Mechanisms Underlying the C3–CAM Photosynthetic Shift in Facultative CAM Plants
Abstract
1. Introduction
2. Signaling Stress Factors
3. Anatomical Variations during the C3–CAM Shift in Facultative CAM Plants
4. Physiological Mechanisms during the C3–CAM Shift in Facultative CAM Plants
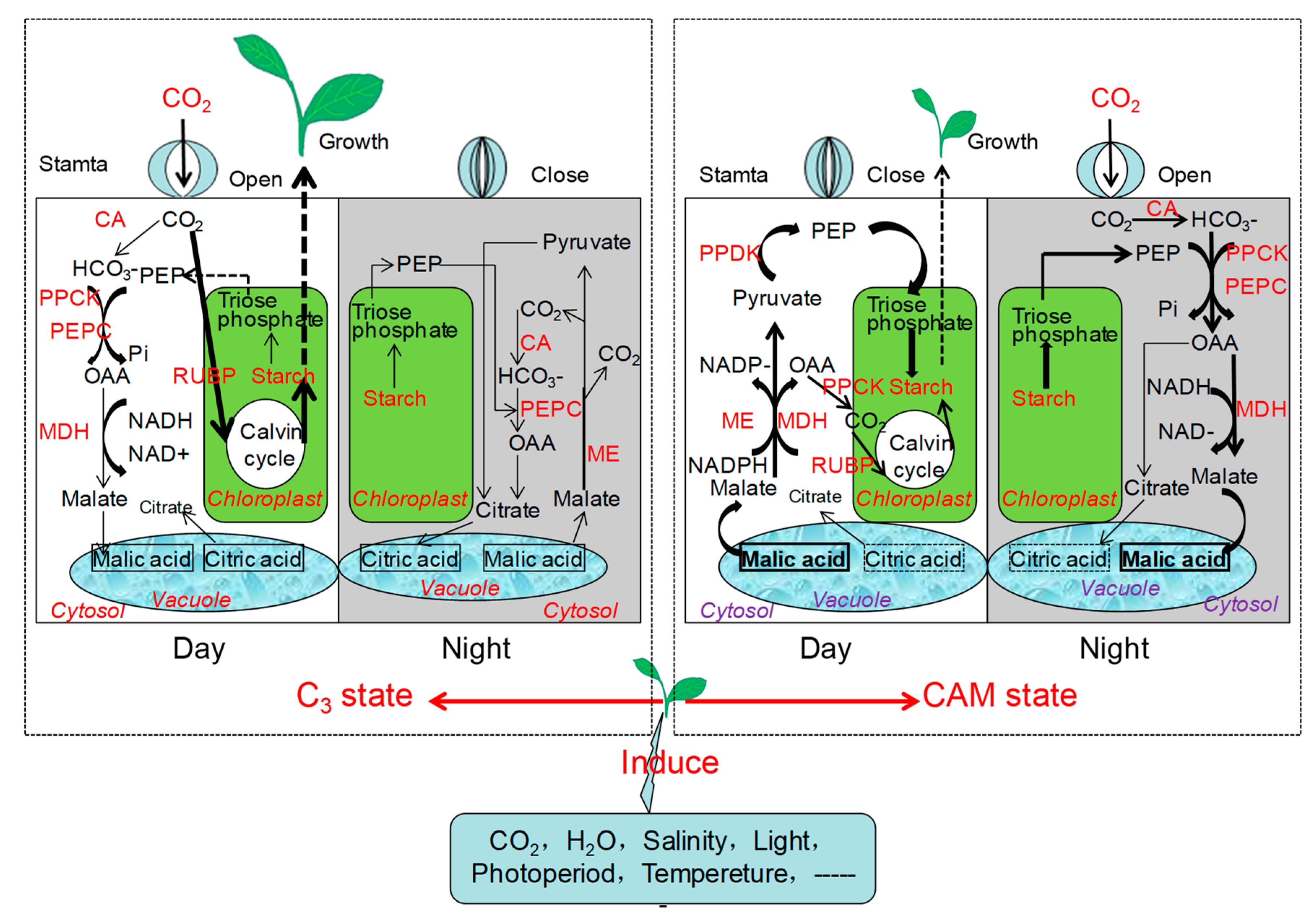
5. Metabolic Mechanism during the C3–CAM Shift in Facultative CAM Plants
6. Molecular Mechanisms during C3–CAM Shift in Facultative CAM Plants
7. DNA Level Regulation
8. Transcriptional Regulation
| Enzyme | Gene | Source/Species | Inducer | Reference |
|---|---|---|---|---|
| Phosphenolpyruvate carboxylase | Ppc1 | M. crystallinum | salt, ABA, drought, cytokinin | [25,100] |
| Kb-1, Kb-2 | K. blossfeldiana | short-day, drought | [101] | |
| Ppc 3 | T. triangulare | ABA | [21] | |
| C3-type PEPCs | C. minor | drought | [102] | |
| Alpha Carbonic Anhydrase 1 | ACA1 | T. triangulare | ABA | [21] |
| Beta Carbonic Anhydrase 5 | BCA5 | T. triangulare | ABA | [21] |
| Malic Enzymes | MEs | T. triangulare | ABA | [21] |
| PEPC Kinase | PPCK1 | M. crystallinum T. triangulare | Salt ABA | [21,103,104] |
| Pyruvate orthophosphate dikinase(PPDK) | Ppdk1 | M. crystallinum T. triangulare | salt, ABA | [21,105] |
| Enolase | Pgh1 | M. crystallinum | salt, drought, cold, hypoxia, ABA, 6-BA | [106] |
| phosphoglyceromutase (PGM) | Pgm1 | M. crystallinum | salt, drought, ABA, 6-BA | [107] |
| GAD-Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | GapC1 | M. crystallinum | salt | [93,108] |
| NADP-Malic enzyme | Mod1 | M. crystallinum T. triangulare | salt ABA | [21,109] |
| Mod4 | T. triangulare | ABA | [21] | |
| NADP-Malate dehydrogenase | MDH1 | M. crystallinum | salt | [80] |
| NAD-Malate dehydrogenase | MDH2 | M. crystallinum | salt | [110] |
| H+-ATPase. c subunit | Atpvc | M. crystallinum, K. daigremontiana | salt, ABA, light | [111,112,113] |
| H+-ATPase, E subunit | AtpvE | M. crystallinum | salt | [114] |
| SNF1 kinase | MK9 | M. crystallinum | salt | [115] |
| RNA-binding protein | Rbp1 | M. crystallinum | salt | [116] |
| Ribosome inactivating proteins | Rip1 | M. crystallinum | salt | [117] |
9. Post-Transcriptional Regulation
10. Protein Level Regulation
11. Implications in Horticultural Crops
12. Future Perspectives
| Omics Approaches | Source/Species | Photosynthesis Type | Year | References |
|---|---|---|---|---|
| Proteomics, Metabolomics | M. crystallinum | facultative CAM plants | 2013 | [141] |
| Transcriptomics | M. crystallinum | facultative CAM plants | 2015 | [142] |
| Metabolomics | M. crystallinum | facultative CAM plants | 2015 | [143] |
| Transcriptomics | M. crystallinum | facultative CAM plants | 2015 | [144] |
| Proteomics, Ionomics | M. crystallinum | facultative CAM plants | 2016 | [145] |
| Transcriptomics, Metabolomics | T. triangulare | facultative CAM plants | 2016 | [6] |
| Transcriptomics | D. catenatum | facultative CAM plants | 2016 | [124] |
| Transcriptomics | Agave (CAM), Polianthes (weak CAM), Manfreda (CAM), Beschorneria (weak CAM) | CAM plants | 2018 | [95] |
| Transcriptomics | D. catenatum | facultative CAM plants | 2018 | [137] |
| Transcriptomics | Erycina pusilla (CAM), Erycina crista-galli(C3), | CAM plants, C3 plants | 2019 | [86] |
| Transcriptomics, Metabolomics | T. triangulare | facultative CAM plants | 2019 | [21] |
| Metabolomics Transcriptomics | Y. gloriosa (C3+ CAM), Y. filamentosa (C3), Y. aloifolia (CAM) | facultative CAM plants, C3 plants, obligate CAM plants | 2019 | [76] |
| Genomics | Sedum album | facultative CAM plants | 2019 | [123] |
| Transcriptomics | M. crystallinum | facultative CAM plants | 2020 | [104] |
| Proteomics, Metabolomics | M. crystallinum | facultative CAM plants | 2021 | [131] |
| Proteomics | M. crystallinum | facultative CAM plants | 2021 | [98] |
| Proteomics, Phosphoproteomics | M. crystallinum | facultative CAM plants | 2022 | [132] |
| Transcriptomics | Tamarix ramosissima | facultative CAM plants | 2022 | [146] |
| Transcriptomics Genomics | M. crystallinum | facultative CAM plants | 2022 | [126] |
| Transcriptomics | 11 species of Agavoideae | facultative CAM plants, C3 plants, obligate CAM plants | 2022 | [147] |
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| CAM | Crassulacean acid metabolism |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| H2O2 | Hydrogen peroxide |
| MDH | Malate dehydrogenase |
| ME | Malic enzyme; |
| NO | Nitric oxide |
| PEPC | Phosphoenolpyruvate carboxylase |
| PEPCK | PEP carboxykinase |
| POD | Peroxidase |
| PFD | Photon flux density |
| Rubisco | Ribulosebisphosphate carboxylase/oxygenase |
| SOD | superoxide dismutase (SOD) |
| TF | Transcription factors |
| TZPs | Tandem zinc knuckle/PLU3 domain encoding genes |
| WUE | Water-use efficiency |
References
- Osmond, C.B. Crassulacean acid metabolism: A curiosity in context. Annu. Rev. Plant Physiol. 1978, 29, 379–414. [Google Scholar] [CrossRef]
- Lüttge, U. Ecophysiology of Crassulacean acid metabolism (CAM). Ann. Bot. 2004, 93, 629–652. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; von Willert, D.J. NaCl-induzierter Crassulaceen säurestoffwechsel bei Mesembryanthemum crystallinum. Z. Pflanzenphysiol. 1972, 67, 166–170. [Google Scholar] [CrossRef]
- Castillo, F.J. Antioxidative protection in the inducible CAM plant Sedum album L. following the imposition of severe water stress and recovery. Oecologia 1996, 107, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Barrera Zambrano, V.A.; Lawson, T.; Olmos, E.; Fernández-García, N.; Borland, A.M. Leaf anatomical traits which accommodate the facultative engagement of Crassulacean acid metabolism in tropical trees of the genus Clusia. J. Exp. Bot. 2014, 65, 3513–3523. [Google Scholar] [CrossRef]
- Brilhaus, D.; Bräutigam, A.; Mettler-Altmann, T.; Winter, K.; Weber, A.P. Reversible burst of transcriptional changes during induction of Crassulacean acid metabolism in Talinum triangulare. Plant Physiology 2016, 170, 102–122. [Google Scholar] [CrossRef]
- Winter, K.; Holtum, J.A.M. Environment or development? Lifetime net CO2 exchange and control of the expression of crassulacean acid metabolism in Mesembryanthemum crystallinum. Plant Physiol. 2007, 143, 98–107. [Google Scholar] [CrossRef]
- Winter, K.; Garcia, M.; Holtum, J.A.M. On the nature of facultative and constitutive CAM: Environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë, and Opuntia. J. Exp. Bot. 2008, 59, 1829–1840. [Google Scholar] [CrossRef]
- Winter, K. Ecophysiology of constitutive and facultative CAM photosynthesis. J. Exp. Bot. 2019, 70, 6495–6508. [Google Scholar] [CrossRef]
- Johnson, G.N.; Lawson, T.; Murchie, E.H.; Raines, C. Photosynthesis in variable environments. J. Exp. Bot. 2015, 66, 2371–2372. [Google Scholar] [CrossRef]
- DePaoli, H.C.; Borland, A.M.; Tuskan, G.A.; Cushman, J.C.; Yang, X.H. Synthetic biology as it relates to CAM photosynthesis: Challenges and opportunities. J. Exp. Bot. 2014, 65, 3381–3393. [Google Scholar] [CrossRef]
- Grams, T.E.E.; Thiel, S. High light-induced switch from C3-photosynthesis to crassulacean acid metabolism is mediated by UV-A/blue light. J. Exp. Bot. 2002, 53, 1475–1483. [Google Scholar] [CrossRef]
- Cushman, J.C. Crassulacean acid metabolism. A plastic photosynthetic adaptation to arid environments. Plant Physiology 2001, 127, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.; He, J.; Yam, T.W. CAM plasticity in epiphytic tropical orchid species responding to environmental stress. Bot. Stud. 2019, 60, 7. [Google Scholar] [CrossRef] [PubMed]
- Borland, A.M.; Hartwell, J.; Weston, D.J.; Schlauch, K.A.; Tschaplinski, T.J.; Tuskan, G.A.; Yang, X.; Cushman, J.C. Engineering crassulacean acid metabolism to improve water-use efficiency. Trends Plant Sci. 2014, 19, 327–338. [Google Scholar] [CrossRef]
- Yang, X.H.; Cushman, J.C.; Borland, A.M.; Edwards, E.J.; Wullschleger, S.D.; Tuskan, G.A.; Owen, N.A.; Griffiths, H.; Smith, J.A.C.; Paoli, H.C.D.; et al. A roadmap for research on crassulacean acid metabolism (CAM) to enhance sustainable food and bioenergy production in a hotter, drier world. New Phytol. 2015, 207, 491–504. [Google Scholar] [CrossRef]
- Liu, D.G.; Chen, M.; Mendoza, B.; Cheng, H.; Hu, R.B.; Li, L.L.; Trinh, C.T.; Tuskan, G.A.; Yang, X.H. CRISPR/Cas9-mediated targeted mutagenesis for functional genomics research of crassulacean acid metabolism plants. J. Exp. Bot. 2019, 70, 6621–6629. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Lee, S.; Choi, W.; Yim, W.; Cushman, J. Laying the foundation for crassulacean acid metabolism (CAM) biodesign: Expression of the C4 metabolism cycle genes of CAM in Arabidopsis. Front. Plant Sci. 2019, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Silvera, K.; Neubig, K.M.; Whitten, W.M.W.; Williams, N.H.; Winter, K.; Cushman, J.C. Evolution along the crassulacean acid metabolism continuum. Funct. Plant Biol. 2010, 37, 995–1010. [Google Scholar] [CrossRef]
- Hartwell, J.; Dever, L.V.; Boxall, S.F. Emerging model systems for functional genomics analysis of crassulacean acid metabolism. Curr. Opin. Plant Biol. 2016, 31, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Maleckova, E.; Brilhaus, D.; Wrobel, T.J.; Weber, A.P.M. Transcript and metabolite changes during the early phase of abscisic acid-mediated induction of crassulacean acid metabolism in Talinum triangulare. J. Exp. Bot. 2019, 70, 6581–6596. [Google Scholar] [CrossRef] [PubMed]
- Cushman, J.C.; Bohnert, H.J. Crassulacean acid metabolism: Molecular Genetics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Lüttge, U. Carbon dioxide and water demand. Crassulacean acid metabolism (CAM), a versatile ecological adaptation exemplifying the need for integration in eco-physiological work. New Phytol. 1987, 106, 593–629. [Google Scholar] [CrossRef]
- Borland, A.M.; Griffiths, H. The regulation of CAM and respiratory recycling by water supply and light regime in the C3-CAM intermediate Sedum telephium. Funct. Ecol. 1990, 4, 33–39. [Google Scholar] [CrossRef]
- Cushman, J.C.; Meyer, G.; Michalowski, C.B.; Schmitt, J.M.; Bohnert, H.J. Salt stress leads to differential expression of two isogenes of phosphoenolpyruvate carboxylase during crassulacean acid metabolism induction in the common ice plant. Plant Cell 1989, 1, 715–725. [Google Scholar]
- Broetto, F.; Lüttge, U.; Ratajczak, R. Influence of light intensity and salt-treatment on mode of photosynthesis and enzymes of the antioxidative response system of Mesembryanthemum crystallinum. Funct. Plant Biol. 2002, 29, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Taybi, T.; Sotta, B.; Gehrig, H.; Giiclii, S.; Kluge, M.; Brulfert, J. Differential effects of abscisic acid on phosphoenolpyruvate carboxylase and CAM operation in Kalanchoe blossfeldiana. Bot. Acta 1995, 108, 240–246. [Google Scholar] [CrossRef]
- Freschi, L.; Rodrigues, M.A.; Domingues, D.S.; Purgatto, E.; Van Sluys, M.A.V.; Magalhaes, J.R.; Kaiser, W.M.; Mercier, H. Nitric oxide mediates the hormonal control of crassulacean acid metabolism expression in young Pineapple plants. Plant Physiology 2010, 152, 1971–1985. [Google Scholar] [CrossRef]
- Wakamatsu, A.; Mori, I.C.; Matsuura, T.; Taniwaki, Y.; Yoshida, R. Possible roles for phytohormones in controlling the stomatal behavior of Mesembryanthemum crystallinum during the salt-induced transition from C3 to crassulacean acid metabolism. J. Plant Physiol. 2021, 262, 153448. [Google Scholar] [CrossRef]
- Ślesak, I.; Karpinska, B.; Surówka, E.; Miszalski, Z.; Karpinski, S. Redox changes in the chloroplast and hydrogen peroxide are essential for regulation of C3-CAM transition and photooxidative stress responses in the facultative CAM plant Mesembryanthemum crystallinum L. Plant Cell Physiol. 2003, 44, 573–581. [Google Scholar] [CrossRef]
- Surówka, E.; Dziurka, M.; Kocurek, M.; Goraj, S.; Rapacz, M.; Miszalski, Z. Effects of exogenously applied hydrogen peroxide on antioxidant and osmoprotectant profiles and the C3-CAM shift in the halophyte Mesembryanthemum crystallinum L. J. Plant Physiol. 2016, 200, 102–110. [Google Scholar] [CrossRef]
- Eastmond, P.J.; Ross, J.D. Evidence that the induction of crassulacean acid metabolism by water stress in Mesembryanthemum crystallinum (L.) involves root signalling. Plant Cell Environ. 1997, 20, 1559–1565. [Google Scholar] [CrossRef]
- Teeri, J.A.; Tonsor, S.J.; Turner, M. Leaf thickness and carbon isotope composition in the Crassulaceae. Oecologia. 1981, 50, 367–369. [Google Scholar] [CrossRef]
- Winter, K.; Wallace, B.J.; Stocker, G.C.; Roksandic, Z. Crassulacean acid metabolism in Australian vascular epiphytes and some related species. Oecologia 1983, 57, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Borland, A.M.; Técsi, L.I.; Leegood, R.C.; Walker, R.P. Inducibility of crassulacean acid metabolism (CAM) in Clusia species; physiological/biochemical characterisation and intercellular localisation of carboxylation processes in three species which show different degrees of CAM. Planta 1998, 205, 342–351. [Google Scholar] [CrossRef]
- Silvera, K.; Santiago, L.S.; Winter, K. Distribution of crassulacean acid metabolism in orchids of Panama: Evidence of selection of weak and strong modes. Funct. Plant Biol. 2005, 32, 397–407. [Google Scholar] [CrossRef]
- Heyduk, K. Evolution of crassulacean acid metabolism in response to the environment: Past, present, and future. Plant Physiol. 2022, 190, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Veste, M.; Herppich, W.B.; von Willert, D.J. Variability of CAM in leaf deciduous succulents from the Succulent Karoo (South Africa). Basic Appl. Biol. 2001, 2, 283–288. [Google Scholar]
- Herrera, A. Crassulacean acid metabolism and fitness under water deficit stress: If not for carbon gain, what is facultative CAM good for? Ann. Bot. 2009, 103, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.J.; Tan, B.; Kelley, T.M.; Tian, J.K.; Chen, S.X. Physiological changes in Mesembryanthemum crystallinum during the C3 to CAM transition induced by salt stress. Front. Plant Sci. 2020, 11, 283. [Google Scholar] [CrossRef]
- De-Santo, A.V.; Alfani, A.; Russo, G.; Fioretto, A. Relationship between CAM and succulence in some species of Vitaceae and Piperaceae. Bot. Gaz. 1983, 144, 342–346. [Google Scholar] [CrossRef]
- Edwards, E.J. Evolutionary trajectories, accessibility and other metaphors: The case of C4 and CAM photosynthesis. New Phytol. 2019, 223, 1742–1755. [Google Scholar] [CrossRef]
- Heyduk, K.; Moreno-Villena, J.J.; Gilman, I.S.; Christin, P.A.; Edwards, E.J. The genetics of convergent evolution: Insights from plant photosynthesis. Nat. Rev. Genet. 2019, 20, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Heyduk, K.; Ray, J.N.; Leebens-Mack, J. Leaf anatomy is not correlated to CAM function in a C3+CAM hybrid species, Yucca gloriosa. Ann. Bot. 2021, 127, 437–449. [Google Scholar] [CrossRef]
- Herrera, A. Are thick leaves, large mesophyll cells and small intercellular air spaces requisites for CAM? Ann. Bot. 2020, 125, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Kliemchen, A.; Schomburg, M.; Galla, H.J.; Lüttge, U.; Kluge, M. Phenotypic changes in the fluidity of the tonoplast membrane of crassulacean acid metabolism plants in response to temperature and salinity stress. Planta 1993, 189, 403–409. [Google Scholar] [CrossRef]
- Jones, M.B. Effect of leaf age on leaf resistance and CO2 exchange of the CAM plant Bryophyllum fedtschenkoi. Planta 1975, 123, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ślesak, I.; Miszalski, Z.; Karpinska, B.; Niewiadomska, E.; Ratajczak, R.; Karpinski, S. Redox control of oxidative stress responses in the C3–CAM intermediate plant Mesembryanthemum crystallinum. Plant Physiol. Biochem. 2002, 40, 669–677. [Google Scholar] [CrossRef]
- Niewiadomska, E.; Bilger, W.; Gruca, M.; Mulisch, M.; Miszalski, Z.; Krupinska, K. CAM-related changes in chloroplastic metabolism of Mesembryanthemum crystallinum L. Planta 2011, 233, 275–285. [Google Scholar] [CrossRef]
- Holtum, J.A.M.; Winter, K. Activities of enzymes of carbon metabolism during the induction of crassulacean acid metabolism in Mesembryanthemum crystallinum. Planta 1982, 155, 8–16. [Google Scholar] [CrossRef]
- O’Leary, B.; Park, J.; Plaxton, W.C. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): Recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem. J. 2011, 436, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Silvera, K.; Winter, K.; Rodriguez, B.L.; Albion, R.L.; Cushman, J.C. Multiple isoforms of phosphoenolpyruvate carboxylase in the Orchidaceae (subtribe Oncidiinae): Implications for the evolution of Crassulacean acid metabolism. J. Exp. Bot. 2014, 65, 3623–3636. [Google Scholar] [CrossRef] [PubMed]
- Mallick, N.; Mohn, F.H. Reactive oxygen species: Response of algal cells. J. Plant Physiol 2000, 157, 183–193. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Aragón, C.; Carvalho, L.; González, J.; Escalona, M.; Amâncio, S. The physiology of ex vitro pineapple (Ananas comosus L. Merr. var MD-2) as CAM or C3 is regulated by the environmental conditions. Plant Cell Rep. 2012, 31, 757–769. [Google Scholar] [CrossRef]
- Aragón, C.; Pascual, P.; González, J.; Escalona, M.; Carvalho, L.; Amancio, S. The physiology of ex vitro pineapple (Ananas comosus L. Merr. var MD-2) as CAM or C3 is regulated by the environmental conditions: Proteomic and transcriptomic profiles. Plant Cell Rep. 2013, 32, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Niewiadomska, E.; Miszalski, Z.; Ślesak, I.; Ratajczak, R. Catalase activity during C3-CAM transition in Mesembryanthemum crystallinum L. leaves. Free Radic. Res. 1999, 31, 251–256. [Google Scholar] [CrossRef]
- Nosek, M.; Gawrońska, K.; Rozpądek, P.; Szechyńska-Hebda, M.; Kornaś, A.; Miszalski, Z. Withdrawal from functional crassulacean acid metabolism (CAM) is accompanied by changes in both gene expression and activity of antioxidative enzymes. J. Plant Physiol. 2018, 229, 151–157. [Google Scholar] [CrossRef]
- Miszalski, Z.; Slesak, I.; Niewiadomska, E.; Baczek-Kwinta, R.; Luttge, U.; Ratajczak, R. Subcellular localization and stress responses of superoxide dismutase isoforms from leaves in the C3-CAM intermediate halophyte Mesembryanthemum crystallinum L. Plant Cell Environ. 1998, 21, 169–179. [Google Scholar] [CrossRef]
- Sanada, Y.; Ueda, H.; Kuribayashi, K.; Andoh, T.; Hayashi, F.; Tamai, N.; Wada, K. Novel light-dark change of proline levels in halophyte (Mesembryanthemum crystallinum L.) and glycophytes (Hordeum vulgare L. and Triticum aestivum L.) leaves and roots under salt stress. Plant Cell Physiol. 1995, 36, 965–970. [Google Scholar] [CrossRef]
- Shevyakova, N.I.; Shorina, M.V.; Rakitin, V.Y.; Kuznetsov, V. Stress-dependent accumulation of spermidine and spermine in the halophyte Mesembryanthemum crystallinum under salinity conditions. Russ. J. Plant Physiol. 2006, 53, 739–745. [Google Scholar] [CrossRef]
- Abreu, M.E.; Carvalho, V.; Mercier, H. Antioxidant capacity along the leaf blade of the C3-CAM facultative bromeliad Guzmania monostachia under water deficit conditions. Funct. Plant Biol. 2018, 45, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Borland, A.M.; Taybi, T. Synchronization of metabolic processes in plants with crassulacean acid metabolism. J. Exp. Bot. 2004, 55, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Black, C.C.; Osmond, C.B. Crassulacean acid metabolism photosynthesis: ‘working the night shift’. Photosynth. Res. 2003, 76, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Weise, S.E.; van Wijk, K.J.; Sharkey, T.D. The role of transitory starch in C3, CAM, and C4 metabolism and opportunities for engineering leaf starch accumulation. J. Exp. Bot. 2011, 62, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Habibi, G.; Hajiboland, R. Comparison of photosynthesis and antioxidative protection in Sedum album and Sedum stoloniferum (Crassulaceae) under water stress. Photosynthetica 2012, 50, 508–518. [Google Scholar] [CrossRef]
- Haider, M.S.; Barnes, J.D.; Cushman, J.C.; Borland, A.M. A CAM- and starch-deficient mutant of the facultative CAM species Mesembryanthemum crystallinum reconciles sink demands by repartitioning carbon during acclimation to salinity. J. Exp. Bot. 2012, 63, 1985–1996. [Google Scholar] [CrossRef]
- Winter, K.; Smith, J.A.C. CAM photosynthesis: The acid test. New Phytol. 2022, 233, 599–609. [Google Scholar] [CrossRef]
- Tay, I.Y.Y.; Odang, K.B.; Cheung, C.Y.M. Metabolic modeling of the C3-CAM continuum revealed the establishment of a starch/sugar-malate cycle in CAM evolution. Front. Plant Sci. 2021, 11, 573197. [Google Scholar] [CrossRef]
- Christopher, J.T.; Holtum, J.A.M. Patterns of carbon partitioning in leaves of crassulacean acid metabolism species during deacidification. Plant Physiol. 1996, 112, 393–399. [Google Scholar] [CrossRef]
- Dodd, A.N.; Borland, A.M.; Haslam, R.P.; Griffiths, H.; Maxwell, K. Crassulacean acid metabolism: Plastic, fantastic. J. Exp. Bot. 2002, 53, 569–580. [Google Scholar] [CrossRef]
- Cushman, J.C.; Agarie, S.; Albion, R.L.; Elliot, S.M.; Taybi, T.; Borland, A.M. Isolation and characterization of mutants of common ice plant deficient in crassulacean acid metabolism. Plant Physiol. 2008, 147, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Borland, A.M.; Dodd, A.N. Carbohydrate partitioning in crassulacean acid metabolism plants: Reconciling potential conflicts of interest. Funct. Plant Biol. 2002, 29, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.E.; Yin, H.; Borland, A.M.; Weighill, D.; Lim, S.D.; De-Paoli, H.C.; Engle, N.; Jones, P.C.; Agh, R.; Weston, D.J.; et al. Transcript, protein and metabolite temporal dynamics in the CAM plant Agave. Nat. Plants 2016, 178, 16178. [Google Scholar] [CrossRef] [PubMed]
- Heyduk, K.; Ray, J.N.; Ayyampalayam, S.; Moledina, N.; Borland, A.; Harding, S.A.; Tsai, C.J.; Leebens-Mack, J. Shared expression of crassulacean acid metabolism (CAM) genes pre-dates the origin of CAM in the genus Yucca. J. Exp. Bot. 2019, 70, 6597–6609. [Google Scholar] [CrossRef]
- Cushman, J.C.; Borland, A.M. Induction of Crassulacean acid metabolism by water limitation. Plant Cell Environ. 2002, 25, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Lüttge, U. Clusia: A Woody Neotropical Genus of Remarkable Plasticity and Diversity; Ecological Studies; Springer: Berlin, Germany, 2007; p. 194. [Google Scholar]
- Borland, A.M.; Hartwell, J.; Jenkins, G.I.; Wilkins, M.B.; Nimmo, H.G. Metabolite control overrides circadian regulation of phosphoenolpyruvate carboxylase knase and CO2 fixation in crassulacean acid metabolism. Plant Physiol. 1999, 121, 889–896. [Google Scholar] [CrossRef]
- Cushman, J.C. Molecular cloning and expression of chloroplast NADP-malate dehydrogenase during crassulacean acid metabolism induction by salt stress. Photosynth. Res. 1993, 35, 15–27. [Google Scholar] [CrossRef]
- Gehrig, H.H.; Wood, J.A.; Cushman, M.A.; Virgo, A.; Cushman, J.C.; Winter, K. Research note: Large gene family of phosphoenolpyruvate carboxylase in the crassulacean acid metabolism plant Kalanchoe pinnata (Crassulaceae) characterised by partial cDNA sequence analysis. Funct. Plant Biol. 2005, 32, 467–472. [Google Scholar] [CrossRef]
- Taybi, T.; Patil, S.; Chollet, R.; Cushman, J.C. A minimal serine/threonine protein kinase circadianly regulates phosphoenolpyruvate carboxylase activity in crassulacean acid metabolism-induced leaves of the common ice plant. Plant Physiol. 2000, 123, 1471–1482. [Google Scholar] [CrossRef]
- Boxall, S.F.; Kadu, N.; Dever, L.V.; Kneřová, J.; Waller, J.L.; Gould, P.J.D.; Hartwell, J. Kalanchoë PPC1 is essential for crassulacean acid metabolism and the regulation of core circadian clock and guard cell signaling genes[CC-BY]. Plant Cell 2020, 32, 1136–1160. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, O.V.; Zakharchenko, N.S.; Shevchuk, T.V.; Bohnert, H.J.; Cushman, J.C.; Buryanov, Y.I. Effect of hypermethylation of CCWGG sequences in DNA of Mesembryanthemum crystallinum plants on their adaptation to salt stress. Biochem. Biokhimiia 2006, 71, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.C.; Li, C.H.; Lee, J.Y.; Yen, H.E. Cytosine methylation changes in the ice plant Ppc1 promoter during transition from C3 to crassulacean acid metabolism. Plant Sci. 2010, 178, 41–46. [Google Scholar] [CrossRef]
- Heyduk, K.; Hwang, M.; Albert, V.A.; Silvera, K.; Lan, T.Y.; Winter, K.; Leebens-Mack, J. Altered gene regulatory networks are associated with the transition from C3 to crassulacean acid metabolism in Erycina (Oncidiinae: Orchidaceae). Front. Plant Sci. 2019, 9, 2000. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.B.; Zhang, J.; Jawdy, S.; Sreedasyam, A.; Sreedasyam, A.; Lipzen, A.; Wang, M.; Ng, V.; Daum, C.; Keymanesh, K.; et al. Comparative genomics analysis of drought response between obligate CAM and C3 photosynthesis plants. J. Plant Physiol. 2022, 277, 153791. [Google Scholar] [CrossRef]
- Schmitt, J.M. Rapid concentration changes of phosphoenolpyruvate carboxylase mRNA in detached leaves of Mesembryanthemum crystallinum. Plant Cell Environ. 1990, 13, 845–850. [Google Scholar] [CrossRef]
- Brulfert, J.; Güclü, S.; Taybi, T.; Pierre, J.N. Enzymatic responses to water stress in detached leaves of the CAM plant Kalanchoë blossfeldiana Poelln. Plant Physiol. Biochem. 1993, 31, 491–497. [Google Scholar]
- Yang, X.H.; Hu, R.B.; Yin, H.F.; Jenkins, J.; Shu, S.Q.; Tang, H.B.; Liu, D.G.; Weighill, D.A.; Yim, W.C.; Ha, J.M.; et al. The Kalanchö genome provides insights into convergent evolution and building blocks of crassulacean acid metabolism. Nat. Commun. 2017, 8, 1899. [Google Scholar] [CrossRef]
- Cushman, J.C.; Vernon, D.M.; Bohnert, H.J. ABA and the transcriptional control of CAM induction during salt stress in the common ice plant. In Control of Plant Gene Expression; Verma, D.P.S., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 287–300. [Google Scholar]
- Schmitt, J.M.; Fißlthaler, B.; Sheriff, A.; Lenz, B.; Bäßler, M.; Meyer, G. Environmental control of CAM induction in Mesembryanthemum crystallinum—A role for cytokinin, abscisic acid and jasmonate? Crassulacean Acid Metabolism. Biochem. Ecophysiol. Evol. 1996, 114, 159–175. [Google Scholar]
- Schaeffer, H.J.; Forstheoefel, N.R.; Cushman, J.C. Identification of enhancer and silencer regions involved in salt-responsive expression of crassulacean acid metabolism (CAM) genes in the facultative halophyte Mesembryanthemum crystallinum. Plant Mol. Biol. 1995, 28, 205–218. [Google Scholar] [CrossRef]
- Amin, A.B.; Rathnayake, K.N.; Yim, W.C.; Garcia, T.M.; Wone, B.; Cushman, J.C.; Wone, B.W.M. Crassulacean acid metabolism abiotic stress-responsive transcription factors: A potential genetic engineering approach for improving crop tolerance to abiotic stress. Front. Plant Sci. 2019, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Heyduk, K.; Ray, J.N.; Ayyampalayam, S.; Leebens-Mack, J. Shifts in gene expression profiles are associated with weak and strong crassulacean acid metabolism. Am. J. Bot. 2018, 105, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Moseley, R.C.; Mewalal, R.; Motta, F.; Tuskan, G.A.; Haase, S.; Yang, X.H. Conservation and diversification of circadian rhythmicity between a model crassulacean acid metabolism plant Kalanchoë fedtschenkoi and a model C3 photosynthesis plant Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 1757. [Google Scholar] [CrossRef]
- Yin, H.B.; Guo, H.B.; Weston, D.J.; Borland, A.M.; Ranjan, P.; Abraham, P.E.; Jawdy, S.S.; Wachira, J.; Tuskan, G.A.; Tschaplinski, T.J.; et al. Diel rewiring and positive selection of ancient plant proteins enabled evolution of CAM photosynthesis in Agave. BMC Genom. 2018, 19, 588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Tan, B.W.; Zhu, D.; Dufresne, D.; Jiang, T.B.; Chen, S.X. Proteomics of Homeobox7 enhanced salt tolerance in Mesembryanthemum crystallinum. Int. J. Mol. Sci. 2021, 22, 6390. [Google Scholar] [CrossRef]
- Cushman, J.C.; Bohnert, H.J. Salt stress alters A/T-rich DNA-binding factor interactions within the phosphoenolpyruvate carboxylase promoter from Mesembryanthemum crystallinum. Plant Mol. Biol. 1999, 20, 411–424. [Google Scholar] [CrossRef]
- McElwain, E.F.; Bohnert, H.J.; Thomas, J.C. Light moderates the induction of phosphoenolpyruvate carboxylase by NaCl and abscisic acid in Mesembryanthemum crystallinum. Plant Physiol. 1992, 99, 1261–1264. [Google Scholar] [CrossRef]
- Gehrig, H.; Taybi, T.; Kluge, M.; Brulfert, J. Identification of multiple PEPC isogenes in leaves of the facultative crassulacean acid metabolism (CAM) plant Kalanchoe blossfeldiana Poelln. cv.Tom Thumb. FEBS Lett. 1995, 377, 399402. [Google Scholar]
- Vaasen, A.; Begerow, D.; Hampp, R. Phosphoenolpyruvate carboxylase genes in C3, crassulacean acid metabolism (CAM) and C3/CAM intermediate species of the genus Clusia: Rapid reversible C3/CAM switches are based on the C3 housekeeping gene. Plant Cell Environ. 2006, 29, 2113–2123. [Google Scholar] [CrossRef]
- Li, B.; Chollet, R. Salt induction and the partial purification/characterization of phosphoenolpyruvate carboxylaseprotein-serine kinase from an inducible crassulacean acid-metabolism (CAM) plant, Mesembryanthemum crystallinum L. Arch. Biochem. Biophys. 1994, 314, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.W.; Yoo, M.J.; Zhu, D.; Noble, J.D.; Kelley, T.M.; Li, J.; Kirst, M.; Assmann, S.M.; Chen, S.X. Molecular changes in Mesembryanthemum crystallinum guard cells underlying the C3 to CAM transition. Plant. Mol. Biol. 2020, 103, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Fissßthaler, B.; Meyer, G.; Bohnert, H.J.; Schmitt, J.M. Age-dependent induction of pyruvate, orthophosphate dikinase in Mesembryanthemum crystallinum L. Planta 1995, 196, 492–500. [Google Scholar]
- Forsthoefel, N.R.; Cushman, M.A.F.; Cushman, J.C. Posttranscriptional and posttranslational control of enolase expression in the facultative crassulacean acid metabolism plant Mesembryanthemum crystallinum L. Plant Physiol. 1995, 108, 1185–1195. [Google Scholar] [CrossRef]
- Forsthoefel, N.R.; Vernon, D.M.; Cushman, J.C. A salinity-induced gene from the halophyte M. crystallinum encodes a glycolytic enzyme, cofactor-independent phosphoglyceromutase. Plant Mol. Biol. 1995, 29, 213–226. [Google Scholar] [CrossRef]
- Ostrem, J.A.; Vernon, D.M.; Bohnert, H.J. Increased expression of a gene coding for NAD: Glyceraldehyde-3-phosphate dehydrogenase during the transition from C3 photosynthesis to crassulacean acid metabolism in Mesembryanthemum crystallinum. J. Biol. Chem. 1990, 265, 3497–3502. [Google Scholar] [CrossRef]
- Cushman, J.C. Characterization and expression of a NADP-malic enzyme cDNA induced by salt stress from the facultative CAM plant, Mesembryanthemum crystallinum. Eur. J. Biochem. 1992, 208, 259–266. [Google Scholar] [CrossRef]
- Ocheretina, O.; Scheibe, R. Cloning and sequence analysis of cDNAs encoding cytosolic malate dehydrogenase. Gene 1997, 199, 145–148. [Google Scholar] [CrossRef]
- Löw, R.; Rockel, B.; Kirsch, M.; Ratajczak, R.; Hörntensteiner, S. Early salt stress effects on the differential expression of vacuolar H+-ATPase genes in roots and leaves of Mesembryanthemum crystallinum. Plant Physiol. 1996, 110, 259–265. [Google Scholar] [CrossRef]
- Bartholomew, D.M.; Rees, D.J.G.; Rambaut, A.; Smith, J.A.C. Isolation and sequence analysis of a cDNA encoding the c subunit of a vacuolar-type H+-ATPase from the CAM plant Kalanchoë diagremontiana. Plant Mol. Biol. 1996, 31, 435–442. [Google Scholar] [CrossRef]
- Tsiantis, M.S.; Bartholomew, D.M.; Smith, J.A.C. Salt regulation of transcript levels for the c subunit of a leaf vacuolar H+-ATPase in the halophyte Mesembryanthemum crystallinum. Plant J. 1996, 9, 729–736. [Google Scholar] [CrossRef]
- Dietz, K.J.; Arbinger, B. cDNA sequence and expression of subunit E of the vacuolar H(+)-ATPase in the inducible crassulacean acid metabolism plant Mesembryanthemum crystallinum. Biochem. Biophys. Acta Gene Struct. Funct. 1996, 1281, 134–138. [Google Scholar] [CrossRef]
- Baur, B.; Fisher, K.; Winter, K.; Dietz, K.J. cDNA sequences of a protein kinase from the halophyte Mesembryanthemum crystallinum L., encoding a SNF-1 homologue. Plant Physiol. 1994, 106, 1225–1226. [Google Scholar] [CrossRef] [PubMed]
- Breiteneder, H.; Michalowski, C.B.; Bohnert, H.J. Environmental stress mediated differential 3′ end formation of chloroplast RNA-binding protein transcripts. Plant Mol. Biol. 1994, 26, 833–849. [Google Scholar] [CrossRef]
- Rippmann, J.F.; Michalowski, C.B.; Nelson, D.E.; Bohnert, H.J. Induction of a ribosome-inactivating protein upon environmental stress. Plant Mol. Biol. 1997, 35, 701–709. [Google Scholar] [CrossRef]
- Cushman, J.C.; Michalowski, C.B.; Bohnert, H.J. Developmental control of crassulacean acid metabolism inducibility by salt stress in the common ice plant. Plant Physiol. 1990, 94, 1137–1142. [Google Scholar] [CrossRef]
- Cushman, J.C.; Bohnert, H.J. Transcriptional activation of CAM genes during development and environmental stress. In Ecological Studies; Smith, J.A.C., Winter, A.C., Eds.; Metabolism: Biochemistry, Ecophysiology and Evolution; Springer: Berlin, Germany, 1996; pp. 135–158. [Google Scholar]
- Bohnert, H.J.; Ayoubi, P.; Borchert, C.; Bressan, R.A.; Burnap, R.L.; Cushman, J.C.; Cushman, M.A.; Deyholos, M.; Fischer, R.; Galbraith, D.W.; et al. A genomics approach towards salt stress tolerance. Plant Physiol. Biochem. 2001, 39, 295–311. [Google Scholar] [CrossRef]
- Cushman, J.C.; Tillett, R.L.; Wood, J.A.; Branco, J.M.; Schlauch, K.A. Large-scale mRNA expression profiling in the common ice plant, Mesembryanthemum crystallinum, performing C3 photosynthesis and crassulacean acid metabolism (CAM). J. Exp. Bot. 2008, 59, 1875–1894. [Google Scholar] [CrossRef] [PubMed]
- Boxall, S.F.; Dever, L.V.; Kneřová, J.; Gould, P.D.; Hartwell, J. Phosphorylation of phosphoenolpyruvate ccarboxylase is essential for maximal and sustained dark CO2 Fixation and core circadian clock operation in the obligate crassulacean acid metabolism species Kalanchoë fedtschenkoi. Plant Cell 2017, 29, 2519–2536. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.M.; Weise, S.E.; Ozersky, P.; Mockler, T.C.; Michael, T.P.; VanBuren, R. Time of day and network reprogramming during drought induced CAM photosynthesis in Sedum album. PLoS Genet. 2019, 15, e1008209. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhang, L.S.; Zhang, G.Q.; Zheng, B.Q.; Liu, Z.J.; Wang, Y. Evolutionary history of PEPC genes in green plants: Implications for the evolution of CAM in orchids. Mol. Phylogenetics Evol. 2016, 94, 559–564. [Google Scholar] [CrossRef]
- Fankhauser, N.; Aubry, S. Post-transcriptional regulation of photosynthetic genes is a key driver of C4 leaf ontogeny. J. Exp. Bot. 2017, 68, 137–146. [Google Scholar] [CrossRef]
- Shen, S.Q.; Li, N.; Wang, Y.J.; Zhou, R.; Sun, P.C.; Lin, H.; Chen, W.; Yu, T.; Liu, Z.; Wang, Z.Y.; et al. High quality ice plant reference genome analysis provides insights into genome evolution and allows exploration of genes involved in the transition from C3 to CAM pathways. Plant Biotechnol. J. 2022, 20, 2107–2122. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.M.; VanBuren, R.; Zhang, J.S.; Huang, L.X.; Miao, W.; Edger, P.P.; Yim, W.C.; Priest, H.D.; Meyers, B.C.; Mockler, T.; et al. Temporal and spatial transcriptomic and microRNA dynamics of CAM photosynthesis in pineapple. Plant J. Cell Mol. Biol. 2017, 92, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.P.; Yim, W.C.; Sun, Y.H.; Ohnishi, M.; Mimura, T.; Cushman, J.C.; Yen, H.E. Identification of ice plant (Mesembryanthemum crystallinum L.) microRNAs using RNA-seq and their putative roles in high salinity responses in seedlings. Front. Plant Sci. 2016, 7, 1143. [Google Scholar] [CrossRef]
- Hu, Z.K.; Nie, Z.Y.; Yan, C.; Huang, H.; Ma, X.J.; Wang, Y.P.; Ye, N.; Tuskan, G.A.; Yang, X.H.; Yin, H.F. Transcriptome and degradome profiling reveals a role of miR530 in the circadian regulation of gene expression in Kalanchoë marnieriana. Cells 2021, 10, 1526. [Google Scholar] [CrossRef] [PubMed]
- Kore-eda, S.; Cushman, M.A.; Akselrod, I.; Bufford, D.; Fredrickson, M.; Clark, E.; Cushman, J.C. Transcript profiling of salinity stress responses by large-scale expressed sequence tag analysis in Mesembryanthemum crystallinum. Gene 2004, 341, 83–92. [Google Scholar] [CrossRef]
- Guan, Q.J.; Kong, W.W.; Zhu, D.; Zhu, W.; Dufresne, C.; Tian, J.K.; Chen, S.X. Comparative proteomics of Mesembryanthemum crystallinum guard cells and mesophyll cells in transition from C3 to CAM. J. Proteom. 2021, 231, 104019. [Google Scholar] [CrossRef]
- Perron, N.; Tan, B.; Dufresne, C.P.; Chen, S.X. Proteomics and phosphoproteomics of C3 to CAM transition in the common ice plant. Methods Enzymol. 2022, 676, 347–368. [Google Scholar]
- Nobe, P. High productivities of certain agronomic CAM species. Crassulacean Acid Metabolism. In Biochemistry, Ecophysiology and Evolution; Springer: Berlin/Heidelberg, Germany, 1996; pp. 255–265. [Google Scholar]
- Töpfer, N.; Braam, T.; Shameer, S.; Ratcliffe, R.G.; Sweetlove, L.J. Alternative crassulacean acid metabolism modes provide environment-specific water-saving benefits in a leaf metabolic model. Plant Cell 2020, 32, 3689–3705. [Google Scholar] [CrossRef]
- Winter, K.; Smith, J.A.C. (Eds.) Taxonomic distribution of crassulacean acid metabolism. In Crassulacean Acid Metabolism. Biochemistry, Ecophysiology and Evolution; Springer: Berlin/Heidelberg, Germany, 1996; pp. 427–436. [Google Scholar]
- Qiu, S.; Sultana, S.; Liu, Z.D.; Yin, L.Y.; Wang, C.Y. Identification of obligate C3 photosynthesis in Dendrobium. Photosynthetica 2015, 53, 168–176. [Google Scholar] [CrossRef]
- Zou, L.H.; Wan, X.; Deng, H.; Zheng, B.Q.; Li, B.J.; Wang, Y. RNA-seq transcriptomic profiling of crassulacean acid metabolism pathway in Dendrobium catenatum. Sci. Data 2018, 5, 180252. [Google Scholar] [CrossRef]
- Zhang, Z.J.; He, D.X.; Gao, R.F. Concomitant CAM and C3 photosynthetic pathways in Dendrobium officinale plants. J. Am. Soc. Hortic. Sci. 2014, 139, 290–298. [Google Scholar] [CrossRef]
- Winter, K.; Holtum, J.A.M. Cryptic crassulacean acid metabolism (CAM) in Jatropha curcas. Funct Plant Biol. 2015, 42, 711–717. [Google Scholar] [CrossRef]
- Yuan, G.L.; Hassan, M.M.; Liu, D.G.; Lim, S.D.; Yim, W.C.; Cushman, J.C.; Markel, K.; Shih, P.M.; Lu, H.W.; Weston, D.J.; et al. Biosystems Design to Accelerate C3-to-CAM Progression. BioDesign Res. 2020, 2020, 3686791. [Google Scholar] [CrossRef]
- Cosentino, C.; Silvestre, D.D.; Fischer-Schliebs, E.; Homann, U.; Palma, A.D.; Comunian, C.; Mauri, P.L.; Thiel, G. Proteomic analysis of Mesembryanthemum crystallinum leaf microsomal fractions finds an imbalance in V-ATPase stoichiometry during the salt-induced transition from C3 to CAM. Biochem. J. 2013, 450, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.H.; Barkla, B.J.; Vera-Estrella, R.; Pantoja, O.; Lee, S.Y.; Bohnert, H.J.; Dassanayake, M. Cell type-specific responses to Psalinity–the epidermal bladder cell transcriptome of Mesembryanthemum crystallinum. New Phytol. 2015, 207, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Barkla, B.J.; Vera-Estrella, R. Single cell-type comparative metabolomics of epidermal bladder cells from the halophyte Mesembryanthemum crystallinum. Front. Plant Sci. 2015, 6, 435. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, H.; Suzuki, T.; Nishikawa, K.; Agarie, S.; Ishiguro, S.; Higashiyama, T. RNA-seq analysis of the response of the halophyte, Mesembryanthemum crystallinum (ice plant) to high salinity. PLoS ONE 2015, 10, e0118339. [Google Scholar] [CrossRef]
- Barkla, B.J.; Vera-Estrella, R.; Raymond, C. Single-cell-type quantitative proteomic and ionomic analysis of epidermal bladder cells from the halophyte model plant Mesembryanthemum crystallinum to identify salt-responsive proteins. BMC Plant. Biol. 2016, 16, 110. [Google Scholar] [CrossRef]
- Yan, X.; Chang, Y.; Zhao, W.J.; Qian, C.J.; Yin, X.Y.; Fan, X.K.; Zhu, X.Y.; Zhao, X.Q.; Ma, X.F. Transcriptome profiling reveals that foliar water uptake occurs with C3 and crassulacean acid metabolism facultative photosynthesis in Tamarix ramosissima under extreme drought. AoB Plants 2022, 14, plab060. [Google Scholar] [CrossRef] [PubMed]
- Heyduk, K.; McAssey, E.V.; Leebens-Mack, J. Differential timing of gene expression and recruitment in independent origins of CAM in the Agavoideae (Asparagaceae). New Phytol. 2022, 235, 2111–2126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Xia, K.; Yang, Y.; Wu, Q.; Zhao, Z. Mechanisms Underlying the C3–CAM Photosynthetic Shift in Facultative CAM Plants. Horticulturae 2023, 9, 398. https://doi.org/10.3390/horticulturae9030398
Qiu S, Xia K, Yang Y, Wu Q, Zhao Z. Mechanisms Underlying the C3–CAM Photosynthetic Shift in Facultative CAM Plants. Horticulturae. 2023; 9(3):398. https://doi.org/10.3390/horticulturae9030398
Chicago/Turabian StyleQiu, Shuo, Ke Xia, Yanni Yang, Qiaofen Wu, and Zhiguo Zhao. 2023. "Mechanisms Underlying the C3–CAM Photosynthetic Shift in Facultative CAM Plants" Horticulturae 9, no. 3: 398. https://doi.org/10.3390/horticulturae9030398
APA StyleQiu, S., Xia, K., Yang, Y., Wu, Q., & Zhao, Z. (2023). Mechanisms Underlying the C3–CAM Photosynthetic Shift in Facultative CAM Plants. Horticulturae, 9(3), 398. https://doi.org/10.3390/horticulturae9030398





