Abstract
Protein hydrolysates and peptides can show biological activities, and pulsed ultrasound improves bioactivities. Among matrices from which protein hydrolysates can be obtain, chickpea is an excellent source. The objective of this research was to evaluate the effect of pulsed ultrasound on globulin concentrate to obtain chickpea hydrolysate (HGb) and peptide fractions and their bioactivity. Antioxidant activity by ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt), FRAP (Ferric Reducing Antioxidant Power) and human erythrocyte assays was determined. The electrophoretic profile, amino acid profile, and antimicrobial activity of hydrolysates were also determined. Two hydrolysates had the highest antioxidant activity: HGb (91.44% ABTS inhibition, 73.04% hemolysis inhibition and 5185.57 µmol TE/g dried sample in FRAP assay) and HGb-20 (48.25% ABTS inhibition, 100% hemolysis inhibition and 2188.53 µmol TE/g dried sample in FRAP assay). Peptide fractions inhibited 100% of the hemolysis on human erythrocytes. The hydrolysates from chickpea proteins obtained with savinase have antioxidant activity through the SET and HAT mechanisms. The application of the obtained compounds for the development of functional foods or for food preservation should be considered.
1. Introduction
During the past decades, the noticeable increase in the prevalence of non-communicable diseases such as cerebrovascular and cardiovascular diseases, cancer, and diabetes has been one of the biggest concerns throughout the world’s population [1,2]. On the other hand, food loss and waste are major problems for food safety, the economy, and the environment [3]. For those reasons, research has focused on identifying bioactive compounds with multiple biological activities, which could be used for health or food preservation methods [4]. Among active compounds that could be employed for this purpose, peptides and protein hydrolysates should be considered, given their multifunctional antioxidant, antihypertensive, antimicrobial, and anti-inflammatory activities, among others [4,5]. These bioactive molecules have gained interest in the food industry—specifically, the ones with antioxidant and antimicrobial activities [6,7]. Antioxidant protein hydrolysates reduce or prevent oxidation, whereas antimicrobial protein hydrolysates insert into the membranes of microorganisms, disrupting them and resulting in the killing of bacteria [7,8].
Identifying protein sources from which protein hydrolysates and peptides can be obtained is an important matter [9]. Several studies indicate that protein hydrolysates and peptides derived from legumes have different bioactive properties [10,11]. In that sense, chickpea peptides have shown antioxidant capacity and antimicrobial capacity [12,13,14]. These biological activities were attributed to the presence of basic, acidic, and hydrophobic amino acids.
Protein hydrolysates and bioactive peptides are inactive within the parent protein; therefore, they must be isolated by processes such as fermentation, gastrointestinal digestion, and enzymatic hydrolysis [8]. In addition, the use of pretreatments enhances the biological activity of the obtained compounds [9]. Pulsed ultrasound prior to enzymatic hydrolysis or ultrasound-assisted hydrolysis generates increases in the bioactivity of the hydrolysates and peptides obtained [15,16]. Increments in biological activity result from the cavitation phenomenon produced by acoustic waves during the application of ultrasound, wherein the microbubbles generated cause conformational changes in the proteins. With the hydrophobic regions exposed, the efficiency of the enzymes increases, allowing for the obtaining of peptides and protein hydrolysates with a more favorable amino acid composition [17,18,19].
Ultrasound treatment on chickpea proteins has been used recently, with a focus on improving the functional properties of chickpea protein isolates, such as emulsification, foaming, and gel properties [20,21,22]. To improve antioxidant activity, ultrasound pretreatment has been used as part of the extraction technique, enhancing phenolic compound extraction from chickpea seeds [23,24]. As far as we know, there are no studies where pulsed ultrasound pretreatment is used to obtain antioxidant peptides and protein hydrolysates from chickpea. Thus, this research is the first to measure the effect of pulsed ultrasound pretreatment on the antioxidant capacity of peptides and protein hydrolysates obtained from chickpea.
The objective of this research was to evaluate the ability of pulsed ultrasound to obtain chickpea peptide fractions by applying ultrasound pretreatment to globulin concentrate and measuring the antioxidant activity, electrophoretic profile, amino acid profile, and antimicrobial activity of the hydrolysates.
2. Materials and Methods
2.1. Materials
Chickpea grains (Cicer arietinum L.) of the kabuli type and Blanco Sinaloa variety harvested in 2017 were obtained from “El Compa” plantations in Hermosillo, Sonora, Mexico (28.596062 N, −111.468929 W) and were provided by Grupo Aliansa (Sonora, Mexico). Savinase (EC 3.4.21.62) obtained from Bacillus sp. was purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents and chemicals used were of analytical or HPLC grade and were also acquired from Sigma-Aldrich, unless otherwise specified.
2.2. Preparation of Chickpea Flour
Chickpea flour was obtained according to the methods by Tovar-Pérez et al. [25] and Tontul et al. [26], with modifications. Five kilograms of raw chickpea seeds were lyophilized. Lyophilized chickpea seeds were ground in a Thomas-Wiley mill (Thomas Scientific, Swedesboro, NJ, USA), and flour was sieved through a 2 mm mesh. The resulting flour was milled in a Braun food processor (Gillette Commercial, Boston, MA, USA) and passed through a 35-mesh sieve with a 0.425 mm pore size. Flour was defatted by extracting twice with petroleum ether (1:5 w/v) for 24 h. Chloroform was used twice (1:5 w/v) for 24 h for remotion of alkaloids. Acetone was used twice (1:3 w/v) for 24 h for removal of polyphenols. Flour was evaporated to dryness and stored at −18 °C until use.
2.3. Preparation of Chickpea Protein Concentrates
Extraction of soluble proteins from chickpea flour was performed according to the method by Tovar-Pérez et al. [25], with modifications. Chickpea flour was extracted successively with 10 mmol/L Tris-HCl (pH 7.5 containing 2 mmol/L EDTA), 0.5 mol/L NaCl (containing 2 mmol/L EDTA and 10 mmol/L Tris-HCl pH 7.5) and 0.1 mol/L NaOH, to obtain albumin (Al), globulin (Gb) and glutelin (Gt) concentrates, respectively. Suspensions (1:2 w/v) were stirred for 5 min and centrifuged at 9000 rpm for 80 min at 4 °C. Each supernatant was dialyzed (molecular weight 12 kDa cutoff) against deionized water for 48 h at room temperature, with a change every 8 h. The contents of the dialysis tubes were centrifuged, lyophilized, and stored at −18 °C. Total protein concentration in chickpea protein extracts was determined as total nitrogen multiplied by 6.25, using micro Kjeldahl [27,28].
2.4. Preparation of Chickpea Protein Hydrolysates
Chickpea hydrolysates were obtained according to the methods by Garcia-Mora et al. [11,27], with some modifications. Freeze-dried chickpea protein concentrates were suspended in deionized water (2% w/v) and equilibrated at 40 °C. The pH value was adjusted to 8 using 0.1 M NaOH. Enzymatic proteolysis employing savinase was carried out at an enzyme–substrate ratio (E/S) of 0.1 U/mg of soluble protein at 40 °C for 2 h and pH 8. The reaction was finalized by heating samples at 80 °C for 15 min. Hydrolysates were centrifuged at 9000 rpm at 10 °C for 20 min, lyophilized, and stored until use, being labeled as albumin hydrolysate (HAl), globulin hydrolysate (HGb) and glutelin hydrolysate (HGt). Soluble protein concentration in hydrolysates was determined according to the method by Bradford [29]. Bovine serum albumin (BSA) was used as a standard at a concentration range from 0 to 1 mg/mL.
2.5. Antioxidant Activity of Protein Concentrates and Protein Hydrolysates
2.5.1. ABTS•+ Scavenging Activity
ABTS assay was carried out according to the method by Re et al. [30], employing 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt. The absorbance of the ABTS radical was adjusted to 0.7 ± 0.02 nm at 734 nm. Samples (20 μL) were mixed with the ABTS radical solution (270 μL) and incubated for 30 min. The absorbance was read at 734 nm using a microplate reader (Thermo Fisher Scientific Inc. Multiskan GO, New York, NY, USA). The results were expressed as the scavenging activity percentage of Abs734 (RSA %). Additionally, for protein hydrolysates, the 50%-inhibitory concentration (IC50) was expressed as the µg/mL of hydrolysate that scavenged half of the ABTS•+ radical.
2.5.2. Evaluation of the Protective Effect on Human Erythrocytes
The protective effect in human erythrocytes was evaluated according to the method by Hernández-Ruiz et al. [31]. In this assay, erythrocyte hemolysis is induced with an AAPH (2,2′-Azobis(2-amidinopropane) dihydrochloride) radical. A suspension of erythrocytes was prepared with PBS (2%). A mixture of erythrocytes (100 μL), sample (100 μL) and AAPH radicals (100 μL) was prepared and incubated at 37 °C under stirring at 30 rpm, in darkness, for 3 h. Afterwards, 1 mL of PBS was added to the mixture and centrifuged at 1500 rpm for 10 min. The absorbance of the supernatant was measured at 540 nm using a microplate reader. The results were expressed as a percentage of hemolysis inhibition (PHI) compared to a similar reaction without sample, and the value was calculated using the following equation:
where AHI was defined as the absorbance of hemolysis induced by AAPH at 540 nm, while AS was defined as the absorbance of the sample at 540 nm.
2.5.3. Ferric Reducing Antioxidant Power (FRAP)
The FRAP assay, in which a ferric ion (Fe+3) is reduced to a ferrous ion (Fe+2), was determined using the method by Benzie and Strain [32], with modifications. The FRAP reagent was prepared in a 1:1:10 ratio of 10 mM TPTZ (2,4,6-tri(2-pyridyl)-s-triazine) in 40 mM HCl, 20 mM FeCl3·6H2O and 0.3 M sodium acetate buffer (pH 3.6), respectively. Samples (20 µL) were mixed with the FRAP reagent (280 µL) and incubated for 40 min. The absorbance was read at 638 nm. Results were expressed as µmol TE/g dried sample.
2.6. Ultrasound Pretreatment on Globulin Concentrate
HGb showed greater antioxidant activity among the different hydrolysates. Furthermore, being that globulin was one of the predominant protein fractions, Gb was chosen to perform pulsed ultrasound pretreatment according to the method by Wang et al. [15], with modifications. A total of 100 mg of freeze-dried globulin concentrate was dissolved in 20 mL of distilled water in a glass beaker to obtain protein solutions with substrate concentration of 5 g/L. Each sample was treated in a probe-type sonicator Branson Digital Sonifier SFX 550 (Branson Ultrasonics Corporation, Brookfield, CT, USA) with a sonotrode with a 12.7 mm diameter tip. The samples were treated with 3 conditions: 10, 20 and 30 min, at powers of 33, 77 and 119 W, respectively, with a pulsed on-time and off-time of 2 and 2 s. The beaker was surrounded by an ice bath to keep the sample at room temperature during the ultrasound treatment. The control consisted of a globulin concentrate without ultrasound pretreatment. Immediately after the application of ultrasound pretreatment, hydrolysis with savinase was carried out according to the conditions described above. The soluble protein concentration and the antioxidant activity of the hydrolysates obtained were determined as previously described.
Samples were coded as globulin concentrate (Gb) or globulin hydrolysate (HGb), followed by time of pulsed ultrasound pretreatment (10, 20, and 30). For example, Gb-20 is the globulin concentrate pretreated with pulsed ultrasound under the condition of 20 min, 77 W; HGb-10 hydrolysates are those produced from globulin concentrate pretreated with pulsed ultrasound under the condition of 10 min, 33 W and hydrolyzed by savinase.
2.7. Ultrafiltration
Hydrolysates with the highest antioxidant activity were used to obtain peptide fractions by ultrafiltration according to the method by Tovar-Pérez et al. [25], with some modifications. The Millipore Amicon® model 8200 agitation cell (EMD Millipore Corporation, Burlington, MA, USA) and ultrafiltration membranes with 10 and 3 kDa molecular weight cutoffs were used, obtaining 3 peptide fractions for each hydrolysate (molecular weight > 10, 10-3 and <3 kDa). Subsequently, the antioxidant activity of the peptide fractions was determined as described above.
2.8. Electrophoretic Profile (SDS-PAGE)
The profiles of chickpea proteins and hydrolysates were analyzed in SDS-PAGE, with 12% resolution gel and 4% stacking gel [33]. A total of 20 μL of sample was loaded into the gel, and protein separation was performed at 100 V. Gels were stained with Coomassie Brilliant Blue R-250 and destained with a methanol: water: acetic acid solution (500:400:100 mL). The same conditions were used to analyze the profile of globulin hydrolysates obtained with pulsed ultrasound pretreatment. The molecular weight of the protein concentrates and hydrolysates was determined by comparison with a wide range molecular weight markers.
2.9. Amino Acid Profile
The amino acid composition of the hydrolysates with the highest antioxidant activity (HGb and HGb-20) was determined by reverse-phase high-performance liquid chromatography (RP-HPLC) using a Hewlett-Packard 1200 series HPLC system (Hewlett-Packard Co., Waldbronn, Germany) according to the method by Vázquez-Ortiz et al. [34]. The samples were hydrolyzed in sealed tubes using 6 M HCl in an evaporator at 150 °C for 12 h. The hydrolyzed samples were resuspended on sodium citrate buffer solution (pH 2.2), and derivatized with O-phthalaldehyde (OPA) for determination of primary amino acids. Chromatographic separation was carried out using a C18 column (4.6 mm ID × 100 mm; Agilent Technologies, Inc., Palo Alto, CA, USA), and the integrations were calculated using ChemStation software (Agilent Technologies Inc., Palo Alto, CA, USA). Fluorescence emission was monitored continuously at 330 and 418 nm. The results were expressed as amino acid residues.
2.10. Antimicrobial Activity of Protein Hydrolysates
2.10.1. Bacterial Strains and Growth Conditions
Staphylococcus aureus (ATCC 65384) and Salmonella enterica subsp. enterica ser. Typhimurium (ATCC 14028) were employed in the experiments. Strains were maintained in tryptic soy broth (TSB) containing glycerol (20%) at 4 °C until use. A loopful of bacteria was transferred to 3 mL of TSB and incubated at 37 °C overnight. A loopful of culture was then transferred to mannitol salt agar or bismuth sulfite agar, and cultures were grown at 37 °C until reaching the desired number of colony-forming units per mL (CFU/mL) for use in inhibitory assays [35].
2.10.2. Plate Preparation and Analysis
Antimicrobial activity was evaluated using the method described by Griffin et al. [36], with some modifications. Mueller–Hinton agar was prepared, and different concentrations of HGb and HGb-20 (10, 5, 1 mg/mL) were added to 15 mL aliquots of molten, tempered agar to give final concentrations of 2.0% v/v. The agar-sample solutions were vortexed for 15 s and immediately poured into Petri dishes.
The plates were inoculated by pipetting 10 µL of a freshly prepared bacterial suspension (104 CFU/mL) onto the agar surface. One agar plate containing the test organism in the absence of the test material was used as a positive control with each experiment. Plates were set up in duplicate, and the entire method was repeated twice. After inoculation, the plates were left to stand for 30 min and then incubated for 24 h at 37 °C. After incubation, the plates were examined for the presence or absence of growth. The minimum inhibitory concentration (MIC) was recorded as the concentration of test material, where at least five out of six readings showed no visible growth or only isolated colonies (≤20).
2.11. Statistical Analysis
Experimental data were subjected to analysis of variance (ANOVA) followed by Tukey test at a 0.95 (p < 0.05) confidence level. Results are presented as mean with standard deviation. All trials were performed in triplicate. Statistical analysis was carried out using JPM software, version 8.0 (SAS Institute Inc., Cary, NC, USA). The graphics were made with SigmaPlot software, version 10.0 (Systat Software Inc., Richmond, IL, USA). Descriptive statistics was applied for the electrophoretic analysis, FTIR and RMN data.
3. Results and Discussion
3.1. Chickpea Protein Concentrates
Three protein concentrates were obtained from the chickpea flour, and their protein content was determined, as shown in Table 1. The amount of concentrate obtained varied from 1.93 to 3.51 g/100 g of flour. Glutelin and globulin concentrates showed higher yields than that of albumin, with a significant difference between glutelin and albumin. Several studies with chickpea seed have reported a higher globulin and glutelin content compared to albumin content, as occurred in this study. Liu, Hung and Bennett [37] reported a content of 28.5% albumin, lower than 43.5% globulin. Singh and Jambunathan [38] reported a content of 56.6% and 18.1% of globulin and glutelin, higher than albumin content (12%). This suggests that there may be variations in the yield of the protein concentrates obtained from chickpea, with those differences being attributed to factors such as seed variety, fractionation technique and storage conditions [25,37].

Table 1.
Protein content of protein concentrates obtained from chickpea seed.
The protein content of the concentrates was determined from 82.73 to 85.23 g/100 g of concentrate and were not significantly different (Table 1). The results of this study are similar to those described in the literature, where values of 83.57 ± 0.22 to 84.8 ± 0.3 g/100 g of chickpea globulin concentrate are reported [39,40]. As a consequence, the process of obtaining protein hydrolysates was favored by the high protein content of the concentrates.
3.2. Antioxidant Activity of Protein Compounds
3.2.1. ABTS•+ Radical Scavenging Activity
Table 2 shows the antioxidant activity of the chickpea protein concentrates and hydrolysates, as determined by the ABTS assay. The protein concentrates displayed values from 10.68 to 37.07% ABTS inhibition, with significant differences between them, wherein they were higher for glutelin concentrate. For hydrolysates, the ABTS inhibition ranges were from 87.75 to 92.37% and were higher for albumin hydrolysate, the difference between hydrolysates being significant. Evangelho, Vanier, Pinto, De Berrios, Guerra Dias and Rosa Zavareze [41] reported 68% ABTS inhibition for bean globulin hydrolysates obtained using alcalase. Ngoh and Gan [42]. determined a 53.33% ABTS inhibition for bean protein hydrolysates obtained with protamex. The ABTS inhibition values determined in the present study were markedly higher than those described above; the difference may be attributed to the enzyme used for the hydrolysis process. Savinase allowed the obtaining of lentil protein hydrolysates with higher antioxidant activity than those obtained with alcalase or protamex [27].

Table 2.
Antioxidant activity of protein concentrates obtained from chickpea seed.
The IC50 values determined from the ABTS assay ranged from 1.54 to 2.12, being lower for albumin (1.54 ± 0.06) and globulin (1.56 ± 0.17) hydrolysates than for glutelin hydrolysate (2.12 ± 0.02) (p < 0.05). Other legume hydrolysates, such as mung bean (Vigna radiata) and poroto (Erythrina edulis), exhibited higher IC50 values—54.65 and 84.14 µg/mL, respectively, thus having lower antioxidant activity than the three chickpea hydrolysates determined in this study [43,44]. The differences in the IC50 value may be due to the enzyme used, given that in the studies described above, alcalase was used. As mentioned previously, the use of savinase allows for the production of hydrolysates with greater antioxidant activity compared to those obtained with alcalase [27].
3.2.2. Evaluation of the Protective Effect on Human Erythrocytes
The results of the protective effect of chickpea protein concentrates and hydrolysates on human erythrocytes are presented in Table 2. In this assay, the AAPH radical causes lysis of the cell membranes of erythrocytes, but if the AAPH radical is reduced by proton donation from an antioxidant compound, hemolysis is inhibited. The ability of these compounds to inhibit the AAPH radical, preventing hemolysis, was measured. The chickpea protein concentrates showed hemolysis inhibition values from 64.89 to 80.38%, with significant differences between them (higher for albumin). For hydrolysates, hemolysis inhibition ranged from 58.76 to 77.40% and were higher for glutelin and globulin hydrolysates, without significant differences between them. The increase in the antioxidant capacity of globulin and glutelin hydrolysates, in comparison to the concentrates from which they were obtained, is attributed to the increase in the exposure of the side chains of the amino acids present in the hydrolysates, which can act by donating protons to stabilize the AAPH radical and stop the chain reaction of the radical, thus preventing damage to erythrocytes [45,46].
Most studies on the protective effect of human erythrocytes investigate compounds such as phenols and ginsenosides [31,45]. In the matter of compounds of protein nature, Zheng, Dong, Su, Zhao and Zhao [47] studied the protective effect of human erythrocytes of different dipeptides against the effects caused by AAPH-induced oxidation, finding hemolysis measurements of 10.64, 3.61, 23.12 and 20.30% for Tyr-Gly, Trp-Gly, Cys-Gly and Met-Gly, respectively, results close to those determined for chickpea hydrolysates in the present study. Zhan, Wang, Liu, Guo, Gong, Hao, et al. [48] evaluated the protective effect of globulin hydrolysates of sachi seeds (Plukenetia volubilis) on erythrocytes, with hemolysis inhibitions of <25% with 500 µg/mL of hydrolysates and >80% with 2000 µg/mL of hydrolysates, concentrations far superior to the ones evaluated in this study (200 µg/mL).
3.2.3. Ferric Reducing Antioxidant Power (FRAP)
The results of the FRAP assay for chickpea protein concentrates and their hydrolysates are shown in Table 2. The concentrates showed FRAP values from 128.07 to 644.27 µmol TE/g dried sample, being higher for globulin. The hydrolysates showed a high reducing power, with FRAP values ranging from 1323.83 to 5185.57 µmol TE/g dried sample. There were significant differences between them with greater values for globulin hydrolysate. Santos Aguilar and Soares de Castro y Sato [49] obtained rice protein hydrolysates using a Bacillus licheniformis protease and alcalase, determining values of 18.78 and 19.31 µmol TE/g sample from a FRAP assay, respectively. Piñuel, Vilcacundo, Boeri, Barrio, Morales, Pinto, et al. [50] obtained a bean protein concentrate with a FRAP value of 95.80 µmol TE/g sample, while the hydrolysate obtained after an in vitro gastrointestinal digestion showed 225.77 µmol TE/g sample from a FRAP assay.
The chickpea hydrolysates obtained in this study, specially HGb, are antioxidant compounds with great electron donor capacity, as evidenced by the amino acids found in abundance in the globulin fraction. A high Asp content was determined in the globulin hydrolysate (HGb, Table 3). Asp is a negatively charged amino acid that possesses electron excess; those electrons can be donated, reducing the ferric ion–TPTZ complex to ferrous ion–TPTZ, a mechanism evaluated in this test [32]. It is known that acidic amino acids can interact with transition metals through their charged residues, avoiding oxidative processes [2].

Table 3.
Amino acid profile (residues/1000 amino acid residues) of globulin hydrolysates.
3.3. Antioxidant Activity of Ultrasound Pretreated Globulin Hydrolysates
Figure 1 presents the antioxidant activity of globulin concentrate and globulin hydrolysate pretreated with pulsed ultrasound determined by three assays. Pulsed ultrasound pretreatment affected the antioxidant capacity of the protein concentrates and hydrolysates in the assays that evaluate the SET mechanism, such as ABTS and FRAP. A reduction in the percentage of ABTS inhibition was observed for globulin concentrate with three pulsed ultrasound pretreatments (<20.9%) and for globulin hydrolysates with ultrasound pretreatment (48.25 to 72.85% ABTS inhibition) compared to the globulin concentrate and hydrolysate without ultrasound pretreatment (Figure 1a).
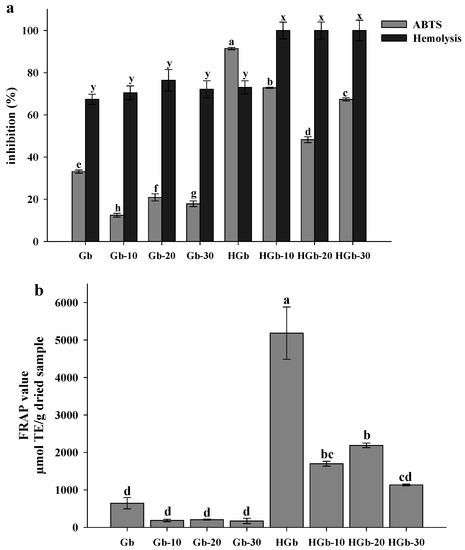
Figure 1.
Antioxidant activity of globulin concentrates and hydrolysates pretreated with pulsed ultrasound. (a) ABTS and Hemolysis inhibition assays; (b) FRAP assay. Results are plotted as mean ± standard deviation of triplicate determinations. Different letters in the same parameter are significantly different (p < 0.05). Concentrations of proteins and hydrolysates were 200 µg/mL. Gb = Globulin; HGb = Hydrolysate of globulin at 10, 20 and 30 min of ultrasound pretreatment.
The same behavior was shown in the FRAP assay; globulin hydrolysate had a greater reducing power than the hydrolysates pretreated with pulsed ultrasound (1134.34 to 2188.53 µmol TE/g dried sample for FRAP value). For the FRAP assay, there were no significant differences between globulin concentrate and the globulin concentrates pretreated with pulsed ultrasound (Figure 1b). Although there was a decrease in the FRAP value for the hydrolysates obtained with ultrasound pretreatment, the results are notoriously superior to that reported for rice and bean hydrolysates, whose FRAP value were determined to be 19.31 and 225.77 µmol TE/g of sample, respectively [49,50]. Xia, Zhai, Huang, Liang, Yang, Song, et al. [51] reported the use of ultrasound pretreatment to obtain antioxidant hydrolysates from microalgae protein (Dunaliella salina), where the use of ultrasound generated a decrease in antioxidant activity (evaluated as % DPPH inhibition) within 30 min, as was what happened in the present study in the ABTS and FRAP assays. Misir and Koral [52] reported a higher percentage of ABTS inhibition for rainbow trout protein hydrolysate obtain by conventional hydrolysis compared to that obtained with ultrasound-assisted hydrolysis.
Figure 1a shows that ultrasound pretreatment greatly favored the protective effect in erythrocytes assay, since 100% erythrocyte hemolysis inhibition was achieved for globulin hydrolysates obtained by pretreatment with ultrasound, a percentage much higher than the one achieved by globulin hydrolysate without ultrasound (73.04%). In addition, there was an increase in the inhibition of hemolysis in the globulin concentrate pretreated with ultrasound (70.47 to 76.39%). Authors have reported that the use of pulsed ultrasound pretreatment increases antioxidant activity in a DPPH inhibition assay and hydroxyl radical inhibition assay [19]. The results observed in the different carried-out assays indicate that the use of ultrasound pretreatment favors obtaining hydrolysates with a proton donation capacity. In the amino acid profile for HGb-20 (Table 3), a high content of Arg was found, a basic amino acid that has the capacity to act as a proton donor [53]. A high content of Ala and Gly was also found. These hydrophobic amino acids confer antioxidant capacity to hydrolysates by increasing their solubility in related substrates. These amino acids could be exposed by the effect of ultrasound pretreatment; as a result, hydrolysates rich in these amino acids were obtained, which could have facilitated the union of globulin hydrolysates with the erythrocyte lipid membranes, donating protons to the AAPH radical and inhibiting their harmful effect [2,18].
3.4. Antioxidant Activity of Peptide Fractions
Figure 2 presents the results of the antioxidant activity evaluation of three peptide fractions of the globulin hydrolysates with the highest antioxidant activity (HGb and HGb-20). In the ABTS assay, a marked decrease in the percentage of inhibition by the peptide fractions of HGb (7.76 to 13.40%) and HGb-20 (3.06 to 6.61%) was observed, compared to the hydrolysates from which they came (Figure 2a). Similar behavior occurred in the FRAP assay, where the peptide fractions of HGb showed FRAP values from 226.08 to 550.62 µmol TE/g dried sample, while the peptide fractions of HGb-20 had FRAP values from 226.08 to 372.02 µmol TE/g dried sample, significantly different from the FRAP value of the hydrolysates HGb and HGb-20 (Figure 2b). Ngoh and Gan [42] reported the same behavior for peptide fractions of bean hydrolysates, where the hydrolysate had a higher percentage of inhibition of the ABTS radical (53.3%) compared to that found in peptide fractions with the same molecular weight as the ones of this study (19.79, 2.61 and 42.18%).
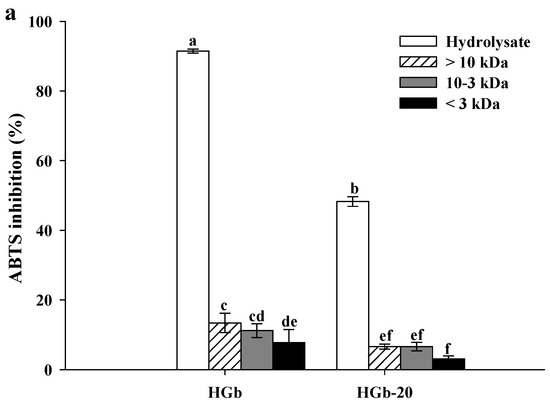
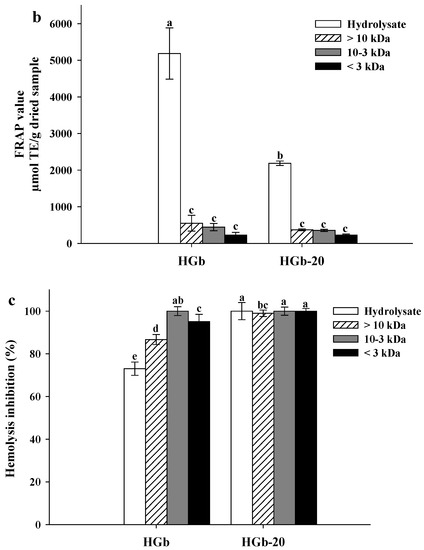
Figure 2.
Antioxidant activity of globulin hydrolysates and peptide fractions. (a) ABTS assay; (b) FRAP assay; (c) Hemolysis inhibition assay. Results are plotted as mean ± standard deviation of triplicate determinations. Bars with different letters are significantly different (p < 0.05). Concentrations of hydrolysates and peptide fractions were 200 µg/mL. HGb = Hydrolysate of globulin at without pulses and HGb-20 Hydrolysate of globulin at 20 min of ultrasound pretreatment.
For the protective effect in the erythrocytes assay (Figure 2c), favorable results were found. Peptide fractions obtained from HGb showed hemolysis inhibitions ranging from 86.68 to 100%, significantly greater than that of hydrolysate (73.04%). Low-molecular-weight peptides, being a short chain of amino acid residues, have smaller steric hindrance compared to hydrolysates, allowing the interaction between hydrophobic and hydrophilic amino acids with free radicals, neutralizing them [51]. The peptide fractions of HGb-20 maintained the ability to inhibit hemolysis, with values from 99 to 100%. Pulsed ultrasound pretreatment favored the obtaining of peptide fractions with hydrophobic amino acids, which facilitated the interaction between peptide fractions and erythrocyte membranes [18]. The peptide fractions obtained from the hydrolysates HGb and HGb-20 showed an antioxidant capacity in which the mechanism of action was HAT, a mechanism measured by the performed assay [45].
3.5. Electrophoretic Profiles
Figure 3a shows the electrophoretic profile of chickpea proteins and hydrolysates obtained in this research. Different electrophoretic profiles were observed for albumin, globulin and glutelin concentrates (Figure 3a; lines B, C, and D). For albumin, bands with molecular weights from 97 to 21 kDa were observed, with three main bands with molecular weights of approximately 97, 48 and 40 kDa (Figure 3a, lane B). Papalamprou, Doxastakis, Biliaderis and Kiosseoglou [54] reported bands with molecular weights of 87, 31.4 and 23.9 kDa, corresponding to the chickpea albumin fraction. Chang [55] reported protein bands with molecular weights of 93.6, 51, 41.7 and 38.9 kDa for chickpea albumin analyzed on a 12% SDS-PAGE gel. Electrophoretic profiles determined in previous studies were similar to the one obtained in the present study. For globulin, a banding pattern from 97 to 21 kDa was observed, with three main bands with molecular weights of approximately 70, 60 and 50 kDa (Figure 3a, lane C). Chang, Alli, Molina, Konishi and Boye [56] identified bands with molecular weights of 70.2, 50.7 and 35 kDa, corresponding to vicilin subunits; bands with molecular weights of 40.6 and 39.5 kDa, corresponding to legumin α-subunits; and bands with molecular weights of 23.5 and 22.5 kDa, corresponding to legumin β-subunits. The bands of 70 and 50 kDa identified in the globulin concentrate in this study corresponds to the chickpea globulin vicilin fraction, while the bands with weights between 30 and 21 kDa corresponds to legumin β-subunits, and the 40 kDa band corresponds to the legumin α-subunit. Legumin and vicilin are the main groups of proteins from chickpea globulin. For glutelin concentrate, an electrophoretic profile with bands from 60 to 25 kDa was observed, with the main protein bands having molecular weights of around 55 and 35 kDa (Figure 3a, lane D). Chang [55] reported molecular weights of 51, 38.1 and 26.2 kDa for chickpea glutelin subunits. The results of this study coincide with a previously reported bibliography since the most intense protein bands for chickpea glutelin were in the range of 55–50 and 40–35 kDa.
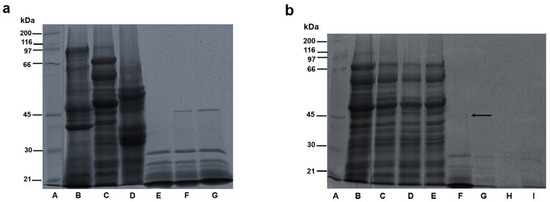
Figure 3.
(a) SDS-PAGE profile of chickpea protein concentrates and hydrolysates. Lane A: molecular weight marker; lane B: Al; lane C: Gb; lane D: Gt; lane E: HAl; lane F: HGb; lane G: HGt. (b) SDS-PAGE profile of globulin concentrates, and hydrolysates pretreated with pulsed ultrasound. Lane A: molecular weight marker; lane B: Gb; lane C: Gb-10; lane D: Gb-20; lane E: Gb-30; lane F: HGb; lane G: HGb-10; lane H: HGb-20; lane I: HGb-30.
The degradation of the protein subunits by the effect of enzymatic hydrolysis on savinase was increased, and there was a presence of bands with molecular weights ranging from 30 to 21 kDa for albumin hydrolysates (Figure 3a, lane E) and bands with molecular weights from 45 to 21 kDa for globulin and glutelin hydrolysates (Figure 3a, lanes F and G). In the study by Garcia-Mora, Peñas, Frias and Martínez-Villaluenga [27], the use of savinase caused a complete degradation of lentil protein subunits within a hydrolysis time of two hours, forming smaller fragments, as was what occurred in this study.
The electrophoretic profile of globulin concentrates and globulin hydrolysates with ultrasound pretreatment obtained in this study is shown in Figure 3b. No differences were found between the banding pattern of globulin concentrate without ultrasound pretreatment and the globulin concentrates pretreated with ultrasound (Figure 3b; lanes B, C, D and E). Results are in accordance with the study by Wang, Wang, Li, Bai, Li and Xu [22], where there was no difference in bands between a chickpea protein isolate with and without ultrasonic treatment. Ultrasound treatment causes changes in the tertiary structure of proteins but not in the secondary structure [19].
The difference in the electrophoretic profile of globulin hydrolysates with ultrasound pretreatment (Figure 3b; lanes G, H, and I) in comparison to the control hydrolysate lies in the disappearance of the 45 kDa band (indicated by an arrow in Figure 3b; lane F). The degradation of the 45 kDa band could be attributed to ultrasound pretreatment, since an ultrasound modifies the structure of proteins, which facilitates bonding between protease and the substrate, increasing hydrolysis efficiency [15,17].
3.6. Amino Acid Composition
The amino acid contents of the two hydrolysates with the highest antioxidant activity, HGb and HGb-20, are presented in Table 3. Both hydrolysates had a high content of Asp, Arg, Ser, Thr, Gly and Ala. Misir and Koral [52] found not differences between the amino acid content of rainbow trout by-product hydrolysates obtained with ultrasound-assisted hydrolysis and those obtained with conventional hydrolysis, using alcalase in both hydrolysis process. These results showed that the amino acid content depends on the protein source and on the ultrasound pretreatment prior to hydrolysis.
Paredes-López, Ordorica-Falomir and Olivares-Vázquez [39] reported high contents of Asp, Glu, Arg, Lys, Ser and Leu for chickpea globulin. Liu, Hung and Bennett [37] determined Asp, Glu, Arg and Leu as the predominant amino acids in a chickpea globulin concentrate. The results of the present study coincide with those described above, finding an abundance of acidic, basic, and hydrophobic amino acids to which the antioxidant activity of globulin hydrolysates can be attributed. Hydrophobic amino acids such as Gly and Ala increase the solubility of protein hydrolysates in lipids due to their nature, causing an interaction with the target organs through hydrophobic interactions with the lipid bilayer of cells, promoting the neutralization of free radicals. In turn, Asp can neutralize free radicals due to its excess of electrons [2,18]. The basic amino acid Arg acts as a proton donor, given its guanidine group [13]. The globulin hydrolysates of the present study showed SET and HAT mechanisms, according to the antioxidant activity assays performed.
3.7. Antimicrobial Activity of Globulin Hydrolysates
Given that protein hydrolysates could display multiple bioactivities, the antimicrobial activity of the globulin hydrolysates with the highest antioxidant activity (HGb and HGb-20) was evaluated. The results are shown in Table 4. A descending trend for the CFU of Salmonella enterica was observed when incrementing HGb concentration, with a value of 38 ± 2.08 CFU for 10 mg/mL, significantly different from other concentrations of the same hydrolysate. By contrast, the lower value of CFU (35 ± 3.05) for HGb-20 was with 5 mg/mL of said hydrolysate. For Staphylococcus aureus, the descending trend occurred when incrementing HGb-20, with a value of 48 ± 2.64 CFU for 10 mg/mL and with significant differences from other concentrations of the same hydrolysate. On the other hand, the lower value of CFU (58 ± 4) for HGb was at 5 mg/mL of the hydrolysate.

Table 4.
Evaluation of the antimicrobial activity of globulin hydrolysates.
MIC values were not determined, given that more than 20 UFCs were counted in all cases. Even still, in this preliminary assay, the inhibition of a Gram+ microorganism by HGb-20 and the inhibition of a Gram- microorganism by HGb was observed, implying differences in the composition of the amino acids present in the peptide sequences of each type of hydrolysate, which could be attributed to differences in the affinity between the hydrolysates and the evaluated microorganisms.
Amino acids present in the HGb sequences have more interactions with the lipopolysaccharides of the outer membrane of Gram-negative bacteria compared to the amino acids present in the HGb-20 sequences, causing the disintegration of microorganisms [6]. On the other hand, the higher affinity of the amino acids present in the HGb-20 sequences with the peptidoglycan cell wall of the Gram-positive bacteria could be because of the ultrasound pretreatment carried out in obtaining the globulin hydrolysates [57]. However, it is necessary to carry out more studies to determine the antimicrobial activity of globulin protein hydrolysates.
4. Conclusions
The application of pulsed ultrasound pretreatment to chickpea globulin allows for the production of hydrolysates and peptide fractions with high antimicrobial activity and antioxidant capacity and a high protective effect in human erythrocytes, improving the proton donation mechanism of hydrolysates and peptide fractions obtained from globulin. Protein hydrolysates obtained with the savinase enzyme display multiple biological activities, given their amino acidic composition, that are antioxidant and antimicrobial in nature and of great relevance to the health and food industry. The compounds identified in this study could be used as ingredients in the elaboration of functional foods, since it is necessary to evaluate their potential beneficial effect using in vivo assays. Moreover, protein hydrolysates could be used for the preservation of food matrices, be applied directly to food products, or be added into films and coatings made with biopolymers. In this manner, the effect of the application of chickpea protein hydrolysates on food preservation should be evaluated.
Author Contributions
Conceptualization, A.C. and E.L.-C.; methodology, J.M.E.-B. and H.S.-V.; validation; W.T.-A. and E.M.-R.; formal analysis, M.F.G.-O. and G.G.-S.; investigation, J.C.R.-F.; resources C.L.D.-T.-S., data curation, F.J.W.-C.; writing—original draft preparation, M.F.G.-O.; supervision, C.L.D.-T.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad de Sonora, grant number USO313007398.
Institutional Review Board Statement
The study was carried out in accordance with the Declaration of Helsinki of 1975. The clinical laboratory is accredited by ISO-IEC 17.025 (NMX-EC-17025) and ISO 15.189 prepared by the technical committee ISO/TC 212 (Clinical Laboratory Testing and In vitro Diagnostic Systems) taking as reference the ISO/IEC 17.025 and ISO 9001 standards.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Informed consent was for blood donation. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The original contributions and data presented in this research are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
María Fernanda González Osuna acknowledges the Consejo Nacional de Ciencia y Tecnología (CONACyT, Mexico) for Ph.D. scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dhaval, A.; Yadav, N.; Purwar, S. Potential applications of food derived bioactive peptides in management of health. Int. J. Pept. Res. Ther. 2016, 22, 377–398. [Google Scholar] [CrossRef]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding food loss and waste -Why are we losing and wasting food? Foods 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef] [PubMed]
- Nasri, R.; Abdelhedi, O.; Nasri, M.; Jridi, M. Fermented protein hydrolysates: Biological activities and applications. Curr. Opin. Food Sci. 2022, 43, 120–127. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Xue, Z.; Jia, Y.; Li, R.; He, C.; Chen, H. The structure-mechanism relationship and mode of actions of antimicrobial peptides: A review. Trends Food Sci. Technol. 2021, 109, 103–115. [Google Scholar] [CrossRef]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A Review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [CrossRef]
- Pan, M.; Liu, K.; Yang, J.; Liu, S.; Wang, S.; Wang, S. Advances on food-derived peptidic antioxidants–A review. Antioxidants 2020, 9, 799. [Google Scholar] [CrossRef]
- Sosalagere, C.; Kehinde, B.A.; Sharma, P. Isolation and functionalities of bioactive peptides from fruits and vegetables: A reviews. Food Chem. 2022, 366, 130494. [Google Scholar] [CrossRef] [PubMed]
- Coscueta, E.R.; Amorim, M.M.; Voss, G.B.; Nerli, B.B.; Picó, G.A.; Pintado, M.E. Bioactive properties of peptides obtained from Argentinian defatted soy flour protein by Corolase PP hydrolysis. Food Chem. 2016, 198, 36–44. [Google Scholar] [CrossRef]
- García-Mora, P.; Martín-Martínez, M.; Bonache, M.A.; González-Múniz, R.; Peñas, E.; Frias, J.; Martínez-Villaluenga, C. Identification, functional gastrointestinal stability and molecular docking studies of lentil peptides with dual antioxidant and angiotensin I converting enzyme inhibitory activities. Food Chem. 2017, 221, 464–472. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Sila, A.; Przybylski, R.; Nedjar-Arroume, N.; Makhlouf, I.; Blecker, C.; Attia, H.; Dhulster, P.; Bougatef, A.; Besbes, S. Purification and identification of novel antioxidant peptides from enzymatic hydrolysate of chickpea (Cicer arietinum L.) protein concentrate. JFF 2015, 12, 516–525. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Del Mar Contreras, M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef]
- Heymich, M.L.; Friedlein, U.; Trollmann, M.; Schwaiger, K.; Böckmann, R.A.; Pischetsrieder, M. Generation of antimicrobial peptides Leg1 and Leg2 from chickpea storage protein, active against food spoilage bacteria and foodborne pathogens. Food Chem. 2021, 347, 128917. [Google Scholar] [CrossRef]
- Wang, B.; Atungulu, G.G.; Khir, R.; Geng, J.; Ma, H.; Li, Y.; Wu, B. Ultrasonic treatment effect on enzymolysis kinetics and activities of ACE-inhibitory peptides from oat-isolated protein. Food Biophys. 2015, 10, 244–252. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, S. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Álvarez, C.; O’Donnell, C.P. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci. Technol. 2015, 46, 60–67. [Google Scholar] [CrossRef]
- Yu, H.C.; Tan, F.J. Optimization of ultrasonic-assisted enzymatic hydrolysis conditions for the production of antioxidant hydrolysates from porcine liver by using response surface methodology. Asian-Australas. J. Anim. Sci. 2017, 30, 1612–1619. [Google Scholar] [CrossRef]
- Shi, R.J.; Chen, Z.J.; Fan, W.X.; Chang, M.C.; Meng, J.I.; Liu, J.Y.; Feng, C.P. Research on the physicochemical and digestive properties of Pleurotus eryngii protein. Int. J. Food Prop. 2018, 21, 2785–2806. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Y.; Xie, L.; Sun, H.; Zheng, Z.; Liu, F. Effects of enzymatic cross-linking combined with ultrasound on the oil adsorption capacity of chickpea protein. Food Chem. 2022, 383, 132641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Li, R.; Wang, Y.; Xiang, Q.; Li, K.; Bai, Y. Effects of combined treatment with ultrasound and pH shifting on foaming properties of chickpea protein isolate. Food Hydrocoll. 2022, 124, 107351. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, K.; Bai, Y.; Li, B.; Xu, W. Effect of high intensity ultrasound on physicochemical, interfacial and gel properties of chickpea protein isolate. LWT 2020, 129, 109563. [Google Scholar] [CrossRef]
- Perez-Perez, L.M.; Huerta-Ocampo, J.A.; Ruiz-Cruz, S.; Cinco-Moroyoqui, F.J.; Wong-Corral, F.J.; Rascón-Valenzuela, L.A.; Robles-García, M.A.; González-Vega, R.I.; Rosas-Burgos, E.C.; Corella-Madueño, M.A.G.; et al. Evaluation of quality, antioxidant capacity, and digestibility of chickpea (Cicer arietinum L. cv Blanoro) stored under N2 and CO2 Atmospheres. Molecules 2021, 26, 2773. [Google Scholar] [CrossRef]
- Hayta, M.; İşçimen, M. Optimization of ultrasound-assisted antioxidant compounds extraction from germinated chickpea using response surface methodology. LWT 2017, 77, 208–216. [Google Scholar] [CrossRef]
- Tovar-Pérez, E.G.; Guerrero-Becerra, L.; Lugo-Cervantes, E. Antioxidant activity of hydrolysates and peptide fractions of glutelin from cocoa (Theobroma cacao L.) seed. CYTA J. Food 2017, 15, 489–496. [Google Scholar] [CrossRef]
- Tontul, I.; Kasimoglu, Z.; Asik, S.; Atbakan, T.; Topuz, A. Functional properties of chickpea protein isolates dried by refractance window drying. Int. J. Biol. Macromol. 2018, 109, 1253–1259. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Martínez-Villaluenga, C. Savinase, the most suitable enzyme for releasing peptides from lentil (Lens culinaris var. Castellana) protein concentrates with multifunctional properties. J. Agric. Food Chem. 2014, 62, 4166–4174. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC: Arlington, VA, USA, 2016. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Protoggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay free radical. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Hernández-Ruiz, K.L.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Gassos-Ortega, L.E.; Ornelas-Paz, J.J.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; López-Mata, M.A.; Rodríguez-Félix, F. Evaluation of antioxidant capacity, protective effect on human erythrocytes and phenolic compound identification in two varieties of plum fruit (Spondias spp.) by UPLC-MS. Molecules 2018, 23, 3200. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ortiz, F.A.; Caire, G.; Higuera-Ciapara, I.; Hernández, G. High performance liquid chromatographic determination of free amino acids in shrimp. J. Liq. Chromatogr. Relat. Technol. 1995, 18, 2059–2068. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.d.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total phenolic, flavonoid, tomatine, and tomatidine contents and antioxidant and antimicrobial activities of extracts of tomato plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.G.; Markham, J.L.; Leach, D.N. An agar dilution method for the determination of the minimum inhibitory concentration of essential oils. J. Essent. Oil Res. 2013, 12, 249–255. [Google Scholar] [CrossRef]
- Liu, L.H.; Hung, T.V.; Bennett, L. Extraction and characterization of chickpea (Cicer arietinum) albumin and globulin. J. Food Sci. 2008, 73, 299–305. [Google Scholar] [CrossRef]
- Singh, U.; Jambunathan, R. Distribution of seed protein fractions and amino acids in different anatomical parts of chickpea (Cicer arietinum L.) and pigeonpea (Cajanus cajan L.). Plant Foods Hum. Nutr. 1982, 31, 347–354. [Google Scholar] [CrossRef]
- Paredes-López, O.; Ordorica-Falomir, C.; Olivares-Vázquez, M.R. Chickpea protein isolates: Physicochemical, functional and nutritional characterization. J. Food Sci. 1991, 56, 726–729. [Google Scholar] [CrossRef]
- Ahmed, M.A. Protein isolates from chickpea (Cicer arietinum L.) and its application in cake. Int. J. Biol. Vet. Agric. Food Eng. 2014, 8, 1101–1107. [Google Scholar]
- Evangelho, J.A.D.; Vanier, N.L.; Pinto, V.Z.; Berrios, J.J.; Dias, A.R.G.; Zavareze, E.D.R. Black bean (Phaseolus vulgaris L.) protein hydrolysates: Physicochemical and functional properties. Food Chem. 2017, 214, 460–467. [Google Scholar] [CrossRef]
- Ngoh, Y.Y.; Gan, C.Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Guerra-A., C.M.; Murillo, W.; Méndez-A., J.J. Antioxidant potential use of bioactive peptides derived from mung bean hydrolysates (Vigna radiata). Afr. J. Food Sci. 2017, 11, 67–73. [Google Scholar] [CrossRef]
- Guerra-Almonacid, C.M.; Torruco-Uco, J.G.; Murillo-Arango, W.; Méndez-Arteaga, J.J.; Rodríguez-Miranda, J. Effect of ultrasound pretreatment on the antioxidant capacity and antihypertensive activity of bioactive peptides obtained from the protein hydrolysates of Erythrina edulis. Emir. J. Food Agric. 2019, 31, 288–296. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Luo, X.Y.; Liu, G.Z.; Chen, Y.P.; Wang, Z.C.; Sun, Y.X. In vitro study of the relationship between the structure of ginsenoside and its antioxidative or prooxidative activity in free radical induced hemolysis of human erythrocytes. J. Agric. Food Chem. 2003, 51, 2555–2558. [Google Scholar] [CrossRef]
- Sbroggio, M.F.; Montilha, M.S.; Figueiredo, V.R.G.; Georgetti, S.R.; Kurozawa, L.E. Influence of the degree of hydrolysis and type of enzyme on antioxidant activity of okara protein hydrolysates. Food Sci. Technol. 2016, 36, 375–381. [Google Scholar] [CrossRef]
- Zheng, L.; Dong, H.; Su, G.; Zhao, Q.; Zhao, M. Radical scavenging activities of Tyr, Trp-, Cys- and Met-Gly and their protective effects against AAPH-induced oxidative damage in human erythrocytes. Food Chem. 2016, 197, 807–813. [Google Scholar] [CrossRef]
- Zhan, Q.; Wang, Q.; Liu, Q.; Guo, Y.; Gong, F.; Hao, L.; Dong, Z. The antioxidant activity of protein fractions from Sacha inchi seeds after a simulated gastrointestinal digestion. LWT 2021, 145, 111356. [Google Scholar] [CrossRef]
- Santos-Aguilar, J.G.; de Castro, R.; Sato, H.H. Optimization of the enzymatic hydrolysis of rice protein by different enzymes using the response surface methodology. 3 Biotech 2018, 8, 372. [Google Scholar] [CrossRef]
- Piñuel, L.; Vilcacundo, E.; Boeri, P.; Barrio, D.A.; Morales, D.; Pinto, A.; Moran, R.; Samaniego, I.; Carrillo, W. Extraction of protein concentrate from red bean (Phaseolus vulgaris L.): Antioxidant activity and inhibition of lipid peroxidation. J. Appl. Pharm. Sci. 2019, 9, 45–58. [Google Scholar] [CrossRef]
- Xia, E.; Zhai, L.; Huang, Z.; Liang, H.; Yang, H.; Song, G.; Li, W.; Tang, H. Optimization and identification of antioxidant peptide from underutilized Dunaliella salina protein: Extraction, in vitro gastrointestinal digestion, and fractionation. BioMed Res. Int. 2019, 2019, 6424651. [Google Scholar] [CrossRef]
- Misir, G.B.; Koral, S. Effects of ultrasound treatment on structural, chemical and functional properties of protein hydrolysate of rainbow trout (Onchorhynchus mykiss) by-products. Ital. J. Food Sci. 2019, 31, 205–223. [Google Scholar]
- Guo, J.; Zhang, T.; Jiang, B.; Miao, M.; Wu, W. The effects of and antioxidative pentapeptide derived from chickpea protein hydrolysates on oxidative stress in Caco-2 and HT-29 cell lines. JFF 2014, 7, 719–726. [Google Scholar] [CrossRef]
- Papalamprou, E.M.; Doxastakis, G.I.; Biliaderis, C.G.; Kiosseoglou, V. Influence of preparation methods on physicochemical and gelation properties of chickpea protein isolates. Food Hydrocoll. 2009, 23, 337–343. [Google Scholar] [CrossRef]
- Chang, Y.W. Isolation and Characterization of Protein Fractions from Chickpea (Cicer arietinum L.) and Oat (Avena sativa L.) Seeds Using Proteomic Techniques. Doctoral Thesis, McGill University, Montreal, QC, Canada, July 2010. [Google Scholar]
- Chang, Y.W.; Alli, I.; Molina, A.T.; Konishi, Y.; Boyce, J.I. Isolation and characterization of chickpea (Cicer arietinum L.) seed protein fractions. Food Bioprocess Technol. 2012, 5, 618–625. [Google Scholar] [CrossRef]
- Shilhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).