Survival and State of Garlic Explants of Two Lithuanian Cultivars after Cryopreservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
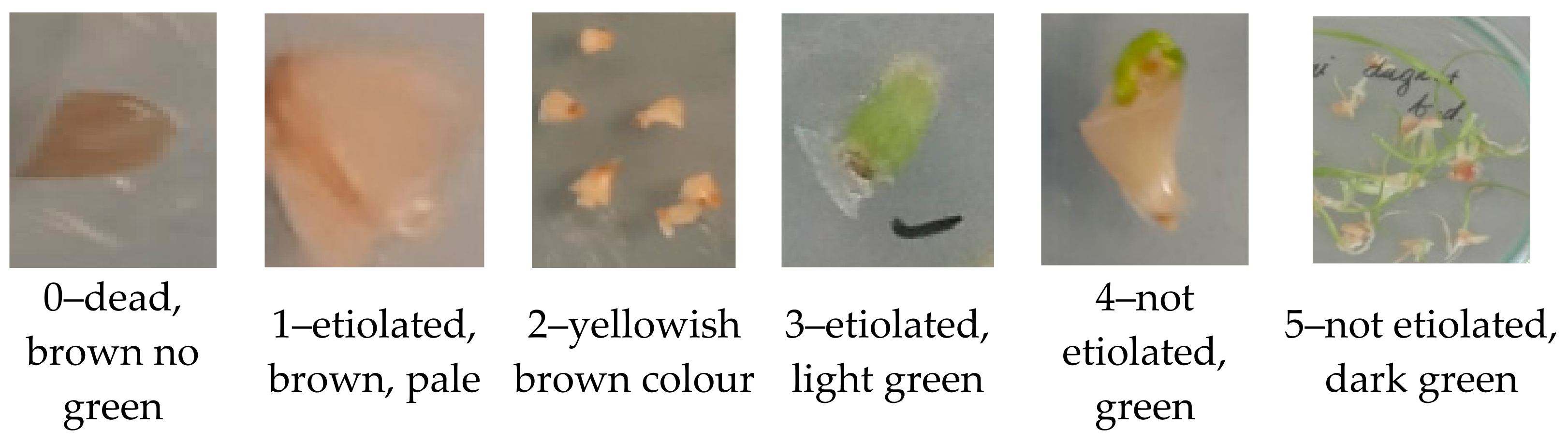
2.2. Assessment of Cryopreservation Efficiency
2.3. Data Analysis
3. Results and Discussion
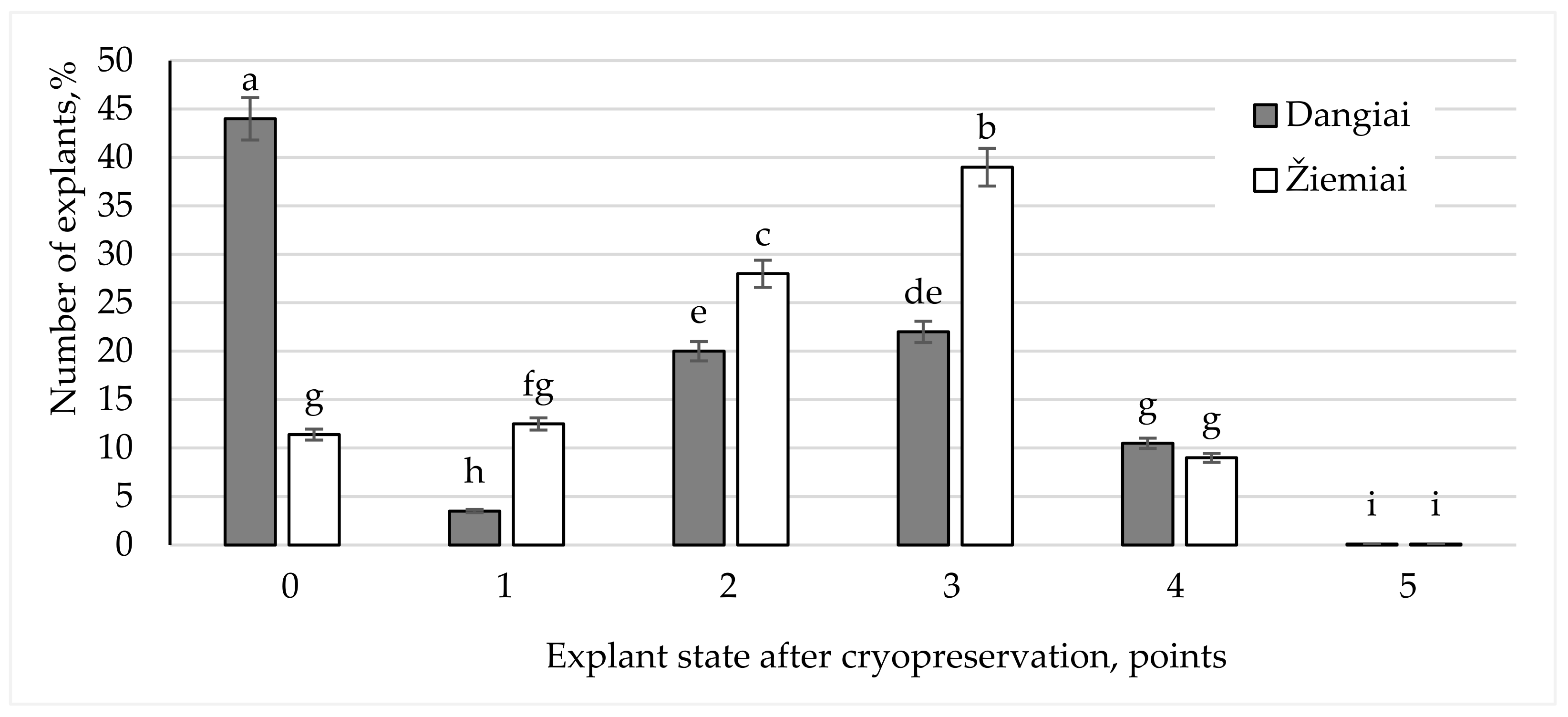
3.1. Influence of Genotype on Survival Rate of Explants
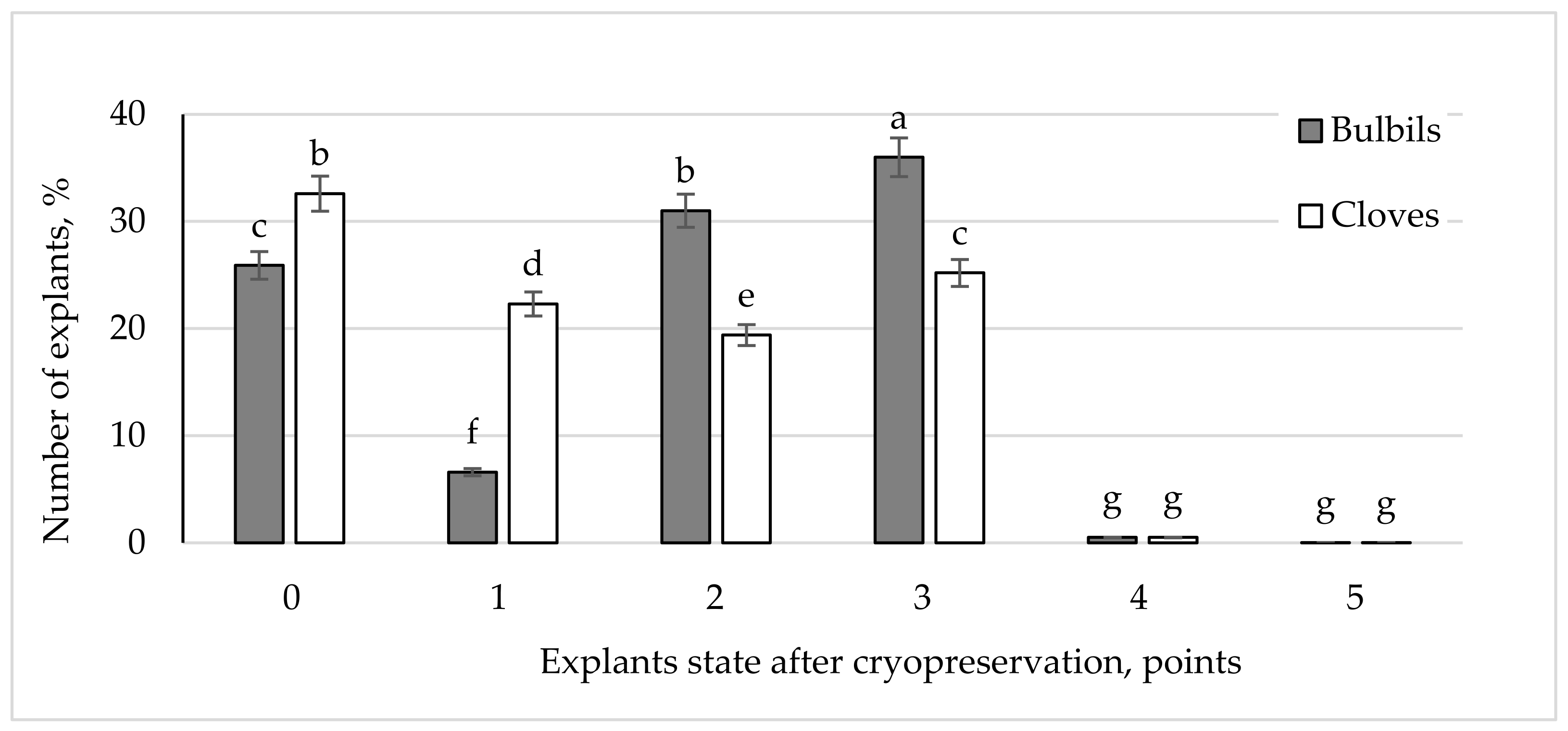
3.2. Influence of Explant Type on the Survival Rate
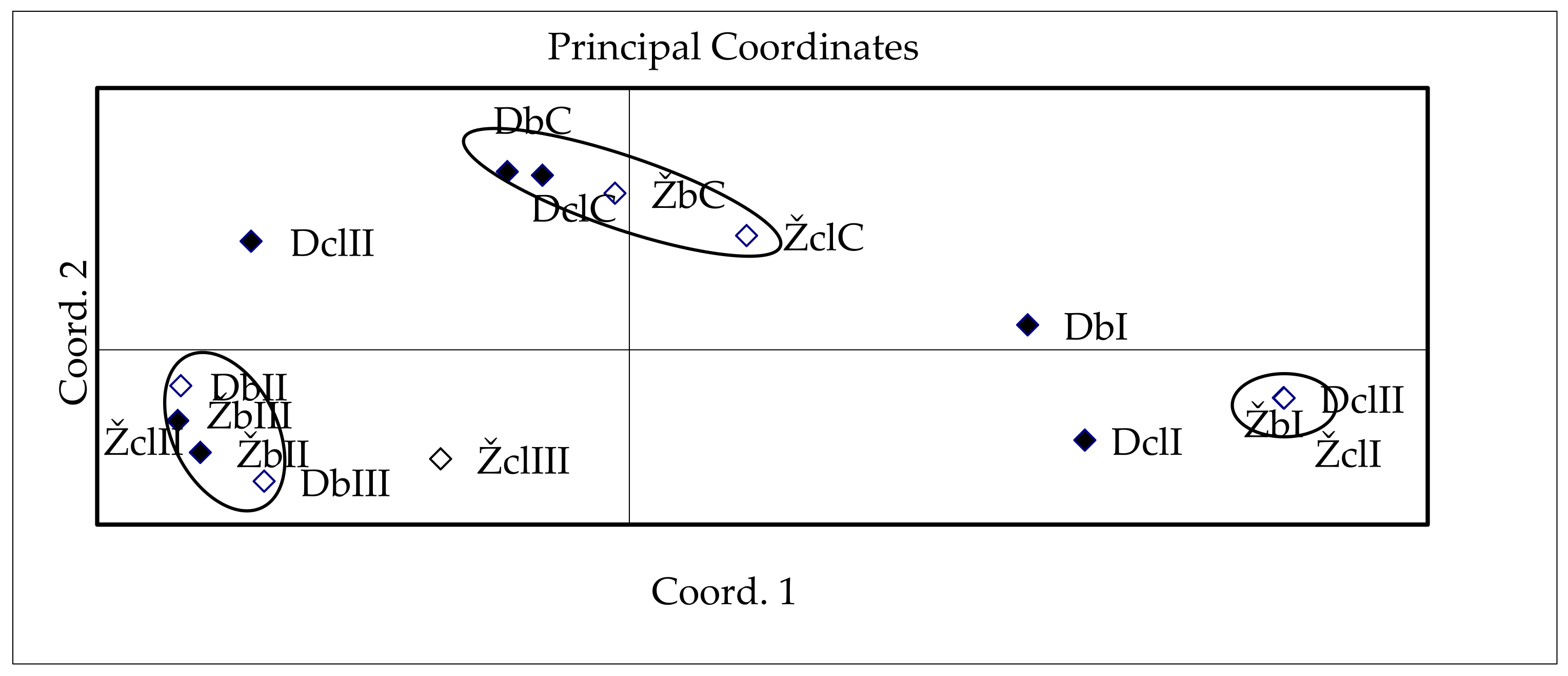
3.3. Influence of Dehydration Conditions on the Survival Rate of Explants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maggioni, L.; Keller, J.; Astley, D. German collections of vegetatively propagated Allium. In Report of Workshop; International Plant Genetic Resources Institute: Rome, Italy; Gatersleben, Germany, 2002; pp. 34–35. [Google Scholar]
- Waterer, D.; Schmitz, D. Influence of variety and cultural practices on garlic yields in Saskatchewan. Can. J. Plant Sci. 1994, 74, 611–614. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Shafir, I.L.; Zemah, H.; Barzilay, A.; Rabinowitch, H.D. Environmental control of garlic growth and florogenesis. J. Am. Soc. Hortic. Sci. 2004, 129, 144–151. [Google Scholar] [CrossRef]
- Volk, G.M.; Stern, D. Phenotypic characteristics of ten garlic cultivars grown at different North American locations. HortScience 2009, 44, 1238–1247. [Google Scholar] [CrossRef]
- Juškevičienė, D.; Karklelienė, R.; Radzevičius, A. Biodiversity and productivity of potato onions (Allium cepa var. Aggregatum G. Don). Acta Hortic. 2019, 1251, 91–96. [Google Scholar] [CrossRef]
- Sasnauskas, A.; Bendokas, V.; Karklelienė, R.; Juškevičienė, D.; Šikšnianas, T.; Gelvonauskienė, D.; Rugienius, R.; Baniulis, D.; Sikorskaitė-Gudžiūnienė, S.; Mažeikienė, I.; et al. Breeding Trends of Fruit and Vegetable Crops for Organic Production in Lithuania. Horticulturae 2017, 3, 1. [Google Scholar] [CrossRef]
- Karklelienė, R.; Juškevičienė, D.; Radzevičius, A.; Sasnauskas, A. Productivity and adaptability of the new carrot and garlic cultivars in Lithuania. Zemdirb. Agric. 2018, 105, 165–170. [Google Scholar] [CrossRef]
- Panis, B. Cryopreservation of monocots. In Plants Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 241–280. [Google Scholar]
- Niwata, E. Cryopreservation of apical meristems of garlic (Allium sativum L.) and high subsequent plant regeneration. Cryoletters 1995, 16, 102–107. [Google Scholar]
- Makowska, Z.; Keller, J.; Engelmann, F. Cryopreservation of apices isolated from garlic (Allium sativum L.) bulbils and cloves. Cryoletters 1999, 20, 175–182. [Google Scholar]
- Keller, E.R.J. Improvement of cryopreservation results in garlic using low temperature preculture and high-quality in vitro plantlets. CryoLetters 2005, 26, 357–366. [Google Scholar]
- Baek, H.; Kim, H.H.; Cho, E.G.; Chae, Y.A.; Engelmann, F. Importance of explants size and origin and of preconditioning treatments for cryopreservation of garlic shoot apices by vitrification. CryoLetters 2003, 24, 381–388. [Google Scholar]
- Ellis, D.; Skogerboe, D.; Andre, C.; Hellier, B.; Volk, G. Implementation of garlic cryopreservation techniques in the national plant germplasm system. CryoLetters 2006, 27, 99–106. [Google Scholar] [PubMed]
- Volk, G.; Maness, N.; Rotindo, K. Cryopreservation of garlic (Allium sativum L.) using plant vitrification solution. CryoLetters 2004, 25, 219–226. [Google Scholar] [PubMed]
- Lynch, P.T.; Souch, G.R.; Harding, K. Effects of post-harvest storage of Allium sativum bulbs on the cryopreservation of stem-discs by encapsulation/ dehydration. J. Hortic. Sci. Biotechnol. 2012, 87, 588–592. [Google Scholar] [CrossRef]
- Kim, H.H.; Cho, E.G.; Park, S.U. Analysis of factors affecting the cryopreservation of garlic shoot tips. J. Biomed. Nanotechnol. 2006, 2, 129–132. [Google Scholar] [CrossRef]
- Liu, X.X.; Wen, Y.B.; Cheng, Z.H.; Mou, S.W. Establishment of a garlic cryopreservation protocol for shoot apices from adventitious buds in vitro. Sci. Hortic. 2017, 226, 10–18. [Google Scholar] [CrossRef]
- Xing, X.; Liu, M.; Zhou, R.; Jiang, F.; Bai, Y.; Wie, H.; Zhang, D.; Wie, J.; Wu, Z. Ascorbic acid addition during dehydration improves garlic shoot tip cryopreservation but does not affect viral load. Cryobiology 2022, 107, 64–73. [Google Scholar] [CrossRef]
- Xing, X.; Liu, M.; Jiang, F.; Zhou, R.; Bai, Y.; Wei., H.; Zhang, D.; Wei, J.; Wu, Z. Abscisic acid induces the expression of AsKIN during the recovery period of garlic cryopreservation. Plant Cell Rep. 2022, 41, 1955–1973. [Google Scholar] [CrossRef]
- Lynch, P.T.; Souch, G.R.; Zamecnik, J.; Harding, K. Optimization of Water Content for the Cryopreservation of Allium sativum in vitro Cultures by Encapsulation-Dehydration. CryoLetters 2016, 37, 308–317. [Google Scholar]
- Keller, E. Cryopreservation of Allium sativum L. (garlic). In Cryopreservation of Plant Germplasm II; Biotechnology in Agriculture and Forestry; Towill, L.E., Bajaj, Y.P.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 50, pp. 37–47. [Google Scholar]
- Kim, H.H.; Lee, J.K.; Hwang, H.S.; Engelmann, F. Cryopreservation of garlic germplasm collections using the droplet-vitrification technique. CryoLetters 2007, 28, 471–482. [Google Scholar]
- Senula, A.; Nagel, M. Cryopreservation of plant shoot tips of potato, mint, garlic, and shallot using plant vitrification solution 3. Methods Mol. Biol. 2021, 2180, 647–661. [Google Scholar] [CrossRef]
- Mikuła, A.; Chmielarz, P.; Hazubska-Przybył, T.; Kulus, D.; Maślanka, M.; Pawłowska, B.; Zimnoch-Guzowska, E. Cryopreservation of Plant Tissues in Poland: Research Contributions, Current Status, and Applications. Acta Soc. Bot. Pol. 2022, 91. [Google Scholar] [CrossRef]
- Lukoševičiūtė, V.; Rugienius, R.; Stanienė, G.; Blažytė, A.; Gelvonauskienė, D.; Bendokas, V.; Gelvonauskis, B.; Sasnauskas, A.; Stanys, V.; Bobinas, Č. Low temperature storage of Rosaceae (Fragaria sp. and Pyrus sp.) genetic resources in vitro. Zemdirb. Agric. 2012, 92, 125–130. [Google Scholar]
- Haimi, P.; Vinskienė, J.; Stepulaitienė, I.; Baniulis, D.; Stanienė, G.; Šikšnianienė, J.B.; Rugienius, R. Patterns of low temperature induced accumulation of dehydrins in Rosaceae crops-Evidence for post-translational modification in apple. J. Plant Physiol. 2017, 218, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sasnauskas, S.A.; Tilvikienė, V.; Mašalaitė, R. Scientific Methodologies for Innovative Research in Horticulture; Lithuanian Research Centre for Agriculture and Forestry: Kaunas, Lithuania, 2013; pp. 157–259. (In Lithuanian) [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Zanke, C.; Zamecnik, J.; Kotlińska, T.; Olas, M.; Keller, E.R.J. Cryopreservation of garlic for the establishment of a European core collection. Acta Hortic. 2011, 908_55. [Google Scholar] [CrossRef]
- Kim, H.H.; Cho, E.G.; Baek, H.J.; Kim, C.Y.; Keller, E.R.J.; Engelmann, F. Cryopreservation of garlic shoot tips by vitrification: Effects of dehydration, rewarming, unloading and regrowth conditions. CryoLetters 2004, 26, 59–70. [Google Scholar]
- Warner, R.M.; Higgins, A.Z. Mathematical Modeling of Protectant Transport in Tissues. Methods Mol. Biol. 2021, 2180, 173–188. [Google Scholar] [CrossRef]
- Kulus, D.; Miler, N. Application of plant extracts in micropropagation and cryopreservation of bleeding heart: An ornamental-medicinal plant species. Agriculture 2021, 11, 542. [Google Scholar] [CrossRef]
- Olas-Sochacka, M. Zabezpieczenie zasobów genowych czosnku pospolitego (Allium sativum L.) w kriobanku genów/Preservation of garlic (Allium sativum L.) genetic resources in cryobank. Zesz. Nauk. Inst. Ogrod. 2017, 25, 85–93. (In Polish) [Google Scholar]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. 2021, 144, 21–24. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juškevičienė, D.; Karklelienė, R.; Radzevičius, A.; Rugienius, R. Survival and State of Garlic Explants of Two Lithuanian Cultivars after Cryopreservation. Horticulturae 2023, 9, 476. https://doi.org/10.3390/horticulturae9040476
Juškevičienė D, Karklelienė R, Radzevičius A, Rugienius R. Survival and State of Garlic Explants of Two Lithuanian Cultivars after Cryopreservation. Horticulturae. 2023; 9(4):476. https://doi.org/10.3390/horticulturae9040476
Chicago/Turabian StyleJuškevičienė, Danguolė, Rasa Karklelienė, Audrius Radzevičius, and Rytis Rugienius. 2023. "Survival and State of Garlic Explants of Two Lithuanian Cultivars after Cryopreservation" Horticulturae 9, no. 4: 476. https://doi.org/10.3390/horticulturae9040476
APA StyleJuškevičienė, D., Karklelienė, R., Radzevičius, A., & Rugienius, R. (2023). Survival and State of Garlic Explants of Two Lithuanian Cultivars after Cryopreservation. Horticulturae, 9(4), 476. https://doi.org/10.3390/horticulturae9040476








