Abstract
Flower color not only determines the quality and commercial value of ornamental plants, but it also plays a vital role in ecological processes such as pollinator attraction. This study aimed to investigate the relationship between flower color and the cellular physicochemical factors of Bletilla striata. The color space values of 21 samples were initially determined, followed by a selection of five samples with significant color differences for testing cell shape, total flavonoid content (ranging from 1.86 to 5.42 mg/g), total anthocyanin content (ranging from 0.52 to 292.62 (A530 − 0.25 ∗ A657)/g), cell pH (varying between 5.03 and 5.74), and metal ion content (including Al3+, Ca2+, Fe3+, K+, Mg2+, Na+, P5+, Zn2+, Mo6+, Cu2+, and Mn2+). The flowers of Bletilla were predominantly purple, pink, and yellow; and distributed in quadrants I, II, and IV on the a* and b* rectangular coordinate. The a* value was identified as the primary color indicator for this species. Total anthocyanin content and Zn2+ showed a significant positive correlation with a*, while Al3+, Ca2+, Fe3+, Mg2+, Na+, and Mn2+ demonstrated negative correlations. Cell shape, flavonoid content, and pH had nonsignificant correlations with a*. In conclusion, the total anthocyanin content and metal ions play crucial roles in determining the flower color of B. striata, which can have implications for pollinator attraction. Future research should focus on understanding the complex interactions between these factors to develop novel ornamental plant varieties with desirable flower colors and enhanced ecological functions.
1. Introduction
Flower color is an important feature when concerning the quality and commercial value of various ornamental plants, as well as playing a vital role in ecology through its natural function in pollinator attraction [1]. When light hits the petals, some light is absorbed as it penetrates the pigment layer, and the other light is reflected in the spongy tissue, passing through the pigment layer again and entering our eyes, thus giving the flower color [2]. The color of the flower is related to many cellular physicochemical factors such as the type and content of pigment present in the petal cells, the shape of the pigment cells, the intracellular pH, and metal ions [3]. These cellular physicochemical factors affect the color presentation of flowers. The main phytochromes found in current research are flavonoids, carotenoids, and betalains [3]. Among them, anthocyanins are a class of flavonoids that produce various colors ranging from red to blue [4]. The surface cell shape of the petal affects the reflection and refraction of light within the cells, thus affecting the brightness of the petal color. Most petal epithelial cells are conical in shape. This structure increases the proportion of the incident light entering the cells and darkens the color of the flower, while flattened cells are lighter in color [5]. The colors of anthocyanins vary significantly at different pH values. In general, anthocyanins are more stable at lower pH levels and appear red in acidic environments. Anthocyanins are unstable in alkaline environments with a high pH, and the flowers are bluish [6]. Not only does the pH vary considerably between different plant species, but also between different tissues and developmental stages of the same plant [6]. Changes in intracellular pH therefore have an important influence on the expression of flower color. Some metal ions can form highly colored and stable complexes with anthocyanins, which can significantly impact the flower color, especially on the bluing of some flower colors [7]. The difference between the blue and purple of the Tulipa gesneriana perianth is due to the accumulation of more Fe3+ in the blue parts than in the purple [8]. In a study of six Gerbera varieties with different colors, it was found that the amounts of metal ions such as Fe2+, Ca2+, and Mg2+ were more effective in influencing flower color parameters. Additionally, these metal ions can affect the stability of the flowers’ final color by altering vacuolar pH and the activities of enzymes involved in the biosynthesis, destruction, accumulation, and transition of pigments [9]. This information highlights the complex relationship between metal ions and flower color, emphasizing the need for further research into the mechanisms underlying these interactions.
Bletilla Rchb.f. is a small genus with six species in Orchidaceae, belonging to the subfamily Epidendroideae, distributed from Myanmar and Indochina, through China to Japan [10]. In China, there are four distinct Bletilla species: B. striata (Thunb.) Rchb. f., with pale purple, purplish red, or pink sepals and petals, rarely whitish, and a lip with undulate mid-lobe; B. ochracea Schltr., featuring pale yellowish-green sepals and petals, rarely whitish, and a lip with obtuse apices; B. formosana (Hayata) Schltr., displaying pale purple, purplish red, or pink sepals and petals, rarely whitish, and a lip with acute or subacute apices; and B. guizhouensis J. Huang and G. Z. Chen, a species resembling B. striata but distinguished by the seven longitudinal lamellae on its lip [10,11]. These plants not only have commercial value due to their low maintenance and adaptability to a wide range of garden conditions, making them perfect for rain gardens, but they also possess significant pharmaceutical properties. They bloom in the spring and summer over a relatively long 10-week period, and are widely used in traditional medicine to treat hemoptysis and traumatic hemorrhage. Pharmacological studies have validated their use, particularly for hemorrhagic diseases, with polysaccharides and stilbenes being the major bioactive chemical constituents of the Bletilla genus according to the literature [12,13]. Despite their importance, all species in Bletilla are currently endangered (EN) due to extensive habitat loss and over-harvesting [14]. Researches on Bletilla mainly involve four directions: pharmacological–chemical and clinical applications, the extraction and identification of secondary metabolites, histoculture fast propagation technology, and novel biomaterial development [15]. However, there is a lack of research on flower color, and a further genetic basis analysis for ornamental applications, which could provide a foundation for future research and conservation efforts.
Bletilla striata have a wide range of flower colors, with color variations on the front and back of the perianths, especially on the lip, with various colors and patterns [16]. The plant has an upright form and lush flowers with high horticultural ornamental value. However, systematic studies on the intrinsic relationship between flower color and important cellular physicochemical factors have not yet been reported. In the present study, the perianth color, surface cell shape, total anthocyanin content, total flavonoid content, pH, and metal ion content of some species of Bletilla were comprehensively measured and analyzed to investigate the relationship between flower color and important cellular physicochemical factors, to provide a new perspective for the in-depth analysis of the physiological and biochemical mechanisms of the flower color formation in B. striata.
2. Materials and Methods
2.1. Plant Materials
Flowers of Bletilla striata and B. ochracea were collected in full bloom at Shanghai Chenshan Botanical Garden from April to May 2022. The majority of these samples are native varieties from different regions, with color variations occurring naturally without any formal registration. The plants were cultivated in sandy soil, and sampling took place under clear skies, with temperatures ranging between 18 and 22 °C. As shown in Figure 1, a total of 21 varieties from different samples were collected for floral color characterization analysis. Based on the perianth and lip colors of their main ornamental parts, they can be classified into five major groups: White B. striata (gW), pure purple B. striata (gP), purple perianth yellow lip B. striata (gPY), purple perianth B. ochracea (gYP), and pure yellow B. ochracea (gY).
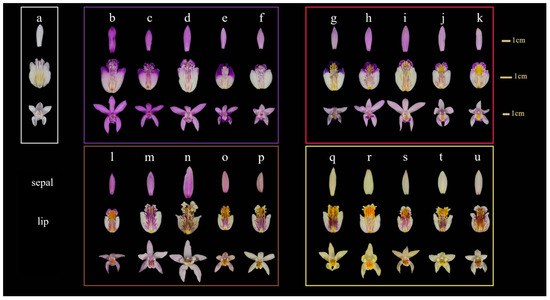
Figure 1.
The phenotypic diversity of floral color in Bletilla of different origins. White group of B. striata (gW): a: Yunnan. Pure purple group of B. striata (gP): b: Qinling, c: Shanghai, d: Japan, e: Wuhu, f: Wuhan. Purple perianth yellow lip group of B. striata (gPY): g: Shanghai, h: Shanxi, i: Hubei, j: Enshi, k: America. Purple perianth group of B. ochracea (gYP): l: Guiyang, m: Liuba, n: Chongqing, o: Liuba, p: Shangluo. Pure yellow group of B. ochracea (gY): q: Sichuan, r: America, s: Jingxi, t: Guizhou, u: Liuba.
Four materials a, c, f, and g (Figure 1), representing different flower colors, were selected for the correlation analysis between flower color and physicochemical factors, with material r as the control. The above materials were assigned the following names or codes based on their color: White (W), Violet (V), Pink (P), Blue (B), and Yellow (Y).
2.2. Methods
2.2.1. Colorimetric Analysis
The color intensities of 21 Bletilla varieties were measured using a colorimeter (CM-2300d, KonicaMinolta, Tokyo, Japan) with a C/2° light source. In this colorimetric analysis, the CIE L* a* b* color space was employed, utilizing the indexes L*, a*, and b* to describe color. These indexes represent lightness (L*), red–green balance (a*), and yellow–blue balance (b*). L* ranges from 0 (black) to 100 (white), with positive a* values indicating red and negative values indicating green, while positive b* values indicate yellow and negative values indicate blue [17,18]. As shown in Figure 2, six different parts were measured. The average values of L*, a*, and b* were obtained. Three samples from each variety were measured as biological replicates. Both the chroma (C*) and hue (h°) were derived from a* and b* using the following equations:
Chroma C* = √(a*2 + b*2),
hue h° = arctan−1(b*/a*).
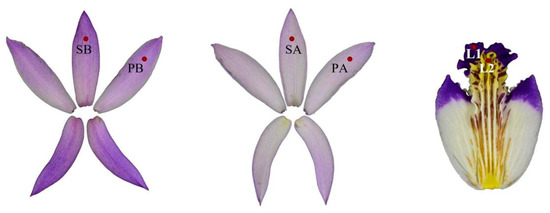
Figure 2.
The illustration of Bletilla flower color measurement location (SB: sepal back, PB: petal back, SA: sepal front, PA: petal front, L1: Lip1, L2: Lip2).
2.2.2. Epidermal Cell Shape Measurements
For each floral part of each variety, five randomly selected epidermal cells were measured using the hand-cut section method, taking into account three angles; that is, α, β, and γ, which describe the degree of bending of the surface of the apical, lateral, and basal cell parts [19]. The S value was calculated for each floral part using the formula:
S = (α1 + α2)/180 ∗ (γ1 + γ2)/180 ∗ βmin/βmax.
In general, the lower the value of S, the more conical the apical and basal parts of the epidermal cells, and the higher the value of S, the flatter the shape of each side of the epidermal cells.
2.2.3. Quantification of Total Flavonoids
The total flavonoid content of Bletilla flowers was quantified regarding the protocol of Yang [20]. The standard curve was plotted using rutin as the standard. The regression equation was obtained as:
y = 18.464x − 0.0341, R2 = 0.995.
The lips, petals, and sepals of the material were ground and weighed 0.2 g, and 2 mL of 65% ethanol extract was added and extracted at 65 °C for 12 h. The extract was obtained through filtration. The absorbance was measured at 510 nm using a full-wavelength enzyme standardizer (TECAN Infinite 200 PRO, Hombrechtikon, Switzerland). The concentration of total flavonoids was calculated by substituting them into the regression equation.
2.2.4. Determination of Total Anthocyanidin
Extraction of anthocyanins from Bletilla flowers was performed following the protocol of Rabino et al. [21]. A 2 mL volume of acidic methanol (1% HCl) to 0.1 g of ground material for the lips, petals, and sepals was added. Samples were incubated for 24 h at 4 °C. The material was sedimented via centrifugation (14,000 rpm, 4 °C, 3 min). The absorptions of the supernatants of the extracts at 530 nm and 657 nm wavelength were determined photometrically using a full-wavelength enzyme standardizer (TECAN Infinite 200 PRO, Switzerland). The quantification of anthocyanins was performed using the following equation:
QAn = (A530 − 0.25 ∗ A657)/g
QAn is the amount of anthocyanins, A530 and A657 are the absorptions at the indicated wavelengths, and g is the weight of the material used for extraction.
2.2.5. pH Measurement
The pH of the perianth and lip was determined with slight modification by referring to Qi et al. [22]. Material (0.5 g) was ground in liquid nitrogen, deionized water was added to 10 mL volume, and centrifugation was performed (12,000 rpm, 4 °C, 15 min). The pH of the supernatant was determined using a pH meter.
2.2.6. Determination of Metal Ions
The concentrations of Al3+, Ca2+, Fe3+, K+, Mg2+, Na+, P5+, Zn2+, Mo6+, Cu2+, and Mn2+ were detected via an inductively coupled plasma mass spectrometer (NexION 300D, Waltham, MA, USA) and an inductively coupled plasma spectrometer (iCAP 7400 ICP-OES, Carlsbad, CA, USA), by referring to the method of Akbari et al. [9].
2.2.7. Correlation between Colorimetric Values and Physicochemical Factors
Using Pearson correlation coefficients and linear regression analysis, correlations among flower color space values (L*, a*, and b*) and cellular physicochemical factors in B. striata were examined. SPSS and OriginPro 8.0 software were utilized for analysis, employing a completely randomized design (CRD) for the ANOVA test to assess significant differences among variables [23].
3. Results
3.1. Measurement of Colorimetric Values of 21 Bletilla Varieties
From the colorimeter measurement data of 21 Bletilla species with a total of 126 ornamental parts (Table 1), it is evident that the L* values of gW and gY were relatively high and concentrated, whereas the L* of gP was primarily lower (Figure 3A). The a* values of gP, gPY, and gYP were generally higher than those of gW and gY. The a* of gP was the highest, gW’s a* was around 0, and gY’s a* was concentrated at between −8.15 and 0 (Figure 3B). The b* values for gW, gP, and gPY were mainly negative, positive for gY, and both positive and negative for gYP. The b* values were the lowest for gP and the highest for gY (Figure 3C). The c* values were the lowest for gW, and relatively higher for gP and gY (Figure 3D).

Table 1.
The distribution range of CIELab colorimetric values (L*, a*, b*, c*, and h) for different Bletilla groups.
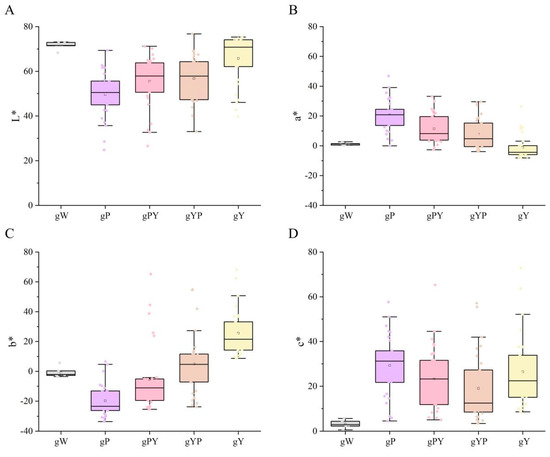
Figure 3.
The box plot, based on flower color phenotype for Bletilla according to CIELab data. (A) L* (darkness to lightness), (B) a* (redness to greenness), (C) b* (yellowness to blueness), and (D) c* (Saturation). gW: White B. striata, gP: Pure purple group of B. striata, gPY: Purple perianth yellow lip group of B. striata, gYP: Purple perianth group of B. ochracea, gY: Pure yellow group of B. ochracea.
In general, the purple family (gP, gPY, and gYP) had lower a L*, higher a*, and lower b* values. For the pure yellow gY, it had the highest L*, the lowest a*, and the highest b*. W had higher L* values, and the a* b* values tended to be close to zero.
The 126 flower color data points in the a* b* hue coordinates were mainly violet, pink, and yellow, distributed in quadrants I, II, and IV on the a* and b* rectangular coordinate system. No distribution was observed in the blue–green quadrant III (Figure 4A). Flower color phenotype data, except for L2, were generally distributed along both sides of the fitted linear equation:
b* = −1.09228a* + 7.55775, (R2 = 0.93497).
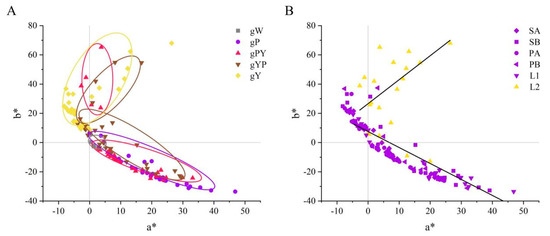
Figure 4.
The flower color distribution of Bletilla in two-dimensional coordinate systems of L*, a*, b*. (A) Five different color groups a* b* distribution (gW: White B. striata, gP: Pure purple group of B. striata, gPY: Purple perianth yellow lip group of B. striata, gYP: Purple perianth group of B. ochracea, gY: Pure yellow group of B. ochracea). (B) Distribution of a* b* in different flower parts (SA: sepal front, SB: sepal back, PA: petal front, PB: petal back, L1: Lip1, L2: Lip2).
Meanwhile, the L2 fitted linear equation was
however, the linear regression fit level was insignificant. L2 was primarily concentrated in quadrant I, while other sites were mainly present in quadrants II and IV, indicating a difference between L2 and the other parts of the color distribution pattern (Figure 4B).
b* = 1.64147a* + 26.57998, (R2 = 0.35209);
3.2. Cell Shape Measurements
An anatomical analysis of White (W), Violet (V), Pink (P), Blue (B), and Yellow (Y) flowers revealed significant differences in cell morphology between different parts of the same flower, and similar cell morphologies in the same parts of different B. striata flowers (Table 2). Lip3 had the highest shape index, followed by Petal and Sepal, which were similar, then Lip1, and finally Lip2 with the lowest shape index:
Lip3 cells were rectangular, Sepal and Petal cells were spherical, Lip1 cells were conical, and Lip2 cells were papillate (Figure 5).
Lip3 > Petal = Sepal > Lip1 > Lip2.

Table 2.
Comparison of cell shape measurements and morphologies among different parts of Bletilla flowers, with White (W), Violet (V), Pink (P), Blue (B), and Yellow (Y) color variations.
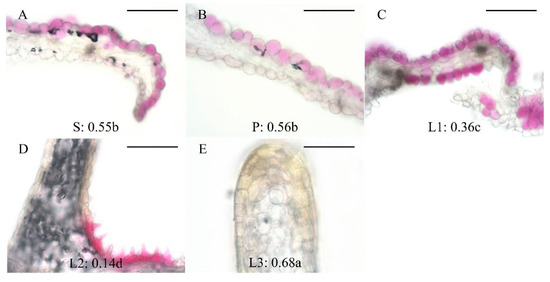
Figure 5.
Cell shapes and shape index. (A) Sepal, (B) Petal, (C) Lip1, (D) Lip2, (E) Lip3 (ridge on the Lip2). Groups annotated with the same letter are not significantly different, according to LSD Test (p < 0.05). Bars represent 200 μm in all panels.
3.3. Quantification of Total Flavonoids and Total Anthocyanidins
The quantifications of total flavonoids (TF) and total anthocyanidins (TA) were conducted in the B. striata flowers of different colors, including White (W), Violet (V), Pink (P), Blue (B), and Yellow (Y). The analysis was performed for three replicates and measured in the sepals (S), petals (P), and lips (L) of each flower color (Figure 6, Table S1).
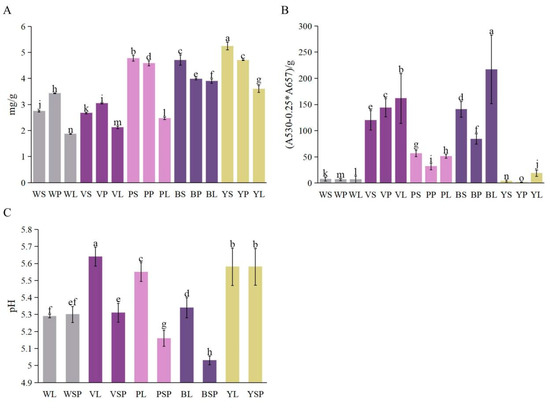
Figure 6.
(A) Quantification of total flavonoids, (B) Determination of total anthocyanin, (C) pH in Bletilla. Error bars represent the standard error of the mean (SEM). Groups annotated with the same letter are not significantly different, according to LSD Test (p < 0.05). WS: White sepal, WP: White petal, WL: White lip, VS: Violet sepal, VP: Violet petal, VL: Violet lip, PS: Pink sepal, PP: Pink petal, PL: Pink lip, BS: Blue sepal, BP: Blue petal, BL: Blue lip, YS: Yellow sepal, YP: Yellow petal, YL: Yellow lip, WSP: White perianth, VSP: Violet perianth, PSP: Pink perianth, BSP: Blue perianth, YSP: Yellow perianth.
For the total flavonoids, the highest concentration (mg/g) was observed in the yellow flowers (YS: 5.25 ± 0.37, YP: 4.70 ± 0.02, YL: 3.60 ± 0.13), followed by pink (PS: 4.77 ± 0.11, PP: 4.58 ± 0.09, PL: 2.47 ± 0.04), blue (BS: 4.71 ± 0.18, BP: 3.98 ± 0.03, BL: 3.90 ± 0.09), white (WS: 2.74 ± 0.03, WP: 3.43 ± 0.01, WL: 1.87 ± 0.01), and violet (VS: 2.67 ± 0.03, VP: 3.04 ± 0.02, VL: 2.12 ± 0.05) flowers (Figure 6A).
The determination of total anthocyanin revealed the highest concentrations (A530 − 0.25 ∗ A657)/g in the blue flowers (BS: 140.70 ± 12.51, BP: 84.33 ± 8.39, BL: 217.14 ± 57.52), followed by violet (VS: 120.19 ± 18.75, VP: 143.94 ± 16.88, VL: 161.62 ± 38.37), pink (PS: 56.57 ± 5.94, PP: 31.92 ± 6.13, PL: 51.21 ± 3.74), white (WS: 7.48 ± 3.47, WP: 6.83 ± 2.13, WL: 7.03 ± 6.19), and yellow (YS: 4.00 ± 1.94, YP: 1.31 ± 0.65, YL: 18.82 ± 6.15) flowers (Figure 6B).
In summary, the analysis of total flavonoids and total anthocyanidins showed significant differences among the B. striata flowers of various colors, with yellow flowers having the highest flavonoid content and blue flowers having the highest anthocyanin content.
3.4. pH Measurement
The pH measurement of Bletilla striata flowers was also conducted in different colors, including white (W), violet (V), pink (P), blue (B), and yellow (Y). The analysis was performed for three replicates and measured in the lips (L) and perianths (SP) of each flower color (Figure 6C, Table S1).
The pH values observed for each flower color are as follows: White flowers showed pH values of 5.28 ± 0.01 in the lips (WL) and 5.32 ± 0.04 in the perianths (WSP). Violet flowers exhibited pH values of 5.68 ± 0.05 in the lips (VL) and 5.36 ± 0.06 in the perianths (VSP). Pink flowers had pH values of 5.49 ± 0.06 in the lips (PL) and 5.14 ± 0.05 in the perianths (PSP). Blue flowers presented pH values of 5.30 ± 0.06 in the lips (BL) and 5.05 ± 0.03 in the perianths (BSP). Lastly, yellow flowers displayed pH values of 5.52 ± 0.10 in the lips (YL) and 5.53 ± 0.10 in the perianths (YSP).
The pH measurement analysis of Bletilla striata flowers revealed variations in pH values among the different colors of flowers, with violet flowers having the highest pH, and blue flowers having the lowest pH.
3.5. Quantification of Metal Ions
The metal ion contents of the five Bletilla varieties are shown in Table 3. Al3+ concentrations ranged from 57.05 mg/kg in violet to 213.36 mg/kg in yellow flowers. Ca2+ concentrations varied, with the highest concentration in pink flowers (2916.15 mg/kg) and the lowest in violet flowers (471.81 mg/kg). Fe3+ concentrations were highest in yellow flowers (277.23 mg/kg) and the lowest in violet flowers (61.68 mg/kg). K+ concentrations were highest in white flowers (17,625.41 mg/kg) and lowest in blue flowers (13,237.87 mg/kg). Mg2+ concentrations ranged from 1238.52 mg/kg in blue flowers to 1764.78 mg/kg in white flowers. Na+ concentrations were highest in white flowers (304.44 mg/kg) and lowest in blue flowers (123.69 mg/kg). P5+ concentrations varied, with the highest concentration in yellow flowers (2592.46 mg/kg) and the lowest in pink flowers (1898.21 mg/kg). Zn2+ concentrations were highest in yellow flowers (34.61 mg/kg) and lowest in blue flowers (18.20 mg/kg). Mo6+ concentrations ranged from 0.25 mg/kg in violet flowers to 1.88 mg/kg in yellow flowers. Cu2+ concentrations were highest in yellow flowers (11.20 mg/kg) and lowest in blue flowers (6.31 mg/kg). Mn2+ concentrations ranged from 8.00 mg/kg in blue flowers to 22.12 mg/kg in yellow flowers.

Table 3.
Comparison of metal ion concentrations (mg/kg) in five Bletilla color varieties: White (W), Violet (V), Pink (P), Blue (B), and Yellow (Y).
The quantification of metal ions in Bletilla striata flowers showed considerable variation among the different color varieties, suggesting that metal ion concentrations may play a role in the flower color expression in B. striata.
3.6. Correlation Analysis
A correlation analysis was performed in this study to investigate the relationships among cellular physicochemical factors and flower color in B. striata. The type of correlation used was Pearson’s correlation (Table 4). Several significant relationships were observed among the color parameters of B. striata flowers. Specifically, there was a significant negative correlation between L* and a* (r = −0.823, p < 0.01), a significant positive correlation between L* and b* (r = 0.546, p < 0.01), and a significant negative correlation between a* and b* (r = −0.853, p < 0.01).

Table 4.
Correlation analysis of cellular physicochemical factors with flower color in B. striata.
Furthermore, the chroma (c*) of B. striata exhibited a highly significant positive correlation with a* (r = 0.833, p < 0.01), and negative correlations with L* (r = −0.877, p < 0.01) and b* (r = −0.468, p < 0.05). These results suggest that the chroma of B. striata is primarily affected by redness, and a* serves as an important indicator for the flower color description of B. striata.
The correlation analysis between the flower color parameters and other factors showed weak correlations for L*, a*, and b* with flower cell shape, suggesting that flower cell shape has a minimal effect on flower color differences in B. striata. Similarly, weak correlations were found between total flavonoids (TF) content and flower color parameters, while the total anthocyanins (TA) content showed a significant positive correlation with a* (r = 0.444, p < 0.05). As the total anthocyanin content increased, the flowers of B. striata became redder and darker.
In this study, pH did not significantly affect the flower color of B. striata. However, metal ions displayed significant correlations with floral parameters. The color of the B. striata flowers became red as the content of Zn2+ increased (r = 0.469, p < 0.05), while the content of Al3+ (r = −0.749, p < 0.01), Ca2+ (r = −0.469, p < 0.05), Fe3+ (r = −0.734, p < 0.01), Mg2+ (r = −0.544, p < 0.01), Na+ (r = −0.454, p < 0.05), and Mn2+ (r = −0.488, p < 0.05) increased, causing the red color of the flowers to diminish.
In summary, total anthocyanidin content and Zn2+ primarily contribute to making B. striata redder, while Al3+, Ca2+, Fe3+, Mg2+, Na+, and Mn2+ cause the red color to fade.
4. Discussion
Flower color is one of the key characteristics of ornamental plants. Novel and bright flower color is prevalent among consumers, and is an important breeding goal for breeders. Flower color is influenced by many factors, such as pigment, epidermal cell shape [5], intracellular pH [6], metal ions [7], and so on, among which the most important factor is the type and content of pigments. Pigments are widely distributed in the cells of plant flowers, leaves, and other tissues as secondary metabolites, giving plants a variety of colors [3]. Moreover, flower color has ecological significance, as it plays a role in attracting pollinators, which are vital for plant reproduction [24]. Different pollinators may be attracted to specific flower colors, creating a connection between the range of pollinators and flower color [1,25]. For example, bees are generally attracted to blue, purple, and yellow flowers, while butterflies prefer red, orange, and pink flowers [1]. Understanding the factors that influence flower color can provide valuable insights for breeders aiming to develop new ornamental plant varieties with desirable characteristics, taking into account the ecological aspect of pollination as well.
Orchidaceae is one of the most species-rich families in the plant kingdom, with about 880 genera and 27,800 species [26]. Orchids have become important ornamental plants with high economic and cultural values, due to their unique flower shape, various flower colors, and strong fragrance [27]. Bletilla is a small genus of Orchidaceae with both medicinal and ornamental values [12]. However, the quantitative evaluation of the floral color of B. striata, the composition of the floral pigments, the physicochemical factors affecting the floral color, the systematic analysis of the floral pigment synthesis pathway, and the molecular regulatory mechanisms have yet to be reported. In this study, we have conducted a quantitative evaluation of floral color in two species of Bletilla and have screened the physicochemical factors affecting floral color in B. striata via correlation analysis.
4.1. Flower Color Characteristics
Identifying flower color efficiently and accurately is the first step in the genetic improvement of ornamental plants. Currently, different models of colorimeter are widely used for ornamental plant color. The CIELab system is widely used for the quantitative description of flower color [17]. Based on colorimeter measurements, the colors of Bletilla ornamental parts were mainly purple, pink, and yellow; rarely red and orange, and with no green flowers. The L2 color variation is specific, and its expression at the molecular level may differ from other ornamental parts, and so it is worthwhile to conduct flower color studies in separate parts in the future. Current studies in Phalaenopsis, Dendrobium [28], and Nigella orientalis [29] have revealed that MYB-like transcription factors often regulate this complex coloration pattern.
Bletilla has a relatively small range of color distribution compared to bulk ornamental flowers such as Chrysanthemum and Rose, but Bletilla possesses delphinidin [30] and cyanidin [31] anthocyanin pathways. The blue flowers of B. striata have been identified as blue due to acylated cyanidin 3, 7-diglucoside [31]. However, in other species, the formation of blue flowers is mainly due to the accumulation of blue–violet delphinidin glycosides in the petals. Given this, B. striata, which possesses the cyanidin bluing pathway and the delphinidin synthesis pathway, has an excellent potential for producing blue varieties with different pigment types in the future.
4.2. Physicochemical Factors Associated with the Main Flower Color of B. striata
Anthocyanins change the color of flowers by affecting the redness (a*) of the flowers; they are the main substances that constitute the red to purple and blue color, and they are present in the plant body in the form of soluble glycosides [3]. The total anthocyanin content of B. striata in the study was significantly and positively correlated with a*. However, among the detected indicators, TA: Blue > Violet > Pink, while a*: Violet > Pink > Blue. It can be seen that the total anthocyanin content and a* are not a single linear variation. Previous studies have shown that flower colors with anthocyanins as the primary pigment show an extensive range of colors, due in large part to minor differences in the chemical structure of the anthocyanins or the fact that the chemical structure, although identical, can also produce variations in hue due to different physical or chemical environmental factors in the vacuoles [3,32]. These include the number of hydroxyl groups carried by anthocyanins, the degree of hydroxyl methylation, the number of glycosylated species, and the position of linkage, etc., all of which can affect the color presentation [32]. The mechanism of anthocyanin presentation in B. striata needs to be further investigated.
The effect of metal ions on flower color is mainly through complexation with flavonoids and other components to form highly colored and stable metal complexes, often called supramolecular pigments. Its influence on flower color is great, and it can especially make some flower colors tend to be blue [7,32]. In blue flower research, it was found that Al3+, Fe3+, Mg2+, etc. [33], can complex with plant flavonoid substances to form blue complexes, such as the blue Centaurea cyanus, formed because Cyanidin-3-O-glucoside complexed with Fe3+ and formed a blue co-color complex, together with flavonols [34]. In the red Chrysanthemum, Al3+, Fe3+, Mg2+, and Ca2+ were found to have a color-enhancing effect on anthocyanins, while no significant effect was found on flavonoids in the white species [35]. Na+ and K+, on the other hand, could increase the vesicular pH through reverse transporter proteins and thus regulate the formation of blue flowers [36]. In B. striata, Zn2+ was significantly correlated with the increase in a*, and Al3+ and Fe3+ were significantly correlated with the increase in b*. K+ was the most abundant metal ion, and it was significantly correlated with the increase in L*. Higher levels of Al3+, Fe3+, Mg2+, Na+, and Mn2+ effectively increased the brightness of B. striata L*, as well as weakening a*, while a higher Ca2+ also inhibited the presentation of B. striata floral color. It can be seen that the same metal ion does not have exactly the same effect on different species or different colors of the same species. B. striata flower color is closely related to the concentration of metal ions in the cells. However, the mechanism of how metal ions affect the flower color of B. striata needs to be further investigated.
4.3. Physicochemical Factors Not Associated with the Main Flower Color of B. striata
The cell morphology affects the lightness L* and saturation C* by influencing the reflection and refraction of light. When the cells are conical, this structure increases the proportion of incident light entering the cells and darkens the flower color. In contrast, when the cells are flat, they reflect more incident light and lighten the flower color [5]. Most petal epithelial cells such as Chrysanthemum [37], Rose [38], Dianthus [39], etc., are conical in shape. B. striata flowers vary significantly in epidermal cell morphology from one ornamental part to another, with three types of epidermal cells on the lip alone. Although different cell morphologies can make differences in flower color presentation, no correlation with cell morphology has been found for the time being for the color differences in the various ornamental parts of B. striata. It has been hypothesized that such cell morphological differences may serve more of an insect pollination purpose [40]. The cell shape of L1, the outermost part of the B. striata lip, is similar to those of petal P and sepal S, which are ellipsoidal. B. striata is a typical insect vector flower [41], and the pollinating insects are mainly bees, so L2 is located in the pollination channel that bees must pass through, and conical cells probably increase the friction and provide landing points for bees.
Flavonoids tend to change the flower color by affecting the yellowness (b*), among which chalcone and aurone are dark yellow; flavonoids and flavonols vary in color from ivory white to light yellow, and most of them act as auxiliary pigments in the process of flower color formation [42]. For example, the main coloring substances of Camellia nitidissima flower color are flavonols, Quercetin 7-glucoside, Quercetin 3-glucoside, etc. [23]. Plant yellow flower presentation is mainly associated with yellow pigments in flavonoids. Total flavonoids also contain colorless substances such as epicatechin, whose relative content interferes with the yellow presentation to varying degrees [23]. In the present study, although B. striata contained high levels of total flavonoids, as did B. ochracea, the correlation between total flavonoids and all three floral parameters L*a*b* of B. striata was weak and did not reach a significant level. The present flavonoid content on B. striata did not significantly affect the floral color differences of B. striata.
The vacuole pH changes the form of anthocyanin, thus changing the flower color [6]. Typically, anthocyanins are red and they are more stable at low pH, and as the vacuole pH increases, the flower color tends to be blue and is easily decomposed. Several forms of anthocyanins are in balance in a given pH solution and they exhibit a specific color [6]. The blue Petunia × atkinsiana (Sweet) D. Don ex W. H. Baxter increases in pH to deepen the blue color of the flower [43]. In Hydrangea macrophylla (Thunb.) Ser., the cellular pH of red flowers is about 0.8 lower than that of blue flowers [33]. It has been pointed out that the pH varies significantly among species and does not vary much within the same species, but it significantly affects the flower color.
In the present study, overall, the redness a* of V, P, and B decreased with decreasing pH, and the correlation between a* and pH was insignificant. This result may be due to the effects of enzymes in the cytoplasm during grinding on the vacuole pH, and the deviation of the measured values. It is also possible that the different color B. striata pH difference is not the key factor causing the variation in a*. Further measurements can be made later using a method that can directly measure the pH inside the vacuoles, such as microelectrodes. Moreover, by part, the pH of the sepal and petals with uniform color distribution was significantly lower than that of the lip with complex color composition, and what factors are responsible for this phenomenon remains to be investigated.
5. Conclusions
In this study, the color space values of 21 flower samples of Bletilla and the cellular physicochemical factors of five different flower colors were determined. The results showed that the flowers of Bletilla present mainly purple, pink and yellow colors. a* was the primary indicator to describe the color of B. striata. The total anthocyanin content and Zn2+ of B. striata showed a significant positive correlation with a*. The redness of B. striata increased significantly with anthocyanins and Zn2+. However, Al3+, Ca2+, Fe3+, Mg2+, Na+, and Mn2+ showed negative correlations, and the increases of these metal ions significantly reduced the redness of B. striata. Meanwhile, the correlation between cell shape, flavonoid content, and pH with a* was insignificant. Therefore, total anthocyanin content and metal ions are the main physicochemical factors of the flower color expression of B. striata.
Future studies should focus on investigating the genetic basis of flower color in Bletilla species, which could help breeders to develop new cultivars with desired color traits, and further our understanding of the mechanisms behind color expression. Additionally, conservation efforts should be put in place to protect these endangered species, which have both ornamental and pharmaceutical value. The findings of this study provide a foundation for future research and conservation strategies in the field of Bletilla flower color and beyond.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9040426/s1, Table S1: Content of total flavonoids, total anthocyanins, and pH.
Author Contributions
Conceptualization, C.X. and C.H.; methodology, C.X.; software, C.X. and W.S.; validation, C.X., C.H. and X.D.; formal analysis, C.X.; investigation, C.X. and Y.G.; resources, W.H.; data curation, C.X.; writing—original draft preparation, C.X. and C.H.; writing—review and editing, C.H., X.D., W.H. and X.S.; visualization, C.X. and X.D.; supervision, W.H. and X.S.; project administration, W.H.; funding acquisition, W.H. and X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Science and Technology Commission, Shanghai Municipality, to Weichang Huang (grant number 19390743600), the project from Shanghai Science and Technology to Develop Agriculture Key Project to Xinhua Zeng (grant number 2021-02-08-00-12-F00778), from the project of Shanghai Landscaping and City Appearance Administrative Bureau to Chao Hu (grant number G202401), and from the Hainan province key research and development plan (grant number ZDYF2020099).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors are grateful to Shanmin Li, Aixian Lu, and Shaofan Luo for the help with measurement and data analyses.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Trunschke, J.; Lunau, K.; Pyke, G.H.; Ren, Z.; Wang, H. Flower color evolution and the evidence of pollinator-mediated selection. Front. Plant Sci. 2021, 12, 617851. [Google Scholar] [CrossRef]
- Vogelmann, T.C. Plant Tissue Optics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 231–251. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- van der Kooi, C.J.; Dyer, A.G.; Kevan, P.G.; Lunau, K. Functional significance of the optical properties of flowers for visual signalling. Ann. Bot. 2019, 123, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Stavenga, D.G.; Leertouwer, H.L.; Dudek, B.; Van der Kooi, C.J. Coloration of flowers by flavonoids and consequences of pH dependent absorption. Front. Plant Sci. 2021, 11, 600124. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Spectral and colorimetric characteristics of metal chelates of acylated cyanidin derivatives. Food Chem. 2017, 221, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.; Miki, N.; Nakajima, N.; Momonoi, K.; Kato, C.; Yoshida, K. Perianth bottom-specific blue color development in Tulip cv. Murasakizuisho requires ferric ions. Plant Cell Physiol. 2007, 48, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Akbari, R.; Hatamzadeh, A.; Sariri, R.; Bakhshi, D. Relationship of flower color parameters and metal ions of petal tissue in fully opened flowers of Gerbera. J. Plant Stud. 2013, 2, 89–96. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Zhu, G.; Lang, K.; Ji, K.; Luo, Y.; Jin, X.; Cribb, P.; Wood, J.; Gale, S.; et al. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2009; Volume 25, pp. 209–210. [Google Scholar]
- Huang, J.I.E.; Wang, M.; Chen, L.-J.; Huang, Z.-C.; Rao, W.-H.; Zhang, Y.-Q.; Chen, J.-B.; Chen, G.-Z. Bletilla guizhouensis (Orchidaceae; Epidendroideae), a new species from Guizhou China: Evidence from morphological and molecular analyses. Phytotaxa 2019, 406, 279–286. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Z.; Jiang, K.; Luo, Y.; Jin, X.; Zhang, Z.; Xu, R.; Muchuku, J.K.; Musungwa, S.S.; Yukawa, T.; et al. Phylogenetic analysis and character evolution of tribe Arethuseae (Orchidaceae) reveal a new genus Mengzia. Mol. Phylogenet. Evol. 2022, 167, 107362. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, M.; Jiang, L.; Xie, Q.; Yuan, H.; Yang, Y.; Zafar, S.; Liu, Y.; Jian, Y.; Li, B.; et al. The medicinal uses of the genus Bletilla in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2021, 280, 114263. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List of Threatened Species. 2019. Available online: https://www.iucnredlist.org (accessed on 1 March 2023).
- Lu, J.; Liu, Y.; Yang, Y.; Sun, L.; Xia, X.; Zhou, M.; Huang, C. The analysis of Bletilla rchb. f. research development review from literatures in china. J. Yunnan Agric. Univ. 2011, 26, 288–292. [Google Scholar]
- Zhu, J.; Huang, W.; Cao, J.; Zhou, X. Evaluation and selection of Bletilla species through AHP method. Chin. J. Trop. Crops 2020, 41, 1553–1559. [Google Scholar]
- Hong, Y.; Bai, X.; Sun, W.; Jia, F.; Dai, S. The numerical classification of Chrysanthemum flower color phenotype. Acta Hortic. Sin. 2012, 39, 1330–1340. [Google Scholar]
- Gonnet, J.-F. Colour effects of co-pigmentation of anthocyanins revisited-1. A colorimetric definition using the CIELAB scale. Food Chem. 1998, 63, 409–415. [Google Scholar] [CrossRef]
- Papiorek, S.; Junker, R.R.; Lunau, K. Gloss, colour and grip: Multifunctional epidermal cell shapes in bee- and bird-pollinated flowers. PLoS ONE 2014, 9, e112013. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, L.; Liu, L.; Xu, R.; He, X.; Chen, L. Optimization of extraction process for total flavonoids from flowers of Rosa xanthina lindle. Chin. J. Trop. Crops 2011, 32, 111. [Google Scholar]
- Rabino, I.; Mancinelli, A.L. Light, temperature, and anthocyanin production. Plant Physiol. 1986, 81, 922–924. [Google Scholar] [CrossRef]
- Qi, Y.; Lou, Q.; Li, H.; Yue, J.; Liu, Y.; Wang, Y. Anatomical and biochemical studies of bicolored flower development in Muscari latifolium. Protoplasma 2013, 250, 1273–1281. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.; Tong, R.; He, L.; Zhang, L.; Li, Z.; Huang, X. Relationship between flower color and important cellular environment elemental factors in yellow Camellia. Guihaia 2019, 39, 1605–1612. [Google Scholar]
- Yan, J.; Wang, G.; Sui, Y.; Wang, M.; Zhang, L. Pollinator responses to floral colour change, nectar and scent promote reproductive fitness in Quisqualis indica (Combretaceae). Sci. Rep. 2016, 6, 24408. [Google Scholar] [CrossRef]
- Reverté, S.; Retana, J.; Gómez, J.M.; Bosch, J. Pollinators show flower colour preferences but flowers with similar colours do not attract similar pollinators. Ann. Bot. 2016, 118, 249–257. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, Y.; Ou, Y.; Ke, Y.; Yao, Y.; Wang, M.; Chen, J.; Ai, Y. Research advances of genes responsible for flower colors in Orchidaceae. Acta Hortic. Sin. 2021, 48, 2057. [Google Scholar]
- Hossain, M.M.; Kant, R.; Van, P.T.; Winarto, B.; Zeng, S.; Teixeira da Silva, J.A. The application of biotechnology to orchids. Crit. Rev. Plant Sci. 2013, 32, 69–139. [Google Scholar] [CrossRef]
- Fan, H.; Cui, M.; Li, N.; Li, X.; Liang, Y.; Liu, L.; Cai, Y.; Lin, Y. Genome-wide identification and expression analyses of R2R3-MYB transcription factor genes from two Orchid species. PeerJ 2020, 8, e9781. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, X.; Yao, X.; Fu, X.; Cheng, J.; Shan, H.; Yin, X.; Kong, H. Mechanisms underlying the formation of complex color patterns on Nigella orientalis (Ranunculaceae) petals. New Phytol. 2022, 237, 2450–2466. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Zhao, M.; Qin, H.; Duan, S.; Li, K.; Liao, C.; Zhou, P. Study on determination of anthocyanin from Bletilla striata flower and antioxidant activity in vitro. Genom. Appl. Biol. 2017, 36, 5269–5276. [Google Scholar]
- Tatsuzawa, F.; Saito, N.; Shigihara, A.; Honda, T.; Toki, K.; Shinoda, K.; Yukawa, T.; Miyoshi, K. An acylated cyanidin 3,7-diglucoside in the bluish flowers of Bletilla striata ‘Murasaki Shikibu’ (Orchidaceae). J. Jpn. Soc. Hortic. Sci. 2010, 79, 215–220. [Google Scholar] [CrossRef]
- Wessinger, C.A.; Rausher, M.D. Lessons from flower colour evolution on targets of selection. J. Exp. Bot. 2012, 63, 5741–5749. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Osakabe, A.; Saito, S.; Furuyama, D.; Tomita, A.; Kojima, Y.; Yamadera, M.; Sakuta, M. Components of protocyanin, a blue pigment from the blue flowers of Centaurea cyanus. Phytochemistry 2005, 66, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, L.; Chen, S.; Cao, J.; Ding, X.; Zheng, C.; Sun, C. Comparative analysis of the effects of internal factors on the floral color of four Chrysanthemum cultivars of different colors. Agriculture 2022, 12, 635. [Google Scholar] [CrossRef]
- Faraco, M.; Spelt, C.; Bliek, M.; Verweij, W.; Hoshino, A.; Espen, L.; Prinsi, B.; Jaarsma, R.; Tarhan, E.; de Boer, A.H.; et al. Hyperacidification of vacuoles by the combined action of two different P-ATPases in the Tonoplast determines flower color. Cell Rep. 2014, 6, 32–43. [Google Scholar] [CrossRef]
- Pu, Y.; Huang, H.; Wen, X.; Lu, C.; Zhang, B.; Gu, X.; Qi, S.; Fan, G.; Wang, W.; Dai, S. Comprehensive transcriptomic analysis provides new insights into the mechanism of ray floret morphogenesis in Chrysanthemum. BMC Genom. 2020, 21, 728. [Google Scholar] [CrossRef]
- Baudino, S.; Caissard, J.-C.; Bergougnoux, V.; Jullien, F.; Magnard, J.-L.; Scalliet, G.; Cock, J.M.; Hugueney, P. Production and emission of volatile compounds by petal cells. Plant Signal Behav. 2007, 2, 525–526. [Google Scholar] [CrossRef]
- Smith, M.T.; Saks, Y.; Staden, J.V. Ultrastructural changes in the petals of senescing flowers of Dianthus caryophyllus L. Ann. Bot. 1992, 69, 277–285. [Google Scholar] [CrossRef]
- Schlüter, P.M.; Schiestl, F.P. Molecular mechanisms of floral mimicry in orchids. Trends Plant Sci. 2008, 13, 228–235. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyake, T. How do rewardless Bletilla striata flowers attract pollinators to achieve pollination? Plant Syst. Evol. 2020, 306, 78. [Google Scholar] [CrossRef]
- Tanikawa, N.; Kashiwabara, T.; Hokura, A.; Abe, T.; Shibata, M.; Nakayama, M. A peculiar yellow flower coloration of Camellia using aluminum-flavonoid interaction. J. Jpn. Soc. Hortic. Sci. 2008, 77, 402–407. [Google Scholar] [CrossRef]
- Morita, Y.; Hoshino, A. Recent advances in flower color variation and patterning of Japanese morning glory and petunia. Breed. Sci. 2018, 68, 128–138. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).