Volatile Organic Compounds: A Review of Their Current Applications as Pest Biocontrol and Disease Management
Abstract
1. Introduction
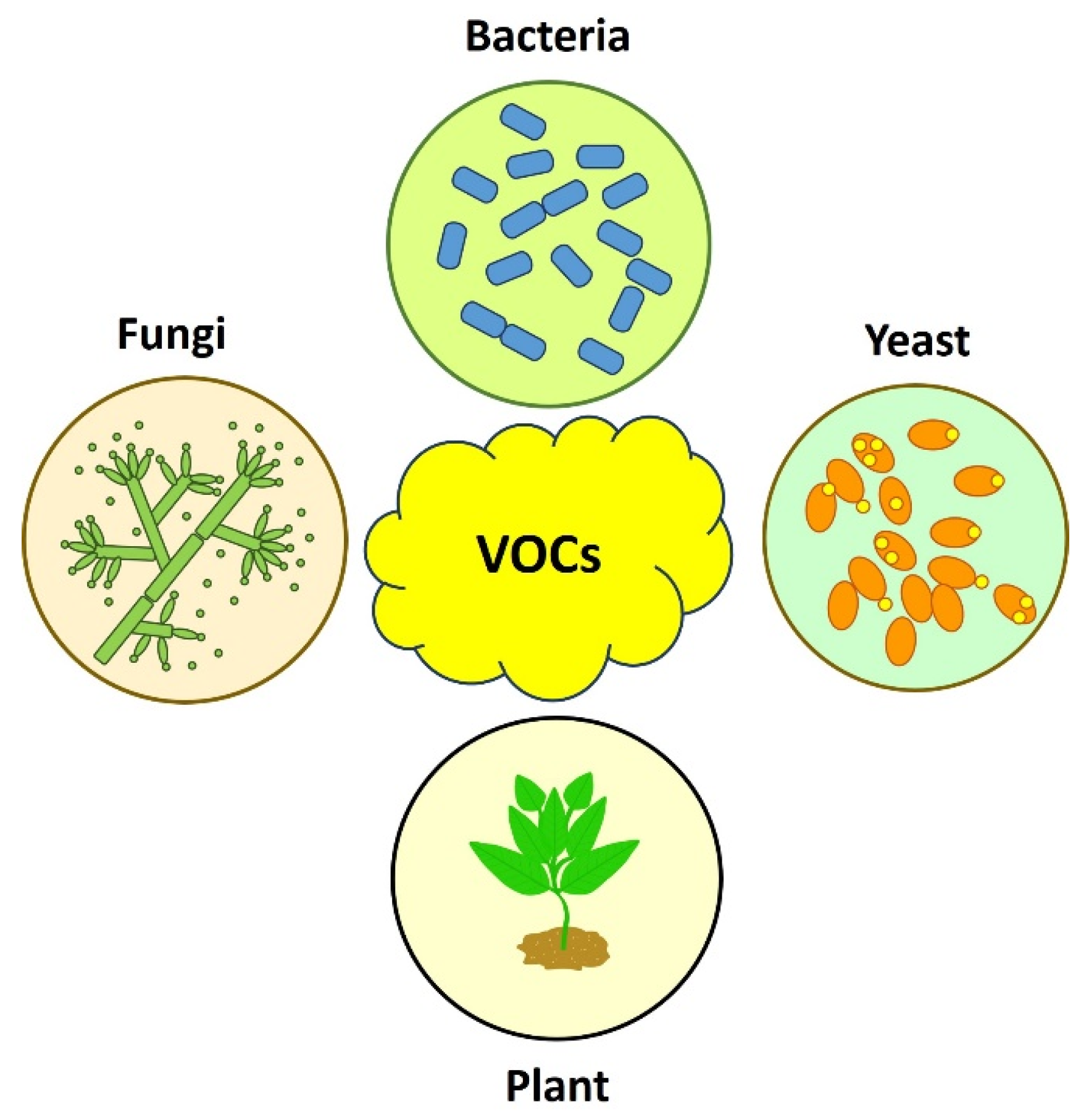
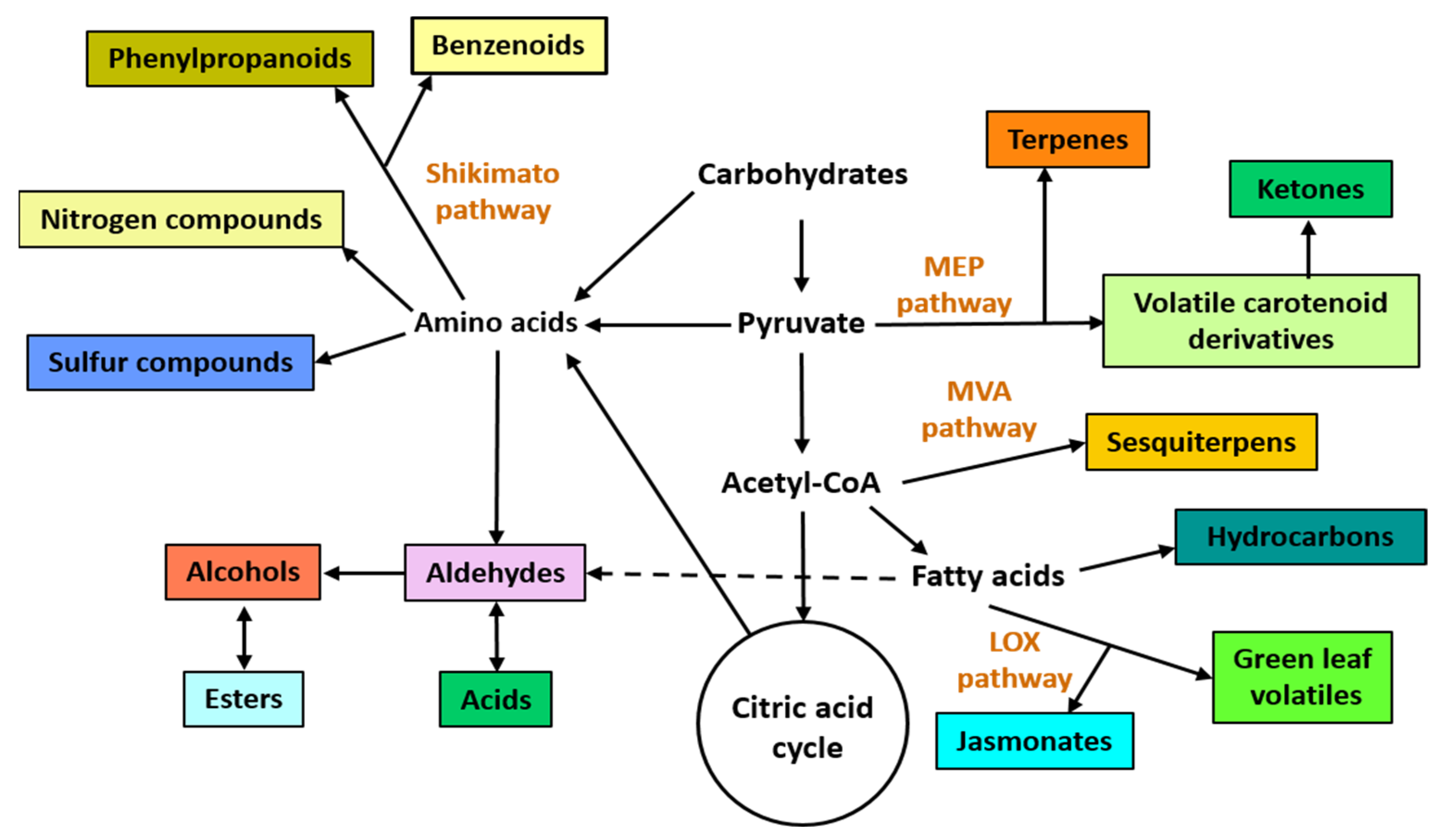
2. General Aspects of VOCs and Possible Biotechnological Applications
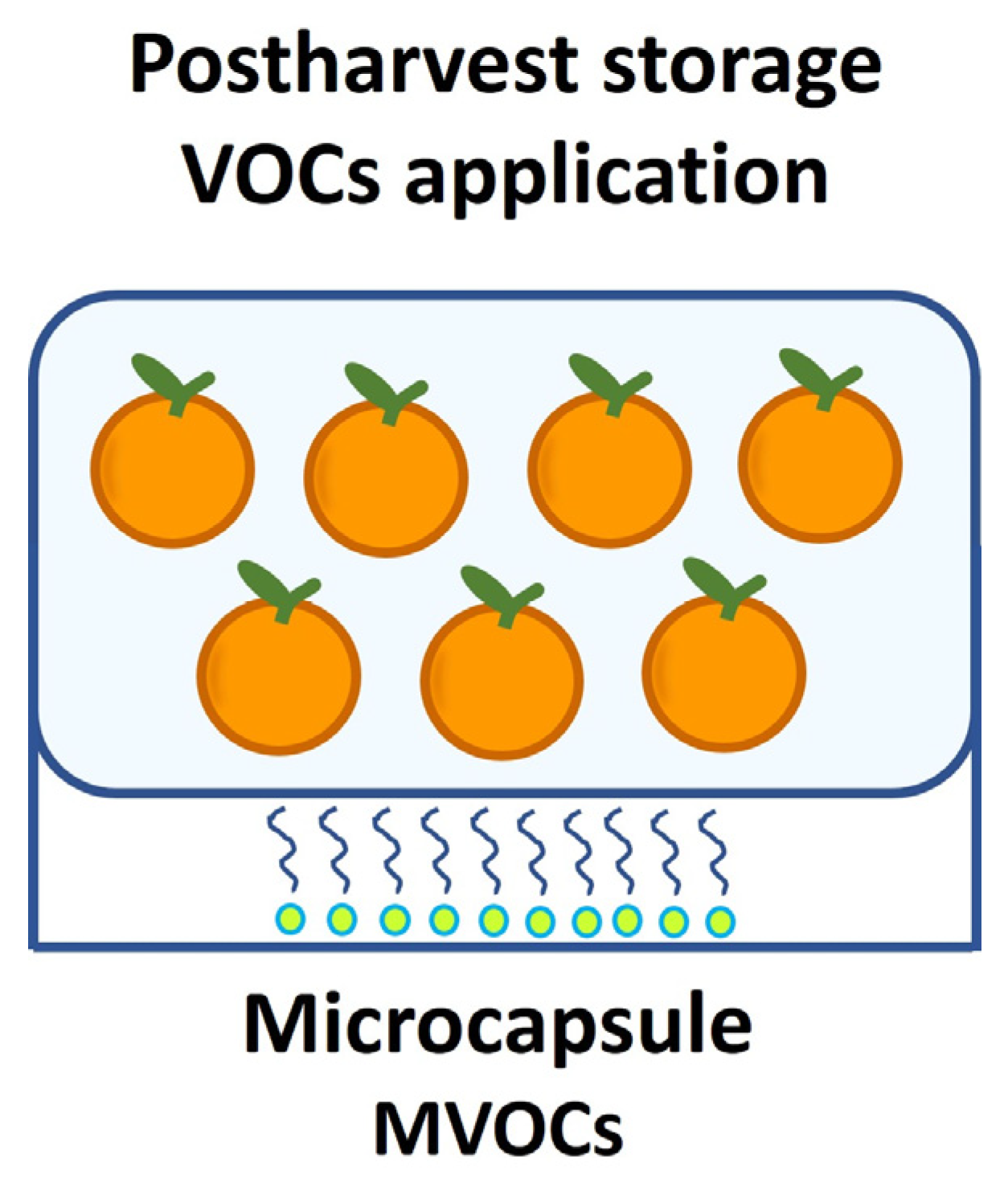
3. Microbial Volatile Organic Compounds as Biocontrol Alternatives for Postharvest Diseases
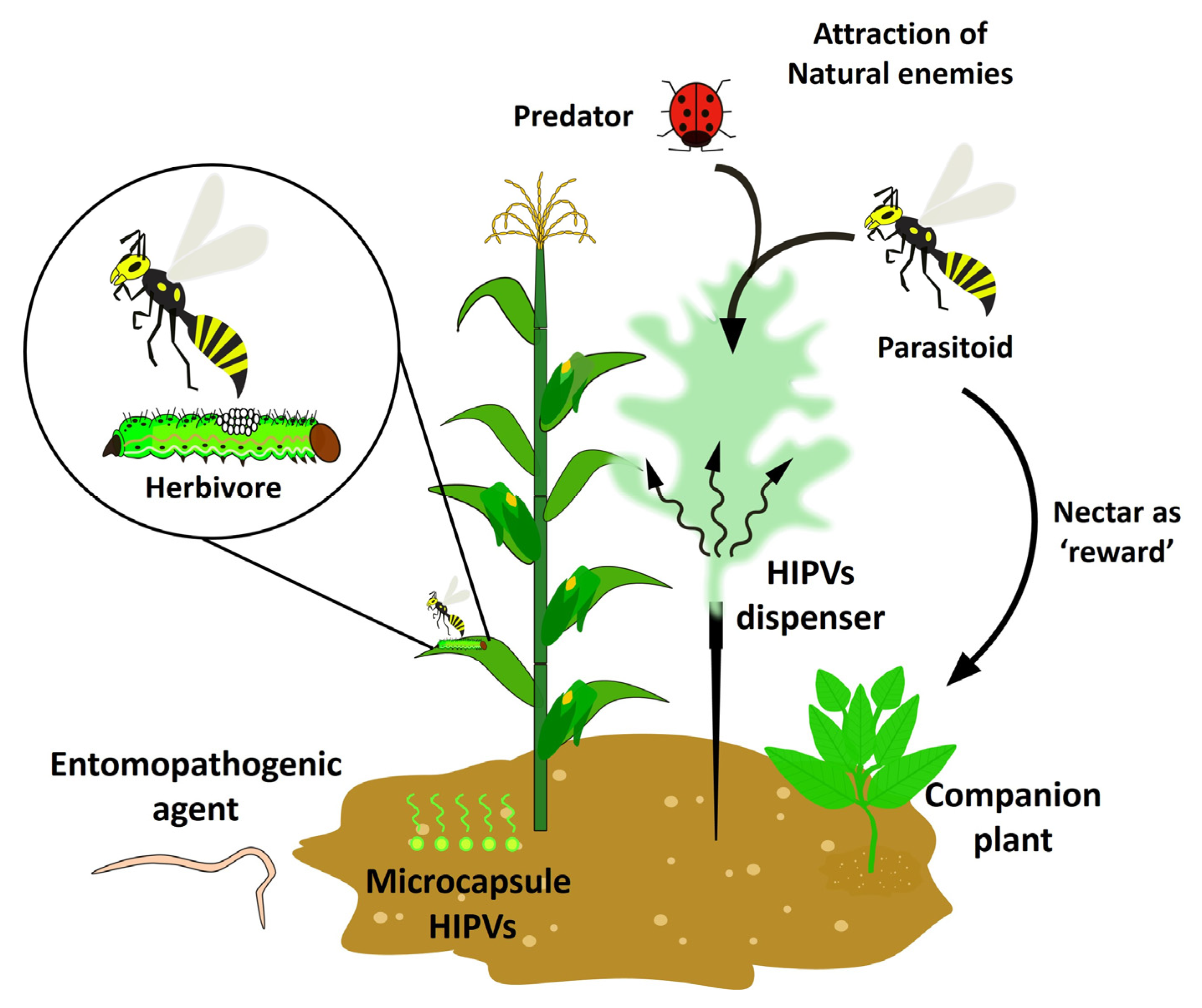
4. Herbivore-Induced Plant Volatiles (HIPVs) as Biocontrol Alternatives in Agriculture
HIPVs as a Tool for Recruitment of Natural Enemies as a Biocontrol of Pests
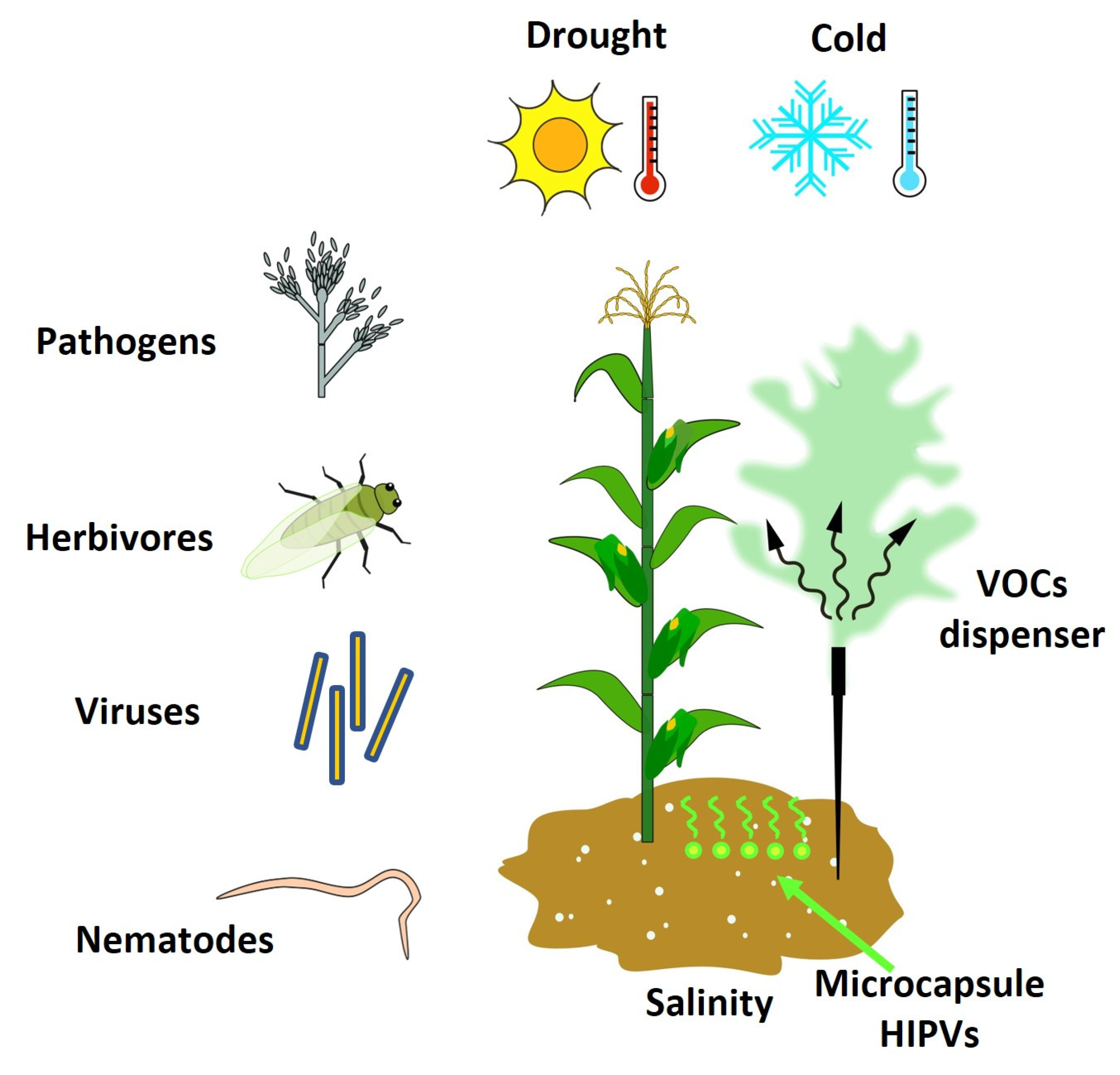
5. VOCs as Inductors of Resistance in Plants against Abiotic and Biotic Stress
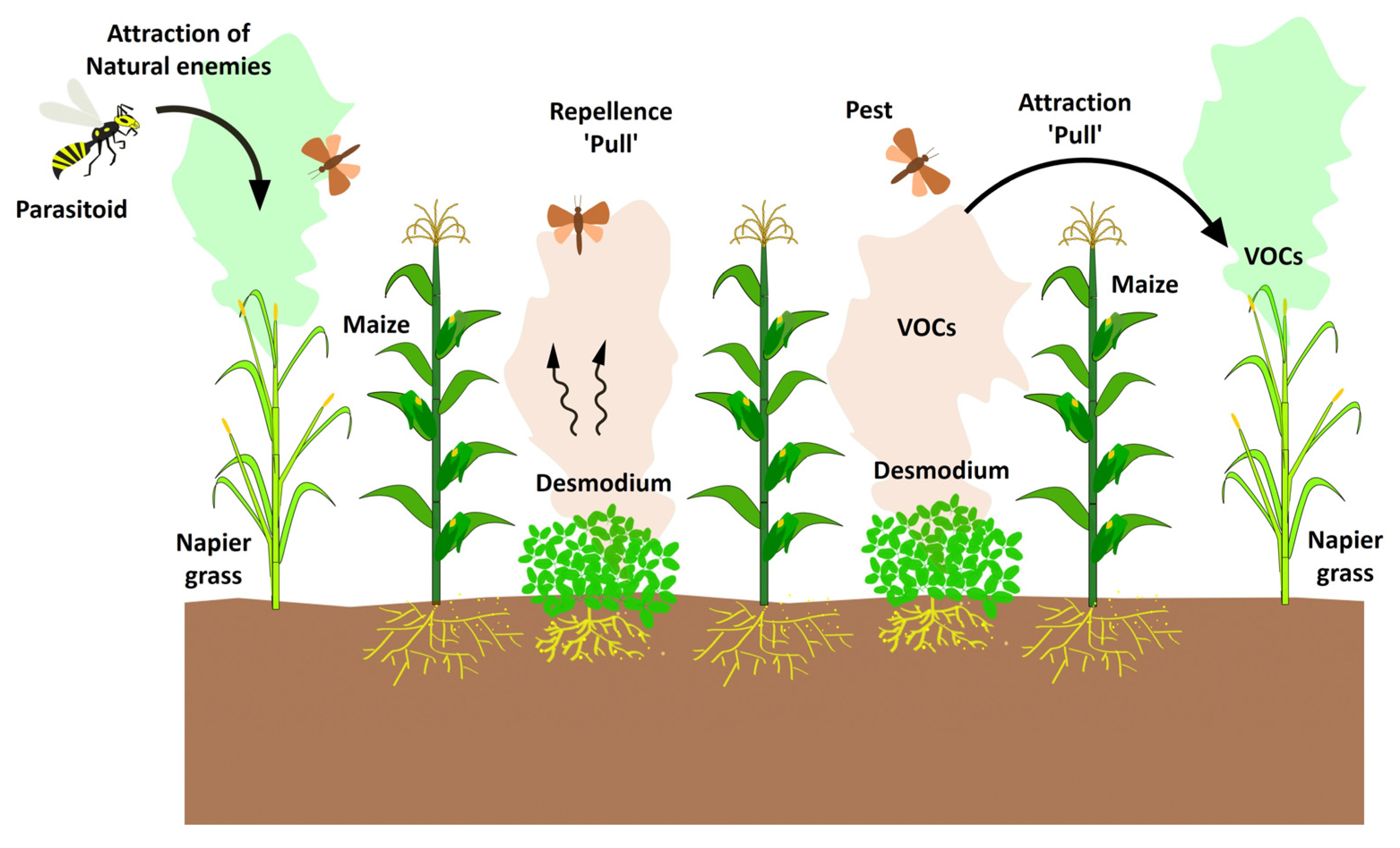
6. Intercropping ‘Push–Pull’ system
7. Application of VOCs in Agricultural Systems
8. Future Trends and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects 2019: Highlights; United Nations: New York, NY, USA, 2019; ISBN 9789211483161. [Google Scholar]
- FAO. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide Residues in European Agricultural Soils—A Hidden Reality Unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Smart, L.E.; Birch, A.N.E.; Blok, V.C.; MacKenzie, K.; Guerrieri, E.; Cascone, P.; Luna, E.; Ton, J. Prospects for Plant Defence Activators and Biocontrol in IPM—Concepts and Lessons Learnt so Far. Crop. Prot. 2017, 97, 128–134. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A Critical Review of Plant Protection Tools for Reducing Pesticide Use on Grapevine and New Perspectives for the Implementation of IPM in Viticulture. Crop. Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P.A. Screening for Novel Biocontrol Agents Applicable in Plant Disease Management—A Review. Biol. Control 2020, 144, 104240. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to Pesticides and the Associated Human Health Effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Ramadan, M.F.A.; Abdel-Hamid, M.M.A.; Altorgoman, M.M.F.; Al Garamah, H.A.; Alawi, M.A.; Shati, A.A.; Shweeta, H.A.; Awwad, N.S. Evaluation of Pesticide Residues in Vegetables from the Asir Region, Saudi Arabia. Molecules 2020, 25, 205. [Google Scholar] [CrossRef]
- Santini, A.; Ghelardini, L. Plant Pathogen Evolution and Climate Change. CAB Rev. 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Brilli, F.; Loreto, F.; Baccelli, I. Exploiting Plant Volatile Organic Compounds (VOCs) in Agriculture to Improve Sustainable Defense Strategies and Productivity of Crops. Front. Plant Sci. 2019, 10, 264. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Gomes, A.; Queiroz, M.; Pereira, O. Mycofumigation for the Biological Control of Postharvest Diseases in Fruits and Vegetables: A Review. Austin J. Biotechnol. Bioeng. 2015, 2, 1–8. [Google Scholar]
- Tilocca, B.; Cao, A.; Migheli, Q. Scent of a Killer: Microbial Volatilome and Its Role in the Biological Control of Plant Pathogens. Front. Microbiol. 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant Volatiles: Production, Function and Pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef] [PubMed]
- Veselova, M.A.; Plyuta, V.A.; Khmel, I.A. Volatile Compounds of Bacterial Origin: Structure, Biosynthesis, and Biological Activity. Microbiology 2019, 88, 261–274. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.L.; Jijakli, M.H.; Sassi, K.; Nasraoui, B. Antifungal Properties of Two Volatile Organic Compounds on Barley Pathogens and Introduction to Their Mechanism of Action. Int. J. Environ. Res. Public Health 2019, 16, 2866. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Amin, B.H.; Aleem, B.; Kingsley, K.L.; Bennett, J.W. Trichoderma Volatile Organic Compounds as a Biofumigation Tool against Late Blight Pathogen Phytophthora Infestans in Postharvest Potato Tubers. J. Agric. Food Chem. 2020, 68, 8163–8171. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Ling, N.; Yang, L.; Huang, Q.; Shen, Q. Response of Tomato Wilt Pathogen Ralstonia Solanacearum to the Volatile Organic Compounds Produced by a Biocontrol Strain Bacillus Amyloliquefaciens SQR-9. Sci. Rep. 2016, 6, 24856. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.M. Biogenic Volatile Compounds for Plant Disease Diagnosis and Health Improvement. Plant Pathol. J. 2018, 34, 459–469. [Google Scholar] [CrossRef]
- Misztal, P.K.; Lymperopoulou, D.S.; Adams, R.I.; Scott, R.A.; Lindow, S.E.; Bruns, T.; Taylor, J.W.; Uehling, J.; Bonito, G.; Vilgalys, R.; et al. Emission Factors of Microbial Volatile Organic Compounds from Environmental Bacteria and Fungi. Environ. Sci. Technol. 2018, 52, 8272–8282. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Gershenzon, J. Multiple Stress Factors and the Emission of Plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, H.; Paré, P.W. Sustained Growth Promotion in Arabidopsis with Long-Term Exposure to the Beneficial Soil Bacterium Bacillus Subtilis (GB03). Plant Signal. Behav. 2009, 4, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of Microbial Antagonists for the Control of Postharvest Diseases of Fruits: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.N.; Tiwari, S.K.; Behera, T.K. Postharvest Diseases of Vegetable Crops and Their Management. In Postharvest Technology—Recent Advances, New Perspectives and Applications; Ahiduzzaman, M., Ed.; IntechOpen: London, UK, 2022; pp. 83–85. [Google Scholar]
- Morita, T.; Tanaka, I.; Ryuda, N.; Ikari, M.; Ueno, D.; Someya, T. Antifungal Spectrum Characterization and Identification of Strong Volatile Organic Compounds Produced by Bacillus pumilus TM-R. Heliyon 2019, 5, e01817. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay Control in the Postharvest System: Role of Microbial and Plant Volatile Organic Compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Sellitto, V.M.; Zara, S.; Fracchetti, F.; Capozzi, V.; Nardi, T. Microbial Biocontrol as an Alternative to Synthetic Fungicides: Boundaries between Pre-and Postharvest Applications on Vegetables and Fruits. Fermentation 2021, 7, 60. [Google Scholar] [CrossRef]
- Kong, W.L.; Rui, L.; Ni, H.; Wu, X.Q. Antifungal Effects of Volatile Organic Compounds Produced by Rahnella Aquatilis JZ-GX1 against Colletotrichum Gloeosporioides in Liriodendron chinense × Tulipifera. Front. Microbiol. 2020, 11, 1114. [Google Scholar] [CrossRef]
- Corcuff, R.; Mercier, J.; Tweddell, R.; Arul, J. Effect of Water Activity on the Production of Volatile Organic Compounds by Muscodor Albus and Their Effect on Three Pathogens in Stored Potato. Fungal Biol. 2011, 115, 220–227. [Google Scholar] [CrossRef]
- Goates, B.J.; Mercier, J. Effect of Biofumigation with Volatiles from Muscodor Albus on the Viability of Tilletia spp. Teliospores. Can. J. Microbiol. 2009, 55, 203–206. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Strobel, G.A.; Moore, E.; Robison, R.; Sears, J. Volatile Antimicrobials from Muscodor Crispans, a Novel Endophytic Fungus. Microbiology 2010, 156, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Pena, L.C.; Jungklaus, G.H.; Savi, D.C.; Ferreira-Maba, L.; Servienski, A.; Maia, B.H.L.N.S.; Annies, V.; Galli-Terasawa, L.V.; Glienke, C.; Kava, V. Muscodor Brasiliensis Sp. Nov. Produces Volatile Organic Compounds with Activity against Penicillium digitatum. Microbiol. Res. 2019, 221, 28–35. [Google Scholar] [CrossRef]
- Pena, L.C.; Jung, L.F.; Savi, D.C.; Servienski, A.; Aluizio, R.; Goulin, E.H.; Galli-Terasawa, L.V.; de Noronha Sales Maia, B.H.L.; Annies, V.; Franco, C.R.C.; et al. A Muscodor Strain Isolated from Citrus Sinensis and Its Production of Volatile Organic Compounds Inhibiting Phyllosticta Citricarpa Growth. J. Plant Dis. Prot. 2017, 124, 349–360. [Google Scholar] [CrossRef]
- Lacey, L.A.; Neven, L.G. The Potential of the Fungus, Muscodor Albus, as a Microbial Control Agent of Potato Tuber Moth (Lepidoptera: Gelechiidae) in Stored Potatoes. J. Invertebr. Pathol. 2006, 91, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Siri-udom, S.; Suwannarach, N.; Lumyong, S. Applications of Volatile Compounds Acquired from Muscodor Heveae against White Root Rot Disease in Rubber Trees (Hevea brasiliensis Müll. Arg.) and Relevant Allelopathy Effects. Fungal Biol. 2017, 121, 573–581. [Google Scholar] [CrossRef]
- Intana, W.; Kheawleng, S.; Sunpapao, A. Trichoderma Asperellum T76-14 Released Volatile Organic Compounds against Postharvest Fruit Rot in Muskmelons (Cucumis melo) Caused by Fusarium Incarnatum. J. Fungi 2021, 7, 46. [Google Scholar] [CrossRef]
- Wonglom, P.; Ito, S.I.; Sunpapao, A. Volatile Organic Compounds Emitted from Endophytic Fungus Trichoderma Asperellum T1 Mediate Antifungal Activity, Defense Response and Promote Plant Growth in Lettuce (Lactuca sativa). Fungal Ecol. 2020, 43, 100867. [Google Scholar] [CrossRef]
- Moya, P.; Girotti, J.R.; Toledo, A.V.; Sisterna, M.N. Antifungal Activity of Trichoderma VOCs against Pyrenophora Teres, the Causal Agent of Barley Net Blotch. J. Plant Prot. Res. 2018, 58, 45–53. [Google Scholar] [CrossRef]
- El-Hasan, A.; Walker, F.; Schöne, J.; Buchenauer, H. Antagonistic Effect of 6-Pentyl-Alpha-Pyrone Produced by Trichoderma harzianum toward Fusarium moniliforme. J. Plant Dis. Prot. 2007, 114, 62–68. [Google Scholar] [CrossRef]
- Ruangwong, O.U.; Pornsuriya, C.; Pitija, K.; Sunpapao, A. Biocontrol Mechanisms of Trichoderma koningiopsis PSU3-2 against Postharvest anthracnose of Chili pepper. J. Fungi 2021, 7, 276. [Google Scholar] [CrossRef]
- Sridharan, A.P.; Sugitha, T.; Karthikeyan, G.; Sivakumar, U. Comprehensive Profiling of the VOCs of Trichoderma longibrachiatum EF5 while Interacting with Sclerotium rolfsii and Macrophomina phaseolina. Microbiol. Res. 2020, 236, 126436. [Google Scholar] [CrossRef]
- Inayati, A.; Sulistyowati, L.; Aini, L.Q.; Yusnawan, E. Antifungal Activity of Volatile Organic Compounds from Trichoderma virens. AIP Conf. Proc. 2019, 2120, 80012. [Google Scholar] [CrossRef]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H.; Gilbert, M.K. The Potential Role of Fungal Volatile Organic Compounds in Aspergillus flavus Biocontrol Efficacy. Biol. Control 2021, 160, 104686. [Google Scholar] [CrossRef]
- Lee, S.O.; Kim, H.Y.; Choi, G.J.; Lee, H.B.; Jang, K.S.; Choi, Y.H.; Kim, J.C. Mycofumigation with Oxyporus Latemarginatus EF069 for Control of Postharvest Apple Decay and Rhizoctonia Root Rot on Moth Orchid. J. Appl. Microbiol. 2009, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Liarzi, O.; Bar, E.; Lewinsohn, E.; Ezra, D. Use of the Endophytic Fungus Daldinia Cf. Concentrica and Its Volatiles as Bio-Control Agents. PLoS ONE 2016, 11, e0168242. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, L.; Hao, J.; Luo, L.; Cao, Y.; Li, J. Biofumigation on Post-Harvest Diseases of Fruits Using a New Volatile-Producing Fungus of Ceratocystis fimbriata. PLoS ONE 2015, 10, e0132009. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiuhong, W. Antifungal Effect of Volatile Organic Compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum. Processes 2020, 8, 1674. [Google Scholar] [CrossRef]
- Calvo, H.; Mendiara, I.; Arias, E.; Gracia, A.P.; Blanco, D.; Venturini, M.E. Antifungal Activity of the Volatile Organic Compounds Produced by Bacillus velezensis Strains against Postharvest Fungal Pathogens. Postharvest Biol. Technol. 2020, 166, 111208. [Google Scholar] [CrossRef]
- Gao, H.; Li, P.; Xu, X.; Zeng, Q.; Guan, W. Research on Volatile Organic Compounds from Bacillus subtilis CF-3: Biocontrol Effects on Fruit Fungal Pathogens and Dynamic Changes during Fermentation. Front. Microbiol. 2018, 9, 456. [Google Scholar] [CrossRef]
- Saleh, A.E.; Ul-Hassan, Z.; Zeidan, R.; Al-Shamary, N.; Al-Yafei, T.; Alnaimi, H.; Higazy, N.S.; Migheli, Q.; Jaoua, S. Biocontrol Activity of Bacillus Megaterium BM344-1 against Toxigenic fungi. ACS Omega 2021, 6, 10984–10990. [Google Scholar] [CrossRef]
- Mannaa, M.; Oh, J.Y.; Kim, K.D. Biocontrol Activity of Volatile-Producing Bacillus megaterium and Pseudomonas Protegens against Aspergillus flavus and Aflatoxin Production on Stored Rice Grains. Mycobiology 2017, 45, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhong, T.; Chen, K.; Du, M.; Chen, G.; Chen, X.; Wang, K.; Zalán, Z.; Takács, K.; Kan, J. Antifungal Activity of Volatile Organic Compounds Produced by Pseudomonas Fluorescens ZX and Potential Biocontrol of Blue Mold Decay on Postharvest Citrus. Food Control 2021, 120, 107499. [Google Scholar] [CrossRef]
- Freitas, C.S.A.; Maciel, L.F.; Corrêa dos Santos, R.A.; Costa, O.M.M.M.; Maia, F.C.B.; Rabelo, R.S.; Franco, H.C.J.; Alves, E.; Consonni, S.R.; Freitas, R.O.; et al. Bacterial Volatile Organic Compounds Induce Adverse Ultrastructural Changes and DNA Damage to the Sugarcane Pathogenic Fungus Thielaviopsis Ethacetica. Environ. Microbiol. 2022, 24, 1430–1453. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Liu, Y.; Li, X.; Zhang, C.; Feng, Z.; Peng, X.; Li, Z.; Qin, S.; Xing, K. Volatile Organic Compounds Produced by Pseudomonas Chlororaphis Subsp. Aureofaciens SPS-41 as Biological Fumigants to Control Ceratocystis Fimbriata in Postharvest Sweet Potatoes. J. Agric. Food Chem. 2019, 67, 3702–3710. [Google Scholar] [CrossRef]
- Yang, M.; Lu, L.; Pang, J.; Hu, Y.; Guo, Q.; Li, Z.; Wu, S.; Liu, H.; Wang, C. Biocontrol Activity of Volatile Organic Compounds from Streptomyces Alboflavus TD-1 against Aspergillus flavus Growth and Aflatoxin Production. J. Microbiol. 2019, 57, 396–404. [Google Scholar] [CrossRef]
- Boukaew, S.; Petlamul, W.; Bunkrongcheap, R.; Chookaew, T.; Kabbua, T.; Thippated, A.; Prasertsan, P. Fumigant Activity of Volatile Compounds of Streptomyces Philanthi RM-1-138 and Pure Chemicals (Acetophenone and Phenylethyl Alcohol) against Anthracnose pathogen in Postharvest Chili Fruit. Crop. Prot. 2018, 103, 1–8. [Google Scholar] [CrossRef]
- Hua, S.S.T.; Beck, J.J.; Sarreal, S.B.L.; Gee, W. The Major Volatile Compound 2-Phenylethanol from the Biocontrol Yeast, Pichia Anomala, Inhibits Growth and Expression of Aflatoxin Biosynthetic Genes of Aspergillus flavus. Mycotoxin Res. 2014, 30, 71–78. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Hernández, A.; Galvan, A.I.; Córdoba, M.G.; Casquete, R.; Serradilla, M.J.; Martín, A. Selection and Application of Antifungal VOCs-Producing Yeasts as Biocontrol Agents of Grey Mould in Fruits. Food Microbiol. 2020, 92, 103556. [Google Scholar] [CrossRef]
- Jaibangyang, S.; Nasanit, R.; Limtong, S. Biological Control of Aflatoxin-Producing Aspergillus flavus by Volatile Organic Compound-Producing Antagonistic Yeasts. BioControl 2020, 65, 377–386. [Google Scholar] [CrossRef]
- Pereyra, M.M.; Garmendia, G.; Rossini, C.; Meinhardt, F.; Vero, S.; Dib, J.R. Volatile Organic Compounds of Clavispora Lusitaniae AgL21 Restrain Citrus Postharvest Pathogens. Biol. Control 2022, 174, 105025. [Google Scholar] [CrossRef]
- Mo, E.K.; Sung, C.K. Phenylethyl Alcohol (PEA) Application Slows Fungal Growth and Maintains Aroma in Strawberry. Postharvest Biol. Technol. 2007, 45, 234–239. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and Distribution of Floral Scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of Plant Volatiles: Nature’s Diversity and Ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Vespermann, A.; Kai, M.; Piechulla, B. Rhizobacterial Volatiles Affect the Growth of Fungi and Arabidopsis thaliana. Appl. Environ. Microbiol. 2007, 73, 5639–5641. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S.; Kunze, B.; Wagner-Dobler, I.; Diestel, R.; Sasse, F. Biological Activity of Volatiles from Marine and Terrestrial Bacteria. Mar. Drugs 2010, 8, 2976–2987. [Google Scholar] [CrossRef]
- War, A.R.; Sharma, H.C.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Herbivore Induced Plant Volatiles: Their Role in Plant Defense for Pest Management. Plant Signal. Behav. 2011, 6, 1973–1978. [Google Scholar] [CrossRef]
- Peñaflor, M.F.G.V.; Bento, J.M.S. Herbivore-Induced Plant Volatiles to Enhance Biological Control in Agriculture. Neotrop. Entomol. 2013, 42, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.; Ton, J. Exploiting Scents of Distress: The Prospect of Manipulating Herbivore-Induced Plant Odours to Enhance the Control of Agricultural Pests. Curr. Opin. Plant Biol. 2006, 9, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; van Loon, J.J.A.; Soler, R. Chemical Complexity of Volatiles from Plants Induced by Multiple Attack. Nat. Chem. Biol. 2009, 5, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Poecke, R.M.P.; Boer, J.G. Inducible Indirect Defence of Plants: From Mechanisms to Ecological Functions. Basic Appl. Ecol. 2003, 42, 27–42. [Google Scholar] [CrossRef]
- Erb, M.; Meldau, S.; Howe, G.A. Role of Phytohormones in Insect-Specific Plant Reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.G.; Heil, M. Unifying Concepts and Mechanisms in the Specificity of Plant—Enemy Interactions. Trends Plant Sci. 2012, 17, 282–292. [Google Scholar] [CrossRef]
- Venkatesan, R. Biosynthesis and Regulation of Herbivore-Induced Plant Volatile Emission. J. Indian Inst. Sci. 2015, 95, 25–34. [Google Scholar]
- Girling, R.D.; Madison, R.; Hassall, M.; Poppy, G.M.; Turner, J.G. Investigations into Plant Biochemical Wound-Response Pathways Involved in the Production of Aphid-Induced Plant Volatiles. J. Exp. Bot. 2008, 59, 3077–3085. [Google Scholar] [CrossRef]
- Leitner, M.; Boland, W. Direct and Indirect Defences Induced by Piercing-Sucking and Chewing Herbivores in Medicago truncatula. New Phytol. 2005, 167, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Köpke, S.; Kunert, M.; Volpe, V.; David, A.; Brand, P.; Dabrowska, P.; Maffei, M.E.; Boland, W. Effects of Feeding Spodoptera Littoralis on Lima Bean Leaves: IV. Diurnal and Nocturnal Damage Differentially Initiate Plant Volatile Emission 1. Plant Physiol. 2008, 146, 965–973. [Google Scholar] [CrossRef]
- Arimura, G.; Ozawa, R.; Shimoda, T.; Nishioka, T.; Boland, W.; Takabayashi, J. Herbivory-Induced Volatiles Elicit Defence Genes in Lima bean Leaves. Nature 2000, 406, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Baldwin, I.T.; Baxter, K.J.; Laue, G.; Felton, G.W. Communication between Plants: Induced Resistance in Wild Tobacco Plants Following Clipping of Neighboring Sagebrush. Oecologia 2000, 125, 66. [Google Scholar] [CrossRef]
- Heil, M. Induction of Two Indirect Defences Benefits Lima bean (Phaseolus lunatus, Fabaceae) in nature. J. Ecol. 2004, 92, 527–536. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ignacimuthu, S.; War, M.Y.; Rashid War, A.; Michael Gabriel Paulraj, B.; Mohd Yousf War, B.; Savarimuthu Ignacimuthu, B. Jasmonic Acid-Mediated-Induced Resistance in Groundnut (Arachis hypogaea L.) Against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J. Plant Growth Regul. 2011, 30, 512–523. [Google Scholar] [CrossRef]
- Mumm, R.; Dicke, M. Variation in Natural Plant Products and the Attraction of Bodyguards Involved in Indirect Plant. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]
- Maurya, A.K. Application of Plant Volatile Organic Compounds (VOCs) in Agriculture. In New Frontiers in Stress Management for Durable Agriculture; Singh, H.B., Singh, A.K., Singh, U.S., Fraceto, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 369–388. ISBN 9789811513220. [Google Scholar]
- Shimoda, T. A Key Volatile Infochemical That Elicits a Strong Olfactory Response of the Predatory Mite Neoseiulus californicus, an Important Natural Enemy of the Two-Spotted Spider Mite. Exp. Appl. Acarol. 2010, 50, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Plant Defense Against Herbivores: Chemical Aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Blande, J.D. Molecular Plant Volatile Communication. Adv. Exp. Med. Biol. 2012, 739, 17–31. [Google Scholar] [CrossRef]
- Ponzio, C.; Gols, R.; Weldegergis, B.T.; Dicke, M. Caterpillar-Induced Plant Volatiles Remain a Reliable Signal for Foraging Wasps during Dual Attack with a Plant Pathogen or Non-Host Insect Herbivore. Plant Cell Environ. 2014, 37, 1924–1935. [Google Scholar] [CrossRef]
- Takemoto, H.; Takabayashi, J. Parasitic Wasps Aphidius Ervi Are More Attracted to a Blend of Host-Induced Plant Volatiles than to the Independent Compounds. J. Chem. Ecol. 2015, 41, 801–807. [Google Scholar] [CrossRef]
- Kang, Z.W.; Liu, F.H.; Zhang, Z.F.; Tian, H.G.; Liu, T.X. Volatile β-Ocimene Can Regulate Developmental Performance of Peach Aphid Myzus Persicae through Activation of Defense Responses in Chinese Cabbage brasasica Pekinensis. Front. Plant Sci. 2018, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, K.; Agarwal, M.; Du, X.B.; Newman, J.; Ren, Y.L. Behavioural Responses of the Parasitoid Aphytis Melinus to Volatiles Organic Compounds (VOCs) from Aonidiella aurantii on Its Host Fruit Tahitian Lime Fruit Citrus Latifolia. Biol. Control 2019, 133, 103–109. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S. Are Synthetic Volatiles, Typically Emitted by Insect-Damaged Peach Cultivars, Navigation Signals for Two-Spotted Lady Beetle (Adalia bipunctata L.) and Green Lacewing (Chrysoperla carnea [Stephens]) Larvae? J. Plant Dis. Prot. 2018, 125, 529–538. [Google Scholar] [CrossRef]
- Chen, C.S.; Zhao, C.; Wu, Z.Y.; Liu, G.F.; Yu, X.P.; Zhang, P.J. Whitefly-Induced Tomato Volatiles Mediate Host Habitat Location of the Parasitic Wasp Encarsia formosa, and Enhance Its Efficacy as a Bio-Control Agent. Pest Manag. Sci. 2021, 77, 749–757. [Google Scholar] [CrossRef]
- Salamanca, J.; Souza, B.; Kyryczenko-Roth, V.; Rodriguez-Saona, C. Methyl Salicylate Increases Attraction and Function of Beneficial Arthropods in Cranberries. Insects 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, C.C.; Xiao, D.; Xu, Q.; Ramirez-Romero, R.; Guo, X.; Wang, S.; Desneux, N. Varying the Spatial Arrangement of Synthetic Herbivore-Induced Plant Volatiles and Companion Plants to Improve Conservation Biological Control. J. Appl. Ecol. 2019, 56, 1176–1188. [Google Scholar] [CrossRef]
- Legaspi, J.C.; Miller, N.; Kanga, L.; Haseeb, M.; Perry, S.; Bldg, P.; Martin, S.; King, L.; Bioagro, D.D.E.; De Viçosa, U.F.; et al. “Attract and Reward” for Syrphid Flies Using Methyl Salicylate and Sweet Alyssum in Kale in North Florida. Subtrop. Agric. Environ. 2020, 71, 49–52. [Google Scholar]
- Stenberg, J.A.; Heil, M.; Åhman, I.; Björkman, C. Optimizing Crops for Biocontrol of Pests and Disease. Trends Plant Sci. 2015, 20, 698–712. [Google Scholar] [CrossRef]
- Simpson, M.; Gurr, G.M.; Simmons, A.T.; Wratten, S.D.; James, D.G.; Leeson, G.; Nicol, H.I.; Orre-gordon, G.U.S. Attract and Reward: Combining Chemical Ecology and Habitat Manipulation to Enhance Biological Control in Field Crops. J. Appl. Ecol. 2011, 48, 580–590. [Google Scholar] [CrossRef]
- Mohammed, K.; Agarwal, M.; Li, B.; Newman, J.; Liu, T.; Ren, Y. Evaluation of D-Limonene and β-Ocimene as Attractants of Aphytis melinus (Hymenoptera: Aphelinidae), a Parasitoid of Aonidiella aurantii (Hemiptera: Diaspididae) on Citrus spp. Insects 2020, 11, 44. [Google Scholar] [CrossRef]
- Ali, M.Y.; Naseem, T.; Zhang, J.; Pan, M.; Zhang, F.; Liu, T.-X. Plant Volatiles and Herbivore Induced Plant Volatiles from Chili pepper Act as Attractant of the Aphid Parasitoid. Plants 2022, 11, 1350. [Google Scholar] [CrossRef]
- Ayelo, P.M.; Yusuf, A.A.; Pirk, W.W.; Chailleux, A. Terpenes from Herbivore-Induced Tomato Plant Volatiles Attract Nesidiocoris tenuis (Hemiptera: Miridae), a Predator of Major Tomato Pests. Pest Manag. Sci. 2021, 77, 5255–5267. [Google Scholar] [CrossRef]
- James, D.G. Synthetic Herbivore-Induced Plant Volatiles as Field Attractants for Beneficial Insects. Environ. Entomol. 2003, 32, 977–982. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Wu, K.; Gao, X.I.W.U.; Guo, Y.U.Y. Field-Testing of Synthetic Herbivore-Induced Plant Volatiles as Attractants for Beneficial Insects. Environ. Entomol. 2008, 37, 1410–1415. [Google Scholar] [CrossRef]
- Ali, J.G.; Alborn, H.T.; Campos-Herrera, R.; Kaplan, F.; Duncan, L.W.; Rodriguez-Saona, C.; Koppenhöfer, A.M.; Stelinski, L.L. Subterranean, Herbivore-Induced Plant Volatile Increases Biological Control Activity of Multiple Beneficial Nematode Species in Distinct Habitats. PLoS ONE 2012, 7, e38146. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.A.; Jiménez-Arias, D.; Expósito-Rodríguez, M.; Sandalio, L.M.; Pérez, J.A. Priming Crops against Biotic and Abiotic Stresses: MSB as a Tool for Studying Mechanisms. Front. Plant Sci. 2014, 5, 642. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus Subtilis: A Plant-Growth Promoting Rhizobacterium that also Impacts Biotic Stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants. In Abiotic and Biotic Stress in Plants; Oliveira, A.B., Ed.; IntechOpen: London, UK, 2019; pp. 3–9. [Google Scholar]
- Pimentel, D.; Peshin, R. (Eds.) Integrated Pest Management: Pesticide Problems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014; ISBN 9789400777958. [Google Scholar]
- Chattopadhyay, C.; Birah, A.; Jalali, B.L. Sustainability in Plant and Crop Protection Natural Resource Management: Ecological Perspectives. In Natural Resource Management: Ecological Perspectives; Peshin, R., Dhawan, A.K., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 133–146. ISBN 9783319997674. [Google Scholar]
- López-Gresa, M.P.; Payá, C.; Ozáez, M.; Rodrigo, I.; Conejero, V.; Klee, H.; Bellés, J.M.; Lisón, P. A New Role for Green Leaf Volatile Esters in Tomato Stomatal Defense against Pseudomonas syringe Pv. tomato. Front. Plant Sci. 2018, 871, 1855. [Google Scholar] [CrossRef]
- Pappas, M.L.; Broekgaarden, C.; Broufas, G.D.; Kant, M.R.; Messelink, G.J.; Steppuhn, A.; Wäckers, F.; van Dam, N.M. Induced Plant Defences in Biological Control of Arthropod Pests: A Double-Edged Sword. Pest Manag. Sci. 2017, 73, 1780–1788. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, L.; Cai, X.; Li, X.; Bian, L.; Luo, Z.; Li, Z.; Chen, Z.; Xin, Z. (E)-Nerolidol Is a Volatile Signal That Induces Defenses against Insects and Pathogens in Tea Plants. Hortic. Res. 2020, 7, 52. [Google Scholar] [CrossRef]
- Tyagi, S.; Lee, K.J.; Shukla, P.; Chae, J.C. Dimethyl Disulfide Exerts Antifungal Activity against Sclerotinia Minor by Damaging Its Membrane and Induces Systemic Resistance in Host Plants. Sci. Rep. 2020, 10, 6547. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Molina-Torres, J.; Ádame-Alvarez, R.-M.; Acosta-Gallegos, J.A.; Heil, M. Plant Volatiles Cause Direct, Induced and Associational Resistance in Common Bean to the Fungal Pathogen Colletotrichum lindemuthianum. J. Ecol. 2015, 103, 250–260. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus Atrophaeus GBSC56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Yang, F.; Zhang, Q.; Tong, H.; Hu, Y.; Zhang, X.; Xie, W.; Wang, S.; Wu, Q.; Zhang, Y. Defence Priming in Tomato by the Green Leaf Volatile (Z)-3-Hexenol Reduces Whitefly Transmission of a Plant Virus. Plant Cell Environ. 2020, 43, 2797–2811. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.G.; Shin, T.S.; Kim, T.H.; Ryu, C.M. Stereoisomers of the Bacterial Volatile Compound 2,3-Butanediol Differently Elicit Systemic Defense Responses of Pepper against Multiple Viruses in the Field. Front. Plant Sci. 2018, 9, 90. [Google Scholar] [CrossRef]
- Taha, M.A.; Ismaiel, A.A.; Ahmed, R.M. 6-Pentyl-α-Pyrone from Trichoderma Koningii Induces Systemic Resistance in Tobacco against Tobacco Mosaic Virus. Eur. J. Plant Pathol. 2021, 159, 81–93. [Google Scholar] [CrossRef]
- Heil, M.; Silva Bueno, J.C. Within-Plant Signaling by Volatiles Leads to Induction and Priming of an Indirect Plant Defense in Nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef]
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile Organic Compounds Produced by Pseudomonas Pseudoalcaligenes Alleviated Drought Stress by Modulating Defense System in Maize (Zea mays L.). Physiol. Plant. 2020, 172, 896–911. [Google Scholar] [CrossRef]
- Li, X.; Ji, Y.; Sheng, Y.; Sheng, L.; Guo, W.; Wang, H.; Zhang, Y. Priming with the Green Leaf Volatile (Z)-3-Hexeny-1-Yl Acetate Enhances Drought Resistance in Wheat Seedlings. Res. Sq. 2021, 10, 785. [Google Scholar] [CrossRef]
- Zhao, M.; Jin, J.; Wang, J.; Gao, T.; Luo, Y.; Jing, T.; Hu, Y.; Pan, Y.; Lu, M.; Schwab, W.; et al. Eugenol Functions as a Signal Mediating Cold and Drought Tolerance via UGT71A59-Mediated Glucosylation in Tea Plants. Plant J. 2022, 109, 1489–1506. [Google Scholar] [CrossRef] [PubMed]
- Cofer, T.M.; Engelberth, M.; Engelberth, J. Green Leaf Volatiles Protect Maize (Zea mays) Seedlings against Damage from Cold Stress. Plant Cell Environ. 2018, 41, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Guo, R.; Zou, X.; Zhang, X.; Yu, X.; Zhan, Y.; Ci, D.; Wang, M.; Wang, Y.; Si, T. Priming with the Green Leaf Volatile (Z)-3-Hexeny-1-Yl Acetate Enhances Salinity Stress Tolerance in Peanut (Arachis hypogaea L.) Seedlings. Front. Plant Sci. 2019, 10, 785. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef]
- Pieterse, M.J.; Does, D.; Zamioudis, C.; Leon-reyes, A.; Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of Jasmonate and Salicylate Signal Crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green Leaf Volatiles: A Plant’s Multifunctional Weapon against Herbivores and Pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef]
- Heil, M. Herbivore-Induced Plant Volatiles: Targets, Perception and Unanswered Questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Jing, T.; Qian, X.; Gao, T.; Li, D.; Schwab, W.; Guo, D.; He, F.; Yu, G.; Li, S.; Wan, X.; et al. Herbivore-Induced Volatiles Influence Moth Preference by Increasing the β -Ocimene Emission of Neighbouring Tea Plants. Plant Cell Environ. 2021, 44, 3667–3680. [Google Scholar] [CrossRef] [PubMed]
- Bertini, L.; Proietti, S.; Focaracci, F.; Sabatini, B.; Caruso, C. Epigenetic Control of Defense Genes Following MeJA-Induced Priming in Rice. J. Plant Physiol. 2018, 228, 166–177. [Google Scholar] [CrossRef]
- Martinez-medina, A.; Flors, V.; Heil, M.; Mauch-mani, B.; Pieterse, C.M.J. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.A. Diseases in Intercropping Systems. Annu. Rev. Phytopathol. 2013, 51, 499–519. [Google Scholar] [CrossRef]
- Maitra, S.; Palai, J.B.; Manasa, P.; Kumar, D.P. Potential of Intercropping System in Sustaining Crop Productivity. Int. J. Agric. Environ. Biotechnol. 2019, 12, 39–45. [Google Scholar] [CrossRef]
- Poveda, J. Beneficial Effects of Microbial Volatile Organic Compounds (MVOCs) in Plants. Appl. Soil Ecol. 2021, 168, 104118. [Google Scholar] [CrossRef]
- Poveda, K.; Kessler, A. New Synthesis: Plant Volatiles as Functional Cues in Intercropping Systems. J. Chem. Ecol. 2012, 38, 1341. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Kebede, Y.; Baudron, F.; Bianchi, F.; Tittonell, P. Agriculture, Ecosystems and Environment Unpacking the Push-Pull System: Assessing the Contribution of Companion Crops along a Gradient of Landscape Complexity. Agric. Ecosyst. Environ. 2018, 268, 115–123. [Google Scholar] [CrossRef]
- Pickett, J.A.; Woodcock, C.M.; Midega, C.A.O.; Khan, Z.R. Push-Pull Farming Systems. Curr. Opin. Biotechnol. 2014, 26, 125–132. [Google Scholar] [CrossRef]
- Hassanali, A.; Herren, H.; Khan, Z.R.; Pickett, J.A.; Woodcock, C.M. Integrated Pest Management: The Push-Pull Approach for Controlling Insect Pests and Weeds of Cereals, and Its Potential for Other Agricultural Systems Including Animal Husbandry. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.R.; Pickett, J.A.; Wadhams, L.; Muyekho, F. Habitat Management Strategies for the Control of Cereal Stemborers and Striga in Maize in Kenya. Int. J. Trop. Insect Sci. 2001, 21, 375–380. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive Function of Herbivore-Induced Plant Volatile Emissions in Nature. Science 2001, 291, 2141–2143. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Tamiru, A.; Morales, X.C.; Nyagol, D.; Cheruiyot, D.; Chidawanyika, F.; Subramanian, S.; Midega, C.A.O.; Bruce, T.J.A.; Khan, Z.R. Bioactive Volatiles From Push-Pull Companion Crops Repel Fall Armyworm and Attract Its Parasitoids. Front. Ecol. Evol. 2022, 10, 883020. [Google Scholar] [CrossRef]
- Tamiru, A.; Bruce, T.J.A.; Woodcock, C.M.; Birkett, M.A.; Midega, C.A.O.; Pickett, J.A.; Khan, Z.R. Chemical Cues Modulating Electrophysiological and Behavioural Responses in the Parasitic Wasp Cotesia sesamiae. Can. J. Zool. 2015, 287, 281–287. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Mutyambai, D.M.; Charles, M.A.O.; Syprine, A.; Nyaga, T.M.; Niassy, S.; Khan, Z.R.; Magara, H.J.O.; Mutyambai, D.M.; Charles, M.A.O.; et al. Responses of Stemborer Chilo partellus to Volatiles Emitted by Maize Landraces Exposed to Signal Grass (Brachiaria brizantha). J. Plant Interact. 2020, 15, 345–357. [Google Scholar] [CrossRef]
- Kigathi, R.N.; Unsicker, S.B.; Reichelt, M.; Kesselmeier, J.; Gershenzon, J.; Weisser, W.W. Emission of Volatile Organic Compounds After Herbivory from Trifolium pratense (L.) Under Laboratory and Field Conditions. J. Chem. Ecol. 2009, 35, 1335–1348. [Google Scholar] [CrossRef]
- Letters, E. Maize Landraces Recruit Egg and Larval Parasitoids in Response to Egg Deposition by a Herbivore. Ecol. Lett. 2011, 14, 1075–1083. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Rambla, J.L.; Granell, A.; Urbaneja, A. Biological Activity and Specificity of Miridae-Induced Plant Volatiles. BioControl 2018, 63, 203–213. [Google Scholar] [CrossRef]
- Uefune, M.; Abe, J.; Shiojiri, K.; Urano, S.; Nagasaka, K.; Takabayashi, J. Targeting Diamondback Moths in Greenhouses by Attracting Specific Native Parasitoids with Herbivory-Induced Plant Volatiles. R. Soc. Open Sci. 2020, 7, 20192. [Google Scholar] [CrossRef] [PubMed]
- Conboy, N.J.A.; Mcdaniel, T.; George, D.; Ormerod, A.; Edwards, M.; Donohoe, P.; Gatehouse, A.M.R.; Tosh, C.R. Volatile Organic Compounds as Insect Repellents and Plant Elicitors: An Integrated Pest Management (IPM) Strategy for Glasshouse Whitefly (Trialeurodes vaporariorum). J. Chem. Ecol. 2020, 46, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Pazouki, L.; Frost, C. Plant Seeds Are Primed by Herbivore-Induced Plant Volatiles. bioRxiv 2019, 17, 522839. [Google Scholar]
- Li, W.; Wang, L.; Zhou, F.; Li, C.; Ma, W.; Chen, H.; Wang, G.; Pickett, J.A.; Zhou, J.-J.; Lin, Y. Overexpression of the Homoterpene Synthase Gene, OsCYP92C21, Increases Emissions of Volatiles Mediating Tritrophic Interactions in Rice. Plant, Cell Environ. 2021, 44, 948–963. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, K.; Jiang, Y.; Xia, B.; Li, P.; Feng, H.; Wyckhuys, K.A.G.; Guo, Y. Mirid Bug Outbreaks in Multiple Crops. Science 2010, 1151, 1151–1154. [Google Scholar] [CrossRef]
- Yin, H.; Li, W.; Xu, M.; Xu, D.; Wan, P. The Olfactory Responses of Mirid Bugs to Six Plant Extracts and Their Volatiles. J. Appl. Entomol. 2021, 145, 125–133. [Google Scholar] [CrossRef]
- Murali-Baskaran, R.K.; Mooventhan, P.; Das, D.; Dixit, A.; Sharma, K.C.; Senthil-Nathan, S.; Kaushal, P.; Ghosh, P.K. The Future of Plant Volatile Organic Compounds (PVOCs) Research: Advances and Applications for Sustainable Agriculture. Environ. Exp. Bot. 2022, 200, 104912. [Google Scholar] [CrossRef]
- Blande, J.D.; Holopainen, J.K.; Niinemets, Ü. Plant Volatiles in Polluted Atmospheres: Stress Responses and Signal Degradation. Plant Cell Environ. 2014, 37, 1892–1904. [Google Scholar] [CrossRef]
- McFrederick, Q.S.; Fuentes, J.D.; Roulston, T.; Kathilankal, J.C.; Lerdau, M. Effects of Air Pollution on Biogenic Volatiles and Ecological Interactions. Oecologia 2009, 160, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.M.C. A Review on Cyclodextrin Encapsulation of Essential Oils and Volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Kaplan, I. Attracting Carnivorous Arthropods with Plant Volatiles: The Future of Biocontrol or Playing with Fire? Biol. Control 2012, 60, 77–89. [Google Scholar] [CrossRef]
| Organism Emitter | Organism Target | Emitted Volatile | Activity | Crop | Reference |
|---|---|---|---|---|---|
| Muscodor albus Worapong et al. | Fusarium sambucinum Fukel, Helminthosporium solani Durieu and Mont, Pectobacterium atrosepticum van Hail, Tilletia horrida Padwick and A. Khan, Tilletia indica Mitra, Tilletia tritici (DC.) Tul. and C. Tul | Volatilome | Fungal growth inhibition | Potato ( Solanum tuberosum L.), Rice (Oryza sativa L.), Wheat ( Triticum aestivum L.) | [32,33] |
| Muscodor crispens. Mitchell et al. | Pythium ultimum Trow, Phytophthora cinnamomic Rands, Sclerotinia sclerotiorum (Lib.) de Bary, Mycosphaerella fijiensis Morelet, Xanthomonas axonopodis pv. Citri Hasse, Yersinia pestis Lehmann & Neumann, Mycobacterium tuberculosis Zopf, Staphylococcus aureus Rosenbach | Volatilome | Fungal and bacterial growth inhibition | Banana (Musa × paradisiaca L.) | [34] |
| Muscodor brasiliensis Pena et al. | Penicillium digitatum Pers | Volatilome | Fungal growth inhibition | Orange (Citrus × sinensis L.) | [35] |
| Muscodor sutura Kudalkar et al. | Phyllosticta citricarpa McAlpine | Volatilome | Fungal growth inhibition | Citrus | [36] |
| Muscodor albus Worapong et al. | Phthorimaea operculella Zeller | Volatilome | Insecticidal effect | Potato (Solanum tuberosum L.) | [37] |
| Muscodor heveae Siri-udom et al. | Rigidoporus microporus Swartz | Volatilome | Fungal growth inhibition | Rubber trees (Hevea brasiliensis Müll. Arg.) | [38] |
| Trichoderma asperellum Samuels et al. | Fusarium incarnatum Desm., Corynespora cassiicola Berk. and M.A. Curtis, Curvularia aeria Bat et al. | Phenylethyl alcohol | Fungal growth inhibition | Muskmelon (Cucumis melo L.), Lettuce (Lactuca sativa L.) | [39,40] |
| Trichoderma harzianum Rifai | Pyrenophora teres Drechsles, Fusarium moniliforme Sheldon | 6-pentyl-alpha- pyrone (6PAP) | Fungal growth inhibition | Barley (Hordeum vulgare L.) | [41,42] |
| Trichoderma spp. Persoon Longibrachiatum Rifai | Sclerotium rolfsii Curzi, Macrophomina phaseolina Tassi | Volatilome | Fungal growth inhibition | Generalist | [44] |
| Trichoderma koningiopsis Samuels et al. | Colletotrichum gloeosporioides Penz | Volatilome | Fungal growth inhibition | Chili pepper (Capsicum annuum L.) | [43] |
| Trichoderma atroviride Bissett | Phytophthora infestans Mont. | 6-pentyl-2- pyrone (6-PP), isoamyl alcohol, isobutyl alcohol | Fungal growth inhibition | Potato (Solanum tuberosum L.) | [19] |
| Trichoderma viridens Pers. | Rhizoctonia solani J.G.Kühn | Volatilome | Fungal growth inhibition | Generalist | [45] |
| Aspergillus flavus Link | Aspergillus flavus Link Aspergillus parasiticus Speare | 3-octanone, trans-2-methyl-2- butenal, 2,3- dihydrofuran, decane | Mycotoxin inhibition | [46] | |
| Daldinia cf. concentrica Bolton | Aspergillus niger P.E.L. van Tieghem, Alternaria alternata Fr., Botrytis cinerea Whetzel, Colletotrichum sp. Corda, Coniella sp. Höhnel, Fusarium euwallaceae Freeman et al., Fusarium mangiferae Britz et al., Fusarium oxysporum Schltdl, Lasiodiplodia theobromae Pat., Penicillium digitatum Pers., Phoma tracheiphila Petri, Pythium ultimum Trow, Pythium aphanidermatum Edson, Rhizoctonia solani J.G.Kühn, Sclerotinia sclerotiorum (Lib.) de Bary | Volatilome; mixture of 4-heptanone and trans-2-octenal | Fungal growth inhibition | Dried fruits, Peanuts (Arachis hypogaea L.) | [48] |
| Oxyporus latemarginatus Durieu & Mont. | Botrytis cinerea Whetzel, Rhyzoctonia solani J.G.Kühn | 5-pentyl-2-furaldehyde | Fungal growth inhibition | Apple (Malus domestica Borkh) | [47] |
| Bacillus subtilis Ehrenberg | Botrytis cinerea Whetzel, Colletotrichum gloeosporioides Penz, Penicillium expansum Link, Monilinia fructicola Winter, Alternaria alternata (Fr.) Keissl, Fusarium oxysporum Schltdl | Volatilome; individual compounds 2,4-di-tert- butylphenol, benzothiazole | Fungal growth inhibition | Peach (Prunus cv. DaJiubao), Litchi (Litchi chinensis Sonn.) | [52] |
| Bacillus amyloliquefaciens Priest et al. | Fusarium solani Mart. | Volatilome | Fungal growth inhibition | [49] | |
| Bacillus velezencis Ruiz-García et.al. | Verticillium dahlia Kleb, Fusarium oxysporum Schltdl, Botrytis cinerea Whetzel, Monilinia fructicola Winter, Monilinia laxa Honey, Penicillium italicum Wehmer, Penicillium expansum Link | Decanal, 3-undecanone, 2-undecanone, 2-undecanol, undecanal, 2,4-dimethyl-6-tert-butylphenol, benzothiazole, benzaldehyde, diacetyl, 1,3-butadiene, N, N-dimethyldodecylamine | Fungal growth inhibition | Strawberry (Fragaria × ananassa Duch.), Apricot (Prunus persica L.), Grape (Vitis vinifera L.), Mandarin (Citrus reticulata L.) | [50,51] |
| Bacillus megaterium de Bary | Aspergillus flavus Link, Penicillium verrucosum Dierckx, Fusarium verticillioides Sacc. | Volatilome | Mycotoxin inhibition | [53,54] | |
| Pseudomonas fluorescens Migula | Penicillium italicum Wehmer | Dimethyl disulfide (DMDS), dimethyl trisulfide (DMTS) | Fungal growth inhibition | Citrus fruits | [55] |
| Pseudomonas protegens Flügge | Aspergillus flavus Link | Volatilome | Mycotoxin Inhibition | Rice (Oryza sativa L.) | [54] |
| Pseudomonas chlororaphis subsp. aureofaciens Kluyver | Ceratocystis fimbriata Ellis and Halst | 3-methyl-1- butanol, phenylethyl alcohol, 2-methyl-1- butanol | Fungal growth inhibition | Sweet potato (Ipomoea batatas L. Lam.) | [57] |
| Streptomyces alboflavus Waksman and Curtis | Aspergillus flavus Link | Dimethyl trisulfide, benzenamine | Mycotoxin Inhibition | [58] | |
| Streptomyces philanthi | Colletotrichum gloeosporioides Penz | Volatilome | Fungal growth inhibition | Chili (Capsicum annuum L.) | [59] |
| Pichia anomala Hansen | Aspergillus flavus Link | 2-phenylethyl ethanol | Mycotoxin Inhibition | Tree nuts | [60] |
| Hanseniaspora uvarum Niehaus | Botrytis cinerea Whetzel | Volatilome | Fungal growth inhibition | Strawberry (Fragaria × ananassa Duch.), Cherries (Prunus subsp. cerasus L.) | [61] |
| Candida nivariensis Alcoba-Florez | Aspergillus flavus Link | Volatilome | Fungal growth inhibition, Mycotoxin inhibition | [62] | |
| Clavispora lusitaniae Uden & Carmo Souza | Penicillium digitatu m Pers. | Volatilome | Fungal growth inhibition | Lemon (Citrus × limon L.) | [63] |
| Volatile Compound | Beneficial Insect | Pest Insect | Crop | Reference |
|---|---|---|---|---|
| β-myrcene, β-caryophyllene | Wasp (Encarsia formosa Gahan) | Whitefly (Bemisia tabaci Gennadius) | Tomato (Solanum lycopersicum L.) | [94] |
| D-limonene, β-ocimene | Wasp (Aphytis melinus DeBach) | California red scale (Aonidiella aurantii Maskell) | Mandarin (Citrus reticulata L.), Orange (Citrus × sinensis L.), Lemon (Citrus × limon L.) | [101] |
| β-ocimene | Wasp (Aphidius gifuensis Ashmaed) | Aphid (Myzus persicae Sulzer) | Chinese cabbage [Brassica rapa L. subsp pekinensis (Lour) Hanelt] | [91] |
| (E)-β-ocimene | Lady beetle (Adalia bipunctata L.), Green lacewing larvae (Chrysoperla carnea Stephens) | Peach (Prunus persica L.) | [93] | |
| α-pinene | Wasp (Aphelinus varipes Foerster) | Aphid (Myzus persicae Sulzer) | Chili pepper (Capsicum annuum L.), Eggplant (Solanum melongena L.), Crown daisy (Glebionis coronaria L.), Chinese cabbage [Brassica rapa L. subsp pekinensis (Lour) Hanelt], Cabbage (Brassica oleracea var. capitata L.) | [102] |
| Mixture (β-pinene, β-phellandrene, 3-carene, β-ocimene) | Mirid (Nesidiocoris tenuis Reuter) | Tomato moth (Tuta absoluta Meyrick), Whitefly (Trialeurodes vaporariorum Westwood) | Tomato (Solanum lycopersicum L.) | [103] |
| (E)-3-hexenyl acetate | Mirid (Deraeocoris brevis Uhler), Anthocorid (Orius tristicolor White), Coccinellid (Stethorus punctum picipes Casey) | [104] | ||
| (Z)-3-hexenyl acetate | Ladybird beetle (Coccinella septempunctata L.) | Cotton (Gossypium L.) | [105] | |
| Nonanal, (Z)-3-hexenyl acetate, methyl salicylate | Linyphiid spider (Erigonidium graminicolum Sundevall) | |||
| Octanal | Bug (Deraeocoris punctulatus Fallen) | |||
| Dimethyl octatriene, nonanal + (Z)-3-hexen-1-ol, octanal | Syrphid fly (Paragus quadrifasciatus Meigen) | |||
| 3,7-dimethyl,1,3,6-octatriene, nonanal, (Z)-3-hexenyl acetate, nonanal + (Z)-3-hexen-1-ol, methyl salicylate | Bug (Orius similis Zheng) | |||
| Pregeijerene | Nematodes Steinernema diaprepesi Nguyen and Duncan, Steinernema sp. glaseri Glaser and Fox, Steinernema riobrave Cabanillas, Poinar and Raulston, Steinernema carpocapsae Weiser, Steinernema feltiae Filipjev, Steinernema kraussei Nikdel and Niknam, Steinernema scapterisci Nguyen and Smart, Heterorhabditis indica Poinar, Karunakar and David, Heterorhabditis zealandica Poinar, Heterorhabditis bacteriophora Poinar | Beetle larvae (Diaprepes abbreviatus L.) Wax moth (Galleria mellonella L.) Beetle (Anomala orientalis Waterhouse) | Citrus | [106] |
| Methyl salicylate | Mite (Neoseiulus californicus McGregor) | Spider mite (Tetranychus urticae C. L. Koch) | [86] | |
| Geocorid (Geocoris pallens Stål.) Hoverflies (Syrphidae Latreille), Coccinellid (Stethorus punctum picipes Casey) Hoverflies (Toxomerus marginatus Say) | Corn borer (Ostrinia nubilalis Hübner) | [95] |
| Volatile Compound | Organism Target | Effect | Crop | Reference |
|---|---|---|---|---|
| Dimethyl disulfide, methyl isovalerate, 2-undecanone | Nematode (Meloidogyne incognita Kofoid and White) | Induce defense response and growth promotion | Tomato (Solanum lycopersicum L.) | [117] |
| (E)-nerolidol | Leafhopper (Empoasca onukii Matsuda), Fungus (Colletotrichum fructicola Prihast et al.) | Induce defense response | Tea plant (Camellia sinensis L.) | [114] |
| Z-3-hexenol | Tomato yellow leaf curl virus | Induces defense response | Tomato (Solanum lycopersicum L.) | [118] |
| 2R,3R-butanediol, 2R,3S- butanediol | Cucumber mosaic virus, Tobacco mosaic virus | Induce defense response | Pepper (Capsicum annum L. cv. Bukwang) | [119] |
| 6-pentyl-α-pyrone (6PP) | Tobacco mosaic virus | Induces systemic resistance | Tobacco (Nicotiana tabacum cv. White Burley) | [120] |
| Dimethyl disulfide (DMDS) | Fungus (Sclerotinia minor Jagger) | Induces systemic resistance | Tomato (Solanum lycopersicum L.) | [115] |
| Nonanal, limonene | Fungus (Colletotrichum lindemuthianum Sacc. and Magnus) | Induce systemic resistance | Common bean (Phaseolus vulgaris L. Sp. Pl.) | [116] |
| Dimethyl disulfide, 2,3-butanediol, 2-pentylfuran | Induces systemic drought tolerance | Maize (Zea mays L.) | [122] | |
| (Z)-3-hexen-1-yl acetate | Induces tolerance against cold stress | Maize (Zea mays L.) | [125] | |
| Induces drought resistance | Wheat (Triticum spp. L.) | [123] | ||
| Protects against salinity stress | Peanut Arachis hypogaea L.) | [126] | ||
| Eugenol | Induces cold and drought tolerance | Tea plant (Camellia sinensis L.) | [124] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razo-Belman, R.; Ozuna, C. Volatile Organic Compounds: A Review of Their Current Applications as Pest Biocontrol and Disease Management. Horticulturae 2023, 9, 441. https://doi.org/10.3390/horticulturae9040441
Razo-Belman R, Ozuna C. Volatile Organic Compounds: A Review of Their Current Applications as Pest Biocontrol and Disease Management. Horticulturae. 2023; 9(4):441. https://doi.org/10.3390/horticulturae9040441
Chicago/Turabian StyleRazo-Belman, Rosario, and César Ozuna. 2023. "Volatile Organic Compounds: A Review of Their Current Applications as Pest Biocontrol and Disease Management" Horticulturae 9, no. 4: 441. https://doi.org/10.3390/horticulturae9040441
APA StyleRazo-Belman, R., & Ozuna, C. (2023). Volatile Organic Compounds: A Review of Their Current Applications as Pest Biocontrol and Disease Management. Horticulturae, 9(4), 441. https://doi.org/10.3390/horticulturae9040441







