Abstract
Olive (Olea europaea L.) fruit and derived products play a pivotal role in the Mediterranean diet, to which they contribute their gastronomic value and their health-promoting properties. The fruit cuticle constitutes the interface between the plant and the surrounding environment, and it modulates relevant traits such as water loss, mechanical resistance, and susceptibility to pests and rots. Hence, a better knowledge of fruit cuticle properties and the impact thereupon of agronomic factors could help improving olive grove management. In this work, time–course changes in fruit cuticle yields and composition were assessed during the on-tree ripening of ‘Arbequina’ olives obtained from irrigated or rain-fed trees grown at a commercial grove located in El Soleràs (Catalonia, Spain), where low annual rainfall occur together with cold winters and hot dry summers. Significantly higher wax contents were observed for rain-fed than for irrigated fruits, both in relative (% over total cuticle) and in absolute terms (from 231 to 840 µg cm−2 and from 212 to 560 µg cm−2, respectively, contingent upon the maturity stage), in agreement with their proposed role as a barrier against water loss. Compositional differences in cuticular waxes and in cutin monomers were also detected between irrigated and rain-fed olives, with major changes involving significantly higher loads per surface area of triterpenoids and ω-hydroxy fatty acids in the latter. In contrast to the load and composition of cuticular wax, no apparent impact of irrigation was observed on either total cuticle yields or cuticle thickness.
“Good morrow, fair ones; pray you, if you know,Where in the purlieus of this forest standsA sheep-cote fenc’d about with olive trees?”William ShakespeareAs You Like It (Act IV, Scene III)
1. Introduction
‘Arbequina’ is the most important olive (Olea europaea L.) cultivar in Catalonia (NE Spain), where it represents over 50% of the total olive-cultivated area [1]. ‘Arbequina’ fruit are used both for oil production and for processing as tables olives. From its original growing region, ‘Arbequina’ production has outspread to other areas of the world, where its groves occupy a total surface of around 60,000 ha [2]. The vigor and productive characteristics of the tree make this cultivar suitable for high-density planting [3] and mechanical harvesting, thereby reducing the cost of the final product.
Olive trees usually grow in Mediterranean-type climates, where the plant must withstand adverse environmental conditions that are becoming increasingly harsher and more stress-inducing due to global climate change. Consequently, it is important to understand the plant–environment interactions. Leaf and fruit cuticles play important roles in plant protection against the surrounding conditions, including the restriction of uncontrolled water loss that challenges plant survival [4].
The plant cuticle is a hydrophobic layer covering the aerial, non-woody plant organs including fruits. Cuticles consist mainly of an insoluble polymeric cutin matrix, rich in hydroxy-, carboxy-, and epoxy-C16 and C18 fatty acid derivatives, in turn covered and embedded with waxes. The wax fraction, present both in amorphous and crystalline form, is composed of very long-chain aliphatic as well as cyclic compounds [5,6,7]. Cuticle constituents are exported and assembled on the external side of the epidermis, hence acting as the first barrier against abiotic (UV radiation, water availability, high temperatures, and mechanical injuries) and biotic (pests and rots) stressors [8]. The limitation of transpirational water loss, which helps protecting plants against desiccation, is one of the important functions exerted by the plant cuticle [9,10].
To date, only a handful of published studies have addressed the cuticles of intact olive fruit, and most of them have focused on cuticular waxes [11,12,13]. Only two studies have also reported the cutin composition of olive fruit cuticles [14,15]. These previous studies revealed that triterpenoid acids (maslinic and oleanolic acids) account for over 65% of cuticular waxes of ‘Arbequina’ fruit, followed by lower amounts of fatty acids, fatty alcohols, n-alkanes, and sterols, while cutin composition is predominated by C18-fatty acids and hydroxy-fatty acids. Even though the main chemical families of cuticle constituents remained the same throughout fruit ripening, significant differences were found in their relative percentages over the total cuticle [14,15], which may impact the cuticle properties and fruit attributes.
Given the relevant roles attributed to plant cuticles on the resistance to biotic and abiotic stressors, the question arises whether an important environmental factor such as water availability may also modulate the profiles of cuticular components in the fruit. This knowledge may also aid the improvement of olive grove management. To this purpose, time–course changes in the cuticle composition of ‘Arbequina’ olive fruits were assessed during on-tree ripening under irrigated or rain-fed conditions. To the best of our knowledge, this information is reported for the first time.
2. Materials and Methods
2.1. Plant Material and Toluidine Blue (TB) Test
‘Arbequina’ olive fruit samples were hand-collected from a commercial grove located in El Soleràs (41°24’ N; 0°39’ E; 450 m altitude), within the geographical area covered by the Protected Designation of Origin (PDO) “Les Garrigues”, and submitted to the usual cultural practices at that producing area, consistent of minimal soil tillage with organic (local farmyard manure) and inorganic (10:10:10 N-P-K) fertilization. No pesticides or fungicides were applied. Trees were planted at 5 × 6 m, resulting in roughly 333 trees/ha. The experimental season was an on-year, largely regulated through pruning. Total rainfall during 2017 at the site amounted to 318 mm, and temperatures are shown in Table 1.

Table 1.
Rainfall and temperatures at the producing site (El Soleràs, 41°24′ N; 0°40′ E) in 2017.
Olive trees either remained rain-fed or were provided with drip irrigation from April to October (1.01 L m−2 day−1, to supply 100% of the estimated daily crop evapotranspiration during the irrigation period). The fruit samples were picked at standard height (around 1.5 m) around different trees in a row, at regular intervals from mid-September to mid-January during the 2017–2018 producing season. Successive picking dates (P) were coded from P1 to P8. No fungal infections were detected, and the fruits bitten by insects were discarded. The fresh weight (g), flesh-to-stone ratio, and water content (%) were determined on three 10-fruit replicates per sampling date and irrigation regime. The fruit length and diameter were individually determined on ten olives using a digital calliper, and the data were expressed as mm. In order to assess the presence of discontinuities on fruit surface, 10 fresh olives per sampling date (as long as the fruit remained green) were stained in a toluidine blue (TB) aqueous solution (0.05%, w/v) for 2 h [16].
2.2. Cuticle Isolation
The fruit cuticles were isolated from around 100 cm2 olive skin per sampling date and irrigation regime. The skin disks (2 disks/fruit) were excised with a cork borer from 50 to 75 olives, depending on the fruit size at each maturation stage, and distributed into two replicate tubes (50 cm2/replicate) for the enzymatic isolation of the cuticular membranes (CM). CM isolation was carried out at 37 °C in 50 mM citrate at pH 4.0, in the presence of 0.2% (w/v) cellulase, 100 U mL−1 pectinase, and 1 mM of NaN3 to prevent microbial growth. When no more material release was apparent, the CM disks were washed in citrate buffer (50 mM, pH 4.0), then in distilled water, and finally dried at 40 °C, weighted, pooled, and kept in hermetically capped vials until further analysis. The cuticle yields were expressed per unit of surface area (mg cm−2).
2.3. Extraction and Analysis of Cuticular Wax
The CM samples (20 mg/replicate × 3 replicates) were extracted in chloroform (2 mg mL−1) with constant shaking during 24 h at room temperature. The chloroform extraction of each sample was carried out three consecutive times, and the resulting extracts were pooled and incubated for 15 min in an ultrasonic bath. The dewaxed cuticular membranes (DCM) were then dried and kept in hermetically capped vials for subsequent cutin monomer analysis. The chloroform extracts were filtered, concentrated in a rotatory evaporator at 40 °C, and transferred to a pre-weighed vial for vacuum concentration until complete dryness. The vials were then weighted, and the total wax yields expressed as μg cm−2. For the wax analysis, the ethers and esters were transformed to free hydroxyl and carboxyl groups, respectively, through derivatization with N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) in the presence of pyridine (3:2, v/v) and dotriacontane (C32) as the internal standard, with shaking during 15 min at 100 °C.
The samples (1 µL) were injected into a gas chromatography–mass spectrometry (GC-MS) system (Agilent 7890N, Santa Clara, CA, USA) equipped with a quadrupole mass selective detector (Agilent 5973N) and a capillary column (DB 5 MS UI, 30 m × 0.25 mm × 0.25 μm; SGE Europe Ltd., Milton Keynes, UK) in the on-column mode. The oven was initially set at 100 °C for 1 min, and the temperature was raised thereafter by 15 °C min−1 to 200 °C, then by 5 °C min−1 to 310 °C, and finally held at this temperature for 10 min. Helium was used as the carrier gas at 1.0 mL min−1. Wax compounds were identified by comparison of their retention times with those of the standards and by matching their electron ionization–mass spectra with those retrieved from a mass spectral library (NIST 11 MS). For the quantitative analysis, a flame ionization detector (FID) was used under the same chromatographic conditions as for the GC-MS, except that the helium flow was 1.3 mL min−1 and that, at the final step, the oven was kept at 310 °C for 13 min. The results were expressed as relative percentage (% over total waxes). The average chain length (ACL) of the acyclic wax compounds was also calculated as the weighted average number of carbon atoms by the following equation:
where Cn is the percentage of each acyclic wax compound with n carbon atoms.
2.4. Extraction and Analysis of Cutin Monomers
For the hydrolysis of cutin monomers, the DCM samples (roughly 10 mg/replicate × 3 replicates) were added to 2 mL 1 M HCL in 100% MeOH and esterified in this solution for 2 h at 80 °C. After cooling down to room temperature, 2 mL saturated NaCl were added, and the cutin monomers were then extracted three consecutive times in 2 mL hexane for 10 min with shaking at 20 °C and finally centrifuged. The supernatants from all three extractions were pooled and dried under vacuum at 40 °C in a pre-weighted vial. The vials containing the dry samples were weighted to calculate total cutin yields (µg cm−2). The procedures for compound derivatization, identification (GC-MS), and quantification (GC-FID), were as described above for the wax analysis, with the exception that heptadecanoate (C17) and tricosanoate (C23) were used as the internal standards.
2.5. Assessment of Cuticle Thickness
Pericarp cubes (1–2 mm) were chopped from the fruit samples and fixed in a formaldehyde–acetic acid (FAA) solution (5% (v/v) formaldehyde and 5% (v/v) glacial acetic acid in 1:1 (v/v) ethanol-distilled water) for 12 h. For the sample dehydration, the FAA solution was changed with different solutions of increasing ethanol concentrations up to 100%. The dehydrated samples were then transferred to Eppendorf tubes for infiltration and polymerization in Technovit 7100® resin (Heraeus Kulzer GmbH, Wehrheim, Germany) and dried during 24 h at 45 °C.
The resin-embedded samples were cut with an ultramicrotome (Leica EM UC6, Leica Microsystems GmbH, Wetzlar, Germany) into 4 µm thick slices, and then stained using the lysochrome Sudan IV (5% (w/v) in 85% (v/v) ethanol) to visualize the lipidic constituents of the olive cuticles. Lysochrome excess was removed by rinsing in 50% (v/v) ethanol, and the stained samples were dried at room temperature. The images were obtained using an optic microscope (Leica DM4000 B) coupled with a camera (Leica DFC300 FX). To measure the cuticle thickness, 5 images per sample were taken with the Fiji image processing software (GNU General Public License) [17].
2.6. Statistical Analysis
The results were expressed as means ± standard deviations. The JMP® Pro 13 software was used to conduct the statistical analysis. A multifactorial analysis of variance (ANOVA) was carried out with the irrigation regime and maturity stage as the factors, and the means were compared with the LSD test (p ≤ 0.05). Principal component analysis (PCA) was also applied to aid the interpretation of the dataset, with full cross-validation as the validation procedure. The PCA models were developed with the Unscrambler software (version 9.1.2, CAMO ASA, Oslo, Norway), after weighing data by the inverse of the standard deviation of each variable to prevent dependence on the measuring units.
3. Results and Discussion
3.1. The Impact of Irrigation on Physical Characteristics of Fruits
The physical characteristics of fruits were assessed after recollection (Table 2). The phenotypic data show that water availability had an impact on the development of ‘Arbequina’ fruit. Weight and size were higher for irrigated than for rain-fed samples, in agreement with higher water content. Different studies have shown that lower water content increases the oil concentration in olive fruits, resulting in higher oil extraction yields [18]. Furthermore, the total content of phenolic compounds was lower in olives grown under irrigation [19], which is in accordance with previous reports [20,21,22,23] and suggests better health-promoting properties for olive fruits and olive oil produced in rain-fed conditions. Consistent with higher water content, the flesh-to-stone ratio was also higher in fruits picked from irrigated trees. Furthermore, the toluidine blue (TB) test (Table 2, Supplementary Figure S1) revealed the existence of pores on the surface of fruit from irrigated trees, while for rain-fed samples these were visible in fruit picked during early October uniquely. The TB test for rain-fed fruit was negative thereafter, which coincided with the combined occurrence of warm temperature episodes (absolute maximum temperature of 28.4 °C in October) and lower rainfall as compared with September, which further dropped during the subsequent months (Table 1). These observations suggest that TB test results might be reflecting the impact of water scarcity, and indeed the presence of cuticular irregularities has been associated with water loss and solute absorption [24,25]. This trend could not be confirmed due to ripening-related color change of the fruit samples that prevented the assessment of TB staining in black fruit. Significant differences in fruit weight and water content between irrigated and rain-fed olives, though, were apparent after mid-October, concomitantly with the positive TB test for the latter (Table 2). Determining the permeability to water of fruit cuticles may help dissecting these aspects in future studies.

Table 2.
Physical characteristics and toluidine blue test of ‘Arbequina’ olives picked at El Soleràs (PDO “Les Garrigues”) during the 2017–2018 season.
3.2. The Impact of Irrigation on Fruit Cuticle Characteristics
A noticeable peak in the total cuticle yield (3.8 mg cm−2) was found at mid-October in rain-fed fruits (Table 3), after the transient occurrence of surface gaps as shown by TB staining in these samples (Table 2). With this exception, no remarkable differences in total cuticle yields were observed during the experimental period between irrigated and rain-fed fruits, with values ranging from 1.7 to 3.8 mg cm−2. For the rain-fed olives, the cutin percentages over the total cuticles roughly ranged from 17 to 35% and displayed an increasing trend throughout maturation up to mid-December (Table 3). When expressed in terms of amount per surface area, total yields ranged from 403.8 to 753.0 µg cm−2, and a steady increase was observed up to mid-January, except for a transient peak at mid-October (Figure 1). This mid-October peak in cutin yields was also found in fruit samples from the irrigated trees, both in absolute (µg cm−2) and relative (% over total cuticle) terms (Table 3, Figure 1). Even though some significant differences in cutin loads were detected between irrigated and rain-fed samples at particular picking dates, no clear trend was apparent.

Table 3.
Total cuticle amounts and thickness, cuticular wax and cutin percentages, and wax-to-cutin ratios in fruit cuticles during ripening of irrigated and non-irrigated ‘Arbequina’ olives.
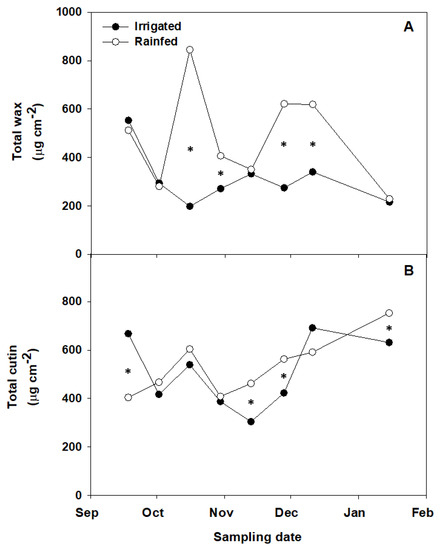
Figure 1.
Total wax (A) and cutin (B) yields in fruit cuticles isolated during the ripening of irrigated and non-irrigated ‘Arbequina’ olives. Values represent means of three replicates. Asterisks stand for significant differences between irrigated and non-irrigated trees at p ≤ 0.05 (LSD test).
Contrarily, the wax yields were significantly higher in rain-fed (231–840 µg cm−2) than in irrigated (213–560 µg cm−2) olives during most of the experimental time (Figure 1) and represented, respectively, 8.9–37.1% and 8.5–20.0% over total cuticle loads (Table 3). The disparities in the wax loads were reflected in wax-to-cutin ratios, which were also generally higher for the rain-fed samples. These data are in accordance with reports on other species, including litchi (Litchi chinensis), longan (Dimocarpus logan) [10], and Arabidopsis [26], for which a relationship between the cuticular waxes and barrier properties under water deficiency conditions has been suggested. The observation of negative TB test for the rain-fed samples (Table 2) concomitantly with increased wax loads (Figure 1) suggests that the up-regulation of the cuticular wax production may have contributed to covering surface discontinuities and hence restricting water loss from non-irrigated fruits. Previous studies on the fruit cuticle of ‘Arbequina’ olives reported higher wax and cutin yields than those found in the present work [14,15], which reflects the year-to-year variation and highlights the convenience of repeating these studies over a range of producing seasons and in various pedo-climatic zones and cultural practices, which can greatly affect water requirements as recently reported for table grapes [27].
For the irrigated fruit, increasing cutin yields in combination with generally decreasing wax percentages over total cuticle in later maturity stages (P5–P8) (Table 3) resulted in lower wax-to-cutin ratios, possibly contributing to lower water contents (Table 2). While no maturity stage-related differences in cuticular permeability to water were detected in a previous study throughout the maturation of non-irrigated ‘Arbequina’ olives produced at the same grove [14], no significant changes in the total amount of cuticular waxes or wax-to-cutin ratios were either detected in that study. However, the wax-to-cutin ratio in leaves more than doubled that in fruits, while the cuticular water permeance of fruits was roughly five-fold higher than that of leaves, which indicates the relevant role of waxes on the barrier properties of the plant organ surface.
While no significant differences in fruit cuticle thickness were observed during the maturation of olives from irrigated trees, a moderately decreasing trend was found for the rain-fed samples. Water availability at early stages of fruit development has been suggested to impact cuticle thickness, and previous studies on Arabidopsis leaves [28] concluded that the deposition of cuticular waxes and cuticle thickness increased in response to water deficit conditions. This is consistent with the observation of thicker cuticles in rain-fed than in irrigated fruits in September (P1), immediately following a period of harsh environmental conditions as indicated by the occurrence of low rainfall and high temperatures during July and August (Table 1). Yet, the idea disagrees with the data indicating that, except for P1 samples, no significant differences were observed in cuticle thickness between irrigated and non-irrigated fruit (Table 3, Supplementary Figure S2), even though the water content was significantly lower in the latter (Table 2). A recent survey of published literature on the role of fruit epidermis as a barrier against water stress [29] concluded that cuticular waxes, rather than cuticle thickness, are pivotal against water loss. The composition of cuticular waxes was hence analyzed in detail.
3.3. Wax Composition of ‘Arbequina’ Olive Fruit Cuticles
The wax fraction comprised cyclic and acyclic compounds (Table 4). The cyclic compounds included large amounts of triterpenoids and minor contents of sterols. As for acyclic waxes, the main chemical families identified were fatty acids, fatty alcohols, and n-alkanes. The acyclic-to-cyclic ratios ranged between 0.15 and 0.29, and no irrigation-related differences were generally detected. However, an increasing trend along fruit maturation was observed. An increasing trend was also reported for rain-fed ‘Arbequina’ fruits produced at the same site [14], even though the values were higher in that previous study (0.27, 0.38, and 0.47 for green, turning, and black fruit, correspondingly), while Diarte et al. [15] did not find significant maturity-related differences. The detected differences are indicative of shifts in the proportions of the different wax compound families throughout fruit maturation. In the present work, higher percentages of triterpenoids and lower relative amounts of fatty alcohols and n-alkanes over total waxes were detected in comparison with those previous reports.

Table 4.
Composition of wax constituents (relative % over total waxes) in fruit cuticles isolated during ripening of irrigated and non-irrigated ‘Arbequina’ olives.
Triterpenoids were the main cuticular wax compounds identified in olive fruit cuticles in agreement with previous studies [12,14,15,30,31,32]. Relative amounts of triterpenoids (ranging from 62.4 to 76.9%) remained largely unchanged along fruit maturation, which aligns with previous reports on ‘Arbequina’ olive [14,15], and no significant differences were detected between irrigated and rain-fed samples (Table 4). Yet, even if the proportion of triterpenoids over total waxes was similar regardless of irrigation, the wax yields were lower in the irrigated samples (Figure 1), meaning that the triterpenoid load was higher in fruits from non-irrigated trees when expressed in absolute terms (µg cm−2) (Figure 2A). The most abundant identified triterpenoids were maslinic and oleanolic acids (Supplementary Table S1), which is in accordance with former reports [14,15,30,31,32]. Even so, the fate of particular compound families throughout fruit development and ripening is cultivar-specific, and may show variability across genotypes [13,15], meaning that each individual cultivar should be examined on a case-by-case basis. For example, the triterpenoid content decreased significantly during maturation of ‘Picual’ olives [15]. Furthermore, even though the percentage of total triterpenoids in the ‘Picual’ fruit was reportedly lower in the irrigated than in the rain-fed olives, the opposite was observed when the data were expressed as mg/fruit, with triterpenoid amounts in the irrigated olives being over three fold those in the non-irrigated samples [32], which contrasts with the data herein. Environmental differences between the growing sites, cultural practices, or cultivar particularities that affect fruit size and metabolism of irrigated vs. rain-fed fruits may also underlie a part of these discrepancies.
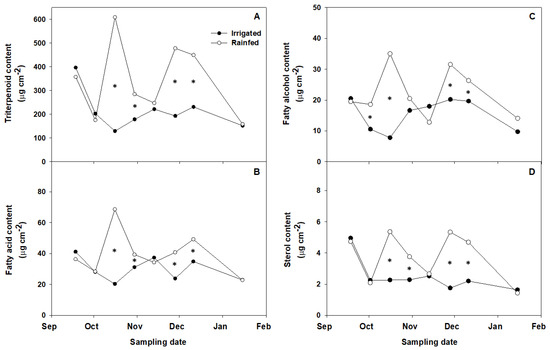
Figure 2.
Total triterpene (A), fatty acid (B), fatty alcohol (C), and sterol (D) amounts in cuticular waxes of fruit cuticles isolated during ripening of irrigated and non-irrigated ‘Arbequina’ olives. Values represent means of three replicates. Asterisks stand for significant differences between irrigated and non-irrigated trees at p ≤ 0.05 (LSD test).
Acyclic wax compounds were present in much lower amounts than triterpenoids, the identified types including fatty acids, fatty alcohols, and minor percentages of n-alkanes (Table 4). Their weighted average chain length (ACL) was similar for irrigated and rain-fed fruits and ranged, respectively, from 24.0 to 24.4 and from 23.3 to 24.8. Significant differences in the ACLs of acyclic compounds between irrigated and rain-fed fruits were detected around the first half of November uniquely, and coincided with lowered rainfall at the producing site (Table 1). These ACL values were lower than those reported in a previous report on ‘Arbequina’ olives picked from the same grove [14], ranging 25.8 to 27.3 contingent on the maturity stage. Because the agronomical practices were the same as in this work, the observed differences may reflect the year-to year variability in climatic conditions.
The percentage of fatty acids in the olive fruit cuticles represented 6.6–11.5% over the total waxes, regardless of irrigation (Table 4). The fatty acid percentages were in general higher at more mature stages, and few differences were detected in response to irrigation. Similar amounts of fatty alcohols (below 10% in all cases) were also observed, regardless of irrigation. Cerotic acid (26:0) quantitatively predominated among the detected fatty acids, while hexacosanol (C26) was the most abundant fatty alcohol, followed by tetracosanol (C24) and octacosanol (C28) (Supplementary Table S1). No clear time–course change trend along fruit maturation was observed for these compounds. As for triterpenoids, however, the irrigation-related differences became more apparent when the data were expressed in absolute terms (µg cm−2), with higher contents corresponding to fruit from non-irrigated trees (Figure 2B,C).
Finally, and regarding n-alkanes, the relative amounts (% over total waxes) were higher in irrigated than in rain-fed fruits (Table 4), in which heptacosane (C27) was detectable at early maturity stages (P1–P2) uniquely (Supplementary Table S1). This was unexpected, since n-alkanes have been often related to the water-proofing functions of the plant cuticle through the ability to establish crystalline structures that would help restrict water loss [33,34,35]. Even so, the very low percentages detected for this wax fraction (around 1% or lower) may indicate that their role in the prevention of water loss from olive fruits may be less important than in other olive organs or in other fruit species. For example, n-alkane amounts in ‘Arbequina’ leaves were found to be around four-fold higher than those in fruits, and the leaf cuticles were moreover observed to display much lower water permeability [14]. The concentrations of n-alkanes in the cuticle of olive fruits have been consistently reported to be much lower than those found for other fruit species (reviewed in [9,36,37]).
3.4. Cutin Monomer Composition of ‘Arbequina’ Olive Fruit Cuticles
Cutin monomers were also analyzed. Overall, the cutin composition showed limited variation in response to irrigation (Table 5). The C18-type predominated over the C16-type monomers, in some instances even doubling the percentages of C16 compounds (Supplementary Table S2). The predominant chemical family of the cutin monomers identified was ω-hydroxy fatty acids, which accounted for 23.5–29.5% and 25.9–29.7% over total cutin in irrigated and rain-fed samples, respectively (Table 5). Monocarboxylic fatty acids and ω-hydroxy fatty acids displaying midchain hydroxy groups were also prominent among the detected cutin monomer types. The highest percentages over total cutin corresponded to 18-hydroxy-octadecenoic acid and 9/10,16-dihydroxy-hexadecanoic acid, which were, therefore, the predominant cutin monomers identified in olive fruit samples (Supplementary Table S2).

Table 5.
Composition of cutin monomers (relative % over total cutin) in fruit cuticles isolated during ripening of irrigated and non-irrigated ‘Arbequina’ olives.
In addition to the mentioned ω-hydroxy acids, considerable relative amounts of the dicarboxylic cis-9-octadecenoic α,ω-diacid were detected, which increased along fruit ripening, particularly in fruits picked from the irrigated trees, to achieve around 11% over total cutin. The monocarboxylic oleic acid (18:1∆9) was more abundant in irrigated than in rain-fed samples, with percentages over total cutin ranging from 6.7 to 14.5% and from 4.8 to 9.2%, correspondingly. Although the main monomer types identified were the same as in previous reports on ‘Arbequina’ olives [14,15], some quantitative discrepancies were detected in comparison with [14], who reported much higher relative amounts (roughly 75% over total cutin) of ω-hydroxy fatty acid monomers with midchain hydroxy groups. As for cuticular waxes, considerable variability in cutin monomer types and relative abundance has been observed across fruit species and cultivars [9,36,37,38,39,40,41]. Additionally, much of the published literature on fruit cuticles has focused preferentially on waxes, and, hence, many knowledge gaps exist regarding cutin composition of fruit species.
Even though cutin yields displayed an increasing trend during the maturation of ‘Arbequina’ olives (Table 3), the time–course change pattern of the different cutin monomer types detected was not the same in all cases (Table 5, Supplementary Table S2). This suggests that the metabolic pathways involved in the biosynthesis, transport, and assembly of cutin monomers were affected differentially during the process.
In order to help visualizing relationships among the factors and many variables considered, PCA was used as a tool to aid the interpretation of the results. Cuticle yield and thickness, wax and cutin loads, and the amounts of the identified compound families were used to characterize the samples (16 samples × 18 variables) (Figure 3). The first two principal components (PC) of this model explained alone 65% of the total variability across the samples. The scores plot shows that samples were distributed along PC1 mainly according to the irrigation regime (Figure 3A), while the effect of the maturity stage was less apparent. The corresponding correlation loadings plot revealed that the rain-fed samples were characterized by higher loads of cuticular waxes and higher contents of some wax types, particularly triterpenes (Figure 3B).
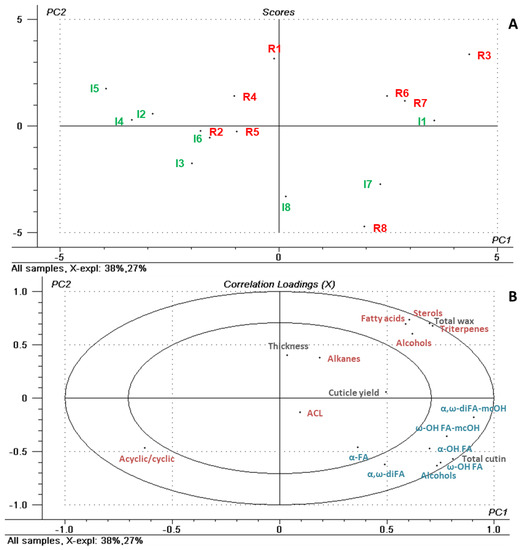
Figure 3.
Scores (A) and correlation loadings (B) plots of PC1 vs. PC2 corresponding to a Principal Component Analysis (PCA) model for components and properties of ‘Arbequina’ fruit cuticles. Codes from 1 to 8 designate successive picking dates throughout on-tree fruit maturation under irrigated (I) and rain-fed (R) conditions. Wax and cutin compound families are indicated, respectively, in red and blue (Abbreviations: ACL, average chain length of the acyclic wax compounds; Acyclic/cyclic, ratio of acyclic to cyclic compounds in cuticular waxes; α-FA, α-monocarboxylic fatty acids; α,ω-diFA, α,ω-dicarboxylic fatty acids; α,ω-diFA-mcOH, α,ω-dicarboxylic fatty acids with midchain hydroxy group; ω-OH FA, ω-hydroxy fatty acids; ω-OH FA-mcOH, ω-hydroxy fatty acids with midchain hydroxy group; and α-OH FA, α-hydroxy fatty acids).
Some cutin monomer families, especially ω- and mid-chain hydroxy acids, were also relevant for sample differentiation along PC1. This observation is significant, since the hydroxyl moieties in cutin monomers are considered important for the proper cross-linking of the cutin polymer, which in turn is essential for the correct arrangement and water-barrier role of cuticular waxes [41]. In contrast, cuticle thickness, the content of n-alkanes and, to a lesser extent, total cuticle yield were located close to the plot center, indicating that these variables had little weight on sample differentiation and, thus, only marginal involvement in response to water scarcity. Even though the n-alkanes in cuticular waxes have been suggested to be a major factor accounting for the water-proofing functions of the plant cuticle, the amounts detected in this study were substantially lower than those reported for other drupe-type fruits such as sweet cherry or peach [36,37], suggesting a minor role of this wax fraction in protecting against water loss in olive fruits.
4. Conclusions
To the best of our knowledge, this is the first report on differences in cuticular composition between irrigated and rain-fed olive fruits. We found substantially higher loads of total waxes and of different cuticular wax compound types in rain-fed fruits in comparison to irrigated samples. The amounts of ω- and mid-chain hydroxy acid-type cutin monomers were also higher in cuticles isolated from rain-fed fruit. These differences highlight the plasticity of the related biosynthetic pathways as a part of the mechanisms involved in the adaptation to environmental conditions.
These results must be regarded as preliminary as they will need to be confirmed in subsequent producing seasons. An additional factor to be considered is the alternate bearing of olive trees. The producing season in this study was an “on” year. Regular pruning aids in minimizing production differences across seasons and, hence, alternate bearing at PDO “Les Garrigues” is currently not a real concern as it traditionally was. Even so, irrigation has been reported to increase the mineral element content in olive fruit during the ‘on’ year uniquely [42], and thus this factor may be relevant for managing olive groves in producing areas where water scarcity is a major climatic characteristic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9030394/s1, Supplementary Figure S1: Toluidine Blue (TB) staining during ripening of irrigated and non-irrigated ‘Arbequina’ olives; Supplementary Figure S2: Sudan-IV-stained pericarp cross-sections observed under bright-field microscopy during ripening of irrigated and non-irrigated ‘Arbequina’ olives; Supplementary Table S1: Cuticular wax constituents (% over total wax) identified in fruit cuticles isolated during ripening of irrigated and non-irrigated ‘Arbequina’ olives; Supplementary Table S2: Cutin monomers (% over total cutin) identified in fruit cuticles isolated during ripening of irrigated and non-irrigated ‘Arbequina’ olives.
Author Contributions
Conceptualization, I.L. and J.G.; investigation, C.D. and A.I.; formal analysis, C.D. and I.L.; data curation, C.D. and I.L.; visualization and writing—original draft preparation, C.D. and I.L.; supervision, I.L. and J.G.; project administration, I.L.; funding acquisition, I.L. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Plan Nacional de I+D, Ministerio de Ciencia e Innovación (MICINN), Spain, grant number AGL2015-64235-R. C.D. was the recipient of a predoctoral scholarship granted by the Universitat de Lleida.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are indebted to Sergi Seró (Cooperativa Agrícola d’El Soleràs, S.C.C.L.) for meticulous field work and for the kind supply of olive fruit samples.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ninot, A.; Hermoso, J.F.; Martí, E.; Rovira, M.; Batlle, I.; Romero, A. Recuperació i Conservació de Varietat Autòctones d’Olivera. Dossier Tècnic; Departament d’Agricultura, Ramaderia i Pesca, Generalitat de Catalunya: Catalonia, Spain, 2015; Volume 80, pp. 6–16. ISSN 1699-5465. [Google Scholar]
- Tous, J. The influence of growing region and cultivar on olives and olive oil characteristics and on their functional constituents. In Olives and Olive Oil as Functional foods: Bioactivity, Chemistry and Processing; Kiritsakis, A., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2017; pp. 45–80. [Google Scholar] [CrossRef]
- Ninot, A.; Howad, W.; Romero, A. Les Varietats Catalanes d’Olivera; Quaderns Agraris (Institució Catalana d’Estudis Agraris): Barcelona, Spain, 2019; Volume 46, pp. 7–36. [Google Scholar] [CrossRef]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef]
- Domínguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The biophysical design of plant cuticles: An overview. New Phytol. 2011, 189, 938–9499. [Google Scholar] [CrossRef]
- Trivedi, P.; Nguyen, N.; Hykkerud, A.L.; Häggman, H.; Martinussen, I.; Jaakola, L.; Karppinen, K. Developmental and Environmental Regulation of Cuticular Wax Biosynthesis in Fleshy Fruits. Front. Plant Sci. 2019, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf Life Potential and the Fruit Cuticle: The Unexpected Player. Front. Plant Sci. 2019, 10, 770. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Technol. 2014, 87, 103–112. [Google Scholar] [CrossRef]
- Riederer, M.; Arand, K.; Burghardt, M.; Huang, H.; Riedel, M.; Schuster, A.-C.; Smirnova, A.; Jiang, Y. Water loss from litchi (Litchi chinensis) and longan (Dimocarpus longan) fruits is biphasic and controlled by a complex pericarpal transpiration barrier. Planta 2015, 242, 1207–1219. [Google Scholar] [CrossRef]
- Bianchi, G. Lipids and phenols in table olives. Eur. J. Lipid Sci. Technol. 2003, 105, 229–242. [Google Scholar] [CrossRef]
- Lanza, B.; Di Serio, M.G. SEM characterization of olive (Olea europaea L.) fruit epicuticular waxes and epicarp. Sci. Hortic. 2015, 191, 49–56. [Google Scholar] [CrossRef]
- Vichi, S.; Cortés-Francisco, N.; Caixach, J.; Barrios, G.; Mateu, J.; Ninot, A.; Romero, A. Epicuticular Wax in Developing Olives (Olea europaea) Is Highly Dependent upon Cultivar and Fruit Ripeness. J. Agric. Food Chem. 2016, 64, 5985–5994. [Google Scholar] [CrossRef]
- Huang, H.; Burghardt, M.; Schuster, A.-C.; Leide, J.; Lara, I.; Riederer, M. Chemical Composition and Water Permeability of Fruit and Leaf Cuticles of Olea europaea L. J. Agric. Food Chem. 2017, 65, 8790–8797. [Google Scholar] [CrossRef]
- Diarte, C.; Lai, P.-H.; Huang, H.; Romero, A.; Casero, T.; Gatius, F.; Graell, J.; Medina, V.; East, A.; Riederer, M.; et al. Insights into Olive Fruit Surface Functions: A Comparison of Cuticular Composition, Water Permeability, and Surface Topography in Nine Cultivars During Maturation. Front. Plant Sci. 2019, 10, 1484. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tanaka, H.; Machida, C.; Watanabe, M.; Machida, Y. A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J. 2004, 37, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Inglese, P.; Famiani, F.; Galvano, F.; Servili, M.; Esposto, S.; Urbani, S. Factors affecting extra-virgin olive oil composition. Hortic. Rev. 2011, 38, 83–147. [Google Scholar] [CrossRef]
- Diarte, C.; Iglesias, A.; Graell, J.; Lara, I. Firmness and cell wall changes during maturation of ‘Arbequina’ olive fruit: The impact of irrigation. Horticulturae 2022, 8, 872. [Google Scholar] [CrossRef]
- Berenguer, M.J.; Vossen, P.M.; Grattan, S.R.; Connell, J.H.; Polito, V.S. Tree Irrigation Levels for Optimum Chemical and Sensory Properties of Olive Oil. Hortscience 2006, 41, 427–432. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Salvador, M.D.; Moriana, A.; Pérez, D.; Olmedilla, N.; Ribas, F.; Fregapane, G. Influence of different irrigation strategies in a traditional Cornicabra cv. olive orchard on virgin olive oil composition and quality. Food Chem. 2007, 100, 568–578. [Google Scholar] [CrossRef]
- Morales-Sillero, A.; García, J.; Torres-Ruiz, J.; Montero, A.; Sánchez-Ortiz, A.; Fernández, J. Is the productive performance of olive trees under localized irrigation affected by leaving some roots in drying soil? Agric. Water Manag. 2013, 123, 79–92. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of drought stress on phenolic accumulation in greenhouse-grown olive trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of Hydrophilic Solutes Through Plant Leaves: Current State of Knowledge and Perspectives of Foliar Fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Fernández, V.; Sotiropoulos, T.; Brown, P.H. Foliar Fertilization: Scientific Principles and Field Practices; International Fertilizer Industry Association: Paris, France, 2013; ISBN 979-10-92366-00-6. [Google Scholar]
- Patwari, P.; Salewski, V.; Gutbrod, K.; Kreszies, T.; Dresen-Scholz, B.; Peisker, H.; Steiner, U.; Meyer, A.J.; Schreiber, L.; Dörmann, P. Surface wax esters contribute to drought tolerance in Arabidopsis. Plant J. 2019, 98, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.; Ferrara, G.; Soto, F.; López, J.A.; Sánchez, F.; Mazzeo, A.; Pastor, A.P.; Domingo, R. Effects of soil and climate in a table grape vineyard with cover crops. Irrigation management using sensors networks. Cienc. Tec. Vitivinic. 2017, 32, 72–81. [Google Scholar] [CrossRef]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lu, S.; Joubes, J.; Jenks, M.A. The impacts of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2003, 151, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, O.; Pinheiro, C.; Misra, C.S.; Fernández, V.; Chaves, M.M. Fleshy fruit epidermis is a protective barrier under water stress. In Water Scarcity and Sustainable Agriculture in Semiarid Environment. Tools, Strategies, and Challenges for Woody Crops; García-Tejero, I.F., Zuazo, V.H.D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 507–533. [Google Scholar] [CrossRef]
- Bianchi, G.; Murelli, C.; Vlahov, G. Surface waxes from olive fruits. Phytochemistry 1992, 31, 3503–3506. [Google Scholar] [CrossRef]
- Guinda, A.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic Triterpenoids from Olive Fruit and Leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef]
- Jiménez-Herrera, R.; Pacheco-López, B.; Peragón, J. Water Stress, Irrigation and Concentrations of Pentacyclic Triterpenes and Phenols in Olea europaea L. cv. Picual Olive Trees. Antioxidants 2019, 8, 294. [Google Scholar] [CrossRef]
- Grncarevic, M.; Radler, F. The effect of wax components on cuticular transpiration-model experiments. Planta 1967, 75, 23–27. [Google Scholar] [CrossRef]
- Leide, J.; Hildebrandt, U.; Reussing, K.; Riederer, M.; Vogg, G. The Developmental Pattern of Tomato Fruit Wax Accumulation and Its Impact on Cuticular Transpiration Barrier Properties: Effects of a Deficiency in a β-Ketoacyl-Coenzyme A Synthase (LeCER6). Plant Physiol. 2007, 144, 1667–1679. [Google Scholar] [CrossRef]
- Leide, J.; Hildebrandt, U.; Vogg, G.; Riederer, M. The positional sterile (ps) mutation affects cuticular transpiration and wax biosynthesis of tomato fruits. J. Plant Physiol. 2011, 168, 871–877. [Google Scholar] [CrossRef]
- Belge, B.; Llovera, M.; Comabella, E.; Gatius, F.; Guillén, P.; Graell, J.; Lara, I. Characterization of Cuticle Composition after Cold Storage of “Celeste” and “Somerset” Sweet Cherry Fruit. J. Agric. Food Chem. 2014, 62, 8722–8729. [Google Scholar] [CrossRef] [PubMed]
- Belge, B.; Goulao, L.F.; Comabella, E.; Graell, J.; Lara, I. Postharvest heat and CO2 shocks induce changes in cuticle composition and cuticle-related gene expression in ‘October Sun’ peach fruit. Postharvest Biol. Technol. 2019, 148, 200–207. [Google Scholar] [CrossRef]
- Kallio, H.; Nieminen, R.; Tuomasjukka, S.; Hakala, M. Cutin Composition of Five Finnish Berries. J. Agric. Food Chem. 2006, 54, 457–462. [Google Scholar] [CrossRef]
- Järvinen, R.; Kaimainen, M.; Kallio, H. Cutin composition of selected northern berries and seeds. Food Chem. 2010, 122, 137–144. [Google Scholar] [CrossRef]
- Peschel, S.; Franke, R.; Schreiber, L.; Knoche, M. Composition of the cuticle of developing sweet cherry fruit. Phytochemistry 2007, 68, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, G.; Beisson, F.; Ohlrogge, J.; Pollard, M. Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: Occurrence of octadeca-cis-6, cis-9-diene-1,18-dioate as the major component. Plant J. 2004, 40, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Bedbabis, S.; Ben Rouina, B.; Boukhris, M.; Ferrara, G. Effects of Irrigation with Treated Wastewater on Root and Fruit Mineral Elements of Chemlali Olive Cultivar. Sci. World J. 2014, 2014, 973638. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).