Yield Response and Antioxidant Activity of Greenhouse Organic Pumpkin (Cucurbita moschata Duch.) as Affected by Soil Solarization and Biofumigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Description and Experimental Design
2.2. Soil Solarization
2.3. Amendments
2.4. Crop Succession after the Solarization
2.5. Pumpkin Cultivation
2.6. Antioxidant Activity
2.7. Statistical Analysis
3. Results
3.1. Soil Solarization
3.2. Yield and Morphological Traits
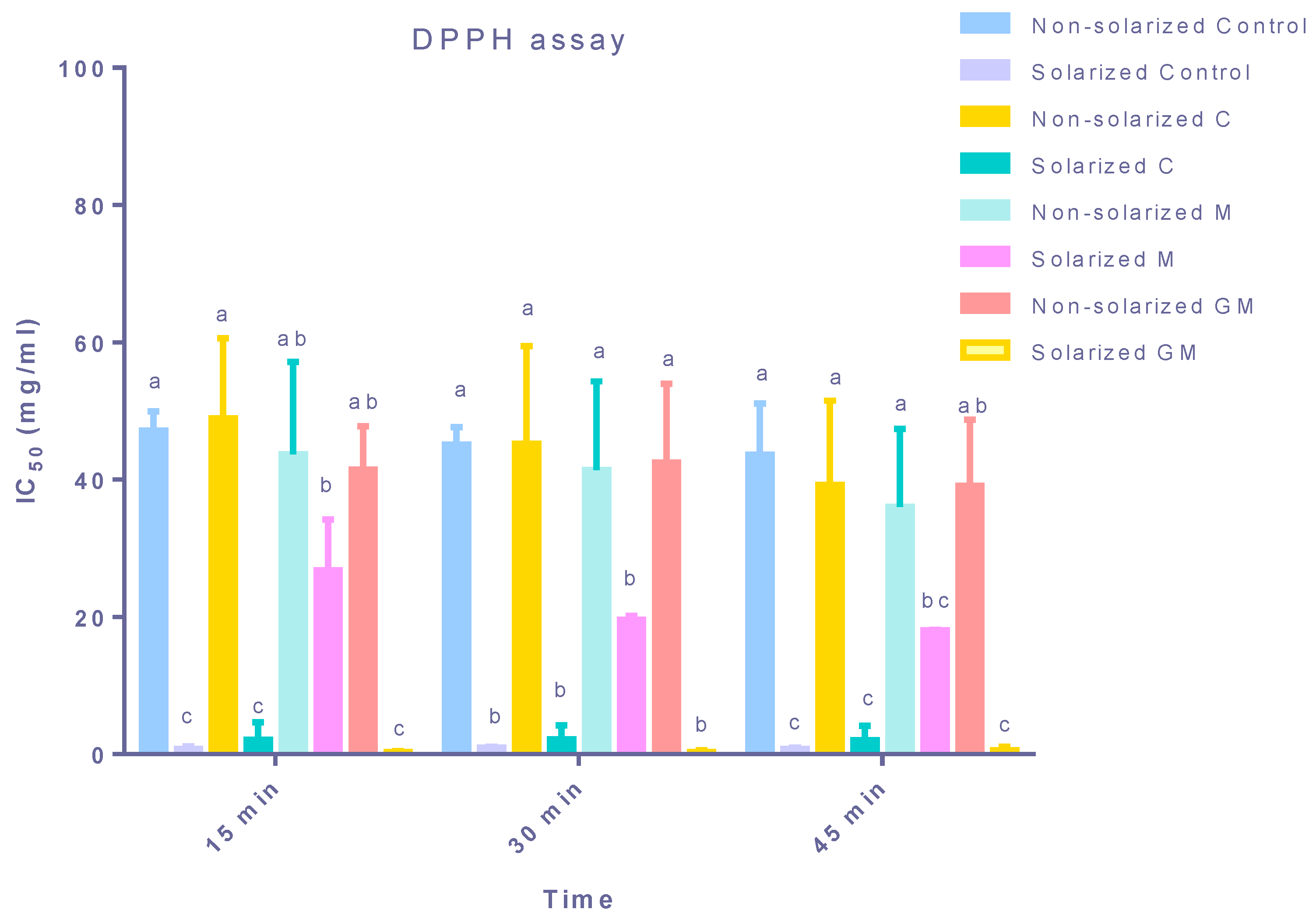
3.3. Antioxidant Activity
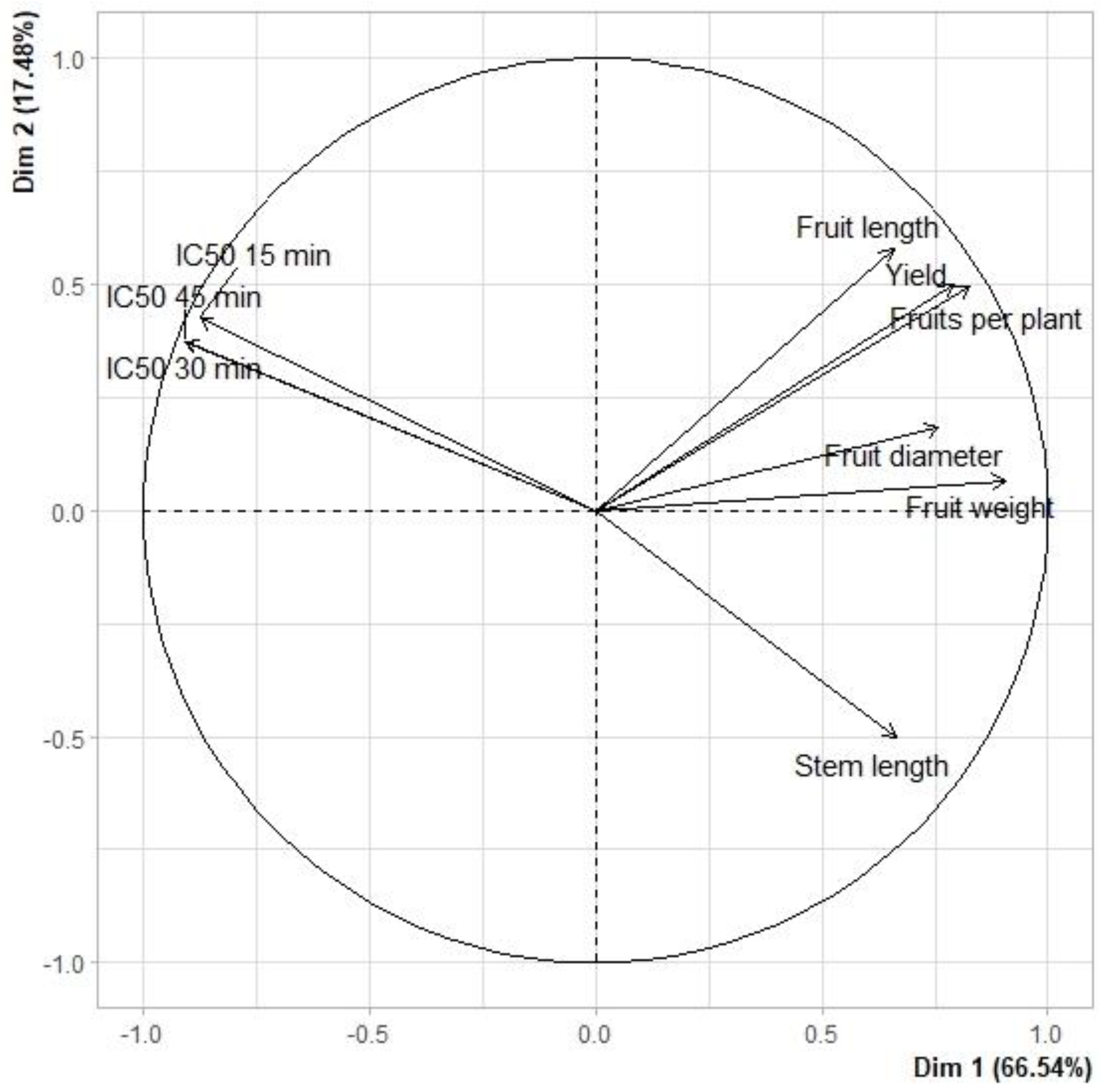
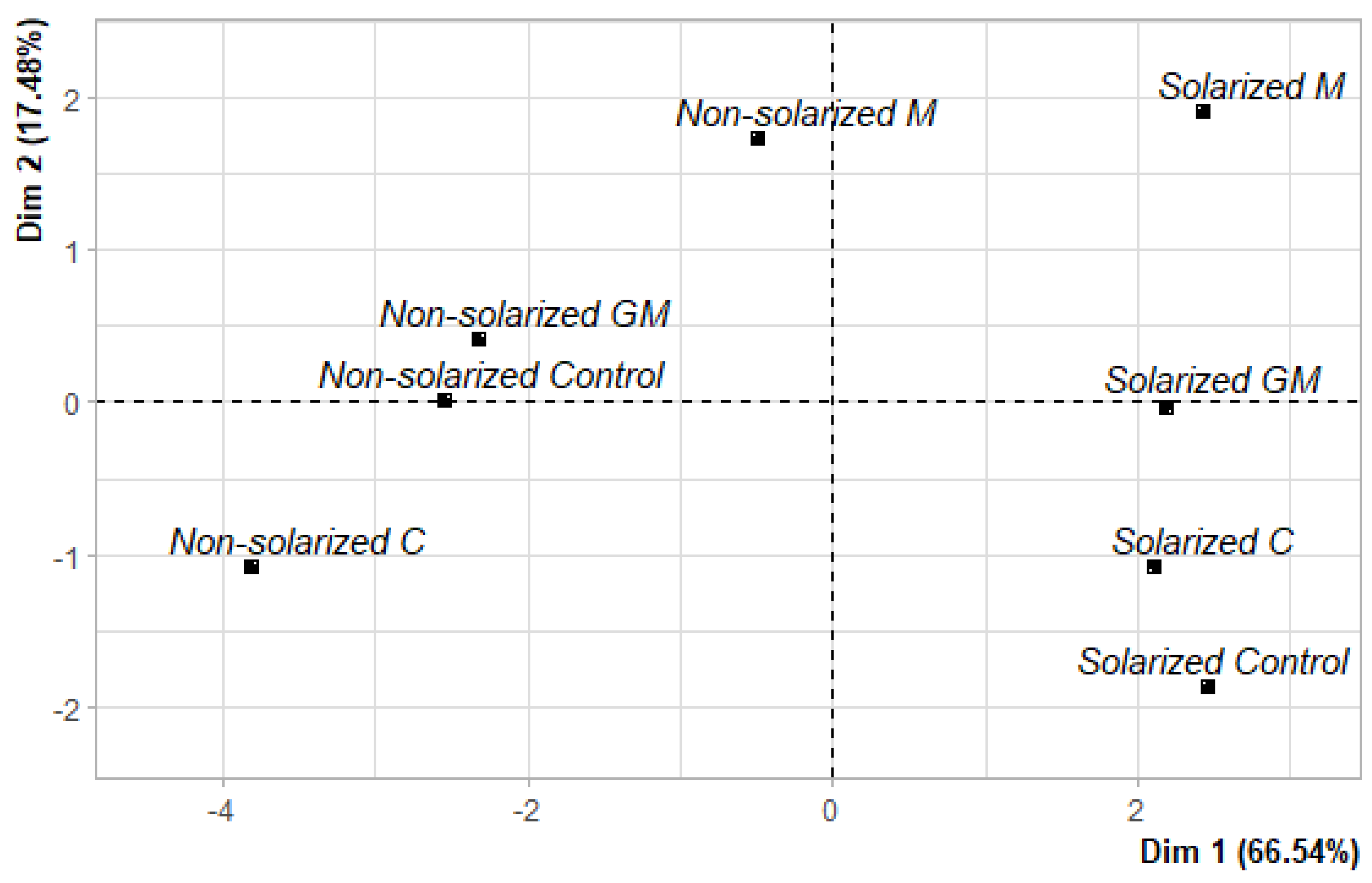
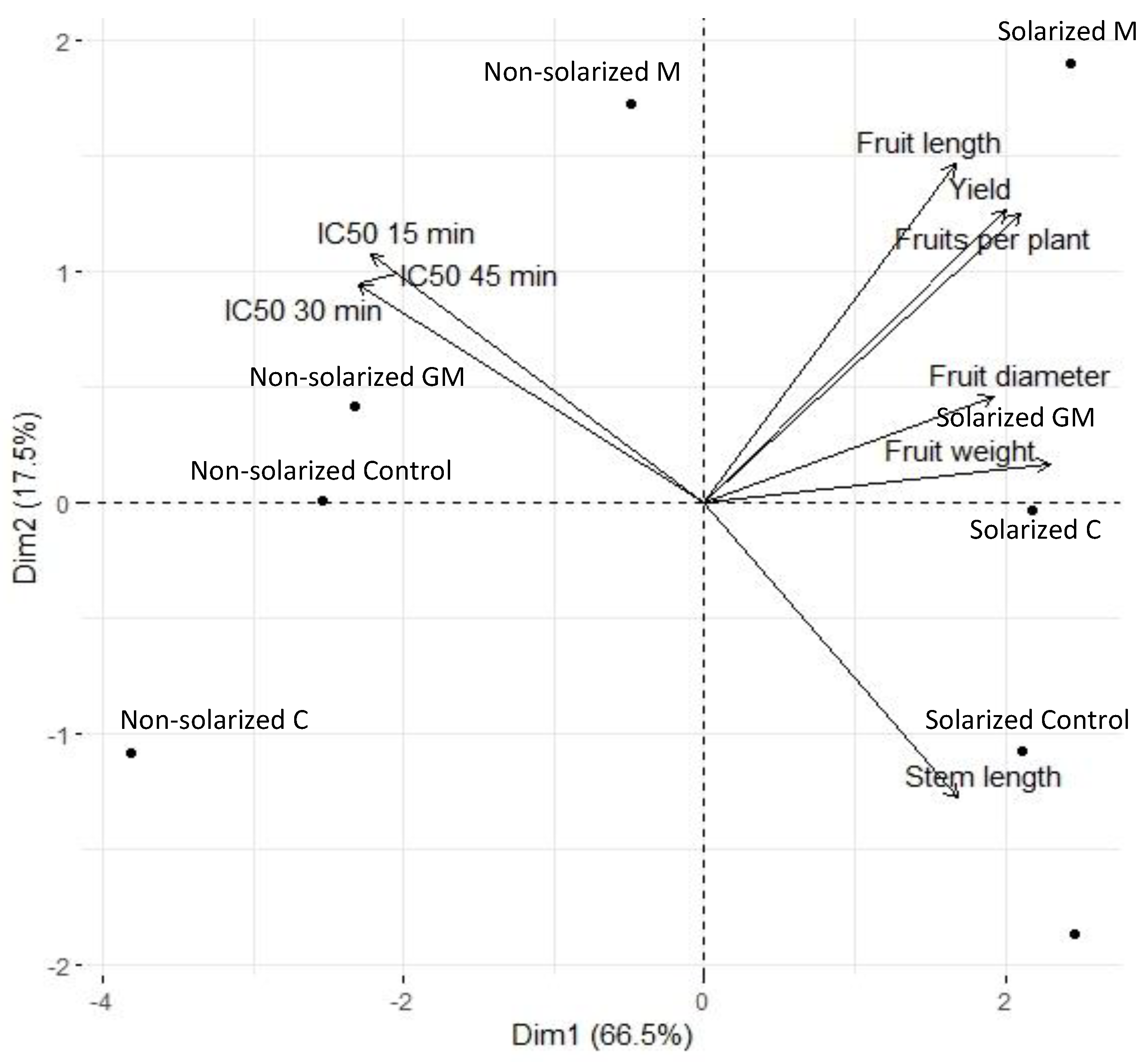
3.4. Principal Component Analysis
4. Discussion
4.1. Soil Temperatures and Yield Response
4.2. Antioxidant Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gullino, M.L.; Garibaldi, A.; Gamliel, A.; Katan, J. Soil disinfestation: From soil treatment to soil and plant health. Plant Dis. 2022, 106, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Katan, J. Three decades of soil solarization: Achievements and limitations. Acta Hortic. 2014, 1015, 69–78. [Google Scholar] [CrossRef]
- Candido, V.; D’Addabbo, T.; Miccolis, V.; Castronuovo, D. Effect of different solarizing materials on weed suppression and lettuce response. Phytoparasitica 2012, 40, 185–194. [Google Scholar] [CrossRef]
- Díaz-Hernandez, S.; Gallo-Llobet, L.; Domínguez-Correa, P.; Rodríguez, A. Effect of repeated cycles of soil solarization and biosolarization on corky root, weeds and fruit yield in screen-house tomatoes under subtropical climate conditions in the Canary Islands. Crop Prot. 2017, 94, 20–27. [Google Scholar] [CrossRef]
- Kanaan, H.; Frenk, S.; Raviv, M.; Medina, S.; Dror, M. Long and short term effects of solarization on soil microbiome and agricultural production. Appl. Soil Ecol. 2018, 124, 54–61. [Google Scholar] [CrossRef]
- Bonanomi, G.; Chiurazzi, M.; Caporaso, S.; Del Sorbo, G.; Moschetti, G.; Felice, S. Soil solarization with biodegradable materials and its impact on soil microbial communities. Soil Biol. Biochem. 2008, 40, 1989–1998. [Google Scholar] [CrossRef]
- Moura, L.; Queiroz, I.; Mourão, I.; Brito, L.M.; Duclos, J. Effectiveness of soil solarization and biofumigation for the control of corky root and root-knot nematode Meloidogyne spp. on tomato. Acta Hortic. 2012, 993, 399–406. [Google Scholar] [CrossRef]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Akhter, K.; Bibi, A.; Rasheed, A.; Rehman, S.; Shafique, U.; Habib, T. Indigenous vegetables of family Cucurbitaceae of Azad Kashmir: A key emphasis on their pharmacological potential. PLoS ONE 2022, 17, e0269444. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, Y.; Zeng, J.; Yao, J.; Lu, A.; Fang, Z.; Wang, G.; Wang, W.; Zhang, Y. Composition analysis of acid hydrolysates from Cucurbita moschata Duch. polysaccharides and their effect on oxidative stress resistance of Caenorhabditis elegans. Food Sci. Hum. Wellness 2023, 12, 795–800. [Google Scholar] [CrossRef]
- Lu, A.; Yu, M.; Fang, Z.; Xiao, B.; Guo, L.; Wang, W.; Li, J.; Wang, S.; Zh, Y. Preparation of the controlled acid hydrolysates from pumpkin polysaccharides and their antioxidant and antidiabetic evaluation. Int. J. Biol. Macromol. 2019, 12, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lee, S.K.; Mbwambo, Z.H.; Chung, H.; Luyengi, L.; Gamez, E.J.; Mehta, R.G.; Kinghorn, A.D.; Pezzuto, J.M. Evaluation of the antioxidant potential of natural products. Comb. Chem. High Throughput Screen. 1998, 1, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Paciulli, M.; Rinaldi, M.; Rodolfi, M.; Ganino, T.; Morbarigazzi, M.; Chiavaro, E. Effects of high hydrostatic pressure on physico-chemical and structural properties of two pumpkin species. Food Chem. 2019, 274, 281–290. [Google Scholar] [CrossRef]
- Candido, V.; D’Addabbo, T.; Basile, M.; Castronuovo, D.; Miccolis, V. Long time effect of soil solarization integrated with dazomet or chicken manure on yield, weeds and root-knot nematodes in tomato and melon. Ital. J. Agron. 2008, 4, 241–252. [Google Scholar] [CrossRef]
- Cuartero, J.; Pascual, J.A.; Vivo, J.M.; Özbolat, O.; Sánchez-Navarro, V.; Egea-Cortines, M.; Zarnoza, R.; Mena, M.M.; Garcia, E.; Ros, M.A. Fist-year melon/cowpea intercropping system improves soil nutrients and changes in soil microbial community. Agric. Ecosyst. Environ. 2022, 328, 107856. [Google Scholar] [CrossRef]
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 2012, 66, 265–283. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Al-Shammary, A.A.G.; Al-Sadoon, J.N.A.; Lahmod, N.R. Influence of the soil solarization management and fertilizer on soil temperature under different soil tillage systems. J. Agric. Sci. 2016, 8, 98. [Google Scholar] [CrossRef]
- Di Mola, I.; Ventorino, V.; Cozzolino, E.; Ottaiano, L.; Romano, I.; Duri, L.G.; Pepe, O.; Mori, M. Biodegradable mulching vs traditional polyethilene film for sustainable solarizarion: Chemical properties and microbial community response to soil management. Appl. Soil Ecol. 2021, 163, 103921. [Google Scholar] [CrossRef]
- Kanaan, H.; Medina, S.; Raviv, M. The effects of soil solarization and compost on soil suppressiveness against Fusarium oxysporum f. sp. melonis. Compost Sci. Util. 2017, 25, 206–210. [Google Scholar] [CrossRef]
- Yanardağ, I.H.; Zornoza, R.; Bastida, F.; Büyükkiliç-Yanardağ, A.; García, C.; Faz, A.; Mermut, A.R. Native soil organic matter conditions the response of microbial communities to organic inputs with different stability. Geoderma 2017, 295, 1–9. [Google Scholar] [CrossRef]
- Abd El-Megid, M.S.; Ibrahim, A.S.; Khalid, S.A.; Satour, M.M. Studies on Vegetable Transplants Using Seed-Bed Solarization: Improvement of Onion Transplant Characters and Smut Disease Control. In Proceedings of the Second International Conference on Soil Solarization and Integrated Management of Soil-Borne Pests, 1998, Aleppo, Syrian Arab Republic, 16–21 March 1997; FAO Plant Protection and Production Paper 147. FAO: Rome, Italy, 1998; pp. 165–174. [Google Scholar]
- Stapleton, J.J.; DeVay, J.E. Soil solarization: A natural mechanism of integrated pest management. In Novel Approaches to Integrated Pest Management; Reuveni, R., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 309–350. [Google Scholar]
- Chen, Y.; Gamliel, A.; Stapleton, J.J.; Aviad, T. Chemical, physical, and microbial changes related to plant growth in disinfested soils. In Soil Solarization; Katan, J., DeVay, J.E., Eds.; CRC: Boca Raton, FL, USA, 1991; pp. 103–129. [Google Scholar]
- Chen, Y.; Katan, J.; Gamliel, A.; Aviad, T.; Schnitzer, M. Involvement of soluble organic matter in increased plant growth in solarized soils. Biol. Fert. Soils 2000, 32, 28–34. [Google Scholar] [CrossRef]
- Gruenzweig, J.M.; Rabinowitch, H.D.; Katan, J. Physiological and developmental aspects of increased plant growth in solarized soils. Ann. Appl. Biol. 1993, 122, 579–591. [Google Scholar] [CrossRef]
- Candido, V.; D’Addabbo, T.; Miccolis, V.; Castronuovo, D. Weed control and yield response of soil solarization with different plastic film in lettuce. Sci. Hortic. 2011, 130, 491–497. [Google Scholar] [CrossRef]
- Coolen, W.A. Methods for the extraction of Meloidogyne spp. and other nematodes from roots and soil. In Root-Knot Nematodes (Meloidogyne Species), Systematics, Biology and Control; Lamberti, F., Taylor, C.E., Eds.; Academic Press: London, UK, 1979; pp. 317–329. [Google Scholar]
- Al-Shammary, A.A.G.; Kouzani, A.; Gyasi-Agyei, Y. A comparison of different solarisation systems and their impacts on soil thermal characteristics-an application in cultivated soils close to Baghdad, a highly populated city in Iraq. Environ. Monit. Assess. 2020, 192, 13. [Google Scholar] [CrossRef]
- Castello, I.; D’Emilio, A.; Raviv, M.; Vitale, A. Soil solarization as a sustainable solution to control tomato Pseudomonads infections in greenhouses. Agron. Sustain. Dev. 2017, 37, 59. [Google Scholar] [CrossRef]
- Candido, V.; Miccolis, V.; Castronuovo, D.; Basile, M.; D’Addabbo, T. Effects of repeated applications of soil solarization in greenhouse in Southern Italy. Acta Hortic. 2005, 698, 187–194. [Google Scholar] [CrossRef]
- Katan, J. Milestones and future expectations for soil disinfestation after 45 years of soil disinfestation symposia (1973–2018). Acta Hortic. 2020, 1270, 1–8. [Google Scholar] [CrossRef]
- Sofi, T.A.; Tewari, A.K.; Razdan, V.K.; Koul, V.K. Long term effect of soil solarization on soil properties and cauliflower vigor. Phytoparasitica 2014, 42, 2–11. [Google Scholar] [CrossRef]
- Candido, V.; D’Addabbo, T.; Basile, M.; Castronuovo, D.; Miccolis, V. Greenhouse soil solarization: Effect on weeds, nematodes and yield of tomato and melon. Agron. Sustain. Dev. 2008, 28, 221–230. [Google Scholar] [CrossRef]
- Putelat, T.; Whitmore, A.P. Optimal control of organic matter applications. Eur. J. Agron. 2023, 143, 126713. [Google Scholar] [CrossRef]
- Larkin, R.P. Long-Term Effects of Compost Amendments and Brassica Green Manures in Potato Cropping Systems on Soil and Crop Health and Productivity. Agronomy 2022, 12, 2804. [Google Scholar] [CrossRef]
- Liu, W.K.; Yang, Q.C.; Du, L. Soilless cultivation for high-quality vegetables with biogas manure in China: Feasibility and benefit analysis. Renew. Agric. Food Syst. 2009, 24, 300–307. [Google Scholar] [CrossRef]
- Yu, F.B.; Luo, X.P.; Song, C.F.; Zhang, M.X.; Shan, S.D. Concentrated biogas slurry enhanced soil fertility and tomato quality. Acta Agric. Scand. B Soil Plant Sci. 2010, 60, 262–268. [Google Scholar] [CrossRef]
- Umavathi, S.; Keerthika, M.; Gopinath, K.; Kavitha, C.; Uddin, M.R.; Alagumanian, S.; Balalakshmi, C. Optimization of aqueous-assisted extraction of polysaccharides from pumpkin (Cucurbita moschata Duch) and their biological activities. Saudi J. Biol. Sci. 2021, 28, 6692–6700. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Santi, S.; Paciulli, M.; Ganino, T.; Pellegrini, N.; Visconti, A.; Vitaglione, P.; Barbanti, D.; Chiavaro, E. Comparison of physical, microstructural and antioxidative properties of pumpkin cubes cooked by conventional, vacuum cooking and sous vide methods. J. Sci. Food Agric. 2021, 101, 2534–2541. [Google Scholar] [CrossRef]
- Márquez-Cardozo, C.J.; Caballero-Gutiérrez, B.L.; Ciro-Velázquez, H.J.; Restrepo-Molina, D.A. Effect of pretreatment and temperature on the drying kinetics and physicochemical and techno-functional characteristics of pumpkin (Cucurbita maxima). Heliyon 2021, 7, e06802. [Google Scholar] [CrossRef]
- Sabatino, L.; D’Anna, F.; Prinzivalli, C.; Iapichino, G. Soil Solarization and Calcium Cyanamide Affect Plant Vigor, Yield, Nutritional Traits, and Nutraceutical Compounds of Strawberry Grown in a Protected Cultivation System. Agronomy 2019, 9, 513. [Google Scholar] [CrossRef]
- Choudhary, R.; Verma, A.; Sharma, S.K.; Yadav, S.K.; Jain, R.K.; Jat, G.; Choudhary, R.S.; Jain, D. Productivity enhancement of sweet corn (Zea mays) through organic weed management practices. Indian J. Agric. Sci. 2021, 91, 1052–1057. [Google Scholar] [CrossRef]
- Fortis-Hernandéz, M.; Preciado-Rangel, P.; Segura-Castruita, M.A.; Mendoza-Tacuba, L.; Gallegos-Robles, M.A.; García Hernandez, J.L.; Vásquez-Vásquez, C. Changes in nutraceutical quality of tomato under different organic substrates. Hortic. Bras. 2018, 36, 189–194. [Google Scholar] [CrossRef]
- Jensen, J.; Styrishave, B.; Gimsing, A.L.; Hansen, H.C.B. The Toxic Effects of Benzyl Glucosinolate and Its Hydrolysis Product, the Biofumigant Benzyl Isothiocyanate, to Folsomia Fimetaria. Environ. Toxicol. Chem. 2010, 29, 359–364. [Google Scholar] [CrossRef] [PubMed]
| Temperature (°C) | Number of Hours | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | ||||||||
| Non-Solarized | Solarized | |||||||
| M | C | GM | Control | M | C | GM | Control | |
| 10 cm depth | ||||||||
| <37 | 1027 | 1105 | 1102 | 806 | 54 | 38 | 180 | 95 |
| ≥37 and <42 | 291 | 213 | 216 | 322 | 557 | 486 | 477 | 483 |
| ≥42 | 0 | 0 | 0 | 190 | 709 | 795 | 662 | 741 |
| 30 cm depth | ||||||||
| <37 | 1318 | 1318 | 1318 | 1318 | 78 | 60 | 75 | 60 |
| ≥37 and <42 | 0 | 0 | 0 | 0 | 1020 | 488 | 1006 | 600 |
| ≥42 | 0 | 0 | 0 | 0 | 222 | 775 | 239 | 660 |
| Treatments (1) | Yield | Fruits Per Plant | Fruit Weight | Fruit Diameter | Fruit Length | Stem Length |
|---|---|---|---|---|---|---|
| (t ha−1) | (n.) | (kg) | (cm) | (cm) | (cm) | |
| Non-solarized M | 28.83 ab | 6.28 a | 2.50 b | 8.83 c | 14.97 | 162.00 |
| Non-solarized C | 23.95 b | 5.17 b | 2.31 c | 8.60 c | 14.60 | 161.33 |
| Non-solarized GM | 27.14 ab | 5.83 ab | 2.45 b | 8.10 e | 14.70 | 160.33 |
| Non-solarized Control | 26.85 ab | 5.70 ab | 2.40 b | 8.33 d | 14.67 | 162.67 |
| Solarized M | 29.53 a | 6.49 a | 2.61 a | 9.87 a | 15.27 | 163.67 |
| Solarized C | 27.78 ab | 6.04 a | 2.49 b | 9.27 b | 15.03 | 166.67 |
| Solarized GM | 29.87 a | 6.50 a | 2.61 a | 8.73 c | 14.80 | 161.66 |
| Solarized Control | 27.90 ab | 6.07 a | 2.68 a | 9.10 b | 14.73 | 167.67 |
| Significance (2) | * | ** | ** | ** | ns | ns |
| Organic Amendments (A) | ||||||
| M | 29.18 | 6.38 a | 2.55 | 9.35 a | 15.12 | 162.33 |
| C | 25.50 | 5.60 b | 2.40 | 8.93 ab | 14.82 | 164.00 |
| GM | 25.50 | 6.17 ab | 2.53 | 8.42 b | 14.75 | 161.00 |
| Control | 27.37 | 5.88 ab | 2.53 | 8.72 ab | 14.70 | 165.17 |
| Significance | ns | * | ns | * | ns | ns |
| Soil solarization (S) | ||||||
| Non-solarized plots | 26.69 b | 5.74 b | 2.41 b | 8.47 b | 14.73 | 161.58 b |
| Solarized plots | 28.77 a | 6.27 a | 2.60 a | 9.24 a | 14.96 | 164.58 a |
| Significance | * | ** | ** | ** | ns | * |
| Interaction | ||||||
| A × S | * | ** | ** | ** | ns | ns |
| Variables | Principal Components | |
|---|---|---|
| 1 | 2 | |
| Yield | 0.8834 | −0.4366 |
| Fruits per plant | 0.9165 | −0.3873 |
| Fruit weight | 0.8761 | −0.0448 |
| Fruit diameter | 0.8139 | 0.4295 |
| Fruit length | 0.8321 | 0.0432 |
| Stem length | 0.5193 | 0.7611 |
| IC50 at 15 min | −0.8751 | 0.4232 |
| IC50 at 30 min | −0.9084 | 0.3723 |
| IC50 at 45 min | −0.9061 | 0.3724 |
| Eigenvalue | 5.99 | 1.57 |
| Total variance (%) | 66.54 | 17.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castronuovo, D.; De Feo, V.; De Martino, L.; Cardone, L.; Sica, R.; Caputo, L.; Amato, G.; Candido, V. Yield Response and Antioxidant Activity of Greenhouse Organic Pumpkin (Cucurbita moschata Duch.) as Affected by Soil Solarization and Biofumigation. Horticulturae 2023, 9, 427. https://doi.org/10.3390/horticulturae9040427
Castronuovo D, De Feo V, De Martino L, Cardone L, Sica R, Caputo L, Amato G, Candido V. Yield Response and Antioxidant Activity of Greenhouse Organic Pumpkin (Cucurbita moschata Duch.) as Affected by Soil Solarization and Biofumigation. Horticulturae. 2023; 9(4):427. https://doi.org/10.3390/horticulturae9040427
Chicago/Turabian StyleCastronuovo, Donato, Vincenzo De Feo, Laura De Martino, Loriana Cardone, Rita Sica, Lucia Caputo, Giuseppe Amato, and Vincenzo Candido. 2023. "Yield Response and Antioxidant Activity of Greenhouse Organic Pumpkin (Cucurbita moschata Duch.) as Affected by Soil Solarization and Biofumigation" Horticulturae 9, no. 4: 427. https://doi.org/10.3390/horticulturae9040427
APA StyleCastronuovo, D., De Feo, V., De Martino, L., Cardone, L., Sica, R., Caputo, L., Amato, G., & Candido, V. (2023). Yield Response and Antioxidant Activity of Greenhouse Organic Pumpkin (Cucurbita moschata Duch.) as Affected by Soil Solarization and Biofumigation. Horticulturae, 9(4), 427. https://doi.org/10.3390/horticulturae9040427









