Abstract
One of the major challenges that global society is facing nowadays is finding sustainable and safe methods for crop growth and development. Besides the traditional crops cultivated worldwide (tomatoes, potatoes, lettuce, strawberries, etc.), there is a general trend in the exploitation of polyvalent plants. Mulberry (Morus spp.) faced no exception; with its undeniable proprieties, it became suitable not only to be used in the sericulture industry, but in the food chain, the pharma industry, and environmental safety. Spare parts of the plants can be used in a very wide range, starting from introducing mulberry leaves in supplements to increase the protein content of a meal to extracting biologically active compounds from fruits and roots to be used in phytotherapy. However, the outstanding proprieties of this plant come with some requirements related to space availability and watering; requirements that can be easily surpassed by using vertical farming methods, such as hydroponic, aeroponic, or aquaponic systems. The present paper aims to evaluate vertical farming techniques’ applicability to mulberry propagation in a controlled environment and their prospects for a more sustainable and safer agricultural practice.
1. Introduction
Mulberry is a deciduous wooden tree, belonging to the Morus genus and Moraceae family. It is a fast-growing plant, widely spread across the globe, from temperate to tropical areas, and used for sericulture and Ayurveda for centuries [1,2]. There are more than 15 species of mulberry with over 500 varieties, out of which M. alba, M. indica, M. nigra, M. bombycis, M. australis, M. cathayana, M. rubra, and M. atropurpurea are the ones with significant importance either in foliage or in fruits production [3]. The mulberry is typically a unisex dioecious plant, meaning that some of its individuals only produce male blooms, while others only produce female blossoms. Additionally, it is possible to find unisexual monoecious plants, which have both male and female flowers on the same plant. Mulberry is a seed-propagated, highly cross-pollinated species, and it can grow in countless varieties or shapes, with distinct fruit and leaf traits [4]. Mulberry leaves are used primarily in sericulture, being the sole feed for Bombyx mori silkworms and are intensively cultivated in countries with great silk production (China, India, Uzbekistan, Thailand, etc.). In contrast, European countries prefer to use mulberry trees for their fruit production and transform them into different food products (jams, vinegar, wine, pastries, etc.).
Besides the nutritional proprieties of mulberry, the physicochemical composition of both leaves and fruits rich in biologically active compounds gives the plant an added value. As such, a considerable quantity of polyphenols, among which the most common are rutin, quercetin, kaempferol, gallic acid, and chlorogenic acid, present in the fruits plays an important role in antioxidative processes and have cardio-protective, anti-inflammatory, antidiabetic effects [5]. In addition, mulberry fruits synthesize unique polyhydroxy alkaloid, 1-Deoxynojirimycin (DNJ), with remarkable applications in medicine and pharmacy. In vivo studies show that patients with cardiovascular diseases reported an amelioration of symptoms after the mulberry leaf powder had been administrated [6,7,8]. Positive results have been also reported when type 2 diabetes model rats were treated with mulberry leaves extracts [9,10,11,12], thus increasing the interest in cultivating it as a nutraceutical plant worldwide (Figure 1).
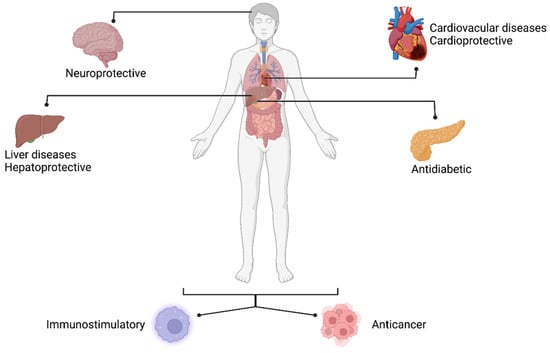
Figure 1.
Illustration of the applicability of mulberry in biomedicine (created with BioRender.com, accessed on 10 February 2023).
Mulberries can be cultivated in a variety of soil types and topographical conditions that are unsuitable for most agricultural crops. However, it is generally known that mulberries develop normally when being exposed to an atmosphere temperature range from 13 °C to 37,7 °C, but the maximum sprout of buds happens when the environmental temperature is 24 °C to 28 °C, with a relative humidity of 65–80% and sunlight duration of 5 to 12 h per day. Mulberry can easily be developed under rainfed conditions when the downfall range is between 600 mm and 2500 mm. If the water conditions are poor due to insufficient rain, an additional irrigation system is necessary to be implemented [13]. Mulberry can grow in various types of soils; it usually thrives in sandy loam soils that range from being slightly acidic to neutral (pH 6.2–6.8) and have significant levels of organic carbon [14]. The general maintenance work required for a mulberry plantation is, in addition to irrigation, fertilizer application, and very effective management of diseases, pests and competitive plants. The application of pesticides and herbicides must be avoided and carried out only in extreme cases, when other treatments are not applicable, due to the compromise of leaves and fruits, that cannot be further used in feeding silkworms, obtaining pharmaceutical extracts, or used in the food industry. This makes mass production susceptible to losses. Moreover, the application of a relatively large amount of water and manure are less environmentally friendly solutions.
Although mulberry is a versatile plant, which can easily adapt to different altitudes, soils, or climates, it represents a challenge for third-millennium agriculture to exploit its potential in unfavorable environments and obtain all-year-round production. Biotechnology played a major role in overcoming biotic and abiotic stress that occurs in mulberry, using genetic engineering techniques. Some recent examples of lowering the biotic stress in Mulberry include transgenic plants that developed resistance to the fungal pathogen Botrytis cinerea, which causes gray mold [15], or to Lasiodiplodia theobromae, which causes black root rot [16]. The use of some antimicrobial peptides for disease control has also been reported [17]. In terms of abiotic factors, caused mainly by cold temperature and soil salinity, stress resistance mediated by MuNPR1, MuNPR4 [18], or miR171 [19] genes have been proven to be efficient in some tested varieties. Furthermore, obtaining mulberry seedlings, regardless of the environmental conditions, has been successfully achieved through in vitro propagation. Murashige and Skoog basal medium, supplemented plant growth regulators, showed performances in cell division, elongation, shoot formation, or callus initiation (auxins), regulate differentiation, stimulate protein and enzymes’ activity (cytokinins), promote flowering, stem elongation, and awaking the seeds or buds when applied to mulberry explants [20,21,22,23]. However, although the micropropagation technique offers the possibility of having seedlings that can develop in the winter months, this solves only part of the problem. Obtaining the mass production of leaves and/or fruits still represents a real challenge for countries with a temperate climate, areas with acidic soils or with a high level of salinity, but also with poor light and rain conditions. Thus, the prospects of using vertical farming technology have been brought into consideration. In comparison to conventional agriculture, vertical farming may produce yields while using less land and water and may not need any pesticides. In order to build resilient crop-producing systems, especially in and around highly populated places, vertical farming technology (VFT) can help producers to meet their expected yield all year round. Fruits, vegetables, and herbs are among the products already produced by VFT, using multi-layer indoor crop cultivation systems such as hydroponic, aquaponic, and aeroponic systems [24].
Based on the latest literature in the field, this paper aims to review the physicochemical composition of mulberry, correlated with their applicability in the medicine and food industry and overall economic importance, as well as the prospects of growing mulberry in more sustainable alternative methods, such as vertical farming technology.
2. Significance of Mulberry (Morus spp.)
2.1. Medicinal Value of Mulberry
Since the beginning of time, humans have had a major need to treat various diseases, and their first instinct was to use plants for this purpose. Nowadays, the pharmaceutical industry and medicine are making tremendous progress. Despite that, therapeutic plants are still being highly used to prevent or treat a wide range of medical conditions. Therefore, numerous scientific laboratories are focused on herbal medicine, and new plant-based drugs are being developed [25]. It is well known that botanical medicine was first used in traditional Chinese and Indian medicine [26], although, as reported by the WHO, nowadays, no less than 80% of the globe’s population uses therapeutic plants for health-related situations [27].
Nowadays, cancer is one of the leading causes of death worldwide. Aiming to decrease morbidity and mortally cancer related rates, the scientific community is constantly developing new treatment approaches [28]. Considering the great potential of plants as a rich reservoir of various bioactive elements, tremendous efforts are being made to develop anticancer plant-based therapeutics. Moreover, a wide range of anticancer agents that are frequently used in clinical practice originated from plants, such as irinotecan or paclitaxel [29,30]. In this direction, due to its complex composition, numerous studies explored the anticancer effects of mulberry [31,32,33,34,35,36].
In 2021, it was explored, in a novel study, the cytotoxic potential of M. nigra fruit extract by using two human cancer lines, specifically, MDA-MB-231 (breast cancer) and PC3 (prostate cancer) [37]. The authors applied the mulberry extract on the cell lines for three days by using five different concentrations, 1%, 1.33%, 2%, 4%, and 10%, respectively. For measuring the cell viability, the MTT assay was used, and the results revealed that mulberry exhibits cytotoxic effect in a concentration-dependent manner. When it comes to the prostate cancer cell line, the cell viability was affected only by applying a mulberry extract of 10% concentration. On the other hand, when applying mulberry extracts of lower concentrations, no cytotoxic effect was registered. As for the breast cancer cell line, similar results were registered. The highest concentration led to the most powerful cytotoxic effect; however, lower anticancer activity was reported at 1%, 1.33%, 2%, and 4%, respectively. Moreover, when comparing the anticancer effect of the same concentrations of the doxorubicin and mulberry extract, the cytotoxic effect of the fruit extract was higher than the outcome of using the chemotherapy drug.
In another study [38], a research team compared the cytotoxic impact of the mulberry flavonoid extract (M. alba) with the effect of doxorubicin on a human colon cancer cell line, specifically HT-29. Furthermore, the authors investigated the cytotoxic action of the two products combined. Aiming at that, they evaluated the adenomatous polyposis coli (APC) expression level induced by the two products, individually and combined. This APC gene expression was assessed due to the fact that it plays a key role in tumor suppression. Furthermore, the poly (ADP-ribose) polymerase (PARP) concentration in the cancer cell line was evaluated after applying the treatments. PARP represents a key protein that plays various roles in certain biological processes, and several studies showed that it is found in higher concentrations in tumor cells. Accordingly, there is a great interest in developing new therapeutics based on PARP inhibitors. Firstly, cancer cell viability was determined by MTT assay after the cell line was exposed to different concentrations of target products for 24 h. The results showed cell viability was affected by administering mulberry flavonoids extract, depending on the applied concentration. Moreover, the effect of the natural extract was similar to the cytotoxic action of doxorubicin. Subsequently, in order to evaluate if cell viability was run-down due to the increased APC gene expression, the authors used the quantitative real-time PCR technique. Their data revealed that all three tested products led to a significant increase in the APC gene expression level in colon cancer cell lines. Pursuing to analyze if the PARP level is correlated with the loss of cell viability, the ELISA assay was performed. Interestingly, in the presence of mulberry leaf extract, the level of PARP not only was reduced, but it was even lower than in the other two groups.
Another major threat, and one of the most widespread chronic diseases, is diabetes. When it comes to this specific disorder, it is crucial to manage blood sugar levels in order to prevent regrettable outcomes [39]. Considering the undesirable side effects of the drugs that are available today, it is imperative to develop new plant-based therapeutics in order to investigate the hypoglycemic potential [40]. It is well documented that mulberry is used especially in Asian countries to treat diabetes.
Yang et al. [41] carried out in vivo research that aimed to evaluate the hypoglycemic action of mulberry leaf extract. For this purpose, the pivotal alkaloid was extracted from mulberry leaves, namely DNJ. For 15 days, the extract was administered to diabetic mice, and the authors observed that DNJ significantly inhibits glucose absorption by suppressing the activity of the enzyme that is responsible for decomposing sugar. Considerably, in the animal model, the blood sugar level was reduced by up to 50%.
In another research, Wistar rats were used to evaluate the potential hypoglycemic effect of mulberry leaf extract (M. alba). For 35 days, the induced-diabetic animals were treated with the target extract and several parameters were measured, for instance, blood glucose, triglyceride level, LDL, HDL, blood urea, the amount of β cells, and so on. Compared with the control group, in the diabetic-induced animals, all studied parameters, except HDL, were highly elevated. However, when applying 600 mg/kg/day, all investigated parameters were restored to the initial values that were determined in the control group. On the other hand, when a smaller amount of mulberry leaf extract was administered, specifically 400 mg/kg/day, hyperglycemia was highly reduced, but the studied levels of certain parameters were not restored [42].
Eo et al. (2014) evaluated the anticancer effect of M. alba root bark. The authors used the mouse macrophage cell line (RAW264.7) to analyze the anti-inflammatory activity, keeping in mind the role of macrophages in producing inflammatory mediators, including NO or TNF-α. NO represents a pivotal factor in controlling the inflammatory response, and in this study, it was shown that mulberry root bark suppresses the over-production of NO. A colorectal cancer cell line (SW480) was used to assess cell viability and apoptosis; two techniques were employed, specifically the MTT assay and Western blot, to evaluate both parameters. Moreover, they investigated if the mulberry root bark treatment included the expression initiation of the activating transcription factor 3 (ATF3), and the regulation of the cyclin D1 level, which are related to the cell growth suppression process and apoptosis. Their study was the first report that confirmed the anti-inflammatory and anticancer effects of mulberry root bark [43].
Yin et al. (2017) [44] used diabetic-induced mice to evaluate the mulberry branch bark potential for regulating insulin secretion. Their data revealed that it is effective in regulating insulin release, but also in controlling glucose levels. In a novel study, Qiu and Zhang (2019) [45] explored the impact of mulberry (M. multicaulis) branch bark on diabetic model mice. The mice were injected with streptozotocin and were fed with a high-fat diet for five weeks. After this time, diabetes symptoms were perceived, including food and fluid intake or high glucose levels, also great insulin resistance was observed. Their results confirmed that mulberry branch bark exhibits a re-balancing impact in diabetic mice by ameliorating the disturbed metabolic processes involved in this endocrine disease.
In addition to mulberry’s antioxidative, anticancer, and hypoglycemic therapeutic effects, a wide range of studies investigated and confirmed other great curative actions of this outstanding plant, highlighting its hypolipidemic, hepatoprotective, antibacterial, and anti-obesity activities. These therapeutic effects are correlated with the main bioactive compounds from leaves, fruits, and root bark, as shown in Table 1.

Table 1.
Main biological compounds from different parts of mulberry and their health benefits.
2.2. Economic Impact of Mulberry
Besides the fact that mulberry exhibits great interest in the scientific community due to its distinctive medicinal values, it plays key roles in numerous industries; therefore, it is a significant player in the global economy (Figure 2).
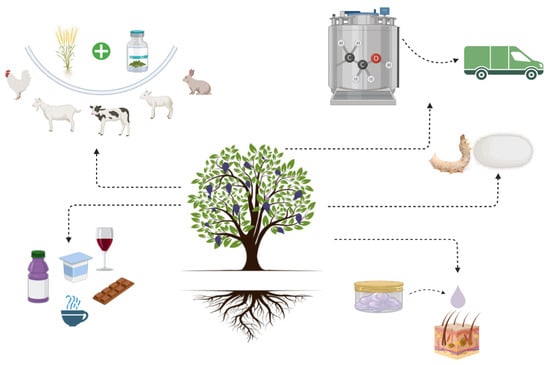
Figure 2.
Main applications of mulberry in various industries (created with BioRender.com, accessed on 10 February 2023).
Mulberry has an indispensable role in sericulture, being the exclusive source of food for silkworms. There is an increasing demand when it comes to the silk produced by B. mori; therefore, the role played by mulberry is even higher for the economic sector [61]. Furthermore, among the most important insects, such as Apis mellifera or Drosophila melanogaster [62], B. mori plays a pivotal role in the biomedicine and pharmaceutical industry. It has been reported that the mulberry leaf content influences silk production. In a particular study, a research team investigated if the moisture content determines certain B. mori parameters, such as larval weight, and filament length, just to mention a few. In terms of mulberry leaves’ moisture level, they demonstrated that by feeding the silkworms with tender leaves that involve higher moisture, all the studied parameters were positively impacted. On the other hand, when the silkworms received coarse leaves that have a lower moisture content, there was observed a negative correlation [63]. In another study, Susanti et al. (2021) [64] evaluated the impact of mulberry leaf quality on silkworms’ nutrition index (growth rate, consumption rate, efficiency of conversion of digested and ingested food, and approximated digestibility). There were defined two groups of newly hatched larvae, one group was fed with unfertilized mulberry leaves and the other group received leaves of fertilized mulberry. Their results showed that by feeding the silkworms with leaves of mulberry plants that were treated with fertilizer, their nutrition index was positively impacted.
Even if the main reason for cultivating mulberry is the silkworms’ rearing, it has been demonstrated that it is a great alternative to replace fodder crops in order to fulfill the high demand of feeding domestic animals. In China, India, but also in Korea, the leftover branches and leaves are used to feed domestic animals. Furthermore, mulberry leaves are currently utilized as supplements to fulfill a balanced diet. Several studies showed that by adding mulberry leaf as a supplement in dairy animals’ nourishment, milk production was increased. Moreover, due to the addition of mulberry leaves, the lipid and fat contents were increased in cow and goat milk. Other researchers successfully used mulberry leaf in order to substitute the commercial concentrate used for pigs and rabbits. In other studies, mulberry leaf was used to supplement hens’ nourishment, and the formulation not only led to bigger eggs, but the yolk color was also improved [65]. Keeping in mind the great potential of the mulberry plant as a promising food supplement, Cai et al. (2019) [66] investigated the toxicity potential of mulberry leaves by using Sprague–Dawley rats. The experimental animals were divided into three groups, as follows: a control group that had not received mulberry leaves, the second group that received 1 g/kg body weight, and the third group that received the highest mulberry quantity, 2 g/kg body weight, respectively. For 28 days, the treatment was administrated once a day, via intragastric gavage. In this study, they analyzed several traits, including rats’ behavior, body weight, feed intake, and numerous blood parameters. By comparing with the control group, neither one of the experimental groups that received mulberry leaves as a food supplement registered any adverse effects. In terms of the livestock feed solution, from an economic point of view, mulberry represents one of the most feasible options for increasing the forage production for livestock due to its extended geological distribution. It is displaying a wide range of ecotypes. On the other hand, by being in the scientific community’s attention for a long period of time, there have been described numerous techniques for mulberry reproduction, cultivation, or pest and disease management. However, there are several studies that certified that mulberry represents a cheaper alternative for supplementing livestock feed [59,60]. For instance, in a study conducted by Islam et al. (2014), a broiler chicken diet was developed, more specifically, a low-cost recipe of a grower diet, which led to the production of low-cholesterol meat [61].
It is well documented that mulberry composition includes various bioactive elements that make mulberry a great functional food. Nowadays, there is a wide range of applications for mulberry in food industries; moreover, it has been processed in different types of products, such as jams, wines, syrups, just to mention a few. For instance, by using M. alba, a group of researchers obtained wine that contained a high level of phenolic compounds. However, in another research, vinegar was obtained from M. alba, and the authors confirmed its antioxidative and antibacterial activities. On another note, by using M. nigra, a research group obtained enriched pasta that displayed hypoglycemic action on human organisms [2]. In a recent study, On-Nom et al. (2020) [67] formulated a mulberry fruit jelly and evaluated its therapeutic effects on 60 dyslipidemia subjects. For seven days, the subjects received one serving size of anthocyanin-rich mulberry fruit jelly (191 mg). After receiving the treatment, various parameters were analyzed, including blood glucose, lipid, insulin, but also oxidative stress. The results revealed that acute consumption of anthocyanins-rich mulberry fruit jelly could lead to a lower risk of cardiometabolic disorders, by significantly decreasing the levels of blood total cholesterol, low-density lipoprotein, and inflammatory markers. Furthermore, by consuming this product, the subjects’ insulin sensitivity was improved [67].
When talking about mulberry’s impact on the global economy, it plays a great role in the cosmetic industry. Mulberry is a component of several creams or bath gels, but, also, it is a constituent of other products that are currently on the market. It is well documented that mulberry has a great contribution in diminishing skin conditions associated with aging [2]. Moreover, it was revealed that mulberry has a great role as a skin whitening agent [2]. It is an inhibitor of tyrosinase which is involved in melanin biosynthesis. There have been described several mulberry-based cosmetic products that aim to counterattack hyperpigmented disorders by inhibiting tyrosinase activity [65].
Another significant role played by mulberry is in the environmental safety area, as shown in Figure 3.
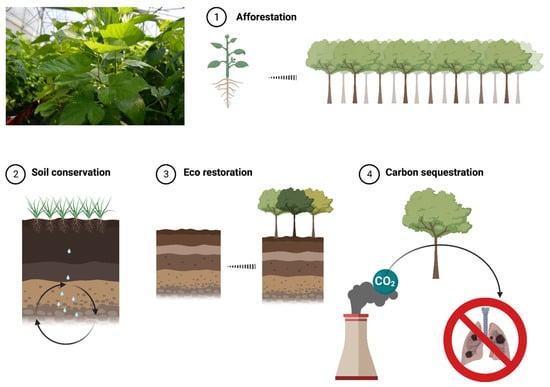
Figure 3.
Importance of mulberry in environmental safety (created with BioRender.com, accessed on 10 February 2023).
Plants have, in general, a high positive impact on reducing global warming by up-taking an important amount of carbon dioxide from the atmosphere and releasing oxygen in return. This process leads to purified air to be used by living organisms. In addition, plants have a so-called “evapotranspiration mechanism of water cycle”, which helps to prevent overheating urban areas and contributes to an improvement of soil health [68]. In this regard, and according to Jiang et al. (2017), mulberry is an excellent green species that can be used to purify the air, as one ha of mulberry trees can absorb around 1000 kg of CO2 a day and release around 730 kg oxygen, the equivalent of 1000 peoples’ breathing demand [69].
Another notable role played by the mulberry tree in the environmental safety is represented by its ability to absorb heavy metals and some toxic organic compounds. Having a well-developed root system (up to 4 m deep into the ground) and increased resistance to environmental conditions, mulberry can grow and develop without any visible changes in soil contaminated with 734 mg/kg of Pb, 1194 mg/kg of Zn, or 53 mg/kg of As. Regarding organic compounds, the phytoremediation power of Morus in soils contaminated with polynuclear aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and trichloroethylene (TCE) [68] has been studied.
Side roles of mulberry in this area are linked to its ability to grow and develop in various environmental conditions, meaning a diverse climate (from very dry areas to temperate countries), altitudes (from below 100 m sea level to over 4000 m above sea level) and soils. Thus, mulberry trees have been planted in desert areas (minimum irrigation required) and salinized lands, aiming for a natural restoration. In both cases, the eco restoration has been successful. Moreover, it has also been demonstrated that mulberry trees can be effectively used in landscaping, as they help prevent floods, drought, and wind currents. Besides the afforestation advantages given by this plant, mulberry plantation allows the cultivation of grass and other crops on the same field [68].
3. Alternative Farming Methods
There is a great interest in food quality and safety among consumers, keeping in mind that conventionally grown foods are known to have negative health effects due to their higher levels of pesticide residue, nitrate, heavy metals, hormones, or antibiotic residues, but these foods also lack nutrients and antioxidants. As a result, the demand for organically grown foods has increased in recent decades due to concerns over food safety and potential health benefits. Organic food production involves cultivating crops without using synthetic fertilizers, weedicides, pesticides, or other chemicals. The popularity of organically grown foods is on the rise because of their positive impact on nutrition and health. Additionally, organic farming practices help to protect soil health and promote biodiversity, leading to more sustainable agricultural systems [70,71,72]. However, it can also be more labor-intensive and may require more land to achieve the same level of productivity as conventional methods. Mulberry organic farming implies the process of growing mulberries by using natural methods such as crop rotation, mulching, composting, vermicomposting, and so on. However, many farmers prefer chemical-based inputs due to their short-term results and cost-effectiveness. On the other hand, the use of synthetic agents can potentially pollute the environment and affect the quality of mulberry by-products; therefore, it negatively impacts the consumer’s health, the silkworms’ welfare, and the beneficial micro-organisms, just to mention a few. Although chemical farming initially results in higher yields, farmers eventually experience a reduction in leaf yield and quality, and in terms of sericulture, it impacts cocoon productivity [73]. Therefore, promoting organic farming is crucial in sericulture to prevent the use of chemicals in mulberry cultivation. Moreover, it has been revealed that by using organic agents, due to soil improvement, the quality of mulberry leaves was enhanced [74]. As such, it is worth considering growing mulberry in alternative systems to ensure proper organic crop management for this species.
Alternative farming methods usually refer to agriculture types other than the conventional ones, such as in vitro micropropagation, vertical farming (including soil-less techniques such as hydroponics, aquaponics, aeroponics, etc.), permaculture, polyculture, and biodynamic farming (Figure 4).
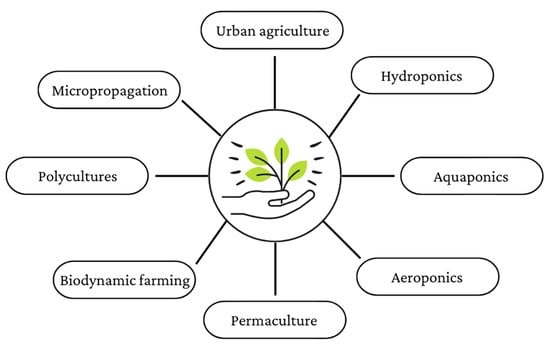
Figure 4.
Schematic representation of the main alternative crop cultivation methods (created with Canva.com, accessed on 10 February 2023).
The subject of the current review is represented by the vertical farming systems, as possible change makers of food chains in urban areas or in areas with infertile ground or excessive dryness. The United Nations estimated a population growth of 2 billion people by 2050 [75]; as such, actions towards achieving the Sustainable Development Goal 2 (“End hunger, achieve food security and improved nutrition and promote sustainable agriculture”) should be taken as soon as possible, worldwide. In terms of food, its production has a direct impact on the environment and consumers; thus, conventional agriculture systems require major updates in making them more sustainable, diverse, environmentally friendly, and accessible.
Vertical farming is an alternative crop cultivation method that aims to ensure global food security by increasing food production per unit of space and resource [76]. Empty industries, contemporary structures built on environmentally damaged sites, and even repurposed ocean freight shipping containers could all be used as alternatives to greater agriculture spaces in our community. Vertical farming is a technique that involves raising crops in controlled indoor conditions with specified lighting, nutrients, and temperatures, Plants are stacked in layers and can reach a height of several floors. Despite the fact that inhabited vertical farming has been around for more than a decade, business-range vertical farming has only recently come under examination. Curiosity about this cutting-edge agricultural technology is rapidly growing, and businesspersons in many places are paying close attention to it [77], not only for its sustainable character, but because even though the initial investment is comparable to a greenhouse set-up, the general profit was higher [76]. Out of the most commonly used vertical farming system, the general hydroponic, aquaponic, and aeroponic systems are further described.
3.1. Hydroponic System
A hydroponic system is a soilless type of crop cultivation, very promising when it comes to geographic areas with limited spaces that can be dedicated to conventional agriculture [78]. The principle of the hydroponic method is described by Lei and Engeseth in 2021, as follows: The plants grow on a liquid substrate (water enriched with essential nutrients) that replaces the soil. The roots are soaked in the mineral-rich solution and absorbed with significantly less effort than soil-based growth methods, which eventually leads to an increased growth rate and yield [79]. There are two main types of hydroponic systems: circulating (closed) and non-circulating. Circulating hydroponic systems, which also can be divided into deep-water culture (DWC) and nutrient film technique (NFT), are proven to give the best results in terms of environmental impact and cost-effectiveness [78]. A basic one-level circulating hydroponic system has, as its main components, a tank full of nutrient solution, a growth tray, an air pump, and a water pump. The water pump is used to generate bubbles that deliver oxygen into the tank and the air pump to force air into the nutritional solution [78,79,80,81], as shown in Figure 5a. For more advanced growing technology, the system can be equipped with UV LEDs linked to a timer, so that plants can have the necessary amount of light during their developing stages when cultivating indoors, as well as an AI device that helps with the controlling and monitoring of the parameters and processes that occur when the hydroponic system is on. In this regard, several pieces of research have been conducted in order to develop the smart farming area, including the applications of Raspberry Pi in modern agriculture [82] or the provision of the IoT framework for an automatic robotic system, designed by Shrivastava et al. in 2021, to route the protocols [83].
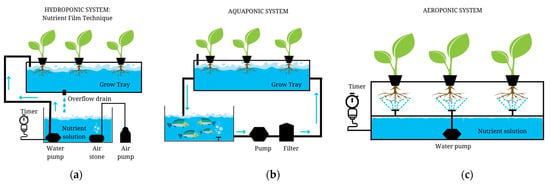
Figure 5.
Hydroponic system (a), aquaponic system (b), and aeroponic system (c). A basic representation of one-layer cultivation techniques (created with Canva.com, accessed on 10 February 2023).
Over the past 50 years, experiments on cultivating plants (Table 1) in the hydroponic system have been conducted with remarkable results in terms of texture, nutritional values, taste, and overall quality of the final product [84]. One of the main influencing components of any hydroponic system is represented by the nutrient solution. It is well known that, in plant cultivation, there are some essential chemical elements that are used for the proper growth and development of any plant. As such, the tank with the nutrient solution should contain basic organic compounds (carbon, hydrogen, and oxygen), macronutrients (nitrogen, phosphorus, potassium, calcium, magnesium, and sulfur), and micronutrients (manganese, iron, boron, zinc, copper, molybdenum, nickel, and chlorine) [85]. As organic elements are widely spread in the environment (in the air and water in the case of hydroponic systems), the mineral elements should be given greater attention when optimizing the nutrient solution. Absorption in the hydroponic crop is normally proportional to the concentration of nutrients in the solution near the roots, and it is highly influenced by environmental parameters, such as pH, salinity, oxygen levels, temperature, and electric conductivity. In addition, the intensity of light, photoperiod, and air humidity have a big impact. Each physicochemical element performs a specific role in the plant, and their excess or deficiency causes symptoms of insufficiency or toxicity [86].
Recently, white mulberry (Morus alba L.) has been successfully reported as grown in hydroponic conditions by Sakurai et al. (2022). The key findings of their study are related to the possibility of growing mulberry in hydroponic conditions and are also related to quality of leaves and roots from obtained biological material. The tasted leaves were noticeably better in terms of the organoleptic measurement, making them edible. When the levels were tested for 1-deoxynojirimycin (DNJ) and polyphenols, the DNJ level increased in the hydroponically grown plants compared to the conventional ones. Contrarily, polyphenol levels dropped in comparison to the field variety. Indicating increased relative levels of lipophilic polyphenols, HPLC profiling found a clear variation in leaf components between hydroponic and field cultivars. When compared to So-Haku-Hi, the root’s polyphenol content—particularly its lipophilic polyphenol content—was exceptionally high (Sang Bai Pi in Chinese). Moreover, anti-obesity efficacy of the hydroponically grown mulberry was further investigated in rats. As a result, the hydroponic cultivar’s leaf and root both demonstrated potential anti-obesity and anti-hyperlipidemic properties via reducing insulin resistance. Differential effects of leaf and root powders suggested that, in addition to DNJ, the lipophilic polyphenols of hydroponically cultivated white mulberry may play a significant part in its anti-diabetic action. In order to cultivate a novel source of mulberry for creating functional foods and medications, hydroponics can become a suitable alternative method [87].
3.2. Aquaponic System
Aquaponics is a technique that falls under the IAAS, meaning the Integrated Agri-Aquaculture Systems. The use of these systems aims to create sustainable farming and food systems through the integration of crop and livestock production principles [88]. This technique integrates the practices of recirculated aquaculture with the manufacture of crops in a hydroponic system [89]. This kind of symbiotic environment was firstly discovered in South China, in the mid-fourteenth century, in a form of a “dike-pond system”. Later on, in sixteenth century Mexico, the chinampas (floating gardens) created by the Aztecs became famous for its unique operating system that consisted of the base of nowadays’ aquaponics [90].
The workflow of a basic aquaponic system is as represented in Figure 5b and has the following components: a growth tray for plants (the hydroponic part), a fish tank, a pump, and a filter [91]. The hydroponic subsystem receives water from the aquaculture subsystem. A bacterial community converts the metabolic waste from fish and unconsumed feed into easily digested nutrients (such as nitrates and phosphates) that plants use to develop. The water might be put back into the fish tanks after being extracted for plant nutrients, when the systems are linked [92]. Some treatment applied to the circulating water system enables the purification and recycling of aquaculture wastewater through the use of machinery for oxygen enrichment, sterilization, and physical and biological filtration. In most cases, sterilization and disinfection are performed by using ozone and UV light [93].
The fish species that can be used in aquaponic systems have a broad spectrum, not being restrictive as long as they provide the necessary nutrients for the development of the plants [94]. Commonly, the Nila tilapia (Oreochromis niloticus) species is extensively used in aquaponics practices, due to its rapid development rate, tolerance for crowded environments, and resistance to temperature variations and poor water quality [95]. Besides the Nila tilapia, several other species have been tested and proved to become well-adapted to the aquaponic system. Love et al. provide some very good examples, based on their international survey that was conducted in 2019, on the topic of aquaponic farming: ornamental fish, shark catfishes (Pangasius spp.), brook trout (Salvelinus fontinalis), black bass (Micropterus spp.), yellow perch (P. flavescens), shrimps, and prawns were successfully used in different countries [96]. In order to maintain an adequate environment for fish development, it is important to constantly maintain good-quality water, to ensure proper nutrient management, and to provide the necessary amount of feed. For fish with a body mass of more than 100 g, the current feeding recommendation for aquaponics is to provide fish feed at 1% of body weight every day [97].
Nutrient management is essential in running aquaponic systems, according to the dynamic that occurs in the solution. Although the wastewater coming from the fish tank contains important amounts of nitrogen, phosphorus, potassium, calcium, magnesium, sodium, and sulfur which serves as a nutrient solution for the plants, the required ratio of micro and macronutrients are not always in normal parameters. A notable example of dysregulation is given by Teng Yang and Hye-Ji Kim in 2020, when cultivating basil, tomato, and lettuce in an aquaponic system. They observed a significant reduction of nitrogen, magnesium, and calcium during the development of the plants, while phosphorus, sodium, and chlorine remained in optimal amounts [98]. Although these processes were conducted manually for several decades, the rapid advances of technology allowed the integration of artificial intelligence in managing the systems. Remarkable examples are shown by Haryanto et al. (2019) [99], Dhal et al. (2022) [100], and Lauguico et al. (2020) [101]. More than that, prospects in using computational tools in assessing the quality of the products obtained have also been approached by Reyes-Yanes et al. (2021) [102].
In terms of economical significance, pilot studies show an increased income when several vegetables were grown in aquaponic system, compared to conventional ones [103,104,105].
3.3. Aeroponic System
The general idea of aeroponics dates back to the 15th century, when random observations about plants that were growing with roots suspended in the air, near waterfalls, were made by scientists [106]. Along the way, they replicated conditions in laboratories for studying the roots’ physiology, but the starting point of modern aeroponics was in 1942, when Carter published his research about “a method of growing plants in water vapor” for a better evaluation of roots [107], cited in [108].
The basic principle of the modern aeroponic system is having a nutrient solution tank from which nozzles linked to a programmable timer sprinkle fine fog onto the plant roots in a given time-frame (Figure 5c) [109]. Although this system represents a variation of hydroponics, the clear advantage is that the roots are constantly aerated, so no additional equipment is required for this purpose. More than that, it allows a lot of flexibility in terms of space required and helps in increasing crop density [110].
The quality of the products obtain in an aeroponic system is nowadays widely studied. Johnson M et al. (2015) evaluated the algal biomass production in aeroponic systems and, interestingly, the lipidic content and fatty acids’ profile were not affected when compared to the conventional growth. Thus, prospects of using such substrate can be explored for increasing the yield that can be further used in biofuel production, feed, or nutraceuticals [111]. Another study reveals that tomatoes maintain higher quality parameters (growth rate, yield and antioxidant capacity, nutrients, etc.) when cultivated in an aeroponic system [112]. Similar results were obtained also by Patil N.L. et al. (2021) when cultivating wheatgrass [113].
The economical aspect of using aeroponics in growing edible plants is also significant. In a study conducted by Pasch J. et al. (2021), the wet and dry leaves weight of basil was up to 40% higher when cultivated in an aeroponic system, compared to a dynamic root-floating system and decoupled aquaponic system. Therefore, the income of selling the final product was also higher [114]. Same results were reported by Tunio and Gao (2020). They obtained not only a higher yield when cultivating lettuce in an aeroponic system, but also the highest shoot and root length, using the less amount of water compared to traditional and hydroponic system [115].
3.4. Plants Adapted to Hydroponic, Aquaponic and Aeroponic Conditions
Several common plants, especially vegetables and spices, have been successfully grown in soil-less vertical farming systems, as shown in the Table 2.

Table 2.
Examples of crops successfully grown in vertical farming systems.
Compared to the other plants mentioned above, mulberry has a biological particularity which makes it suitable for intensive growth: the excitability of dormant buds. Annually, a large number of buds form, but just a part of them start to grow, while others (from the bottom of the tree crown) stay in a dormant state. They are the ones that, stimulated by the production cuttings, start in the vegetation. This process leads to the development of new vigorous shoots, ensuring leaf production for the following year [129]. Further exploitation of mulberry could be possible if its availability would not be dependent on the climate, type of soils, or land size. As such, it is safe to consider mulberry as one the most potent plants while using alternative farming methods in completion of the conventional ones to ensure year-round production and maximize income [68].
However, there are several disadvantages and challenges that are associated with vertical farming systems, the most important being energy dependency. These kinds of systems are dependent on LED lighting, and not on sunlight, but also are dependent on CO2 management. These throwbacks include constant monitoring, which implies certain technical knowledge. One of the biggest challenges is the cost involved in the use of vertical farming systems, which are higher than the costs implied in traditional agriculture [130].
4. Conclusions
Among a wide range of medicinal plants, mulberry is one of the most important major players when it comes to bioactive composition. Recently, there is a high demand for this specific plant, as numerous researchers obtained a wide range of mulberry-based products that exhibit constructive biological effects. It is well documented that oxidative stress plays a crucial role in multiple diseases, which represent death-leading causes worldwide, such as cardiovascular diseases or cancer. As stated by previous research, mulberry exhibits strong antioxidant activity, and it could play a key role in treating the aforementioned target diseases. Furthermore, mounting evidence revealed that mulberry could be used in the treatment of hyperlipidemia-based diseases by contributing to the prevention of lipid accumulation. This event occurs via the stimulation of lipolysis and the repression of lipid synthesis. These results revealed that the mulberry plant represents a powerful prophylactic agent for fatty liver disease. In addition to mulberry’s role in alternative medicine, it constitutes the unique nourishment source for B. mori. However, fresh leaves are not the only way of feeding silkworms. There are described several recipes for artificial diets that facilitate the silkworms’ rearing when the climate does not permit its rearing during the whole year. These recipes mainly consist of mulberry leaf powder and represent a great advantage for sericulture. Besides the pivotal role of mulberry in various industries, it also represents a key player in the environmental safety area.
In terms of global food security, achieving sustainable development goals requires new technologies and strategies to make agriculture and food production, in general, more resilient. One of the most recommended solutions is the transition from conventional agriculture to alternative systems, such as indoor hydroponics, aquaponics, and aeroponics. Several plants have been successfully cultivated in such environments independent of the outside climate, free from pesticides and insecticides, and with a minimum amount of water. Therefore, the applicability of these techniques may be extrapolated to a larger collection of functional plants, especially to mulberry, when cultivating bush-type for a maximum yield per surface unit. The aforementioned use of mulberry, not only in sericulture but also in medicine, pharmacy, and the food industry, makes it a very suitable candidate for year-round production in countries that do not benefit from it due to cold weather, inappropriate soil, or limited available land.
Author Contributions
Conceptualization, E.-D.B., G.-M.B. and D.S.D.; resources, E.-D.B., G.-M.B., A.R.M. and D.S.D.; data curation, E.-D.B., G.-M.B. and A.R.M.; writing—original draft preparation, E.-D.B. and G.-M.B.; writing—review and editing, A.R.M. and D.S.D.; visualization, E.-D.B. and G.-M.B.; supervision, D.S.D.; project administration, A.R.M. and D.S.D.; funding acquisition, A.R.M. and D.S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the AGENȚIA PENTRU FINANȚAREA INVESTIȚIILOR RURALE, PNDR, Programul Naţional pentru Dezvoltare Rurală 2014–2020 pentru acordarea ajutorului financiar nerambursabil /Măsura 16.1 şi 16.1a (grant number C161A0000011861300012).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Memete, A.R.; Timar, A.V.; Vuscan, A.N.; Miere, F.; Venter, A.C.; Vicas, S.I. Phytochemical Composition of Different Botanical Parts of Morus Species, Health Benefits and Application in Food Industry. Plants 2022, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, A.; Thakur, J.; Murali, S.; Bali, K. Mulberry as a Life Savior—A Review. J. Pharmacogn. Phytochem. 2020, 9, 2445–2451. [Google Scholar]
- Paşca, I.; Morar, R.; Dezmirean, D.; Matei, A.; Marghitaş, L. Sericicultură Teoretică şi Practică; Risoprint: Cluj-Napoca, Romania, 2008. [Google Scholar]
- Bhattacharjya, D.; Sadat, A.; Dam, P.; Buccini, D.F.; Mondal, R.; Biswas, T.; Biswas, K.; Sarkar, H.; Bhuimali, A.; Kati, A.; et al. Current concepts and prospects of mulberry fruits for nutraceutical and medicinal benefits. Curr. Opin. Food Sci. 2021, 40, 121–135. [Google Scholar] [CrossRef]
- Chen, X.; Sohouli, M.H.; Nateghi, M.; Melekoglu, E.; Fatahi, S. Impact of mulberry consumption on cardiometabolic risk factors: A systematic review and meta-analysis of randomized-controlled trials. J. Clin. Pharm. Ther. 2022, 47, 1982–1993. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, W.; Gu, Y.; Yu, S. 1-Deoxynojirimycin in mulberry (Morus indica L.) leaves ameliorates stable angina pectoris in patients with coronary heart disease by improving antioxidant and anti-inflammatory capacities. Front. Pharmacol. 2019, 10, 569. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.; Jiang, J.; Li, Y.; Yu, S. Mulberry leaf attenuates atherosclerotic lesions in patients with coronary heart disease possibly via 1-Deoxynojirimycin: A placebo-controlled, double-blind clinical trial. J. Food Biochem. 2021, 45, e13573. [Google Scholar] [CrossRef]
- Zhang, L.; Su, S.; Zhu, Y.; Guo, J.; Guo, S.; Qian, D.; Ouyang, Z.; Duan, J.-A. Mulberry leaf active components alleviate type 2 diabetes and its liver and kidney injury in db/db mice through insulin receptor and TGF-β/Smads signaling pathway. Biomed. Pharmacother. 2019, 112, 108675. [Google Scholar] [CrossRef]
- Duan, Y.; Dai, H.; An, Y.; Cheng, L.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; He, C.; Zhang, H.; et al. Mulberry Leaf Flavonoids Inhibit Liver Inflammation in Type 2 Diabetes Rats by Regulating TLR4/MyD88/NF-B Signaling Pathway. Evid. Based Complement. Altern. Med. 2022, 2022, 3354062. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Liu, Q.H.; Liu, Z.; Tang, J.W.; Chua, E.G.; Li, F.; Xiong, X.S.; Wang, M.M.; Wen, P.B.; Shi, X.Y.; et al. Ethanol extract of mulberry leaves partially restores the composition of intestinal microbiota and strengthens liver glycogen fragility in type 2 diabetic rats. BMC Complement. Med. Ther. 2021, 21, 172. [Google Scholar] [CrossRef]
- Thaipitakwong, T.; Supasyndh, O.; Rasmi, Y.; Aramwit, P. A randomized controlled study of dose-finding, efficacy, and safety of mulberry leaves on glycemic profiles in obese persons with borderline diabetes. Complement. Ther. Med. 2020, 49, 102292. [Google Scholar] [CrossRef]
- Sericulture Technology: Mulberry Cultivation. Available online: https://agritech.tnau.ac.in/sericulture/seri_mulberry%20cultivation.html (accessed on 10 February 2023).
- Kumar Yadav, V.; Padhan, D.; Sobhana, V.; Sen, S.; Josepha, M.; Santha, P.C.; Chandrashekar, M.N.; Praveen Kumar, K.; Tewary, P. Chemical Science Review and Letters Effect of Organic vis-à-vis Conventional Cultivation Practices on Growth and Yield of Mulberry (Morus alba L.). Chem. Sci. Rev. Lett. 2020, 9, 571–577. [Google Scholar]
- Xin, Y.; Meng, S.; Ma, B.; He, W.; He, N. Mulberry genes MnANR and MnLAR confer transgenic plants with resistance to Botrytis cinerea. Plant Sci. 2020, 296, 110473. [Google Scholar] [CrossRef] [PubMed]
- Gnanesh, B.N.; Arunakumar, G.S.; Tejaswi, A.; Supriya, M.; Manojkumar, H.B.; Devi, S.S. Characterization and Pathogenicity of Lasiodiplodia theobromae Causing Black Root Rot and Identification of Novel Sources of Resistance in Mulberry Collections. Plant Pathol. J. 2022, 38, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Chowdhury, T.; Saha, S. Antimicrobial peptide: A competent tool for plant disease control in mulberry—A review. Vegetos 2022. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Wang, H.; Qin, R.L.; Fang, L.J.; Liu, Z.; Yuan, S.S.; Gai, Y.P.; Ji, X.L. Characterization of NPR1 and NPR4 genes from mulberry (Morus multicaulis) and their roles in development and stress resistance. Physiol. Plant. 2019, 167, 302–316. [Google Scholar] [CrossRef]
- Sun, Z.; Kumar, R.M.S.; Li, J.; Yang, G.; Xie, Y. In Silico search and biological validation of MicroR171 family related to abiotic stress response in mulberry (Morus alba). Hortic. Plant J. 2022, 8, 184–194. [Google Scholar] [CrossRef]
- Litwińczuk, W.; Jacek, B. Micropropagation of mountain mulberry (Morus bombycis Koidz.) ‘kenmochi’ on cytokinin-free medium. Plants 2020, 9, 1533. [Google Scholar] [CrossRef]
- Sarkar, T.; Ravindra, K.N.; Doss, S.G.; Kumar, P.M.P.; Tewary, P. In vitro regeneration of mulberry plants from seedling explants of Morus indica cv. G4 through direct organogenesis. Trees 2022, 36, 113–125. [Google Scholar] [CrossRef]
- Vijayan, K.; Tikader, A.; Teixeira da Silva, J.A. Application of Tissue Culture Techniques for Propagation and Crop Improvement in Mulberry (Morus spp.). Tree For. Sci. Biotechnol. 2011, 5, 1–13. [Google Scholar]
- Dubey, V.; Khan, S.; Shah, K.W.; Raghuwanshi, R.K. Standardization of Protocol for In Vitro Micropropagation of Morus alba L., an Important Economical and Medicinal Plant. Pharm. Biosci. J. 2020, 8, 46–51. [Google Scholar] [CrossRef]
- Van Delden, S.H.; SharathKumar, M.; Butturini, M.; Graamans, L.J.A.; Heuvelink, E.; Kacira, M.; Kaiser, E.; Klamer, R.S.; Klerkx, L.; Kootstra, G.; et al. Current status and future challenges in implementing and upscaling vertical farming systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef]
- Khan, H. Medicinal Plants in Light of History: Recognized Therapeutic Modality. J. Evid. Based Complement. Altern. Med. 2014, 19, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, B.; Warude, D.; Pushpangadan, P.; Bhatt, N. Ayurveda and Traditional Chinese Medicine: A Comparative Overview. Adv. Access Publ. 2005, 2, 465–473. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed. Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Dasari, S.; Njiki, S.; Mbemi, A.; Yedjou, C.G. Pharmacological Effects of Cisplatin Combination with Natural Products in Cancer Chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef]
- Huang, M.; Jian, J.; Jian, L. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Elrayess, R.A.; El-Hak, H.N.G. Anticancer Natural Products: A Review. Cancer Stud. Mol. Med. 2019, 5, 11–22. [Google Scholar] [CrossRef]
- Deepa, M.; Sureshkumar, T.; Satheeshkumar, P.K.; Priya, S. Purified mulberry leaf lectin (MLL) induces apoptosis and cell cycle arrest in human breast cancer and colon cancer cells. Chem. Biol. Interact. 2012, 200, 38–44. [Google Scholar] [CrossRef]
- Sik, J.; Lee, D.; Rak, S.; Wook, J.; Choi, C.; Su, T.; Sung, K.; Hyun, K. Chemical characterization of cytotoxic indole acetic acid derivative from mulberry fruit (Morus alba L.) against human cervical cancer. Bioorg. Chem. 2018, 76, 28–36. [Google Scholar]
- Erden, Y. Sour black mulberry (Morus nigra L.) causes cell death by decreasing mutant p53 expression in HT-29 human colon cancer cells. Food Biosci. 2021, 42, 101113. [Google Scholar] [CrossRef]
- Cheng, K.; Wang, C.; Chang, Y. Mulberry fruits extracts induce apoptosis and autophagy of liver cancer cell and prevent hepatocarcinogenesis in vivo. J. Food Drug Anal. 2019, 28, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Liu, J.; Xu, M.; Zheng, J. Evaluation of the Liver Cancer Prevention of Anthocyanin Extracts from Mulberry (Morus alba L.) Variety PR-01. Adv. Biosci. Biotechnol. 2018, 9, 423–442. [Google Scholar] [CrossRef]
- Ding, B.; Lv, Y.; Zhang, Y. Anti-tumor effect of morusin from the branch bark of cultivated mulberry in Bel-7402 cells via the MAPK pathway. R. Soc. Chem. 2016, 6, 17396–17404. [Google Scholar] [CrossRef]
- Dalkiliç, S.; Dalkiliç, L.K.; İnci, Ş.; Korkmaz, İ.; Kırbağ, S. Investigation of cytotoxic effect of black mulberry (Morus nigra L.) fruit. Indian J. Tradit. Knowl. 2021, 20, 54–58. [Google Scholar]
- Fallah, S.; Karimi, A.; Panahi, G.; Nejad, S.G.; Fadaei, R.; Seifi, M. Human colon cancer HT-29 cell death responses to doxorubicin and Morus Alba leaves flavonoid extract. Cell Mol. Biol. 2016, 62, 72–77. [Google Scholar]
- Pranata, R.; Henrina, J.; Matthew, W.; Lawrensia, S.; Huang, I. Diabetes and COVID-19: The past, the present, and the future. Metabolism 2021, 121, 154814. [Google Scholar] [CrossRef]
- Marlen, S.; Mata, R.; Andrade-Cetto, A. Molecules Isolated from Mexican Hypoglycemic Plants: A Review. Molecules 2020, 25, 4145. [Google Scholar]
- Yang, C.; Deng, X.; Tang, B.; Wang, X. Study on Hypoglycemic Effect of Mulberry Leaf Extract Based on Big Data analysis. J. Phys. Conf. Ser. 2021, 1774, 022105. [Google Scholar] [CrossRef]
- Mohammadi, A.; Naik, P.R. Evaluation of hypoglycemic effect of Morus alba in an animal model. Indian J. Pharmacol. 2008, 40, 15–18. [Google Scholar]
- Eo, H.J.; Park, J.H.; Park, G.H.; Lee, M.H.; Lee, J.R.; Koo, J.S.; Jeong, J.B. Anti-inflammatory and anti-cancer activity of mulberry (Morus alba L.) root bark. BMC Complement. Altern. Med. 2014, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.L.; Liu, H.Y.; Zhang, Y.Q. Mulberry branch bark powder significantly improves hyperglycemia and regulates insulin secretion in type II diabetic mice. Food Nutr. Res. 2017, 61, 1368847. [Google Scholar] [CrossRef]
- Qiu, F.; Zhang, Y.Q. Metabolic effects of mulberry branch bark powder on diabetic mice based on GC-MS metabolomics approach. Nutr. Metab. 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, D.S.; Vidhathri, B.S.; Vidyashree, D.N.; Narayanaswamy, T.K.; Subbarayappa, C.T.; Muthuraju, R. Biochemical Composition and Pharmacological Properties of Mulberry (Morus spp.)—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2207–2217. [Google Scholar] [CrossRef]
- Dhiman, S.; Kumar, V.; Mehta, C.M.; Gat, Y.; Kaur, S. Bioactive compounds, health benefits and utilisation of Morus spp.—A comprehensive review. J. Hortic. Sci. Biotechnol. 2020, 95, 8–18. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.; Guo, X.; Chen, D.; Zhou, W.; Chen, X.; Zhang, Q. Flavonoid Levels and Antioxidant Capacity of Mulberry Leaves: Effects of Growth Period and Drying Methods. Front. Plant Sci. 2021, 12, 684974. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The neuroprotective effects of phenolic acids: Molecular mechanism of action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef]
- Flaczyk, E.; Kobus-Cisowska, J.; Przeor, M.; Korczak, J.; Remiszewski, M.; Korbas, E.; Buchowski, M. Chemical characterization and antioxidative properties of Polish variety of Morus alba L. leaf aqueous extracts from the laboratory and pilot-scale processes. Agric. Sci. 2013, 4, 141–147. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef]
- Islam, A.; Islam, M.S.; Rahman, M.K.; Uddin, M.N.; Akanda, M.R. The pharmacological and biological roles of eriodictyol. Arch. Pharm. Res. 2020, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Mohamed Jalaludeen, A. Protective role of sinapic acid against arsenic—Induced toxicity in rats. Chem. Biol. Interact. 2011, 194, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, M.; Muradoglu, F.; Sensoy, R.I.G.; Yilmaz, H. Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci. Hortic. 2011, 132, 37–41. [Google Scholar] [CrossRef]
- Du, Q.; Zheng, J.; Xu, Y. Composition of anthocyanins in mulberry and their antioxidant activity. J. Food Compos. Anal. 2008, 21, 390–395. [Google Scholar] [CrossRef]
- Thomas Pannakal, S.; Eilstein, J.; Prasad, A.; Ekhar, P.; Shetty, S.; Peng, Z.; Bordier, E.; Boudah, S.; Paillat, L.; Marrot, L.; et al. Comprehensive characterization of naturally occurring antioxidants from the twigs of mulberry (Morus alba) using on-line high-performance liquid chromatography coupled with chemical detection and high-resolution mass spectrometry. Phytochem. Anal. 2022, 33, 105–114. [Google Scholar] [CrossRef]
- Yulistiani, D.; Jelan, Z.A.; Liang, J.B.; Yaakub, H.; Abdullah, N. Effects of supplementation of mulberry (Morus alba) foliage and urea-rice bran as fermentable energy and protein sources in sheep fed urea-treated rice straw based diet. Asian-Australas J. Anim. Sci. 2015, 28, 494–501. [Google Scholar] [CrossRef]
- Salinas-Chavira, J.; Castillo-Martínez, O.; Ramirez-Bribiesca, J.E.; Mellado, M. Effect of increasing levels of white mulberry leaves (Morus alba) on ruminal dry matter degradability in lambs. Trop. Anim. Health Prod. 2011, 43, 995–999. [Google Scholar] [CrossRef]
- Islam, M.R.; Siddiqui, M.N.; Khatun, A.; Siddiky, M.N.A.; Rahman, M.Z.; Bostami, A.B.M.R.; Selim, A.S.M. Dietary effect of mulberry leaf (Morus alba) meal on growth performance and serum cholesterol level of broiler chickens. SAARC J. Agric. 2014, 12, 79–89. [Google Scholar] [CrossRef]
- Attila, C.R.; Cristian, R.; Alina, N.; Mihaela, R.M.; Veronica, L.; Alexandru, A.E. Molecular and bioinformatics analysis of the relative expression profiles of dorsal, Toll-1, Relish and Duox genes in young versus old diutinus workers of Apis mellifera. Rom. Biotechnol. Lett. 2016, 21, 11513–11526. [Google Scholar]
- Rahmathulla, V.K.; Tilak, R.; Rajan, R.K. Influence of Moisture Content of Mulberry Leaf on Growth and Silk Production in Bombyx mori L. Caspian J. Env. Sci. 2006, 4, 25–30. [Google Scholar]
- Susanti, S. and Tanjung, M. Effect Of The Quality Of Mulberry Leaves Morus alba Against The Silkworm Nutrition Index Bombyx mori L. (Lepidoptera:Bombicidae). Int. J. Ecophysiol. 2021, 3, 1. [Google Scholar]
- Gug, K. Physiological and Whitening Effects of Morus alba Extracts. J. Chosun Nat. Sci. 2012, 5, 46–52. [Google Scholar] [CrossRef]
- Cai, M.; Mu, L.; Wang, Z.L.; Liu, J.Y.; Liu, T.L.; Wanapat, M.; Huang, B.Z. Assessment of mulberry leaf as a potential feed supplement for animal feeding in P.R. China. Asian-Australas. J. Anim. Sci. 2019, 32, 1145–1152. [Google Scholar] [CrossRef]
- On-Nom, N.; Suttisansanee, U.; Tongmai, J.; Khemthong, C.; Chamchan, R.; Prangthip, P.; Hanboonkunupakarn, B.; Chupeerach, C. Consumption of Anthocyanin-Rich Mulberry Fruit Jelly with a High-Fat Meal Decreases Postprandial Serum Cardiometabolic Risk Factors in Dyslipidemia Subjects. J. Nutr. Metab. 2020, 2020, 1370951. [Google Scholar] [CrossRef]
- Rohela, G.K.; Shukla, P.; Kumar, R.M.; Chowdhury, S.R. Mulberry (Morus spp.): An ideal plant for sustainable development. Trees For. People 2020, 2, 100011. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, R.; Yan, X.; Jia, C.; Jiang, S.; Long, T. Mulberry for environmental protection. Pak. J. Bot. 2017, 49, 781–788. [Google Scholar]
- Das, S.; Chatterjee, A.; Pal, T.K. Organic farming in India: A vision towards a healthy nation. Food Qual. Saf. 2021, 4, 69–76. [Google Scholar] [CrossRef]
- Durán-Lara, E.F.; Valderrama, A.; Marican, A. Natural organic compounds for application in organic farming. Agriculture 2010, 10, 41. [Google Scholar] [CrossRef]
- Łuczka, W.; Kalinowski, S. Barriers to the development of organic farming: A polish case study. Agriculture 2020, 10, 536. [Google Scholar] [CrossRef]
- Sakthivel, N.; Ravikumar, J.; Chikkanna; Mukund, V.K.; Bindroo, B.B.; Sivaprasad, V. Organic Farming in Mulberry: Recent Breakthrough; Regional Sericultural Research Station: Salem, India, 2014. [Google Scholar]
- Chakraborty, B.; Kundu, M.; Chattopadhyay, R.N. Organic Farming with Bio-mulching—A New Paradigm for Sustainable Leaf Yield & Quality of Mulberry (Morus alba L.) under Rainfed Lateritic Soil Condition. Agric. Agric. Sci. Procedia 2016, 11, 31–37. [Google Scholar]
- Population. Available online: https://www.un.org/en/global-issues/population (accessed on 4 June 2022).
- Zhang, Z.; Rod, M.; Hosseinian, F. A Comprehensive Review on Sustainable Industrial Vertical Farming Using Film Farming Technology. Sustain. Agric. Res. 2020, 10, 46. [Google Scholar] [CrossRef]
- Saxena, N.N. The Review on Techniques of Vertical Farming. Int. J. Mod. Agric. 2021, 10, 732–738. [Google Scholar]
- Gumisiriza, M.S.; Ndakidemi, P.; Nalunga, A.; Mbega, E.R. Building sustainable societies through vertical soilless farming: A cost-effectiveness analysis on a small-scale non-greenhouse hydroponic system. Sustain. Cities Soc. 2022, 83, 103923. [Google Scholar] [CrossRef]
- Lei, C.; Engeseth, N.J. Comparison of growth characteristics, functional qualities, and texture of hydroponically grown and soil-grown lettuce. LWT 2021, 150, 111931. [Google Scholar] [CrossRef]
- Sronsri, C.; Sittipol, W.; U-yen, K. Quantity and quality of lettuce (Lactuca sativa L.) grown by a circulating hydroponic method with a Halbach array magnetizer. J. Food Compos. Anal. 2022, 108, 104460. [Google Scholar] [CrossRef]
- Rattan, S.; Partap, M.; Kanika; Kumar, S.; Warghat, A.R. Nutrient feeding approach enhances the vegetative growth biomass, volatile oil composition, and myristicin content in hydroponically cultivated Petroselinum crispum (Mill.) Nyman. J. Appl. Res. Med. Aromat. Plants 2022, 26, 100359. [Google Scholar] [CrossRef]
- Musa, A.; Hassan, M.; Hamada, M.; Aliyu, F.; Aliyu, F.M. Low-Power Deep Learning Model for Plant Disease Detection for Smart-Hydroponics Using Knowledge Distillation Techniques. J. Low Power Electron. Appl. 2022, 12, 24. [Google Scholar] [CrossRef]
- Shrivastava, A.; Nayak, C.K.; Dilip, R.; Samal, S.R.; Rout, S.; Ashfaque, S.M. Automatic robotic system design and development for vertical hydroponic farming using IoT and big data analysis. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364. [Google Scholar] [CrossRef]
- Asao, T. Hydroponics—A Standard Methodology for Plant Biological Researches; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Domingues, D.S.; Takahashi, H.W.; Camara, C.A.P.; Nixdorf, S.L. Automated system developed to control pH and concentration of nutrient solution evaluated in hydroponic lettuce production. Comput. Electron. Agric. 2012, 84, 53–61. [Google Scholar] [CrossRef]
- Sakurai, M.; Sato, S.; Fukushima, T.; Konishi, T. Characteristics of Morus alba L. Cultured by In-Room Hydroponics. Am. J. Plant Sci. 2022, 13, 91–108. [Google Scholar] [CrossRef]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell Editors, G.M. Aquaponics Food Production Systems Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Diver, S. Aquaponics-Integration of Hydroponics with Aquaculture; ATTRA Publication: Davis, CA, USA, 2009. [Google Scholar]
- Kledal, P.R.; Thorarinsdottir, R. Aquaponics: A Commercial Niche for Sustainable Modern Aquaculture. In Sustainable Aquaculture; Springer International Publishing: Cham, Switzerland, 2018; pp. 173–190. [Google Scholar]
- Danner, R.I.; Mankasingh, U.; Anamthawat-Jonsson, K.; Thorarinsdottir, R.I. Designing aquaponic production systems towards integration into greenhouse farming. Water 2019, 11, 2123. [Google Scholar] [CrossRef]
- Lobillo-Eguíbar, J.; Fernández-Cabanás, V.M.; Bermejo, L.A.; Pérez-Urrestarazu, L. Economic sustainability of small-scale aquaponic systems for food self-production. Agronomy 2020, 10, 1468. [Google Scholar] [CrossRef]
- Wei, Y.; Li, W.; An, D.; Li, D.; Jiao, Y.; Wei, Q. Equipment and Intelligent Control System in Aquaponics: A Review. IEEE Access 2019, 7, 169306–169326. [Google Scholar] [CrossRef]
- Baganz, G.F.M.; Junge, R.; Portella, M.C.; Goddek, S.; Keesman, K.J.; Baganz, D.; Staaks, G.; Shaw, C.; Lohrberg, F.; Kloas, W. The aquaponic principle—It is all about coupling. Rev. Aquac. 2022, 14, 252–264. [Google Scholar] [CrossRef]
- Ghamkhar, R.; Hartleb, C.; Wu, F.; Hicks, A. Life cycle assessment of a cold weather aquaponic food production system. J. Clean. Prod. 2020, 244, 118767. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.J. Nutrient management regime affects water quality, crop growth, and nitrogen use efficiency of aquaponic systems. Sci. Hortic. 2019, 256, 108619. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.J. Characterizing nutrient composition and concentration in tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Ulum, M.H.; Ibadillah, A.F.; Alfita, R.; Aji, K.; Rizkyandi, R. Smart Aquaponic System Based Internet of Things (IoT); Institute of Physics Publishing: Bristol, UK, 2019. [Google Scholar]
- Dhal, S.B.; Jungbluth, K.; Lin, R.; Sabahi, S.P.; Bagavathiannan, M.; Braga-Neto, U.; Kalafatis, S. A Machine-Learning-Based IoT System for Optimizing Nutrient Supply in Commercial Aquaponic Operations. Sensors 2022, 22, 3510. [Google Scholar] [CrossRef] [PubMed]
- Lauguico, S.C.; Concepcion, R.S.; Alejandrino, J.D.; Tobias, R.R.; Macasaet, D.D.; Dadios, E.P. A comparative analysis of machine learning algorithms modeled from machine vision-based lettuce growth stage classification in smart aquaponics. Int. J. Environ. Sci. Dev. 2020, 11, 442–449. [Google Scholar] [CrossRef]
- Reyes-Yanes, A.; Martinez, P.; Ahmad, R. Towards automated aquaponics: A review on monitoring, IoT, and smart systems. J. Clean. Prod. 2020, 263, 121571. [Google Scholar] [CrossRef]
- Asciuto, A.; Schimmenti, E.; Cottone, C.; Borsellino, V. A financial feasibility study of an aquaponic system in a Mediterranean urban context. Urban For. Urban Green. 2019, 38, 397–402. [Google Scholar] [CrossRef]
- Greenfeld, A.; Becker, N.; Bornman, J.F.; Spatari, S.; Angel, D.L. Monetizing environmental impact of integrated aquaponic farming compared to separate systems. Sci. Total Environ. 2021, 792, 148459. [Google Scholar] [CrossRef]
- Suárez-Cáceres, G.P.; Lobillo-Eguíbar, J.; Fernández-Cabanás, V.M.; Quevedo-Ruiz, F.J.; Pérez-Urrestarazu, L. Polyculture production of vegetables and red hybrid tilapia for self-consumption by means of micro-scale aquaponic systems. Aquac. Eng. 2021, 95, 102181. [Google Scholar] [CrossRef]
- Weathers, P.J.; Zobel¢, R.W. Aeroponics for the culture of organisms, tissues and cells. Biotechnol. Adv. 1992, 10, 93–115. [Google Scholar] [CrossRef]
- Carter, W. A method of growing plants in water vapor to facilitate examination of roots. Phytopathology 1942, 32, 623–625. [Google Scholar]
- Buckseth, T.; Sharma, A.K.; Pandey, K.K.; Singh, B.P.; Muthuraj, R. Methods of pre-basic seed potato production with special reference to aeroponics—A review. Sci. Hortic. 2016, 204, 79–87. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Graves, W.R.; Gladon, R.J. Aeroponic system for control of root-zone atmosphere. Environ. Exp. Bot. 2006, 55, 70–76. [Google Scholar] [CrossRef]
- Gwynn-Jones, D.; Dunne, H.; Donnison, I.; Robson, P.; Sanfratello, G.M.; Schlarb-Ridley, B.; Hughes, K.; Convey, P. Can the optimisation of pop-up agriculture in remote communities help feed the world? Glob. Food Secur. 2018, 18, 35–43. [Google Scholar] [CrossRef]
- Johnson, M.; Villani, T.S.; Azmat, A.; Simon, J.E.; Both, A.J. Evaluation of algal biomass production on vertical aeroponic substrates. Algal Res. 2015, 10, 240–248. [Google Scholar] [CrossRef]
- Wang, M.; Dong, C.; Gao, W. Evaluation of the growth, photosynthetic characteristics, antioxidant capacity, biomass yield and quality of tomato using aeroponics, hydroponics and porous tube-vermiculite systems in bio-regenerative life support systems. Life Sci. Space Res. 2019, 22, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.L.; Kulkarni, A.A.; Amalnerkar, D.; Kamble, S.C. Exploration of wheatgrass as functional food by using urban agriculture models for regulating growth & nutrients. S. Afr. J. Bot. 2022, 151, 284–289. [Google Scholar]
- Pasch, J.; Appelbaum, S.; Palm, H.W.; Knaus, U. Growth of Basil (Ocimum basilicum) in Aeroponics, DRF, and Raft Systems with Effluents of African Catfish (Clarias gariepinus) in Decoupled Aquaponics (s.s.). AgriEngineering 2021, 3, 559–574. [Google Scholar] [CrossRef]
- Tunio, M.H.; Hussain Tunio, M.; Gao, J. Technological Modernization and Its Influence on Agriculture Sustainability, Aeroponics Systems in Belt and Road Countries. In Proceedings of the 10th Sino-Foreign Postgraduate Academic Forum Jiangsu University, Zhenjiang, China, 22 November 2020. [Google Scholar]
- De Miranda, F.R.; da Silva, V.B.; dos Santos, F.S.R.; Rossetti, A.G.; da Silva, C.d.F.B. Production of strawberry cultivars in closed hydroponic systems and coconut fibre substrate. Rev. Ciência Agronômica 2014, 45, 833–841. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A.; Asao, T.; Asaduzzaman, M.; Raihan Talukder, M.; Tanaka, H.; Ueno, M.; Kawaguchi, M.; Yano, S.; Ban, T. Production of Low-Potassium Content Melon Through Hydroponic Nutrient Management Using Perlite Substrate. Front. Plant Sci. 2018, 9, 1382. [Google Scholar]
- Bakiu, R.; Tafaj, C.; Taci, J. First Study about Aquaponic Systems in Albania. J. Mar. Biol. Aquac. Res. 2018, 1, 1–7. [Google Scholar] [CrossRef]
- Fernández-Cabanás, V.M.; Delgado, A.; Lobillo-Eguíbar, J.R.; Pérez-Urrestarazu, L. Early production of strawberry in aquaponic systems using commercial hydroponic bands. Aquac. Eng. 2022, 97, 102242. [Google Scholar] [CrossRef]
- Rodríguez-Delfín, A.; Molina, L. Advances of Hydroponics in Latin America. Acta Hortic. 2012, 947, 23–32. [Google Scholar] [CrossRef]
- Krastanova, M.; Sirakov, I.; Ivanova-Kirilova, S.; Yarkov, D.; Orozova, P. Aquaponic systems: Biological and technological parameters. Biotechnol. Biotechnol. Equip. 2022, 36, 305–316. [Google Scholar] [CrossRef]
- Hayden, A.L. Aeroponic and Hydroponic Systems for Medicinal Herb, Rhizome, and Root Crops. HortScience 2006, 41, 536–538. [Google Scholar] [CrossRef]
- Kumar Naik, P. Review-Production and utilisation of hydroponics fodder. Indian J. Anim. Nutr. 2015, 32, 1–9. [Google Scholar]
- Amankwaa-Yeboah, P.; Bessah, E. Assessment of Aquaponics-based Food Production System Effluents on the Performance of Maize. Int. J. Agric. Res. Rev. 2017, 5, 615–627. [Google Scholar]
- Germer, J.; Sauerborn, J.; Asch, F.; de Boer, J.; Schreiber, J.; Weber, G.; Müller, J. Skyfarming an ecological innovation to enhance global food security. J. Verbrauch. Lebensm. 2011, 6, 237–251. [Google Scholar] [CrossRef]
- Wimmerova, L.; Keken, Z.; Solcova, O.; Bartos, L.; Spacilova, M. A Comparative LCA of Aeroponic, Hydroponic, and Soil Cultivations of Bioactive Substance Producing Plants. Sustainability 2022, 14, 2421. [Google Scholar] [CrossRef]
- Hilmi, E.; Sari, L.K.; Cahyo, T.N.; Mahdiana, A.; Soedibya, P.H.T.; Sudiana, E. Survival and growth rates of mangroves planted in vertical and horizontal aquaponic systems in North Jakarta, Indonesia. Biodiversitas 2022, 23, 687–694. [Google Scholar] [CrossRef]
- Mangaiyarkarasi, R. Aeroponics System for Production of Horticultural Crops. Madras Agric. J. 2020, 1, 32–40. [Google Scholar]
- Mărghitaș, L.; Dezmirean, D.; Pașca, I. Sericicultura; Mediamira: Cluj-Napoca, Romania, 2003. [Google Scholar]
- Jürkenbeck, K.; Heumann, A.; Spiller, A. Sustainability matters: Consumer acceptance of different vertical farming systems. Sustainability 2019, 11, 4052. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).