brief Introduction. J. Food Nutr. Popul. Health 2017, 1, 1–5. [Google Scholar]
- Tomić, J.; Štampar, F.; Glišić, I.; Jakopič, J. Phytochemical assessment of plum (Prunus domestica L.) cultivars selected in Serbia. Food Chem. 2019, 299, 125113. [Google Scholar] [CrossRef] [PubMed]
- Murcia, M.A.; Jiménez, A.M.; Martínez-Tomé, M. Evaluation of the antioxidant properties of Mediterranean and tropical fruits compared with common food additives. J. Food Protect. 2001, 64, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.E.; Singh, D.P.; Clingeleffer, P.R. Micronutrient mineral and folate content of Australian and imported dried fruit products. Crit. Rev. Food Sci. Nutr. 2010, 51, 38–49. [Google Scholar] [CrossRef]
- Karasawa, K.; Miyashita, R.; Otani, H. Anti-allergic properties of a fruit extract of prune (Prunus domestica L.) in mite-sensitized BALB/c mice. Food Sci. Technol. Res. 2012, 18, 755–760. [Google Scholar] [CrossRef]
- Igwe, E.; Charlton, K. A systematic review on the health effects of plums (Prunus domestica and Prunus salicina). Phytother. Res. 2016, 30, 701–731. [Google Scholar] [CrossRef]
- Arion, C.M.; Tabart, J.; Kevers, C.; Niculaua, M.; Filimon, R.; Beceanu, D.; Dommes, J. Antioxidant potential of different plum cultivars during storage. Food Chem. 2014, 146, 485–491. [Google Scholar] [CrossRef]
- Murathan, Z.T.; Arslan, M.; Erbil, N. Analyzing Biological Properties of Some Plum Genotypes Grown in Turkey. Int. J. Fruit Sci. 2020, 20 (Suppl. S3), S1729–S1740. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Ansary, A.E.; Mostafa, M.A.; Kamel, T.A.; Safwat, G. Evaluation of the fitochemical, antioxidant, antibacterial and anticancer activity of Prunus domestica fruit. Not. Bot. Horti. Agrobo. 2019, 47, 395–404. [Google Scholar] [CrossRef]
- Belhadj, F.; Marzouki, M.N. Antioxidant, antihemolitic and antibacterial effects of dried and fresh Prunus domestica L. Int. J. Pharm. Res. Bio. Sci. 2014, 3, 191–207. [Google Scholar]
- Miljić, U.; Puškaš, V.; Cvetković, D.; Velićanski, A.; Vujić, J. Chemical composition and in vitro antimicrobial and cytotoxic activities of plum (Prunus domestica L.) wine. J. Inst. Brew. 2016, 122, 342–349. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Nešović, M.; Ćirić, I.; Tešić, Ž.; Pezo, L.; Tosti, T.; Gašić, U.; Dojčinović, B.; Lončar, B.; Meland, M. Polyphenolics and Chemical Profiles of Domestic Norwegian Apple (Malus × domestica Borkh.) Cultivars. Front. Nutr. 2022, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fotirić Akšić, M.; Nešović, M.; Ćirić, I.; Tešić, Ž.; Pezo, L.; Tosti, T.; Gašić, U.; Dojčinović, B.; Lončar, B.; Meland, M. Chemical composition of different domestic raspberries from Norway. Horticulturae 2022, 8, 765. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N. Factors influencing mineral composition of plum fruits. J. Elem. 2012, 17, 453–464. [Google Scholar] [CrossRef]
- Çalişir, S.; Hacýseferoðullarý, H.; Özcan, M.; Arslan, D. Some nutritional and technological properties of wild plum (Prunus spp.) fruits in Turkey. J. Food Eng. 2005, 66, 233–237. [Google Scholar] [CrossRef]
- Motyleva, S.; Upadysheva, G.; Tumaeva, T. Influence of rootstocks on the productivity and chemical composition of Prunus domestica L. fruits. Potr. S. J. F. Sci. 2021, 15, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Jaroszewska, A. Quality of fruit cherry, peach and plum cultivated under different water and fertilization regimes. J. Elementol. 2011, 16, 51–58. [Google Scholar]
- Nergiz, C.; Yildiz, H. Research on chemical composition of some varieties of European plums (Prunus domestica) adapted to the Aegean district of Turkey. J. Agr. Food Chem. 1997, 45, 2820–2823. [Google Scholar] [CrossRef]
- Stacewicz–Sapuntzakis, M.; Bowen, P.E.; Hussain, E.A.; Damayanti-Wood, B.I.; Farnsworth, N.R. Chemical composition and potential health effects of prunes: A functional food? Crit. Rev. Food Sci. Nutr. 2001, 41, 251–286. [Google Scholar] [CrossRef]
- Rato, E.A.; Agulheiro, C.A.; Barroso, M.J.; Riquelme, F. Soil and rootstock influence on fruit quality of plums (Prunus domestica L.). Sci. Hort. 2008, 118, 218–222. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Lever, E.; Scott, S.M.; Louis, P.; Emery, P.W.; Whelan, K. The effects of prunes on stool output, gut transit time and gastrointestinal microbiota: A randomized controlled trial. Clin. Nutr. 2019, 38, 165–173. [Google Scholar] [CrossRef]
- Sheet, B.S.; Artik, N.; Ayed, M.; Fawzi, O.A. Some Alternative Sweeteners (Xylitol, Sorbitol, Sucralose and Stevia): Review. Karaelmas Sci. Eng. J. 2014, 4, 63–70. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar signaling during fruit ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef]
- Woznicki, T.L.; Heide, O.M.; Sønsteby, A.; Måge, F.; Remberg, S.F. Climate warming enhances flower formation, earliness of blooming and fruit size in plum (Prunus domestica L.) in the cool Nordic environment. Sci. Hortic. 2019, 257, 108750. [Google Scholar] [CrossRef]
- Kang, H.K.; Kang, H.R.; Lee, Y.S.; Song, H.S. Characteristics of organic acid contents and fermentation solution of Prunus mume in South Korea. Korean J. Plant Res. 2020, 33, 194–199. [Google Scholar] [CrossRef]
- Mertoğglu, K.; Gülbandilar, A.; Bulduk, İ. Growing conditions effect on fruit phytochemical composition and anti-microbial activity of plum (cv. Black Diamond). Int. J. Agric. For. Life Sci. 2020, 4, 56–61. [Google Scholar]
- Berüter, J. Carbohydrate metabolism in two apple genotypes that differ in malate accumulation. J. Plant Physiol. 2004, 161, 1011–1029. [Google Scholar] [CrossRef]
- Vangdal, E.; Flatland, S.; Nordbø, R. Fruit quality changes during marketing of new plum cultivars (Prunus domestica L.). Hort. Sci. 2007, 34, 91–95. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Okulevičiūtė, R.; Lanauskas, J.; Kviklys, D.; Zymonė, K.; Rendyuk, T.; Žvikas, V.; Uselis, N.; Janulis, V. Variability in the content of phenolic compounds in plum fruit. Plants 2020, 9, 1611. [Google Scholar] [CrossRef]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Polyphenolic characterization and antioxidant activity of Malus domestica and Prunus domestica cultivars from Costa Rica. Foods 2018, 7, 15. [Google Scholar] [CrossRef]
- Aitchison, J. Principal component analysis of compositional data. Biometrika 1983, 1, 57–65. [Google Scholar] [CrossRef]
- Aitchison, J. Reducing the dimensionality of compositional data sets. Math. Geol. 1984, 16, 617–635. [Google Scholar] [CrossRef]
- Reig, G.; Font i Forcada, C.; Mestre, L.; Jiménez, S.; Betrán, J.A.; Moreno, M.Á. Horticultural, leaf mineral and fruit quality traits of two ‘Greengage’ plum cultivars budded on plum based rootstocks in Mediterranean conditions. Scientia Horticulturae 2018, 232, 84–91. [Google Scholar] [CrossRef]
- Dugalic, K.; Sudar, R.; Viljevac, M.; Josipovic, M.; Cupic, T. Sorbitol and Sugar Composition in Plum Fruits Influenced by Climatic Conditions. J. Agric. Sci. Tech. 2014, 16, 1145–1155. [Google Scholar]
- Yu, X.M.; Rizwan, H.M.; Li, P.; Luo, S.X.; Sherameti, I.; Wu, W.F.; Lin, J.; Zheng, S.X.; Oelmüller, R.; Chen, F.X. Comparative studies on the physiochemical properties, phenolic compounds and antioxidant activities in 13 japanese plum cultivars grown in the subtropical region of China. Appl. Ecol. Environ. Res. 2020, 18, 3147–3159. [Google Scholar] [CrossRef]
- Moriguchi, T.; Ishizawa, Y.; Sanada, T. Differences in Sugar the Classification Composition by the Princi in pal Prunus persica Fruit and Component Analysis. J. Japan Soc. Hort. Sci. 1990, 59, 307–312. [Google Scholar] [CrossRef]
- Bae, H.; Yun, S.K.; Jun, J.H.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H. Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. J. Appl. Bot. Food Qual. 2014, 87, 24–29. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ivanova, V.; Trusheva, B.; Kamenova-Nacheva, M.; Tabakov, S.; Simova, S. Chemical Composition and Antioxidant Capacity of the Fruits of European Plum Cultivar “Čačanska Lepotica” Influenced by Different Rootstocks. Foods 2022, 11, 2844. [Google Scholar] [CrossRef]
- Celik, F.; Gundogdu, M.; Alp, S.; Muradoglu, F.; Ercişli, S.; Gecer, K.M.; Canan, I. Determination of Phenolic Compounds, Antioxidant Capacity and Organic Acids Contents of Prunus domestica L., Prunus cerasifera Ehrh. and Prunus spinosa L. Fruits by HPLC. Acta Chromatogr. 2017, 29, 507–510. [Google Scholar] [CrossRef]
- Stojanov, D.; Milošević, T.; Mašković, P.; Milošević, N. Impact of fertilization on the antioxidant activity and mineral composition of red raspberry berries of cv. ‘Meeker’. Mitt. Klosterneubg. Rebe Und Wein Obstbau Und Früchteverwertung 2019, 69, 184–195. [Google Scholar]
- de Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Fotirić Akšić, M.; Tosti, T.; Nedić, N.; Marković, M.; Ličina, V.; Milojković Opsenica, D.; Tešić, Ž. Influence of frost damage on the sugars and sugar alcohol composition in quince (Cydonia oblonga Mill) Floral nectar. Acta Physiol. Plant 2015, 37, 1701. [Google Scholar] [CrossRef]
- Carbone, K.; Giannini, B.; Picchi, V.; Lo Scalzo, R.; Cecchini, F. Phenolic composition and free radical scavenging activity of different apple varieties in relation to the cultivar, tissue type and storage. Food Chem. 2011, 127, 493–500. [Google Scholar] [CrossRef]





| Njøs Fruit and Berry Centre | NIBIO Ullensvang | NMBU | |||||
|---|---|---|---|---|---|---|---|
| 1 | Admiral Rigny | 22 | Ontario | 43 | Edda * | 51 | Blue Rock |
| 2 | Reine Claude d’Althanns | 23 | Opal * | 44 | Jubileum * | 52 | Czar # |
| 3 | Anita | 24 | Reine Claude d’Oullins ‘Henjum | 45 | Čačanska lepotica | 53 | Diamond |
| 4 | Avalon | 25 | Prosser 84 | 46 | Mallard * | 54 | Edwards # |
| 5 | Bleue de Belgique | 26 | R5 | 47 | Opal * | 55 | Emil |
| 6 | Czar # | 27 | Raud Eplevik | 48 | Reeves * | 56 | Excalibur # |
| 7 | Diana | 28 | Reeves * | 49 | Valor * | 57 | Experimentalfältets sviskon |
| 8 | Edda * | 29 | Reine Claude Althanns | 50 | Victoria * | 58 | Herman # |
| 9 | Edwards # | 30 | Reine Claude Noire # | 59 | Ive | ||
| 10 | Excalibur # | 31 | Reine Claude Souffriau # | 60 | Mount Royal # | ||
| 11 | Frostaplomme | 32 | Rivers Early Prolific # | 61 | Reine Claude Noire # | ||
| 12 | Grand Duke | 33 | Rød Victoria | 62 | Reine Claude d’Oullins | ||
| 13 | Haganta | 34 | Ruth Gerstetter | 63 | Reine Claude Souffriau # | ||
| 14 | Helgøyplomme | 35 | Sanctus Hubertus | 64 | Rivers Early Prolific # | ||
| 15 | Herman # | 36 | Sviske frå Tveit # | 65 | Sinikka | ||
| 16 | Jefferson | 37 | Thames Cross | 66 | Søgne | ||
| 17 | Jubileum * | 38 | Valor * | 67 | Sviske frå Tveit # | ||
| 18 | Kirkes | 39 | Victoria * | 68 | Traneplommer | ||
| 19 | Mallard * | 40 | Vinterplomme | ||||
| 20 | Mount Royal # | 41 | Washington | ||||
| 21 | Njøs II | 42 | Yakima | ||||
| Norwegian Area | Njøs | NIBIO Ullensvang | NMBU | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD |
| Al | 2.65 | 35.52 | 9.80 | 6.24 | 4.73 | 24.38 | 14.22 | 6.77 | 5.38 | 42.77 | 15.23 | 11.33 |
| B | 0.68 | 15.56 | 5.70 | 4.01 | 0.56 | 6.58 | 2.21 | 2.21 | 4.15 | 12.70 | 7.56 | 2.47 |
| Ca | 42.70 | 482.97 | 172.50 | 109.02 | 120.27 | 540.67 | 356.06 | 130.79 | 56.71 | 280.22 | 111.19 | 54.02 |
| Cu | 1.50 | 6.23 | 3.26 | 1.03 | 1.44 | 5.81 | 3.80 | 1.40 | 1.28 | 2.96 | 2.27 | 0.48 |
| Fe | 2.58 | 41.26 | 12.31 | 10.76 | 3.17 | 54.72 | 22.97 | 15.42 | 2.98 | 13.78 | 7.60 | 2.44 |
| K | 1503.75 | 11,545.75 | 4177.48 | 2626.83 | 2745.22 | 11,696.62 | 8055.80 | 3287.93 | 1562.42 | 3118.25 | 2194.73 | 413.38 |
| Mg | 76.76 | 513.84 | 173.49 | 96.55 | 121.49 | 547.76 | 367.31 | 144.64 | 63.57 | 137.99 | 99.70 | 21.18 |
| Mn | 0.69 | 9.42 | 2.86 | 2.51 | 1.79 | 9.48 | 5.77 | 2.75 | 0.64 | 1.52 | 1.10 | 0.26 |
| Na | 0.50 | 101.24 | 42.86 | 27.82 | 37.23 | 101.33 | 72.07 | 20.49 | 15.35 | 50.10 | 28.09 | 9.63 |
| P | 4563.32 | 10,225.25 | 7054.28 | 1436.21 | 3312.55 | 8540.89 | 6394.22 | 1885.68 | 5581.36 | 12,198.01 | 7878.88 | 2025.18 |
| S | 124.00 | 367.56 | 252.83 | 63.44 | 74.26 | 404.39 | 242.07 | 117.90 | 130.50 | 385.43 | 203.98 | 61.07 |
| Zn | 2.71 | 24.60 | 8.41 | 4.02 | 2.56 | 11.70 | 6.92 | 3.06 | 4.90 | 23.99 | 8.94 | 4.12 |
| N (%) | 2.68 | 3.65 | 3.10 | 0.17 | 2.95 | 3.21 | 3.11 | 0.09 | 2.96 | 3.29 | 3.07 | 0.08 |
| Norwegian Area | Njøs | NIBIO Ullensvang | NMBU | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD |
| Sorbitol | 4.16 | 164.54 | 81.98 | 31.42 | 67.79 | 114.32 | 92.53 | 16.19 | 52.47 | 144.01 | 98.02 | 25.12 |
| Trehalose | 0.10 | 9.70 | 1.59 | 1.79 | 0.29 | 34.66 | 7.09 | 11.60 | 0.32 | 5.15 | 1.88 | 1.47 |
| Arabinose | 0.05 | 3.34 | 0.94 | 0.70 | 0.37 | 1.51 | 0.76 | 0.40 | 0.30 | 3.28 | 1.08 | 0.68 |
| Glukose | 156.69 | 349.79 | 229.91 | 41.90 | 223.43 | 278.16 | 244.46 | 18.73 | 138.26 | 319.13 | 222.53 | 43.44 |
| Fructose | 60.95 | 275.64 | 173.66 | 34.07 | 150.04 | 244.51 | 192.98 | 28.43 | 142.18 | 285.38 | 197.92 | 37.17 |
| Sucrose | 76.78 | 227.29 | 157.38 | 42.28 | 97.03 | 279.02 | 208.25 | 58.47 | 67.43 | 233.16 | 136.52 | 57.38 |
| Turanose | 2.31 | 31.55 | 13.09 | 6.96 | 6.75 | 29.90 | 19.22 | 9.04 | 1.47 | 29.30 | 12.33 | 6.77 |
| Glycerol | 0.25 | 7.20 | 1.17 | 1.12 | 0.22 | 2.15 | 1.27 | 0.64 | 0.24 | 2.18 | 0.90 | 0.58 |
| Galactitol | 0.75 | 6.85 | 2.63 | 1.23 | 1.54 | 9.80 | 3.88 | 2.67 | 0.48 | 7.62 | 2.42 | 1.66 |
| Galactose | 16.17 | 39.07 | 27.92 | 6.89 | 20.48 | 45.58 | 33.80 | 8.06 | 11.65 | 37.62 | 24.86 | 7.23 |
| Ribose | 0.60 | 11.82 | 4.89 | 2.76 | 2.44 | 13.12 | 8.73 | 3.88 | 0.42 | 7.98 | 3.39 | 2.41 |
| Isomaltose | 0.26 | 4.66 | 1.73 | 1.11 | 1.17 | 3.68 | 2.27 | 0.78 | 0.22 | 3.50 | 1.47 | 1.00 |
| Isomaltotriose | 0.23 | 4.48 | 2.14 | 0.99 | 0.62 | 4.65 | 3.01 | 1.23 | 0.21 | 3.42 | 1.93 | 0.91 |
| Maltose | 1.56 | 32.12 | 10.78 | 8.51 | 2.86 | 37.54 | 18.26 | 12.91 | 1.12 | 12.87 | 5.58 | 3.36 |
| Maltotriose | 0.17 | 1.59 | 0.53 | 0.28 | 0.33 | 1.33 | 0.69 | 0.32 | 0.12 | 0.81 | 0.36 | 0.20 |
| Mannitol | 0.07 | 7.81 | 2.08 | 1.66 | 1.21 | 3.70 | 2.37 | 0.84 | 0.06 | 6.28 | 1.94 | 2.07 |
| Xylose | 0.24 | 12.23 | 5.03 | 2.11 | 2.50 | 11.56 | 6.82 | 3.01 | 2.11 | 13.36 | 5.46 | 2.78 |
| Melibiose | 1.96 | 10.46 | 5.97 | 1.92 | 4.58 | 7.11 | 5.97 | 0.99 | 2.06 | 9.46 | 5.78 | 2.11 |
| Panose | 0.04 | 20.56 | 3.55 | 3.94 | 0.17 | 6.66 | 2.00 | 2.23 | 1.33 | 15.76 | 6.17 | 4.36 |
| Rhamnose | 0.93 | 18.51 | 5.20 | 3.46 | 2.24 | 9.51 | 5.02 | 2.41 | 1.07 | 14.68 | 5.78 | 4.59 |
| Raffinose | 0.53 | 4.34 | 2.02 | 0.90 | 1.54 | 3.38 | 2.54 | 0.61 | 1.96 | 6.55 | 3.55 | 1.40 |
| Stachyose | 0.33 | 10.37 | 1.87 | 1.62 | 0.87 | 3.98 | 1.71 | 0.98 | 1.00 | 11.17 | 4.19 | 2.97 |
| Sum of sugars | 435.13 | 757.08 | 648.21 | 71.87 | 694.56 | 830.55 | 763.56 | 46.99 | 532.55 | 760.83 | 640.77 | 65.22 |
| Sum of sugar alcohols | 15.45 | 168.85 | 87.85 | 31.04 | 72.75 | 128.03 | 100.05 | 17.61 | 61.52 | 147.83 | 103.29 | 24.02 |
| Norwegian Area | Njøs | NIBIO Ullensvang | NMBU | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD |
| Citric | 0.13 | 2.56 | 1.01 | 0.48 | 0.16 | 1.78 | 0.99 | 0.66 | 0.09 | 1.64 | 0.90 | 0.41 |
| Maleic | 0.26 | 2.33 | 1.06 | 0.42 | 0.69 | 2.23 | 1.03 | 0.50 | 0.24 | 1.22 | 0.91 | 0.25 |
| Malic | 6.70 | 31.59 | 21.52 | 6.67 | 17.68 | 32.52 | 23.66 | 4.56 | 15.61 | 31.39 | 24.06 | 4.80 |
| Pyruvic | 0.26 | 2.63 | 1.02 | 0.45 | 0.69 | 2.18 | 1.04 | 0.48 | 0.24 | 1.22 | 0.91 | 0.25 |
| Shikimic | 0.08 | 0.37 | 0.15 | 0.06 | 0.10 | 0.30 | 0.15 | 0.06 | 0.08 | 0.20 | 0.12 | 0.03 |
| Lactic | 0.08 | 0.27 | 0.12 | 0.05 | 0.08 | 0.11 | 0.10 | 0.01 | 0.07 | 0.10 | 0.08 | 0.01 |
| Propionic | 0.001 | 0.01 | 0.002 | 0.002 | 0.001 | 0.003 | 0.002 | 0.001 | 0.0003 | 0.002 | 0.001 | 0.001 |
| Butiric | 0.07 | 0.42 | 0.13 | 0.08 | 0.08 | 0.10 | 0.09 | 0.01 | 0.07 | 0.09 | 0.08 | 0.01 |
| Quinic | 2.28 | 9.06 | 4.73 | 1.88 | 2.59 | 5.50 | 4.01 | 0.85 | 1.64 | 6.17 | 4.35 | 1.40 |
| Oxalic | 0.05 | 10.19 | 1.53 | 2.60 | 0.11 | 1.92 | 0.49 | 0.61 | 0.04 | 0.22 | 0.12 | 0.06 |
| Fumaric | 0.01 | 2.25 | 0.44 | 0.47 | 0.07 | 0.32 | 0.18 | 0.09 | 0.07 | 0.66 | 0.28 | 0.14 |
| Norwegian Area | Njøs | NIBIO Ullensvang | NMBU | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD |
| Protocatechuic acid | 0 | 12.82 | 0.86 | 2.30 | 1.40 | 173.61 | 32.71 | 58.30 | 2.06 | 24.16 | 7.10 | 5.03 |
| 5-O-Caffeoylquinic acid | 0 | 161.84 | 24.94 | 43.11 | 13.50 | 54.89 | 23.11 | 14.10 | 18.33 | 137.53 | 66.31 | 33.39 |
| p-Hydroxybenzoic acid | 0 | 8.48 | 1.01 | 2.15 | 1.63 | 11.49 | 4.83 | 3.53 | 0.00 | 20.77 | 8.15 | 5.63 |
| Caffeic acid | 0 | 7.67 | 2.52 | 1.85 | 0.93 | 3.58 | 1.51 | 0.86 | 1.21 | 13.13 | 5.26 | 3.21 |
| Rutin | 0.57 | 122.38 | 21.33 | 33.59 | 7.37 | 33.75 | 17.36 | 9.43 | 15.39 | 132.82 | 58.06 | 30.42 |
| p-Coumaric acid | 0 | 2.18 | 0.33 | 0.63 | 0.48 | 4.72 | 2.56 | 1.67 | 0.22 | 17.56 | 3.88 | 4.36 |
| Quercetin 3-O-glucoside | 0.24 | 22.35 | 4.60 | 5.04 | 0.58 | 5.78 | 2.68 | 1.62 | 2.11 | 20.99 | 10.67 | 5.42 |
| Isorhamnetin 3-O-rutinoside | 0 | 35.92 | 1.71 | 5.79 | 1.08 | 27.68 | 8.43 | 8.71 | 0 | 28.94 | 8.42 | 7.14 |
| Ferulic acid | 0 | 3.99 | 0.60 | 1.20 | 0 | 2.41 | 0.30 | 0.85 | 0 | 4.89 | 2.65 | 1.27 |
| Isorhamnetin 3-O-glucoside | 0 | 4.93 | 0.15 | 0.76 | 0 | 1.13 | 0.31 | 0.38 | 0 | 3.27 | 1.07 | 1.06 |
| Quercetin 3-O-rhamnoside | 0 | 1.20 | 0.04 | 0.19 | 0.14 | 0.75 | 0.33 | 0.20 | 0 | 19.06 | 1.63 | 4.70 |
| Kaempferol 7-O-glucoside | 0 | 1.14 | 0.16 | 0.33 | 0.16 | 1.73 | 0.72 | 0.48 | 0 | 3.98 | 0.86 | 0.86 |
| Phlorizin | 0 | 1.13 | 0.15 | 0.32 | 0.57 | 1.40 | 0.94 | 0.29 | 0 | 102.16 | 6.43 | 23.90 |
| Quercetin | 1.89 | 16.47 | 6.59 | 2.59 | 4.54 | 6.81 | 5.55 | 0.83 | 1.97 | 26.01 | 5.20 | 5.79 |
| Phloretin | 0 | 0.47 | 0.04 | 0.11 | 0 | 0.25 | 0.21 | 0.08 | 0 | 0.83 | 0.17 | 0.19 |
| Acacetin | 0 | 0.91 | 0.05 | 0.16 | 0 | 0.71 | 0.28 | 0.26 | 0 | 10.02 | 0.73 | 2.33 |
| Syringic acid | 0 | 9.46 | 0.37 | 1.73 | 0 | 11.03 | 1.38 | 3.90 | 0 | 14.81 | 6.69 | 5.06 |
| Aesculetin | 0 | 5.28 | 0.90 | 0.96 | 0.30 | 1.72 | 0.66 | 0.44 | 0 | 7.48 | 2.65 | 2.58 |
| Kaempferol 3-O-glucoside | 0 | 1.21 | 0.27 | 0.28 | 0.03 | 0.58 | 0.18 | 0.21 | 0 | 0 | 0 | 0 |
| Sum of phenolic compounds | 8.24 | 305.00 | 66.63 | 85.11 | 42.14 | 234.42 | 104.07 | 63.80 | 89.83 | 383.11 | 195.93 | 85.93 |
| TPC (g GAE/kg) | 3.47 | 17.09 | 8.71 | 2.93 | 4.77 | 20.59 | 9.39 | 4.99 | 4.30 | 13.84 | 7.77 | 4.99 |
| RSA (mol TE/kg) | 12.14 | 103.52 | 42.72 | 20.74 | 18.11 | 39.13 | 31.10 | 7.30 | 22.33 | 58.48 | 36.48 | 7.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotirić Akšić, M.; Tešić, Ž.; Kalaba, M.; Ćirić, I.; Pezo, L.; Lončar, B.; Gašić, U.; Dojčinović, B.; Tosti, T.; Meland, M. Breakthrough Analysis of Chemical Composition and Applied Chemometrics of European Plum Cultivars Grown in Norway. Horticulturae 2023, 9, 477. https://doi.org/10.3390/horticulturae9040477
Fotirić Akšić M, Tešić Ž, Kalaba M, Ćirić I, Pezo L, Lončar B, Gašić U, Dojčinović B, Tosti T, Meland M. Breakthrough Analysis of Chemical Composition and Applied Chemometrics of European Plum Cultivars Grown in Norway. Horticulturae. 2023; 9(4):477. https://doi.org/10.3390/horticulturae9040477
Chicago/Turabian StyleFotirić Akšić, Milica, Živoslav Tešić, Milica Kalaba, Ivanka Ćirić, Lato Pezo, Biljana Lončar, Uroš Gašić, Biljana Dojčinović, Tomislav Tosti, and Mekjell Meland. 2023. "Breakthrough Analysis of Chemical Composition and Applied Chemometrics of European Plum Cultivars Grown in Norway" Horticulturae 9, no. 4: 477. https://doi.org/10.3390/horticulturae9040477
APA StyleFotirić Akšić, M., Tešić, Ž., Kalaba, M., Ćirić, I., Pezo, L., Lončar, B., Gašić, U., Dojčinović, B., Tosti, T., & Meland, M. (2023). Breakthrough Analysis of Chemical Composition and Applied Chemometrics of European Plum Cultivars Grown in Norway. Horticulturae, 9(4), 477. https://doi.org/10.3390/horticulturae9040477












