Abstract
Catechins are essential phenolic compounds in persimmon. However, the catechin content in the leaves of persimmon germplasms has not been systematically and comprehensively evaluated. To systematically assess catechin variation in different growth stages and astringency types of persimmon leaves, the young and adult leaves catechin content of 249 persimmon germplasms from Korea, Japan, and 16 provinces in China was quantified using an HPLC method. The results showed that the content of (−)-epicatechin (EC) and (−)-gallocatechin gallate (GCG) had higher coefficient of variation (CV) values in persimmon young leaves (106.74%) and adult leaves (91.49%), respectively. The genetic diversity H’ of (+)-catechin (CA) and (+)-gallocatechin (GC) content was higher in young leaves (2.00 and 2.03), and the H’ value of (−)-epigallocatechin (EGC) and CA content was higher in adult leaves (1.98 and 1.92). The non-galloylated catechins, EGC, EC, and CA, were significantly positively correlated with each other in both young and adult leaves. Catechins of persimmon leaves showed different accumulation patterns in different growth stages and astringency types; (−)-epigallocatechin gallate (EGCG) and CA had the highest average content in the persimmon germplasms of Guangxi and Shanxi provinces of China, respectively. The content of (+)-catechin gallate (CG) was found to be significantly higher in Japanese pollination-constant non-astringent (J-PCNA) persimmon leaves, and EC, GCG had the highest levels in Chinese PCNA (C-PCNA) type. The 249 germplasms were classified into five clusters (Cluster I-V) by SOM clustering based on the content of nine catechins, with EC, CA, and GC having the highest content in Cluster V of the adult leaves. In addition, four excellent germplasms of Cluster III had the highest EGC and GC content in young leaves. Thus, the critical period for catechin utilization of persimmon leaves can be further determined, and provide theoretical references for excellent germplasm screening.
1. Introduction
Persimmon (Diospyros kaki Thunb.) is a horticultural plant that belongs to the Ebenaceae family and is one of the essential fruit trees that has a high commercial and medicinal value in Asian countries [1,2]. According to the Food and Agriculture Organization of the United Nations (FAO), persimmon fruit production in China was approximately 3,429,438 tonnes in 2021, which accounted for 79.16% of the total production worldwide. The total production and planting area of persimmon have grown and expanded rapidly in recent years, indicating that persimmon is becoming an important fruit crop around the world [3]. In addition to the substantial economic value of persimmon fruit, persimmon leaves, flowers, skins, roots, and peels have the potential to be used in medicine, especially persimmon leaves, which have significant healthcare value [4].
Persimmon leaves are rich in vitamin C, choline, carotenoids, amino acids, and other beneficial active ingredients [5]. In addition, persimmon leaves also contain zinc, calcium, iron, and other mineral elements that benefit human health [6]. Persimmon leaves have long been used in traditional Chinese medicine [4]. Flavonoids, phenylpropanoids, terpenoids, and other valuable metabolites have been extracted from persimmon leaves, which have antibacterial, antioxidative, hypolipidemic, hemostatic, and antidiabetic properties [5,7]. Proanthocyanidins (PAs) are secondary flavonoid metabolites and important functional components of foods [8]. At present, persimmon PAs are mainly extracted from fruit—the utilization rate of persimmon leaves is low. Han et al. [9] have shown that the average annual soluble tannin content of persimmon leaves is higher than that of fruit in some varieties. Therefore, persimmon leaves can be used as a raw material for extracting proanthocyanidins and reduce waste of fruit resources.
Persimmon PAs have numerous properties, such as relief from snake venom [10] and adsorption of heavy metals [11]. PAs are formed by flavan-3-ols polymerization and can be divided into monomeric PAs and polymeric PAs [12,13]. Monomeric PAs, also known as catechins, are a cluster of polyphenolic bioactive components, including (+)-gallocatechin (GC), (−)-gallocatechin gallate (GCG), (−)-epigallocatechin (EGC), (−)-epigallocatechin gallate (EGCG), (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (+)-catechin (CA), and (+)-catechin gallate (CG) [14,15]. Gallic acid (GA) and catechins are essential polyphenolic compounds in many plants, such as the tea plant (Camellia sinensis) [16,17]. Based on the presentation or not of a galloyl group on the C-ring, the catechins are classified into two categories: galloylated catechins (EGCG, ECG, CG, and GCG) and non-galloylated catechins (GC, EGC, EC, and CA) [18,19]. Catechins are higher in tea leaves, accounting for 12–24% of dry weight and 60–80% of total polyphenols, with EGCG, EGC, ECG, and EC content being higher [20].
Persimmon can be divided into pollination-constant non-astringent (PCNA) and non-PCNA, based on the natural deastringency of mature fruits on trees and their genetic characteristics [21]. PCNA persimmon was divided into Chinese PCNA persimmon (C-PCNA), which is controlled by a dominant allele, and Japanese PCNA persimmon (J-PCNA), which is controlled by a recessive allele [22]. Non-PCNA persimmon can be divided into the following types: pollination-variant non-astringent (PVNA), pollination-variant astringent (PCA), and pollination-variant astringent (PVA) types [23]. Analysis with phloroglucinol in different astringency types of persimmons showed that the PA composition of persimmon during fruit development consisted of six monomers: CA, GC, EC, ECG, EGC, and EGCG [12,24]. The PA composition of persimmon fruit differed between the different astringency types. For example, CA content was found to be higher in the early stage of fruit development, and GA content was found to be significantly higher in PCA compared to PCNA types during fruit development [25]. In the BC1 generation, the content of EGC and EGCG in the non-PCNA persimmon fruit was higher than that in the PCNA persimmon [12]. C-PCNA type persimmon fruit showed a higher content of CA and GA than J-PCNA fruit [25].
Plant germplasm resources are the genetic sources and material basis for genetic improvement [26]. Germplasm resource evaluation is the foundation and premise for breeding and hybridizing [27]. Research on the genetic diversity of germplasm resources is important for resource conservation and utilization [28]. Genetic diversity is characterized by analyzing variations at the DNA level, phenotypes, and physiological characteristics [3,29,30]. In recent years, the genetic diversity in persimmon germplasm resources has been reported, mainly focusing on the phenotypic diversity of leaves and fruits [31,32], the flavonoid and polyphenol content diversity [33], and the ascorbic acid content diversity of leaves [34]. In addition, a variety of molecular markers has been used to investigate genetic diversity and population structure in persimmon germplasm resources [35,36]. Catechins, as monomeric PAs, are essential active ingredients in persimmon leaves. However, catechin composition and content diversity in the leaves of persimmon germplasms have not been evaluated systematically and comprehensively.
This study quantified the young and adult leaf catechin content of 249 persimmon germplasms from Korea, Japan, and 16 provinces and municipalities of China using a high-performance liquid chromatography (HPLC) method. Through Shannon–Weiner diversity, correlation, variance, and clustering analysis, the genetic variation of the catechin content was systematically analyzed in the leaves of persimmon germplasms. Catechin composition and content differences between young and adult leaves could be obtained. Thus, the critical period for the utilization of active component catechins in persimmon leaves can be further determined. This also provides theoretical references for excellent trait exploitation and germplasm screening.
2. Materials and Methods
2.1. Plant Materials
Leaves of 249 persimmon cultivars were collected at the forest planting base of the Research Institute of Non-timber Forestry (Yuanyang County, Henan Province, China, 34°55′18″–34°56′27″ N, 113°46′14″–113°47′35″ E), with each cultivar represented by three individuals. These materials included 206 PCA, 3 PVA, 36 PCNA, and 4 PVNA varieties (Supplementary Materials, Table S1). The astringency type and geographic distribution of samples are shown in Supplementary Materials, Table S1. These 10-year-old cultivars were managed using conventional cultivation measures, with a row spacing of 3 × 4 m. Young and adult leaf samples of each cultivar were collected from the three individuals in March and June, respectively. The third leaves of annual branches were randomly collected from the three clones with each replicate consisting of 20 leaves. Three clones for each germplasm were selected as three biological replicates. Fresh leaves were immediately frozen in liquid nitrogen and stored at −80 °C until the catechin content was determined.
2.2. Instruments and Reagents
Experiments were performed using high-performance liquid chromatography coupled with a 2998 photodiode array (PDA) detector (Waters ACQUITY Arc system, USA), XS204 analytical balance (Mettler Toledo, Switzerland), HT-300 BQ ultrasonic cleaner (Tianhua, Jinan, China), 5430 R high-speed freezing centrifuge (Eppendorf, Germany), and a Milli-Q Integral water purification system (Merck Millipore, USA). Standards of (+)-gallocatechin (GC), (−)-gallocatechin gallate (GCG), (−)-epigallocatechin (EGC), (−)-epigallocatechin gallate (EGCG), (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (+)-catechin (CA), (+)-catechin gallate (CG), and gallic acid (GA), with purity = 98%, were purchased from Beijing Solarbio Science and Technology Co., Ltd. HPLC grade methanol and acetonitrile were purchased from FTSCI Co., Ltd. (Wuhan, China), and formic acid was purchased from Macklin., Ltd. (Shanghai, China).
2.3. Sample Preparation and Chromatographic Conditions
Catechins were extracted and measured in the leaves following the method of Meng et al. [37] and Wang et al. [38], with some modifications. After vacuum freeze-drying, all leaf samples were ground to a fine powder and sieved. The powdered samples were weighed (200.0 mg) and mixed with 80% (v/v) methanol in an ultrasonic bath for 30 min; the leaves: extract ratio was 1:20. The mixture was then centrifuged at 8000 rpm for 5 min after storage at 4 °C for overnight, and the supernatant was filtered through a 0.22 µm membrane and transferred into the injection bottle. Chromatographic separation was performed on a Thermo Syncronis C18 column (250 mm × 4.6 mm, particle size 5 µm). The mobile phase included eluent A, acetonitrile, and eluent B, 0.2% formic acid water. The analysis conditions were as follows: column temperature, 30 °C; injection volume, 5 μL; flow rate, 0.8 mL/min; detection wavelength, 275 nm. The gradient program was 5% A at 0~3 min, 5→17% A at 3~22 min, 17% A at 22~40 min, 17→20% A at 40~42 min, and 20→5% A at 42~43 min. Nine catechins were identified by comparing the retention time to the corresponding standard, and their concentrations were determined according to the external standard method (Supplementary Materials, Table S2). The HPLC chromatograms of the mixed standard and sample are shown in Supplementary Materials, Figure S1.
2.4. Statistical Analysis
Data analysis was performed using Excel 2019 and SPSS 24.0 software. Average values were divided into ten grades based on the standard deviations (σ) and means (μ), from the first [Xi < (X−2σ)] to the tenth [Xi > (X+2σ)] grade, at increments of 0.5σ. The Shannon–Wiener index (H′) is defined as H′ = −∑PilnPi, where Pi is a percentage at grade i for one trait measured in total numbers. Principal components analysis (PCA) was conducted using SIMCA-P (V14.0) software [39]. The differences between young and adult persimmon leaves were evaluated by orthogonal partial least squares discriminant analysis (OPLS-DA), in which the Q2Y (cum) and R2Y (cum) values were used to judge the validity of the model [40]. SOM analysis was conducted using Python 3.6. The evaluation of persimmon germplasms was performed using subordinate function analysis, which is based on the following formula: Tij = (Xij − Xjmin)/(Xjmax − Xjmin), where Tij is the j indicator subordinate function value of i germplasm, Xij is the j indicator actual value of i germplasm, Xjmin is the minimum value of the j indicator, and Xjmax is the maximum value of the j indicator [41].
3. Results
3.1. Variation and Genetic Diversity of Catechin Contents
The catechin content in young and adult leaves of 249 germplasms is presented in Supplementary Materials, Table S1. The content of nine catechins was diverse: for example, GC had the highest average content in both young and adult leaves (1143.82 and 359.16 mg/100 g); however, average EGCG content was the lowest at 0.79 and 0.98 mg/100 g, respectively.
Catechin content was compared between young and adult leaves (Figure 1). In comparison to adult leaves, young leaves showed high levels of GC, ECG, CA, and CG content; The average content of EGCG and EC in adult persimmon leaves was higher than that in young leaves (p < 0.05). In addition, GA content ranged from 4.28 to 239.08 mg/100 g in young leaves, with an average of 47.45 mg/100 g. The distribution of GA content ranged from 0.52 to 18.59 mg/100 g in adult leaves, with an average of 3.22 mg/100 g and a 14.7-fold decrease compared to adult leaves.
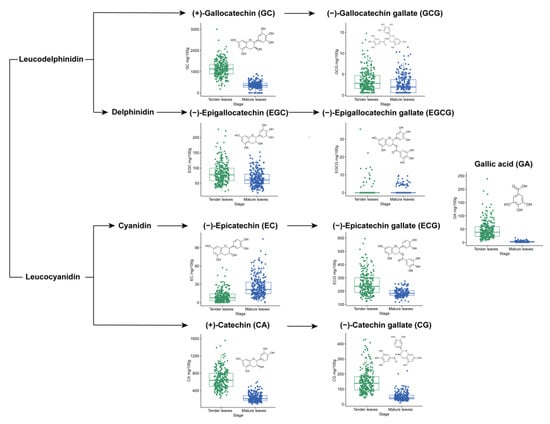
Figure 1.
Content of catechins in young and adult leaves among 249 persimmon germplasms. The green (left) and blue (right) boxplots represent the catechin content in young and adult leaves, respectively.
The varied content of nine catechins in young and adult leaves is shown in Supplementary Materials, Table S2. In the 249 persimmon germplasms, there was considerable variation in the content of catechins, with coefficients of variation (CV) ranging from 21.24% to 67.78% (young leaves) and 39.16 to 461.07% (adult leaves). Notably, a few germplasms contained high levels of EGCG, resulting in a large CV value and a low Shannon–Wiener diversity index (H’). Furthermore, the EC and GCG content exhibited higher CV values in young (106.74%) and adult (91.49%) persimmon leaves, respectively, indicating a rich diversity of catechin content within the germplasms. The genetic diversity H’ of the CA and GC content was higher in the young leaves (2.00 and 2.03), and the H’ value of the EGC and CA content was higher in adult persimmon leaves (1.98 and 1.92); these results showed that catechins were evenly distributed in each grade. These results suggest that persimmon leaves are rich in catechins, and that catechin content differs at different growth stages.
3.2. Pearson Correlation Analysis of Catechin Content
Pearson correlation analysis was performed on the catechin content in young (Figure 2A) and adult (Figure 2B) leaves. The results showed significant correlation relationships for all 20 pairs; most showed a highly significant positive correlation. For example, the non-galloylated catechins, EGC, EC, and CA, were significantly positively correlated with each other in both young and adult persimmon leaves (all correlations p < 0.01). Furthermore, significant negative relationships were found for three pairs of catechin compositions. In young leaves, GC and ECG were negatively correlated; the correlation coefficient was −0.127. In adult leaves, the correlation coefficients between GA and CG, and CG and EGC, were −0.271 and −0.174, respectively.
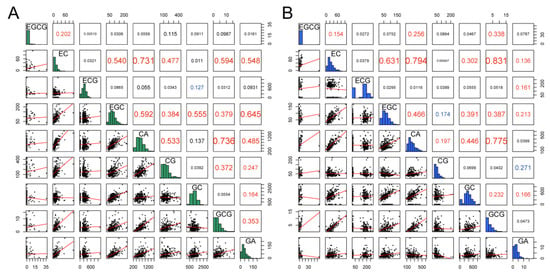
Figure 2.
Pearson correlation analysis of catechin compositions in the leaves of persimmon germplasm leaves ((A) young leaves; (B) adult leaves). The bottom half of the diagram is a scatter diagram and a fitting curve (red line). The correlation coefficient in the upper corner is blue, which means a very significant negative correlation; red, means a very significant positive correlation; the larger the font, the more significant the correlation. The diagonals show histograms and probability density curves.
3.3. Differences in Catechin content between Young and Adult Persimmon Leaves
Data simplification was conducted using principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) to construct a sample-catechin composition matrix (498 × 9) (Figure 3).
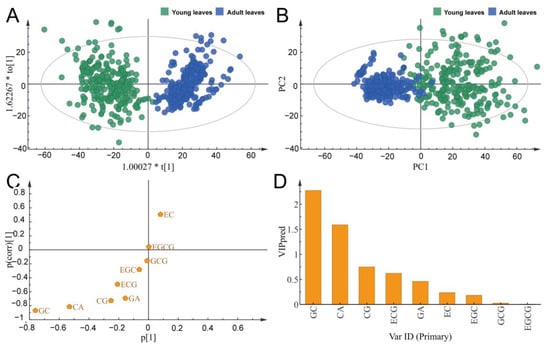
Figure 3.
Differences in catechin content between young and adult leaves ((A): PCA model; (B): OPLS-DA model; (C): S-plot; (D): VIP plot).
Four principal components were obtained and accounted for 95.6% of the total variance (Figure 3A). PC1 and PC2 accounted for 70.7% and 12.6% of the total variance, respectively. In the score plot of PC1, persimmon germplasms were well separated between young and adult leaves. It was possible to distinguish persimmon leaves from two growing periods, which showed that the metabolites accumulation pattern of persimmon leaves was different at different development stages, and different growth stages greatly influenced the catechin content. Furthermore, an OPLS-DA model was constructed to measure the catechin differences between young and adult leaves of 249 persimmon germplasms. The results further confirmed that persimmon germplasms had obviously different catechin content between adult and young leaves (Figure 3B).
Each point in the S-plot of the OPLS-DA represents a kind of catechin, and the greater the distance of a point on the x-axis, the greater the contribution of the point to the overall sample differences; the larger the differences on the y-axis, the better the relevance of the points within the group (Figure 3C). As a result, EC made a larger contribution to adult leaves, while GC and CA made a larger contribution to young leaves (Figure 3D). These three indicators could effectively discriminate persimmon adult and young leaves. The catechin component differed between young and adult leaves. Based on the above results, the critical period of catechin component utilization of persimmon leaves can be determined.
3.4. Differences in Catechin Composition of Persimmon Leaves from Different Sources and Astringency Types of Persimmon
The catechin indexes of persimmon germplasms from different sources (sample size > 5) were compared (Supplementary Materials, Table S3). The results showed that EGCG and CA had the highest average content in the persimmon germplasms of Guangxi and Shanxi provinces of China, respectively. In young leaves, the EC, CA, and GC content were the highest in Shanxi germplasms. EGC, CG, and GA were the highest in Zhejiang, and the galloylated catechins, ECG and GCG, were the highest in Guangxi and Hubei, respectively. In adult persimmon leaves, the contents of EC, EGC, GC, and GCG were the highest in Hebei germplasms. ECG and GA contents were highest in Zhejiang and Shaanxi, respectively, and CG content was highest in Japanese germplasms.
The catechin content of different astringency types of persimmon leaves was compared (Figure 4). In both young (Figure 4A) and adult (Figure 4B) leaves, the CG content was significantly higher in the J-PCNA type, and EC and GCG had the highest levels in C-PCNA. CA had the highest average content in both young C-PCNA and adult PVNA leaves. The content of GA was significantly higher in PVA adult leaves than in the other types.
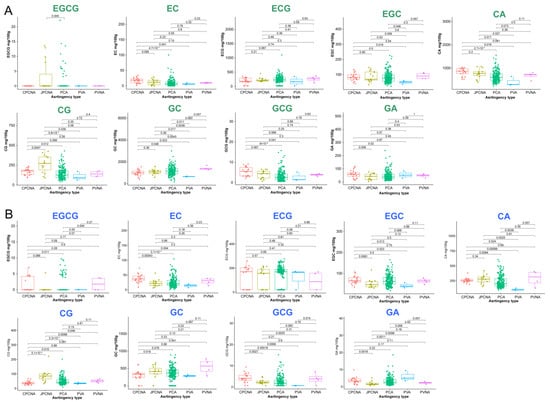
Figure 4.
The catechin composition of different astringency types of persimmon leaves ((A) young leaves; (B) adult leaves).
3.5. Cluster Analysis and Excellent Germplasms Screening
The subordinate function value was calculated based on the content of nine catechins in young and adult persimmon leaves. The subordinate function value was aggregated and averaged for comprehensive evaluation. The top ten best-performing germplasms with the highest scores were selected, including Shuishi 103, Xiuning biantashi, Haian xiaofangshi, Lishui No.3, Mengjin zhaijiahong, Caoqishi, Lishui No.13, Dongyang, Kishu, and Heishi (Supplementary Materials, Table S4). These ten germplasms originated from Zhejiang province, Henan province, Shanxi province, Jiangsu province, Anhui province of China, and Japan, with the Zhejiang province containing four excellent germplasms.
The 249 persimmon germplasms were classified into five clusters by SOM clustering according to the catechin content in young and adult leaves. To demonstrate the SOM clustering results, a 249 × 9 sample catechin composition dataset was projected into a low-dimensional space in young (Figure 5A) and adult (Figure 5B) persimmon leaves. After SOM trained 100 iterations of the data matrix, a rectangular topological structure of 6 × 6 was selected as the final output layer, and topological mapping maps of 249 persimmon germplasms were obtained. In the same neurons, different persimmon germplasms exhibited similar catechin content patterns. The results of SOM clustering showed that the five groups classified according to catechins had small relationships between young and adult leaves (Supplementary Materials, Table S5). There were no significant differences in EGCG and ECG content in the young leaves among five persimmon clusters, while the other catechin compositions showed significant differences among these groups (Figure 5C). EC, CA, and GC had the highest content in Cluster V of adult leaves (Figure 5D). Cluster III of young leaves had the highest content of EGC and GC, which contained four germplasms, including Lishui No. 1, Zheye, Xiaohuoguan, and Dahuoguan. These germplasms could represent better materials for catechin EGC and GC utilization.
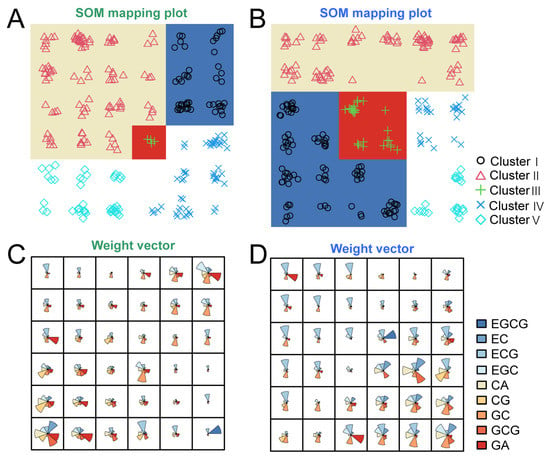
Figure 5.
SOM cluster results of 249 persimmon germplasms according to content of 9 catechins. The 6 × 6 grid containing 36 neurons and 5 clusters represents the SOM cluster results of 249 persimmon young leaves (A) and adult leaves (B). Different background color and symbols represents different clusters. Catechin distribution patterns of 249 persimmon young leaves (C) and adult leaves (D) in 36 neurons.
4. Discussion
Phenotypic variation is the most direct consequence of genetic variation and gene-environment interaction [42]. Persimmon is a typical subtropical and deciduous fruit tree that is believed to have originated in southern China and was later cultivated in Korea, Japan, Brazil, and Italy [23,43]. Plants are highly varied after long-term evolution [44,45]. In this study, the changes in catechin content in 249 persimmon young and adult leaves were analyzed, to evaluate the genetic diversity of persimmon germplasms. The results showed that catechins in persimmon resources exhibit great genetic diversity and that the population contains many evolved types.
Due to the multidimensionality of the population data and the high correlation between some variables, traditional single-variable analysis cannot quickly, fully, and accurately explore the potential information contained in the data [46]. PCA is a method of unsupervised data analysis that linearly recombines all variables into a set of variables and selects a few complex variables from them to reflect as much information as possible about the original variables based on the questions analyzed, thus achieving dimensional reduction. PCA of the variables also reflects the inter-group and intra-group changes in the sample [39]. Based on 249 catechin content data points from young and adult leaves, this study visualized the catechin accumulation patterns of persimmon leaves at different development stages using PCA. It was found that the developmental stage of persimmon leaves greatly influenced the catechin content. Metabolite content has been found to be closely related to the development stage; for example, the metabolite content of rice seeds at different growth stages is more different than that of different rice seeds at the same development stage [47], similar to the findings in our study. Supervised OPLS-DA models can identify similarities and differences between groups with designated subgroups and evaluate model classification using R2X, R2Y, Q2Y, and OPLS-DA score diagrams [48]. In this study, OPLS-DA models were able to effectively differentiate persimmon samples from different leaf growth periods, and potentially different chemical constituents were selected based on the VIP value. When evaluating the quality of persimmon leaves, the EC, GC, and CA contents should be taken as the main catechin indexes to distinguish young and adult leaves.
Metabolite accumulation profiles usually differ at different plant growth stages [49,50]. Significant differences in the metabolism of Camellia sinensis leaves in different seasons were observed, with significant seasonal fluctuations in flavanols, polyester catechins, proanthocyanidins, quercetin glycosides, and amino acids [51]. During the development of persimmon leaves, there are significant differences in catechin content from young leaves to maturity. For example, the young leaves had higher GC, ECG, CA, and CG content than the adult leaves in this study. Previous studies suggested that polyphenol and tannin contents were higher in the leaves of all species in the early stage [52]. Moreover, persimmon leaves harvested in late May had the highest amount of antioxidants content compared to late June, possibly associated with movement of antioxidants from leaves to fruits during fruiting [53]. It is suggested that the accumulation of catechins during persimmon leaf development may have the same regulation mechanisms.
SOM clustering is an unsupervised artificial neural network approach with a high generalization capability, which can learn the distribution of eigenvectors in space by itself. Data with similar features are clustered in the same region of the network topology; thus, target data with similar features are classified into homologous clusters [54]. The results can be intuitively expressed by corresponding visualization techniques, which are very suitable for visualizing high-dimensional data, providing improved guidance for excellent germplasm selection and resource utilization. In this study, 249 persimmon germplasms were divided into five clusters; nine catechins were found to have significant differences among five clusters, and the CA of Cluster Ⅴ showed better comprehensive performance. We also found that the relationships of the five clusters between young and adult leaves was weak, which showed that the metabolite patterns of different persimmon germplasms leaves were different in the same year. After comprehensive evaluation of catechins in persimmon leaves, the top 10 best-performing germplasms were selected, including Shuishi 103, Xiuning biantashi, Haian xiaofangshi, Lishui No.3, Mengjin zhaijiahong, Caoqishi, Lishui No.13, Dongyang, Kishu, and Heishi. These germplasms can be considered materials which may be preferred for use in breeding in the future. At the same time, the accumulation of catechins in persimmon leaves of different sources and astringency types was also quite different, indicating that the distribution of persimmon germplasms varies geographically, which may be related to the high adaptive capacity following the long-term introduction of persimmon.
The comprehensive analysis of the contents of nine catechins in young and adult persimmon leaves in 249 germplasms indicated that catechins had different metabolic accumulation patterns at different growth stages and for different astringency types of persimmon leaves. The catechin components were also closely associated with the geographical environment. The results of this study provide support for the development of persimmon, the utilization of excellent varieties, and hybrid breeding in the future.
5. Conclusions
In conclusion, there was a high diversity of leaf catechin contents among 249 persimmon germplasms. When determining the composition of nine catechins in persimmon young and adult leaves, it was found that catechins showed different accumulation patterns at different growth stages and for different astringency types of persimmon leaves. The young leaves had higher GC, ECG, CA, and CG contents than the adult leaves, CG content was found to be significantly higher in J-PCNA persimmon leaves, and EC and GCG had the highest levels in C-PCNA leaves. In addition, the catechin components were also closely associated with the geographic environment. EGCG and CA had the highest average contents in the germplasms of Guangxi and Shanxi provinces of China, respectively. This work provides a theoretical basis for germplasm screening and excellent trait exploitation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9040464/s1, Figure S1: HPLC chromatograms of mixed standard (A) and sample (B); Table S1: Origins and catechin content of young and adult leaves in 249 persimmon germplasms; Table S2: Diversity and variation of catechin content in young and adult persimmon leaves; Table S3: One-way analysis of variance (ANOVA) of catechin content (mean ± SD) in persimmon leaves among different regions; Table S4: Excellent germplasms screening based on subordinate function value of each catechin in persimmon leaves; Table S5: One-way ANOVA of catechin content (mean ± SD) in persimmon leaves among different SOM clusters.
Author Contributions
Conceptualization, Y.W. and Y.S.; methodology, Y.W. and Y.S.; validation, H.L., W.H. and P.S.; formal analysis, Y.W. and Y.S.; investigation, Y.W., H.L. and Y.S.; data curation, Y.W. and Y.S.; writing—original draft preparation, Y.W. and Y.S.; writing—review and editing, F.L. and J.F; funding acquisition, J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (2018YFD1000600).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Ali, S.; Hussain, S.; Ercisli, S.; Ilhan, G.; Marc, R.A.; Skrovankova, S.; Mlcek, J. Improvement of Postharvest Quality and Bioactive Compounds Content of Persimmon Fruits after Hydrocolloid-Based Edible Coating Application. Horticulturae 2022, 8, 1045. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Pu, T.; Suo, Y.; Han, W.; Diao, S.; Li, H.; Sun, P.; Fu, J. Transcriptomic profiling analysis to identify genes associated with PA biosynthesis and insolubilization in the late stage of fruit development in C-PCNA persimmon. Sci. Rep. 2022, 12, 19140. [Google Scholar] [CrossRef]
- Han, W.; Zhang, Q.; Pu, T.; Wang, Y.; Li, H.; Luo, Y.; Li, T.; Fu, J. Diversity of Fruit Quality in Astringent and Non−Astringent Persimmon Fruit Germplasm. Horticulturae 2023, 9, 24. [Google Scholar] [CrossRef]
- Xie, C.; Xie, Z.; Xu, X.; Yang, D. Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015, 163, 229–240. [Google Scholar] [CrossRef]
- Hossain, A.; Shahidi, F. Persimmon Leaves: Nutritional, Pharmaceutical, and Industrial Potential—A Review. Plants 2023, 12, 937. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-M.; Wang, J.; Gao, R.; Gong, B.-C.; Ai, C.-X. Integrated Metabolomic-Transcriptomic Analysis Reveals Diverse Resource of Functional Ingredients from Persimmon Leaves of Different Varieties. Front. Plant Sci. 2022, 13, 904208. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Huang, T.; Tian, B.; Zhan, J. Advances in Biosynthesis and Biological Functions of Proanthocyanidins in Horticultural Plants. Foods 2020, 9, 1774. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Li, J.; Li, H.; Liang, Y.; Sun, P.; Fu, J. Annual variation of total polyphenol and flavonoid contents in leaves of differ-ent species (varieties) of Diospyros. J. China Agric. Univ. 2016, 21, 31–40. [Google Scholar]
- Zhang, Y.; Zhong, L.; Zhou, B.; Chen, J.-Y.; Li, C.-M. Interaction of characteristic structural elements of persimmon tannin with Chinese cobra PLA2. Toxicon 2013, 74, 34–43. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Choi, H.-J. Persimmon leaf bio-waste for adsorptive removal of heavy metals from aqueous solution. J. Environ. Manag. 2018, 209, 382–392. [Google Scholar] [CrossRef]
- Akagi, T.; Ikegami, A.; Suzuki, Y.; Yoshida, J.; Yamada, M.; Sato, A.; Yonemori, K. Expression balances of structural genes in shikimate and flavonoid biosynthesis cause a difference in proanthocyanidin accumulation in persimmon (Diospyros kaki Thunb.) fruit. Planta 2009, 230, 899–915. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kong, A.-N.T. Biological Properties of Monomeric and Polymeric Catechins: Green Tea Catechins and Procyanidins. Pharm. Biol. 2004, 42, 84–93. [Google Scholar] [CrossRef]
- Oliveira, J.; Mateus, N.; de Freitas, V. Flavanols: Catechins and proanthocyanidins. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1753–1801. [Google Scholar]
- Wangkarn, S.; Grudpan, K.; Khanongnuch, C.; Pattananandecha, T.; Apichai, S.; Saenjum, C. Development of HPLC Method for Catechins and Related Compounds Determination and Standardization in Miang (Traditional Lanna Fermented Tea Leaf in Northern Thailand). Molecules 2021, 26, 6052. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, L.; Chen, Y.; Wang, X.; Liao, Y.; Xiao, Y.; Fu, X.; Yang, Z. Metabolism of Gallic Acid and Its Distributions in Tea (Camellia sinensis) Plants at the Tissue and Subcellular Levels. Int. J. Mol. Sci. 2020, 21, 5684. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Li, J.; Li, Y.; Yuan, L.; Liu, S.; Huang, J.; Liu, Z. Dynamic changes in catechin levels and catechin biosynthesis-related gene expression in albino tea plants (Camellia sinensis L.). Plant Physiol. Biochem. 2013, 71, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Li, P.; She, G.; Xia, E.; Benedito, V.A.; Wan, X.C.; Zhao, J. Genome-wide analysis of serine carboxypeptidase-like acyltransferase gene family for evolution and characterization of enzymes involved in the biosynthesis of galloylated catechins in the tea plant (Camellia sinensis). Front. Plant Sci. 2020, 11, 848. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.-Q.; Fu, Y.-Q.; Liu, Y.-Y.; Qin, Y.; Chen, J.-X.; Yin, J.-F.; Xu, Y.-Q. A targeted and nontargeted metabolomics study on the oral processing of epicatechins from green tea. Food Chem. 2022, 378, 132129. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Akagi, T.; Katayama-Ikegami, A.; Yonemori, K. Proanthocyanidin biosynthesis of persimmon (Diospyros kaki Thunb.) fruit. Sci. Hortic. 2011, 130, 373–380. [Google Scholar] [CrossRef]
- Ikegami, A.; Eguchi, S.; Yonemori, K.; Yamada, M.; Sato, A.; Mitani, N.; Kitajima, A. Segregations of Astringent Progenies in the F1 Populations Derived from Crosses between a Chinese Pollination-constant Nonastringent (PCNA) ‘Luo Tian Tian Shi’, and Japanese PCNA and Pollination-constant Astringent (PCA) Cultivars of Japanese Origin. Hortscience 2006, 41, 561–563. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Z. Origin, Evolution, taxonomy and germplasm. In The Persimmon Genome; Tao, R., Luo, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 11–27. [Google Scholar]
- Akagi, T.; Suzuki, Y.; Ikegami, A.; Kamitakahara, H.; Takano, T.; Nakatsubo, F.; Yonemori, K. Condensed Tannin Composition Analysis in Persimmon (Diospyros kaki Thunb.) Fruit by Acid Catalysis in the Presence of Excess Phloroglucinol. J. Jpn. Soc. Hortic. Sci. 2010, 79, 275–281. [Google Scholar] [CrossRef]
- Fei, X.; Zhou, L.; Gong, B. Differences of the components of tannin among three types of persimmon fruits and characteristics of tannin from ‘Luotian Tianshi’. For. Res. 1999, 12, 369–373. [Google Scholar]
- Gong, H.; Rehman, F.; Ma, Y.; A, B.; Zeng, S.; Yang, T.; Huang, J.; Li, Z.; Wu, D.; Wang, Y. Germplasm Resources and Strategy for Genetic Breeding of Lycium Species: A Review. Front. Plant Sci. 2022, 13, 802936. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Li, S.; Chen, Q.; da Silva, J.A.T.; Wang, A.; Yu, X.; Wang, L. Germplasm resources and genetic breeding of Paeonia: A systematic review. Hortic. Res. 2020, 7, 107. [Google Scholar] [CrossRef]
- Rao, V.R.; Hodgkin, T. Genetic diversity and conservation and utilization of plant genetic resources. Plant Cell. Tissue Organ Cult. 2002, 68, 1–19. [Google Scholar] [CrossRef]
- Lukanda, M.M.; Dramadri, I.O.; Adjei, E.A.; Arusei, P.; Gitonga, H.W.; Wasswa, P.; Edema, R.; Ssemakula, M.O.; Tukamuhabwa, P.; Tusiime, G. Genetic Diversity and Population Structure of Ugandan Soybean (Glycine max L.) Germplasm Based on DArTseq. Plant Mol. Biol. Rep. 2023, 1–10. [Google Scholar] [CrossRef]
- Wang, C.; Gong, H.; Feng, M.; Tian, C. Phenotypic Variation in Leaf, Fruit and Seed Traits in Natural Populations of Eucommia ulmoides, a Relict Chinese Endemic Tree. Forests 2023, 14, 462. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Gong, B.; Xie, Q. Fruit and Seed Phenotypic diversity of two species of Diospyros spp. in dabie mountains. For. Res. 2021, 35, 188–196. [Google Scholar] [CrossRef]
- Diao, S.; Li, F.; Duan, W.; Han, W.; Sun, P.; Fu, J. Genetic diversity of phenotypic traits of leaves in F1 progeny of persimmon. J. China Agric. Univ. 2017, 22, 32–44. [Google Scholar]
- Ercisli, S.; Akbulut, M.; Ozdemir, O.; Sengul, M.; Orhan, E. Phenolic and antioxidant diversity among persimmon (Diospyrus kaki L.) genotypes in Turkey. Int. J. Food Sci. Nutr. 2008, 59, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, Y.; Liang, J.; Fu, J. Annual variation and diversity of vitamin C in leaves of persimmon germplasm. J. Hunan Agric. Univ. (Nat. Sci.) 2014, 40, 288–293. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Chachar, S.; Xu, J.; Yang, Y.; Guan, C. A comprehensive evaluation of genetic diversity in persimmon (Diospyros kaki Thunb.) germplasms based on large-scale morphological traits and SSR markers. Sci. Hortic. 2023, 313, 111866. [Google Scholar] [CrossRef]
- Naval, M.D.M.; Zuriaga, E.; Pecchioli, S.; Llácer, G.; Giordani, E.; Badenes, M.L. Analysis of genetic diversity among persimmon cultivars using microsatellite markers. Tree Genet. Genomes 2010, 6, 677–687. [Google Scholar] [CrossRef]
- Meng, Y.; Du, Q.; Du, H.; Wang, Q.; Wang, L.; Du, L.; Liu, P. Analysis of chemotypes and their markers in leaves of core collections of Eucommia ulmoides using metabolomics. Front. Plant Sci. 2023, 13, 1029907. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, X.; Wang, L.; Shao, J.; Chen, X.; Liu, H.; Mei, W. Determination and analysis of multifunctional components in tea. J. Food Saf. Qual. 2019, 10, 7779–7786. [Google Scholar] [CrossRef]
- Saccenti, E.; Hoefsloot, H.C.J.; Smilde, A.K.; Westerhuis, J.A.; Hendriks, M.M.W.B. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2014, 10, 361–374. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Brychkov, Y.A.; Savischenko, N.V. Application of Hypergeometric Functions of Several Variables in the Mathematical Theory of Communication: Evaluation of Error Probability in Fading Singlechannel System. Lobachevskii J. Math. 2020, 41, 1971–1991. [Google Scholar] [CrossRef]
- Pham, B.; McConnaughay, K. Plant phenotypic expression in variable environments. In Ecology and the Environment; Monson, R.K., Ed.; Springer: New York, NY, USA, 2014; pp. 119–141. [Google Scholar]
- Veberic, R.; Jurhar, J.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Comparative study of primary and secondary metabolites in 11 cultivars of persimmon fruit (Diospyros kaki L.). Food Chem. 2010, 119, 477–483. [Google Scholar] [CrossRef]
- Zhang, Y.; Fischer, M.; Colot, V.; Bossdorf, O. Epigenetic variation creates potential for evolution of plant phenotypic plasticity. New Phytol. 2013, 197, 314–322. [Google Scholar] [CrossRef]
- Casacuberta, J.M.; Jackson, S.; Panaud, O.; Purugganan, M.; Wendel, J. Evolution of Plant Phenotypes, from Genomes to Traits. G3 Genes Genome Genet. 2016, 6, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, L.; Du, Q.; Du, H. Chemotype classification and biomarker screening of male Eucommia ulmoides Oliv. flower core collections using UPLC-QTOF/MS-based non-targeted metabolomics. PeerJ 2020, 8, e9786. [Google Scholar] [CrossRef]
- Li, K. Dissection of the Genetic Bases Underlying the Metabolic Diversity in Rice Seeds Across Different Developmental Stages. Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Ding, M.; Jiang, Y.; Yu, X.-A.; Zhang, D.; Li, J.; Wang, H.; Shen, J.; Gao, X.-M.; Chang, Y.-X. Screening of Combinatorial Quality Markers for Natural Products by Metabolomics Coupled with Chemometrics. A Case Study on Pollen Typhae. Front. Pharmacol. 2018, 9, 691. [Google Scholar] [CrossRef]
- Lawal, U.; Mediani, A.; Maulidiani, H.; Shaari, K.; Ismail, I.S.; Khatib, A.; Abas, F. Metabolite profiling of Ipomoea aquatica at different growth stages in correlation to the antioxidant and α-glucosidase inhibitory activities elucidated by 1H NMR-based metabolomics. Sci. Hortic. 2015, 192, 400–408. [Google Scholar] [CrossRef]
- Wang, Y.; Suo, Y.; Han, W.; Li, H.; Wang, Z.; Diao, S.; Sun, P.; Fu, J. Comparative transcriptomic and metabolomic analyses reveal differences in flavonoid biosynthesis between PCNA and PCA persimmon fruit. Front. Plant Sci. 2023, 14, 1130047. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Qi, D.; Yang, T.; Lv, H.; Guo, L.; Zhang, Y.; Zhu, Y.; Peng, Q.; Xie, D.; Tan, J.; et al. Nontargeted Analysis Using Ultraperformance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry Uncovers the Effects of Harvest Season on the Metabolites and Taste Quality of Tea (Camellia sinensis L.). J. Agric. Food Chem. 2015, 63, 9869–9878. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Moon, K.; Kim, J.; Seong, J.; Sohn, T.-H. Changes of chemical components in persimmon leaves during growth for processing persimmon leaves tea. Korean J. Food Sci. Technol. 1994, 26, 141–146. [Google Scholar]
- Hossain, A.; Moon, H.K.; Kim, J.-K. Antioxidant properties of Korean major persimmon (Diospyros kaki) leaves. Food Sci. Biotechnol. 2018, 27, 177–184. [Google Scholar] [CrossRef]
- Sen, P.; Lamichhane, S.; Mathema, V.B.; McGlinchey, A.; Dickens, A.M.; Khoomrung, S.; Orešič, M. Deep learning meets metabolomics: A methodological perspective. Briefings Bioinform. 2020, 22, 1531–1542. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
