Analysis of Aroma Volatiles from Michelia crassipes Flower and Its Changes in Different Flower Organs during Flowering
Abstract
1. Introduction
2. Materials and Methods
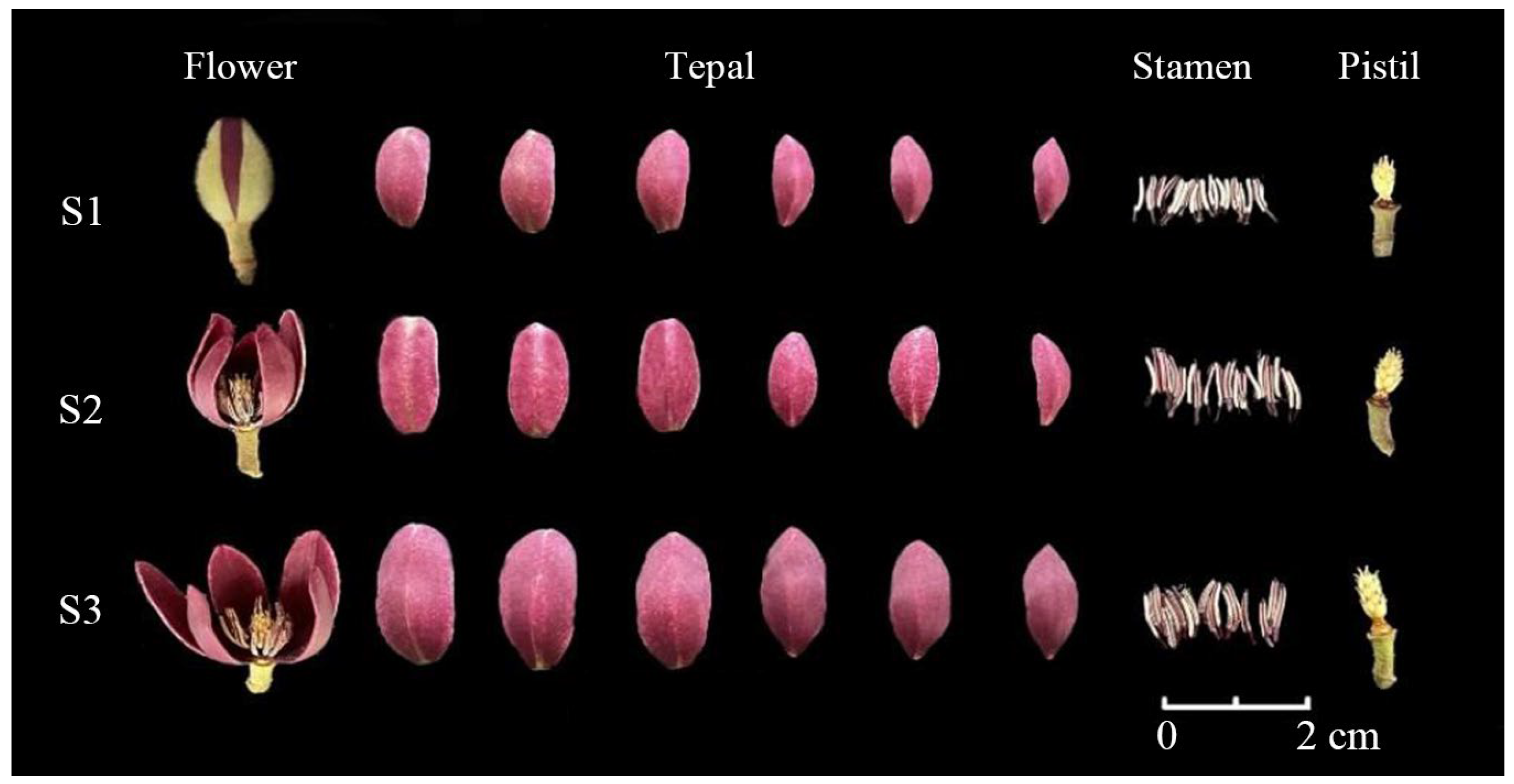
2.1. Plant Materials and Sampling
2.2. HS-SPME Analysis
2.3. GC-HRMS Analysis
2.4. Data Analysis
2.5. Paraffin Section Detection
3. Results
3.1. Volatile Compounds Identification in M. crassipes Flower
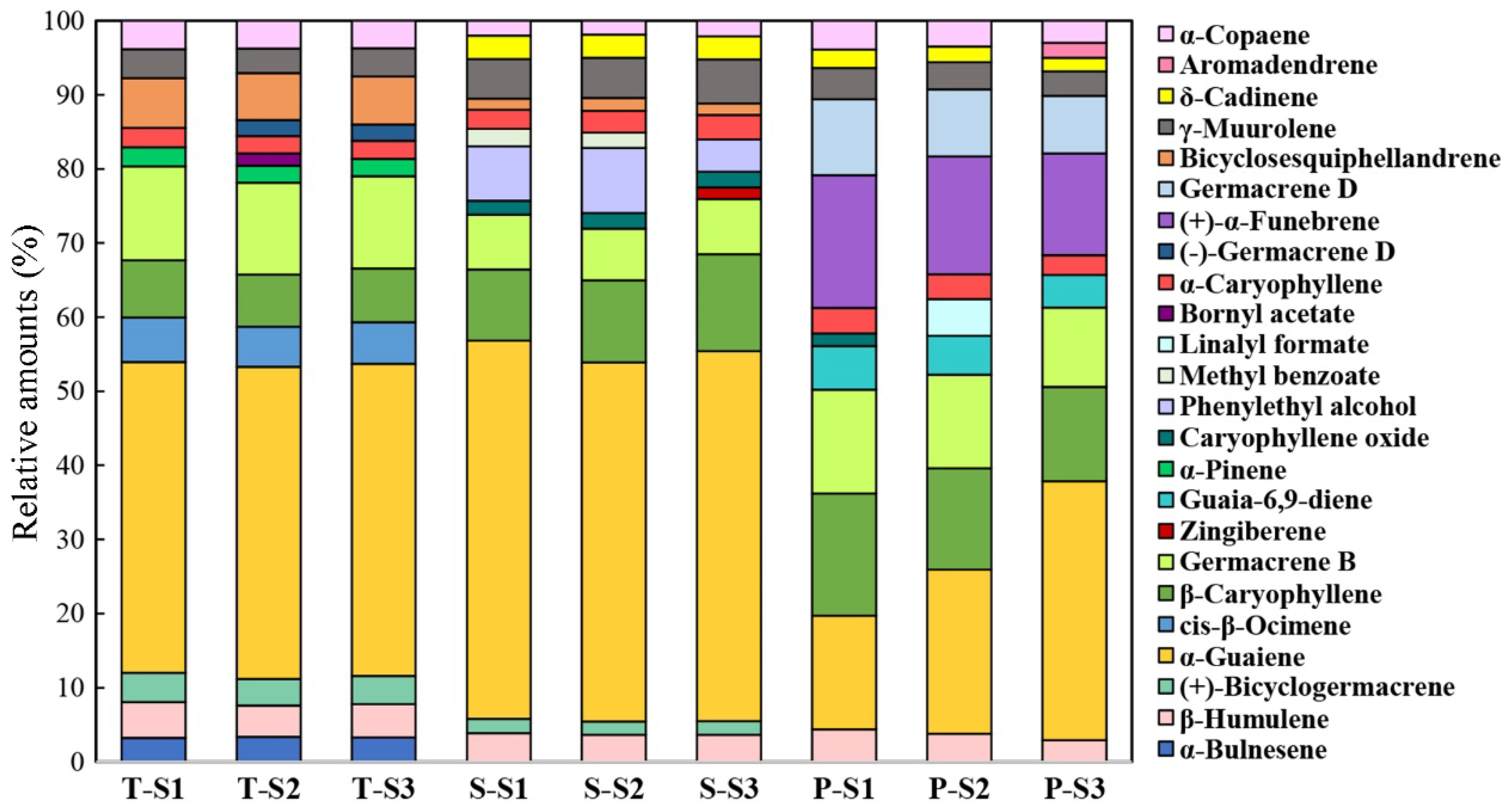
3.2. Temporal and Spatial Patterns of Scent Emission in M. crassipes Flowers
3.3. Oil Cells in Different Floral Parts of M. crassipes at Different Flowering Stages
4. Discussion
4.1. Floral Aroma Characteristics of M. crassipes
4.2. Spatiotemporal Variation of Floral Scent of M. crassipes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, D.; Zhang, H.; Qiu, X.; Jian, H.; Wang, Q.; Zhou, N.; Ye, Y.; Lu, J.; Yan, H.; Tang, K. Comparative transcriptomic and metabonomic analysis revealed the relationships between biosynthesis of volatiles and flavonoid metabolites in Rosa rugosa. Ornam. Plant. Res. 2021, 1, 5. [Google Scholar] [CrossRef]
- Meng, L.; Shi, R.; Wang, Q.; Wang, S. Analysis of floral fragrance compounds of Chimonanthus praecox with different floral colors in Yunnan, China. Separations 2021, 8, 122. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H.; Wan, Y.; Li, T.; Liu, X.; Sun, Z.; Li, Z. Volatile organic compounds emissions from Luculia pinceana flower and its changes at different stages of flower development. Molecules 2016, 21, 531. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Han, Y.; Wang, H.; Wang, X.; Li, K.; Dong, M.; Li, Y.; Zhu, Q.; Shang, F. Mechanism of floral scent production in Osmanthus fragrans and the production and regulation of its key floral constituents, β-ionone and linalool. Hortic. Res. 2019, 6, 106. [Google Scholar] [CrossRef]
- Tian, J.; Ma, Z.; Zhao, K.; Zhang, J.; Xiang, L.; Chen, L. Transcriptomic and proteomic approaches to explore the differences in monoterpene and benzenoid biosynthesis between scented and unscented genotypes of wintersweet. Physiol. Plant 2019, 166, 478–493. [Google Scholar] [CrossRef]
- Fan, Z.; Li, J.; Li, X.; Yin, H. Composition analysis of floral scent within genus Camellia uncovers substantial interspecific variations. Sci. Hortic. 2019, 250, 207–213. [Google Scholar] [CrossRef]
- Kong, Y.; Bai, J.; Lang, L.; Bao, F.; Dou, X.; Wang, H.; Shang, H. Variation in floral scent compositions of different lily hybrid groups. J. Am. Soc. Hortic. Sci. 2017, 142, 175–183. [Google Scholar] [CrossRef]
- Oyama-Okubo, N.; Tsuji, T. Analysis of floral scent compounds and classification by scent quality in tulip cultivars. J. Jpn. Soc. Hortic. Sci. 2013, 82, 344–353. [Google Scholar] [CrossRef]
- Zhang, T.; Bao, F.; Yang, Y.; Hu, L.; Ding, A.; Ding, A.; Wang, J.; Cheng, T.; Zhang, Q. A comparative analysis of floral scent compounds in intraspecific cultivars of Prunus mume with different corolla colours. Molecules 2019, 25, 145. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, W.; Zhang, D.; Wang, G.; Cao, F. Flowering stage and daytime affect scent emission of Malus ioensis “Prairie Rose”. Molecules 2019, 24, 2356. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.W.; Hao, C.Y.; He, S.Z.; Wu, G.; Tan, L.H.; Xu, F.; Hu, R.S. Volatile organic compound emissions from different stages of Cananga odorata flower development. Molecules 2014, 19, 8965–8980. [Google Scholar] [CrossRef]
- Steenhuisen, S.L.; Raguso, R.A.; Jürgens, A.; Johnson, S.D. Variation in scent emission among floral parts and inflorescence developmental stages in beetle-pollinated Protea species (Proteaceae). S. Afr. J. Bot. 2010, 76, 779–787. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, G.; Wang, S.; Lin, Q.; Zhang, J.; Li, X. Analysis of the variation in scent components of Hosta flowers by HS-SPME and GC–MS. Sci. Hortic. 2014, 175, 57–67. [Google Scholar] [CrossRef]
- Balao, F.; Herrera, J.; Talavera, S.; Dotterl, S. Spatial and temporal patterns of floral scent emission in Dianthus inoxianus and electroantennographic responses of its hawkmoth pollinator. Phytochemistry 2011, 72, 601–609. [Google Scholar] [CrossRef]
- Ghosh, D.; Chaudhary, N.; Uma Kumari, K.; Singh, J.; Tripathi, P.; Meena, A.; Luqman, S.; Yadav, A.; Chanotiya, C.S.; Pandey, G.; et al. Diversity of essential oil-secretory cells and oil composition in flowers and buds of Magnolia sirindhorniae and its biological activities. Chem. Biodivers. 2021, 18, e2000750. [Google Scholar] [CrossRef]
- Hadacek, F.; Weber, M. Club-shaped organs as additional osmophores within the Sauromatum inflorescence: Odour analysis, ultrastructural changes and pollination aspects. Plant Biol. 2002, 4, 367–383. [Google Scholar] [CrossRef]
- Xu, H.; Li, F.; Pan, Y.; Gong, X. Interspecific hybridization processes between Michelia yunnanensis and M. crassipes and embryogenesis of the heterozygote. HortScience 2017, 52, 1043–1047. [Google Scholar] [CrossRef]
- Cheng, K.K.; Nadri, M.H.; Othman, N.Z.; Rashid, S.; Lim, Y.C.; Leong, H.Y. Phytochemistry, bioactivities and traditional uses of Michelia × alba. Molecules 2022, 27, 3450. [Google Scholar] [CrossRef]
- Báeza, D.; Moralesa, D.; Pinob, J.A. Volatiles from Michelia champaca flower: Comparative analysis by simultaneous distillation-extraction and solid phase microextraction. Nat. Prod. Commun. 2012, 7, 659–660. [Google Scholar] [CrossRef]
- Lesueur, D.; Serra, D.d.R.; Bighelli, A.; Hoi, T.M.; Ban, N.K.; Thai, T.H.; Casanova, J. Chemical composition and antibacterial activity of the essential oil of Michelia foveolata Merryll ex Dandy from Vietnam. Flavour Fragr. J. 2007, 22, 317–321. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kao, C.L.; Chen, H.C.; Huang, M.H.; Li, H.T.; Wu, M.D.; Cheng, M.J. A morpholine dimer of Michelia crassipes. Chem. Nat. Compd. 2021, 57, 468–470. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Q.; Li, Z.; Jin, X.; Xing, W. Metabolic and transcriptomic analysis related to flavonoid biosynthesis during the color formation of Michelia crassipes tepal. Plant Physiol. Biochem. 2020, 155, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Perez, A.; Romero-Gonzalez, R.; Garrido Frenich, A. Feasibility of applying untargeted metabolomics with GC-Orbitrap-HRMS and chemometrics for authentication of black pepper (Piper nigrum L.) and identification of geographical and processing markers. J. Agric. Food Chem. 2021, 69, 5547–5558. [Google Scholar] [CrossRef]
- Shi, S.; Duan, G.; Li, D.; Wu, J.; Liu, X.; Hong, B.; Yi, M.; Zhang, Z. Two-dimensional analysis provides molecular insight into flower scent of Lilium ‘Siberia’. Sci. Rep. 2018, 8, 5352. [Google Scholar] [CrossRef]
- Du, X.; Plotto, A.; Baldwin, E.; Rouseff, R. Evaluation of volatiles from two subtropical strawberry cultivars using GC-olfactometry, GC-MS odor activity values, and sensory analysis. J. Agric. Food Chem. 2011, 59, 12569–12577. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools—An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Gao, H. The Forming and Releasing and Chemical Components of Fragrance of Three Species in Michelia Linn. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2009. [Google Scholar]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Methven, L. Techniques in sensory analysis of flavour. In Flavour Development, Analysis and Perception in Food and Beverages; Parker, J.K., Elmore, J.S., Methven, L., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 353–368. [Google Scholar] [CrossRef]
- Azuma, H.; Toyota, M.; Asakawa, Y.; Yamaoka, R.; Garciía-Franco, J.G.; Dieringer, G.; Thien, L.B.; Kawano, S. Chemical divergence in floral scents of magnolia and allied genera (Magnoliaceae). Plant Species Biol. 1997, 12, 69–83. [Google Scholar] [CrossRef]
- Azuma, H.; Thien, L.B.; Kawano, S. Floral scents, leaf volatiles and thermogenic flowers in Magnoliaceae. Plant Species Biol. 1999, 14, 121–127. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, X.; Xing, J.; Li, N.; Li, J.; Su, Q.; Chen, Y.; Zhang, B.; Zhu, B. Accurate Determination of 12 Lactones and 11 Volatile Phenols in Nongrape Wines through Headspace-Solid-Phase Microextraction (HS-SPME) Combined with High-Resolution Gas Chromatography-Orbitrap Mass Spectrometry (GC-Orbitrap-MS). J. Agric. Food Chem. 2022, 70, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Romera-Torres, A.; Arrebola-Liébanas, J.; Vidal, J.L.M.; Frenich, A.G. Determination of Calystegines in Several Tomato Varieties Based on GC-Q-Orbitrap Analysis and Their Classification by ANOVA. J. Agric. Food Chem. 2019, 67, 1284–1291. [Google Scholar] [CrossRef]
- Scalliet, G.; Piola, F.; Douady, C.J.; Réty, S.; Raymond, O.; Baudino, S.; Bordji, K.; Bendahmane, M.; Dumas, C.; Cock, J.M.; et al. Scent evolution in Chinese roses. Proc. Natl. Acad. Sci. USA 2008, 105, 5927–5932. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–633. [Google Scholar] [CrossRef]
- Hu, M.L.; Li, Y.Q.; Bai, M.; Wang, Y.L.; Wu, H. Variations in volatile oil yields and compositions of Magnolia zenii Cheng flower buds at different growth stages. Trees 2015, 29, 1649–1660. [Google Scholar] [CrossRef]
- Fanourakis, D.; Papadopoulou, E.; Valla, A.; Tzanakakis, V.A.; Nektarios, P.A. Partitioning of transpiration to cut flower organs and its mediating role on vase life response to dry handling: A case study in chrysanthemum. Postharvest Biol. Technol. 2021, 181, 111636. [Google Scholar] [CrossRef]
- Dobson, H.E.M.; Danielson, E.M.; Wesep, I.D.V. Pollen odor chemicals as modulators of bumble bee foraging on Rosa rugosa Thunb. (Rosaceae). Plant Species Biol. 1999, 14, 153–166. [Google Scholar] [CrossRef]
- Howell, A.D.; Alarcón, R. Osmia bees (Hymenoptera: Megachilidae) can detect nectar-rewarding flowers using olfactory cues. Anim. Behav. 2007, 74, 199–205. [Google Scholar] [CrossRef]
- Raguso, R.A. Why are some floral nectars scented? Ecology 2004, 85, 1486–1494. [Google Scholar] [CrossRef]


| Category | Relative Content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T-S1 | T-S2 | T-S3 | S-S1 | S-S2 | S-S3 | P-S1 | P-S2 | P-S3 | |
| terpene | 71.8 ± 3.54 | 72.06 ± 3.56 | 74.16 ± 3.68 | 70.24 ± 3.47 | 65.17 ± 3.23 | 74.5 ± 3.69 | 78.58 ± 3.87 | 76.16 ± 3.73 | 83.87 ± 4.13 |
| alcohol | 4.12 ± 0.2 | 3.1 ± 0.16 | 2.37 ± 0.12 | 7.23 ± 0.36 | 7.66 ± 0.38 | 4.63 ± 0.22 | 1.71 ± 0.08 | 1.63 ± 0.08 | 1.58 ± 0.06 |
| ester | 0.65 ± 0.03 | 1.64 ± 0.09 | 1.23 ± 0.07 | 1.96 ± 0.1 | 2.23 ± 0.11 | 1.47 ± 0.08 | 0.36 ± 0.02 | 3.46 ± 0.17 | 0.35 ± 0.01 |
| benzenoid | 0.7 ± 0.03 | 0.66 ± 0.04 | 0.44 ± 0.02 | 6.61 ± 0.33 | 6,73 ± 0.33 | 3.68 ± 0.18 | 0.51 ± 0.03 | 0.51 ± 0.03 | 0.52 ± 0.02 |
| aldehyde | 0.05 ± 0 | 0.05 ± 0 | Tr | 0.2 ± 0.01 | 0.55 ± 0.03 | 0.21 ± 0.01 | 0.15 ± 0 | 0.16 ± 0 | 0.15 ± 0 |
| phenol | Tr | Tr | 0.17 ± 0.01 | Tr | Tr | 0.14 ± 0.01 | 0.17 ± 0.01 | Tr | 0.12 ± 0.01 |
| ketone | Tr | Tr | Tr | Tr | Tr | Tr | 0.04 ± 0 | 0.04 ± 0 | 0.04 ± 0 |
| Compound | RT | Odor Threshold * | Odor Description *2 | OAVs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T-S1 | T-S2 | T-S3 | S-S1 | S-S2 | S-S3 | P-S1 | P-S2 | P-S3 | ||||
| Ethyl 3-methyl valerate | 18.084 | 0.008 | fruit, pineapple | 0.29 | 0.01 | 0.16 | 15.66 | 31.30 | 28.00 | 20.99 | 32.64 | 33.22 |
| Methyl benzoate | 9.071 | 0.52 | fruit, sweet | 2.15 | 1.60 | 1.70 | 6.00 | 7.48 | 4.50 | 2.94 | 3.78 | 4.06 |
| β-Damascone | 42.968 | 0.009 | fruit, berry | Tr *3 | Tr | Tr | Tr | Tr | Tr | 2.92 | 3.73 | 4.00 |
| β-Caryophyllene | 29.443 | 64 | wood, pepper | 0.41 | 0.45 | 0.48 | 0.20 | 0.33 | 0.45 | 2.20 | 2.54 | 2.87 |
| α-Pinene | 32.331 | 6 | wood, pine | 1.44 | 1.52 | 1.64 | 0.23 | 0.40 | 0.52 | 1.69 | 1.91 | 2.40 |
| β-Ionone | 45.309 | 0.1 | flower, iris | Tr | 1.20 | 1.29 | Tr | Tr | Tr | Tr | Tr | Tr |
| Phenylethyl alcohol | 13.697 | 15 | flower, rose | 0.16 | 0.21 | 0.13 | 0.64 | 1.11 | 0.64 | 0.37 | 0.51 | 0.60 |
| Butanal | 6.049 | 2 | spice, cocoa | Tr | 0.15 | Tr | 0.12 | 0.38 | Tr | 0.46 | 0.73 | 0.66 |
| Benzyl acetate | 30.737 | 2 | sweet, jasmine | Tr | Tr | 0.66 | Tr | 0.05 | 0.06 | 0.02 | 0.02 | 0.01 |
| cis-β-Ocimene | 29.388 | 34 | flower, nasturtium | 0.60 | 0.64 | 0.69 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 | 0.03 |
| Linalool | 12.978 | 6 | flower, rose | 0.40 | 0.42 | 0.35 | Tr | Tr | Tr | Tr | Tr | Tr |
| β-Selinene | 31.489 | 1 | herb | Tr | 0.25 | 0.34 | Tr | Tr | Tr | Tr | Tr | Tr |
| Bornyl acetate | 21.698 | 75 | wood, mint | Tr | Tr | 0.08 | 0.31 | 0.06 | 0.14 | 0.15 | 0.05 | 0.14 |
| α-Caryophyllene | 31.388 | 160 | wood, lilac | 0.06 | 0.06 | 0.06 | 0.02 | 0.04 | 0.05 | 0.18 | 0.25 | 0.24 |
| D-Limonene | 10.823 | 10 | green, lemon | 0.05 | 0.06 | 0.07 | 0.01 | Tr | 0.04 | 0.18 | 0.21 | 0.22 |
| Nerolidol | 36.983 | 15 | flower, citrus | 0.19 | 0.21 | 0.19 | 0.02 | 0.03 | 0.05 | Tr | Tr | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, Y.; Yuan, J.; Jin, X.; Huang, Y.; Zhang, Z.; Chen, Y.; Zhang, M. Analysis of Aroma Volatiles from Michelia crassipes Flower and Its Changes in Different Flower Organs during Flowering. Horticulturae 2023, 9, 442. https://doi.org/10.3390/horticulturae9040442
Yong Y, Yuan J, Jin X, Huang Y, Zhang Z, Chen Y, Zhang M. Analysis of Aroma Volatiles from Michelia crassipes Flower and Its Changes in Different Flower Organs during Flowering. Horticulturae. 2023; 9(4):442. https://doi.org/10.3390/horticulturae9040442
Chicago/Turabian StyleYong, Yubing, Jieli Yuan, Xiaoling Jin, Yu Huang, Zhe Zhang, Yan Chen, and Minhuan Zhang. 2023. "Analysis of Aroma Volatiles from Michelia crassipes Flower and Its Changes in Different Flower Organs during Flowering" Horticulturae 9, no. 4: 442. https://doi.org/10.3390/horticulturae9040442
APA StyleYong, Y., Yuan, J., Jin, X., Huang, Y., Zhang, Z., Chen, Y., & Zhang, M. (2023). Analysis of Aroma Volatiles from Michelia crassipes Flower and Its Changes in Different Flower Organs during Flowering. Horticulturae, 9(4), 442. https://doi.org/10.3390/horticulturae9040442





