Abstract
Fresh blueberries are prone to softening and dehydration during postharvest, which limits their competitiveness when reaching the final markets. Commercial cultivars ‘Duke’ and ‘Brigitta’ exhibit contrasting softening patterns. Although ‘Duke’ berries usually show higher firmness levels at harvest as compared to ‘Brigitta’, they display higher softening and weight loss rates after cold storage. The aim of this study was to evaluate the physicochemical changes and modifications in cuticle composition of ‘Duke’ and ‘Brigitta’ blueberries across five developmental stages: green (G), 25 and 50% pink (25P, 50P), and 75 and 100% blue (75B, 100B), to determine those characters with the most influence on their postharvest behavior. For each developmental stage, maturity parameters, respiration, and ethylene production rates were assessed, and cuticular wax and cutin were analyzed. Principal component analysis (PCA) revealed that ‘Duke’ berries were characterized by higher respiration and ethylene production rates, while ‘Brigitta’ showed higher contents of oleanolic acid and α-amyrin over total waxes. The results suggest that larger surface/volume ratios and higher amounts of ursolic acid and lupeol in ripe fruit may underlie higher weight and firmness loss rates of ‘Duke’ berries as compared to ‘Brigitta’.
1. Introduction
The highbush blueberry (Vaccinium corymbosum L.) is a native northern hemisphere species belonging to the Ericaceae family []. The fruit, a spherical berry, exhibits a double sigmoid-type growth pattern [,] with the following three phases: (i) rapid cell division and weight gain [,]; (ii) slight fruit growth rate in favor of seed development []; and (iii) rapid increase in volume due to cell elongation [,]. In phase iii the fruit begins to ripen, with associated changes in color, increases in total soluble solids (TSS) and reduction in organic acids, commonly expressed as titratable acidity (TA). The TSS/TA ratio, as for many other species, is used in the blueberry industry as an indicator of maturity and postharvest potential [].
The ripening process of blueberry fruit is usually described based on changes in fruit skin color from green to blue []. Berries are extremely firm in the immature green stage; firmness decreases considerably as the fruit turn to a green-pink stage and continue to soften at a slower rate until reaching the ripe blue stage [,]. Changes in fruit coloration are mainly attributed to the decrease in chlorophyll and subsequent anthocyanin synthesis in epidermal cells, which advances progressively from the calyx to the pedicel of the fruit [,]. On the other hand, although blueberry is considered a non-climacteric fruit, certain studies have evidenced an increase in metabolic rate (i.e., respiration and ethylene production) during fruit ripening, which argues for potentially climacteric behavior [,].
In the fresh blueberry export industry, postharvest softening is one of the most limiting traits for the competitiveness of each particular cultivar []. Although blueberry fruit softening at early ripening stages has been associated with enzymatic degradation of cell wall components [], very minor cell wall modifications are detectable from the stage in which the fruit reaches 75% blue coloration until full ripeness [,], which suggests that these processes are almost complete at harvest [,]. Given the above, other factors must be contributing to the postharvest softening of blueberries. These may involve the properties of the fruit cuticle, that has been shown to exert a relevant influence on postharvest quality in some species []. Moisture loss is a relevant cause of firmness changes during the storage of blueberries [], and important differences have been reported between cultivars during long refrigerated storage [,,,,].
Additionally, there is a close relationship between fruit firmness and softening [], which is a critical attribute for consumers’ acceptance [], especially after shipments to distant countries (45–60 d) []. Recent studies have shown high variability in initial firmness and percentage of soft fruit within each particular batch of blueberries [,,]. The amount of very soft fruit (<1.4 N) at harvest ranged from 0 to 67%, with the cultivar and maturity stage being the most relevant factors in subsequent softening during storage [].
In this regard, and according to the recent literature on blueberries [,,], the composition of the waxy layer of the fruit, generally known as the “bloom”, could be critical for genotype-related differences in softening rates during blueberry shipment overseas. This waxy layer is the outermost part of the fruit cuticle, an extracellular membrane covering all aerial, non-lignified plant structures (i.e., stems, fruit, and flowers) []. The cuticle is composed of a cutin matrix (an insoluble polymer of fatty acid derivatives), embedded in a complex of intra- and extra-cuticular waxes and a small fraction of phenolic compounds []. Despite being a thin layer, it is an essential component of the stabilizing structure of primary epidermal tissues, and it modulates water permeability during postharvest life [], among other fruit traits.
‘Duke’ and ‘Brigitta’ are the two most planted blueberry cultivars in Chile, and they exhibit substantial differences in terms of the following features: (i) length of ripening window (30 d for ‘Duke’ and 60 d for ‘Brigitta’), (ii) pedigree (‘Duke’ is 100% V. corymbosum; ‘Brigitta’ is 96% V. corymbosum and 4% V. angustifolium []), (iii) precocity (early and mid-season cultivars, respectively) and (iv) postharvest softening patterns; indeed, even though ‘Duke’ berries may reach the harvesting date at higher firmness levels, they consistently exhibit higher softening and weight loss rates after cold storage when compared to ‘Brigitta’ [,,,]. Therefore, it is hypothesized that the study of physicochemical changes and modifications in cuticle composition across developmental stages of fruit (green, 25 and 50% pink, and 75 and 100% blue) may provide clues on those characters with the most influence on genotype-related differences in postharvest behavior.
2. Materials and Methods
2.1. Plant Material and Study Setup
During the producing season 2015/2016, highbush blueberries (Vaccinium corymbosum L., cvs. ‘Brigitta’ and ‘Duke’) were picked from 11- and 10-year old plants, respectively, at a commercial orchard located in Río Claro, Maule Region, Chile (35°15′35.16″ S; 71°14′22.53″ W). Fruit maturity and quality characteristics were studied across fruit development every four to five days. Samples were collected by picking fruit at the most predominant stage based on visual assessment of external color. Maturity stages were designated as G (100% green), 25P (75% green-25% pink), 50P (50% green-50% pink), 75B (25% pink-75% pink-blue) and 100B (90 to 100% blue) (Figure 1). For each developmental stage, four replicates (25 fruits/replicate) were collected to assess maturity parameters, respiration and ethylene production rates. Another three replicates (25 fruits/replicate) were sampled for cuticular wax and cutin analyses.
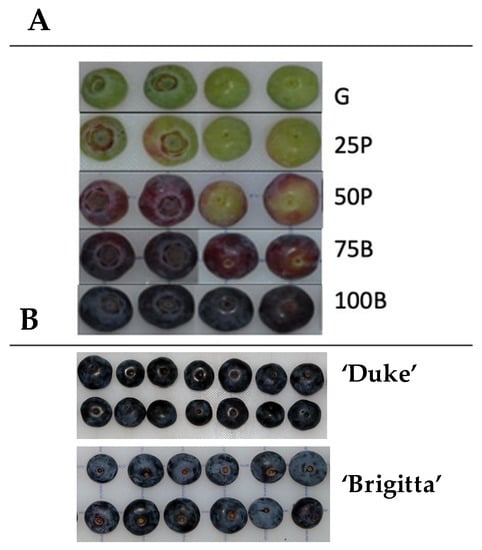
Figure 1.
(A), Fruit developmental stages considered in this study (G, green; 25P, 25% pink; 50P, 50% pink; 75B, 75% blue; 100B, 100% blue). (B), Fully ripe ‘Duke’ and ‘Brigitta’ fruit.
2.2. Maturity Parameters, Respiration Rate and Ethylene Production
Skin color, flesh firmness and equatorial fruit diameter were measured on four replicates (25 berries/replicate) at each developmental stage considered. Surface color was assessed on the equatorial zone of each berry, using a Minolta Chroma Meter (CR210, Osaka, Japan) calibrated with a white panel; chroma (C*) and lightness (L*) were recorded []. Fruit weight (g) and equatorial diameter (mm) were measured with an electronic scale and a digital caliper, respectively. Total surface was estimated from the average diameter of each fruit, assuming a spheroidal shape. The same batch of fruit was used for the determination of fruit firmness (N) using a FirmTech 2 instrument (BioWorks, Inc., Wamego, KS, USA), with minimum and maximum compression forces set at 0.15 and 1.96 N, respectively, and loading cell adjusted to 6 mm s−1 []. To analyze total soluble solids (TSS, %) and titratable acidity (TA, % citric acid), five fruits per replicate were blended. TSS were measured on 2 mL of juice with a digital refractometer (Pocket PAL-1, Atago, Tokyo, Japan). For TA determination, 10 mL of juice were diluted to 100 mL with distilled water and titrated with NaOH (0.1 mol L−1) to pH 8.2. TSS/TA ratios were calculated from TSS and TA values. To determine the respiration rate (RRCO2, µg kg−1 s−1) and ethylene production (EP, ng kg−1 s−1), 4 replicates (three berries/replicate) were placed in sealed glass containers (28 mL) at 18 °C for 2 h. For RRCO2 assessment, CO2 accumulation was measured using a gas analyzer (Quantek 902P, Quantek Instruments Inc., Grafton, MA, USA) equipped with a thermal conductivity detector, after calibration with a standard (2.1% CO2 and 2.2% O2 in N2 balance). For EP determination, 1 mL of the gas within each container was extracted with a syringe and injected into a gas chromatograph (GC-2014, Shimadzu, Kyoto, Japan) fitted with a flame ionization detector and an activated alumina column (3 mm i.d., 80/100 mesh). The injector, oven, and detector temperatures were set at 75 °C, 100 °C, and 170 °C, respectively, with helium as the carrier gas (0.67 mL s−1), in the presence of hydrogen and air (0.67 and 6.67 mL s−1, correspondingly). An ethylene standard (1 μL L−1) was used for calibration.
2.3. Extraction, Identification and Quantification of Cuticular Waxes of Fruit
The analysis of cuticular waxes and the identification of triterpenoids were carried out as described previously for blueberries []. To prevent wax removal during sampling, entire clusters were collected into bags and, once at the lab, the berries were gently pulled with tweezers by holding them from the pedicel. Cuticular wax was obtained from three replicates (25 fruits/replicate) by immersing each one in 50 mL distilled dichloromethane, with constant stirring for one minute. After filtering and drying under reduced pressure at 30 °C in a rotatory evaporator, the solid waste was weighed to determine the wax yield (g m−2). Samples were then treated with diazomethane in diethyl ether to extract the methyl esters of triterpene acids.
For triterpene determination and quantification, 1 g L−1 of solid waste was treated with 1 mL of diazomethane solution in diethyl ether to obtain the methyl esters of the triterpene acids. After evaporation to dryness, the derivatized samples were diluted in isopropanol and examined using a gas chromatography–mass spectrometer (GC–MS) as described below. Major triterpenes were identified by thin layer chromatography (TLC), GC–MS and nuclear magnetic resonance (NMR) spectroscopy before and after derivatization as the corresponding methyl esters. Compound identification was performed with authentic standards of α-amyrin, oleanolic acid, ursolic acid, and lupeol.
Subsequent determinations were performed by GC. Compound analysis was performed by GC (Trace 1300, Thermo Fisher Scientific, Milan, Italy) connected to a mass selective detector equipped with an ionization single quadrupole []. A capillary column (0.25 μm film thickness × 30 m length, 0.25 mm i.d.) was considered (Rtx-5, Restek Corporation, Bellefonte, PA, USA). The operating temperature of the oven was controlled at 240 °C for 3 min; raised to 280 °C at 20 °C min−1, for a total operating duration of 60 min. The head pressure was 124 kPa. The temperature of the injector and detector was 290 °C, with 0.2 min split-less injection mode. Samples (1 µL) were injected into a GC–MS system using helium as the carrier gas at 25 μL s−1. For mass spectrometric (MS) analyses, the ion source temperature was 230 °C (70 eV, m/z 50−700). The retention time (Rt) of the internal standard and triterpenes was as follows: cholesterol (12 min), α-amyrin (17 min), lupeol (18 min), oleanolic acid methyl ester (23 min) and ursolic acid methyl ester (25 min).
For triterpene quantification, cholesterol (Sigma-Aldrich C 8667, purity > 99%, Saint Louis, MO, USA) was considered as the internal standard. A GC (Trace 1300, Thermo Fisher Scientific, Milan, Italy) was coupled to a flame ionization detector (FID). A capillary column (0.25 μm film thickness × 30 m length, 0.25 mm i.d.) (Elite-5MS, Perkin Elmer, Waltham, MA, USA) was installed. The temperature of the oven was kept for 3 min at 240 °C and raised to 280 °C at 20 °C min−1, for a total operating duration of 45 min. Helium was the carrier gas (25 μL s−1). A volume of 1 µL was injected and the detector maintained at 290 °C, running for 0.2 min in a splitless injection mode. Hydrogen (0.58 mL s−1) and air (5.83 mL s−1) were used as vector gas. The quantification was performed by comparing the total area of each chromatographic peak with cholesterol as the internal standard at a concentration of 1 g L−1. Data from each compound are presented both in surface units (g m−2) and as a percentage over total wax.
2.4. Extraction and Analysis of Cutin Monomers
Cutin components were extracted and analyzed as follows. Dewaxed cuticular membranes were hydrolyzed in 3 mL HCl (1 mol L−1 in 100% MeOH) and esterified in the same solvent for 2 h at 80 °C. The methanolysate was allowed to cool at room temperature and then 2 mL of saturated NaCl was added. Cutin monomers were extracted 3 times in 2 mL hexane for 10 min, thoroughly mixed, centrifuged at 20 °C, and the resulting extracts pooled and evaporated to dryness in a N2 steam. Cutin yields were established gravimetrically as g m−2, and each compound was estimated as a relative percentage (% over total cutin yield). Dry cutin samples were derivatized with N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) for 15 min at 100 °C, then heptadecanoate (C17) and tricosanoate (C23) were added as the internal standards, and injected (1 μL) into a GC-FID for quantitative determination. Cutin compounds were determined based on their electron ionization (EI) mass spectra after GC–MS analysis, following the procedure described for wax analysis. The chromatographic conditions were as described in previous work [].
2.5. Statistical Analysis
For each cultivar, a completely randomized design was considered in one factor experiment, considering the developmental stages as treatments. Data were first subjected to analyses of variance (ANOVA) and Tukey’s honestly significant difference (HSD) test (p ≤ 0.05) when ANOVA showed significant differences. Additionally, to facilitate the understanding of the relationship of all variables on fruit development for both cultivars, principal components analysis (PCA) was performed. Analyses were performed using the statistical software R (R version 3.0.0, R Development Core Team, Vienna, Austria, 2008).
3. Results
For both cvs. ‘Duke’ and ‘Brigitta,’ most of the physicochemical characteristics considered showed highly significant differences across the maturity stages (Table 1). Among the variables assessed, fruit weight (~18–56%), TSS (~16–38%), TSS/TA (~20–119%), ethylene production (~27–230%) and respiration rate (~19–39%) showed the most remarkable changes. Although firmness values were similar (slightly higher than 4 N) for both cultivars at the beginning of fruit development (G stage), firmness evolution differed up to stage 50P, and then continued with a similar trend up to 100B (Table 1). On the other hand, when genotypic differences for TSS/TA ratios were studied, ‘Brigitta’ and ‘Duke’ displayed a similar trend up to the 50P stage, with the former cultivar increasing over the latter at 75B and 100B (Table 1). In contrast, TA, ethylene production, respiration rate, and wax content were always greater in ‘Duke’ than in ‘Brigitta’ (Table 1).

Table 1.
Physicochemical characteristics of ‘Duke’ and ‘Brigitta’ fruit, sampled at five developmental stages: green (G), 25% pink (25P), 50% pink (50P), 75% blue (75B), 100% blue (100B).
Statistical differences in the composition of the triterpene fraction among the developmental stages for both cultivars were not found when expressed as g m−2 (Figure 2A), but significance was apparent when considered as a percentage over total cuticular waxes (i.e., ursolic acid, oleanolic acid, α-amyrin, and lupeol) (Table 2). However, the mean separations did not show clear trends in the time-course evolution of these compounds. As for genotypic differences, ‘Duke’ showed similar levels of ursolic acid in comparison to ‘Brigitta’, but four times more lupeol, one-fourth less oleanolic acid, and α-amyrin was not detectable. Although no statistical comparisons were made between the cultivars, lupeol stood out quantitatively among the four triterpenes detected in the ‘Duke’ samples, whereas oleanolic acid predominated in ‘Brigitta’ (Figure 1A, Table 2).
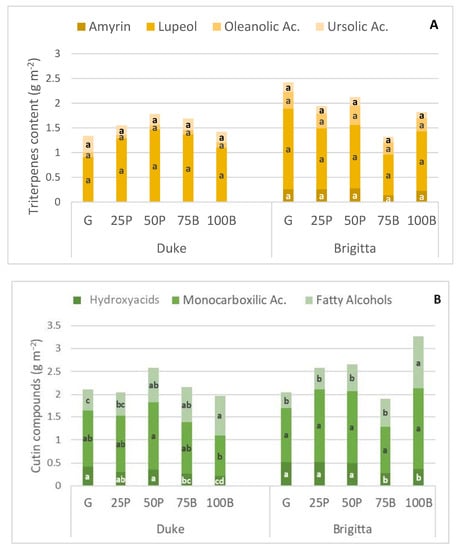
Figure 2.
Amounts of cuticle triterpene (A) and cutin (B) components (g m−2) of ‘Duke’ and ‘Brigitta’ fruit, sampled at five developmental stages: green (G), 25% pink (25P), 50% pink (50P), 75% blue (75B), 100% blue (100B). For each cultivar and component, means with different letters across developmental stages, differ at p < 0.05 (Tukey’s test).

Table 2.
Relative % of cuticle triterpene and cutin components from ‘Duke’ and ‘Brigitta’ fruit, sampled at five developmental stages: green (G), 25% pink (25P), 50% pink (50P), 75% blue (75B), 100% blue (100B).
Regarding the main cutin compounds identified, hydroxyacids generally decreased and fatty alcohols increased as the fruit developed, regardless of cultivar (Table 2). Even though, for both cultivars, the highest amount (g m−2) and proportion (%) corresponded to monocarboxylic acids, a different trend was observed for each cultivar: whereas they tended to decrease in ‘Duke’, no significant changes were detected in ‘Brigitta’ among maturity stages. Statistical differences were similar whether data were expressed as g m−2 or as a percentage (Figure 2B, Table 2).
For data exploration by means of PCA, triterpenes and cutin compounds were expressed as a percentage. The PCA model developed showed that 92% of sample variability could be explained by three principal components (PC1: 45%; PC2: 36% and PC3: 11%). The relative weight (%) of each variable (Table 3) indicates that sample differentiation along PC1 was mainly influenced by the physicochemical variables (weight, firmness TSS, TA and TSS/TA), total triterpenes, and specific cutin components (hydroxyacids and fatty alcohols). Separation along PC2 (36%) was associated mainly with triterpenes, ethylene production and respiration rates. Finally, ursolic acid and monocarboxylic acids’ contents had the most weight for separation along PC3.

Table 3.
Contribution of physicochemical variables, triterpenes and cutin monomers to differentiation between ‘Duke’ and ‘Brigitta’ fruit, sampled at five developmental stages: green (G), 25% pink (25P), 50% pink (50P), 75% blue (75B), 100% blue (100B).
The scores plot of PC1 and PC2 (Figure 3A) showed that samples of both cultivars distributed progressively along PC1 from the most immature stage (G) to stage 100B, with the physicochemical characteristics (i.e., TSS, TA, TSS/TA, weight, wax yields, and firmness) as the most relevant variables for sample differentiation (Figure 3B). A clear separation between cultivars was observed along PC2, which was associated with two physicochemical variables (ethylene production and respiration rates) and with some triterpenes (α-amyrin, lupeol and oleanolic acid). When PC2 was contrasted with PC3 (Figure 3C), cultivar differentiation along PC2 was also observed. No clear trend was found across PC3, except that ursolic acid and monocarboxylic acids were the variables showing the most weight for sample separation along this axis (Figure 3D).
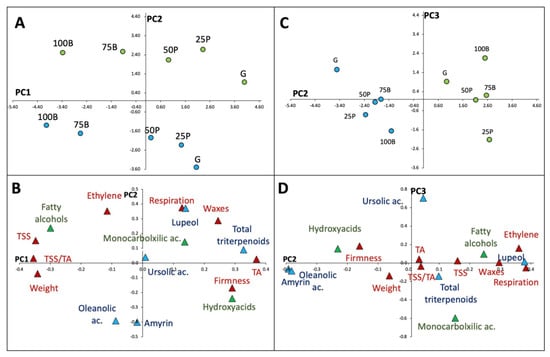
Figure 3.
Principal component analysis (PCA) of ‘Duke’ and ‘Brigitta’ fruit (green and blue circles, respectively) sampled at five developmental stages: green (G), 25% pink (25P), 50% pink (50P), 75% blue (75B), 100% blue (100B). (A,C), scores plots for PC1 vs. PC2 and PC2 vs. PC3, respectively. (B,D), loadings plots for PC1 vs. PC2 and PC2 vs. PC3, in the same order. Physicochemical parameters, triterpene compounds and cutin monomers are denoted as red, light blue and green triangles, correspondingly. PC1, PC2, and PC3 accounted for 45, 36, and 11% of total sample variability, respectively.
4. Discussion
The final quality at the destination depends on fruit characteristics at harvest and on post-harvest handling, but it is also influenced by metabolic changes that occur during growth and maturation. Postharvest softening is the main limiting factor that affects the shelf life of blueberries, but only small changes in cell wall components have been reported in blueberry fruit during the late developmental stages [,]. Additionally, firmness loss during the storage of blueberries has been associated with weight loss [,], which in turn relates to the composition of the fruit cuticle. Therefore, the present study has provided hints that ripening-related modifications in other fruit traits, such as those in physicochemical characteristics and in cuticle composition, could partly explain the contrasting postharvest behavior of ‘Duke’ and ‘Brigitta’ blueberries.
4.1. Differences between Cultivars
PCA analysis revealed that ‘Duke’ fruit were characterized by higher respiration and ethylene production rates, while ‘Brigitta’ samples displayed higher contents of oleanolic acid and α-amyrin (Figure 2B). For both cultivars, fruit samples were separated according to the maturation stage along PC1, which alone represented 45% of the total variance (Figure 2A). Even though PC3 explained only 11% of the total variability, ursolic acid and monocarboxylic acid contents were the variables showing the most weight on sample differentiation along this axis.
Blueberries show an increase in respiration and ethylene production at the middle stage of ripening, which coincides with the fruit color shift from green-pink to pink-blue [,]. Harvesting is usually carried out at a post-climacteric stage, and fruit quality does not improve further during post-harvest handling and storage [,]. Previous studies [] demonstrated that lower postharvest quality of ‘Duke’ was also associated with higher respiration and ethylene production rates during fruit maturation that resulted in 25% firmness loss and 10 to 21% weight loss from harvest to the end of cold storage, whereas firmness and weight loss rates were 4% and less than 10% in ‘Brigitta’, respectively. Moreover, respiration rate and ethylene production peaks were higher and earlier in ‘Duke’ (between G and 25P stages) than in ‘Brigitta’ (between 50P and 75B). Additionally, ‘Duke’ showed twice more weight loss when compared to ‘Brigitta’ in a study where nine blueberry cultivars were evaluated [].
As reported before [], the highest weight loss of ‘Duke’ could be associated with a larger surface/volume ratio of this cultivar, since ‘Brigitta’ berries were larger than ‘Duke’ fruit in diameter (11–12%) and weight (42–47%) at the 75B and 100B stages, respectively (Table 1). Both cultivars were followed carefully at harvest, so the fact that ‘Duke’ exhibits a shorter ripening window compared to ‘Brigitta’ (~30 and ~60 d, respectively) implies that a larger number of ripe fruit are removed from a single cluster at each harvest for the former. This factor would account for smaller final fruit size in ‘Duke’, as reported in a recent study []. Interestingly, ‘Brigitta’ berries exhibited higher TSS/TA ratios than ‘Duke’ (26.7 and 20.9 at 100B stage, respectively), but this was not related to more accentuated weight loss.
Although fruit cuticle is a very thin interface, it constitutes an important structural stabilization component for epidermal primary tissues, and it influences fruit postharvest quality in the following three main aspects: water permeability and consequent tissue dehydration, susceptibility to infections, and susceptibility to physiological disorders []. Fruit cuticular waxes usually comprise triterpenes and n-alkanes in a variable, genotype-dependent proportion. Furthermore, fatty alcohols, aldehydes and ketones are also present [,]. Triterpenoid components may include mainly triterpenoid acids, such as ursolic and oleanolic acids, while in other fruit species, triterpenoid alcohols, such as amyrins, may predominate. Among triterpenols, lupeol has been also reported to be a prominent compound in Asian pear [], citrus (reviewed in []), tomato, grape, bell pepper, eggplant, and grapefruit [], as well as in blueberries [,].
It is known that cuticle thickness and water permeability coefficients do not necessarily correlate [], so specific compounds should be involved in cuticular water barrier functions. Higher ursolic acid levels in ‘Duke’ than in ‘Brigitta’ fruit would favor more intense postharvest dehydration []. ‘Duke’ fruit have been reported to display the highest lupeol levels, together with the highest water loss rates, among the four blueberry cultivars []. Although studies in tomatoes [], bell peppers [], sweet cherries [] and peaches [] have shown a positive relationship between weight loss and the ratio between n-alkanes and triterpenoids, alkane proportion in fruit cuticles exhibited no association with dehydration in blueberries [].
High contents of ursolic acid have been suggested to account for low resistance to transpirational water loss in Aspidosperma pyrifolium leaves []. Additionally, early deposition of oleanolic acid and its gradual decrease throughout berry development could be related to decreased mechanical toughness of the cuticle in grape berries []; therefore, higher oleanolic acid amounts in ‘Brigitta’ fruit would contribute to more rigid cuticles, possibly restricting water loss.
No clear differences were found between the cultivars for time-course changes in cutin monomers, with the exception of monocarboxylic acids, which generally decreased in ‘Duke’ samples along fruit ripening, while remaining steady in ‘Brigitta’ fruit. Only minor quantitative differences in fruit cutin composition were observed at harvest between two sweet cherry cultivars [], with the main constituent compounds being the same, and limited changes were found during shelf-life at 20 °C, despite the different water loss rates after cold storage.
4.2. Differences between Developmental Stages
For both cultivars, immature berries displayed higher levels of total triterpenes, total wax loads, TA, firmness and hydroxyacid amounts; in contrast, mature fruit were characterized by higher fatty alcohol contents, TSS/TA ratios and weight. Although the epicuticular wax layer and cuticle thickness have been observed to increase progressively during blueberry fruit development, concomitantly with the thickening of the fruit epidermis and hypodermal cells [], increased thickness of these waxy deposits had no significant effect on water loss rates [,,]. Consistent with these previous observations, total wax yield (g/m2) was relevant for sample differentiation along PC1 uniquely and characterized green fruit of both cultivars considered in this study (Figure 3A,B).
The cuticular membrane comprises crystalline and amorphous waxes that encrust and enclose the cutin matrix, a polyester composed of hydroxylated and epoxy-hydroxylated fatty acids, which largely establishes the scaffold for the arrangement of cuticular waxes [] and is constituted by C16 and C18 fatty acid derivatives []. In our study, hydroxyacids were more abundant in immature berries, whereas monocarboxylic acids and fatty alcohols were predominant in mature fruit. This could indicate some de-esterification of the cutin matrix, which would result in the release of the fatty acids and alcohols that constitute the polyester [].
Total waxes decreased in both cultivars along fruit development (Table 1), which is possibly related to fruit enlargement as maturity advances. Indeed, fruit surface increased from 4.2 to 5.8 cm2 between G and 100B stages in ‘Duke’, and from 4.9 to 7.5 cm2 between the same stages in ‘Brigitta’, resulting in lower amounts of wax per surface unit. This has been reported for cherries where the cuticular membrane is submitted to a notable strain in the latest stages of development as the fruit surface enlarges [,]. The resulting strain of the cuticle may form microscopic cracks that would further increase water movement out of the fruit. This should be included in further studies. Additionally, the integrity of the cutin polyester depends, in part, on the proportion of hydroxyacids that allow the formation of ester bonds, and hence of the cutin network, this process being essential for proper arrangement of cuticular waxes, and hence for their role on limiting transpiration and water loss. Since higher amounts of hydroxyacids were found in immature fruit, data suggest that the structure of the cutin matrix be altered during fruit ripening, thus compromising the barrier functions of the cuticle. Finally, since transpiration is the main route for water loss and the cuticle is considered a major barrier for this process, the characteristics of the stem scar should be also included in future studies.
5. Conclusions
Since blueberry fruit softening during storage is associated with higher weight loss during this period, the present study focused on the surface characteristics of two blueberry cultivars intended for long-term overseas shipment and displaying contrasting postharvest performance. The results indicate that higher rates of weight and firmness loss in ‘Duke’ berries as compared to ‘Brigitta’ could be explained by larger surface/volume ratios and higher amounts of ursolic acid and lupeol in ripe fruit. Therefore, it is suggested that cuticular composition is a trait that should be taken into consideration for the selection of new cultivars for long-term storage. Furthermore, to the best of our knowledge, this is the first report on the composition of the cutin matrix of fruit cuticles in blueberries.
Author Contributions
C.M., I.L., J.G. and G.A.L. contributed to the conception and design of the work. C.M. and G.A.L. performed the field experiment, acquisition, statistical analyses, and interpretation of data. I.L., J.G., G.S.-H. and S.T.-V. contributed to the performance of the chemical analyses. C.M., I.L., J.G., G.A.L., G.S.-H. and S.T.-V. collaborated to generate and validate the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
In Chile, the study was funded by the National Agency of Research and Development (ANID; FONDECYT 1191818) and PCHA/Doctorado Nacional/2013-63130042. In Spain, this work was partially supported by “Fundación Carolina” and “Programa de Doctorado en Ciencia y Tecnología Agraria y Alimentaria”, Universitat de Lleida.
Data Availability Statement
Data are contained within the article. More information can be provided upon request.
Acknowledgments
Authors truly thank AMS Family S.A. (Curicó, Chile) for facilitating commercial fields, and North Bay Chile S.p.a. for permanent technical support. We acknowledge Irene Manríquez (Instituto de Química de Recursos Naturales, Universidad de Talca, Chile) and Montserrat Llovera (Scientific-Technical Services, Universitat de Lleida, Spain) for their skillful technical work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Lobos, G.A.; Hancock, J.F. Breeding blueberries for a changing global environment: A review. Front. Plant Sci. 2015, 6, 782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, T.W.; Sherman, W.B.; Sharpe, R.H. Fruit development in short and long cycle blueberries. HortScience 1970, 5, 274–275. [Google Scholar]
- Coombe, B.G. The development of fleshy fruits. Annu. Rev. Plant Physiol. 1976, 27, 207–228. [Google Scholar] [CrossRef]
- Birkhold, K.T.; Koch, K.E.; Darnell, R.L. Carbon and nitrogen economy of developing rabbiteye blueberry fruit. J. Am. Soc. Hort. Sci. 1992, 117, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Cano-Medrano, R.; Darnell, R. Cell number and cell size in parthenocarpic vs. pollinated blueberry (Vaccinium ashei) fruits. Ann. Bot. 1997, 80, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Galletta, G. Blueberries and cranberries. In Advances in Fruit Breeding; Janick, J., Moore, J.N., Eds.; Purdue University Press: West Lafayette, IN, USA, 1975; pp. 154–196. [Google Scholar]
- Gough, R. The Highbush Blueberry and its Management; Food Products Press, Haworth Press Inc.: New York, NY, USA, 1994; 272p. [Google Scholar]
- Galletta, G.J.; Ballinger, W.E.; Monroe, R.J.; Kushman, L.J. Relationships between fruit acidity and soluble solids level of highbush blueberry clones and fruit keeping quality. J. Am. Soc. Hortic. Sci. 1971, 6, 758–762. [Google Scholar]
- Shutak, V.G.; Gough, R.E.; Windus, N.D. The Cultivated Highbush Blueberry: Twenty Years of Research. Rhode Island Agricultural Experiment Station Bulletin; University of Rhode Island: Kingston, NY, USA, 1980; 48p. [Google Scholar]
- Ballinger, W.E.; Kushman, L.J.; Hamann, D.D. Factors affecting the firmness of highbush blueberries. J. Am. Soc. Hortic. Sci. 1973, 98, 583–587. [Google Scholar]
- Ballinger, W.E.; Maness, E.P.; Kushman, L.J. Anthocyanins in ripe fruit of the highbush blueberry, Vaccinium corymbosum L. J. Am. Soc. Hortic. Sci. 1970, 95, 283–285. [Google Scholar]
- Ballinger, W.E.; Maness, E.P.; Kushman, L.J.; Galletta, G.J. Anthocyanins in ripe fruit of a ‘pink-fruited’ hybrid of highbush blueberry, Vaccinium corymbosum L. J. Am. Soc. Hortic. Sci. 1972, 97, 381–384. [Google Scholar]
- El-Agamy, S.Z.A.; Aly, M.; Biggs, R.H. Fruit maturity as related to ethylene in ‘Delite’ blueberry. Proc. Fla. State Hort. Soc. 1982, 95, 245–246. [Google Scholar]
- Moggia, C.; González, C.; Lobos, G.A.; Valdés, M.; Lara, I.; Graell, J. Changes in quality and maturity of ‘Duke’ and ‘Brigitta’ blueberries during fruit development: Postharvest implications. Acta Hortic. 2018, 1194, 1495–1501. [Google Scholar] [CrossRef] [Green Version]
- Vicente, A.R.; Ortugno, C.; Rosli, H.; Powell, A.L.T.; Greve, L.C.; Labavitch, J.M. Temporal sequence of cell wall disassembly events in developing fruits 2. Analysis of blueberry (Vaccinium species). J. Agric. Food Chem. 2007, 55, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Proctor, A.; Miesle, T.J. Polygalacturonase and pectinmethylesterase activities in developing highbush blueberries. HortScience 1991, 26, 579–581. [Google Scholar] [CrossRef] [Green Version]
- Angeletti, P.; Castagnasso, H.; Miceli, E.; Terminiello, L.; Concellón, A.; Chaves, A.; Vicente, A.R. Effect of preharvest calcium applications on postharvest quality, softening and cell wall degradation of two blueberry (Vaccinium corymbosum) varieties. Postharvest Biol. Technol. 2010, 58, 98–103. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Technol. 2014, 87, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Paniagua, A.C.; East, R.; Hindmarsh, J.P.; Heyes, J.A. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]
- Sargent, S.A.; Brecht, J.K.; Forney, C.F. Blueberry harvest and postharvest operations: Quality maintenance and food safety. In Blueberries for Growers, Gardeners, Promoters; Childers, N.F., Lyrene, P.M., Eds.; E.O. Painter Printing Co.: DeLeon Springs, FL, USA, 2006; pp. 139–151. [Google Scholar]
- Alsmairat, N.; Contreras, C.; Hancock, J.; Callow, P.; Beaudry, R. Use of combinations of commercially relevant O2 and CO2 partial pressures to evaluate the sensitivity of nine highbush blueberry fruit cultivars to controlled atmospheres. HortScience 2011, 46, 74–79. [Google Scholar] [CrossRef]
- Paniagua, A.C.; East, R.; Heyes, J.A. Interaction of temperature control deficiencies and atmosphere conditions during blueberry storage on quality outcomes. Postharvest Biol. Technol. 2014, 95, 50–59. [Google Scholar] [CrossRef]
- NeSmith, D.S.; Prussia, S.E.; Tetteh, M.; Krewer, G. Firmness losses of rabbiteye blueberries (Vaccinium ashei Reade) during harvesting and handling. Acta Hort. 2002, 574, 287–293. [Google Scholar] [CrossRef]
- Lobos, G.A.; Bravo, C.; Valdés, M.; Graell, J.; Lara, I.; Beaudry, R.M.; Moggia, C. Within-plant variability in blueberry (Vaccinium corymbosum L.): Maturity at harvest and position within the canopy influence fruit firmness at harvest and postharvest. Postharvest Biol. Technol. 2018, 146, 26–35. [Google Scholar] [CrossRef]
- Moggia, C.; Graell, J.; Lara, I.; Schmeda-Hirschmann, G.; Thomas-Valdés, S.; Lobos, G.A. Fruit characteristics and cuticle triterpenes as related to postharvest quality of highbush blueberries. Sci. Hortic. 2016, 211, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Moggia, C.; Graell, J.; Lara, I.; González, G.; Lobos, G.A. Firmness at harvest impacts postharvest fruit softening and internal browning development in mechanically damaged and non-damaged highbush blueberries (Vaccinium corymbosum L.). Front. Plant Sci. 2017, 8, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Castellarin, S.D. Blueberry water loss is related to both cuticular wax composition and stem scar size. Postharvest Biol. Technol. 2022, 188, 111907. [Google Scholar] [CrossRef]
- Domínguez, E.; Cuartero, J.; Heredia, A. An overview on plant cuticle biomechanics. Plant Sci. 2011, 181, 77–84. [Google Scholar] [CrossRef]
- Jetter, S.; Schäffer, S.; Riederer, M. Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: Evidence from Prunus laurocerasus L. Plant Cell Environ. 2000, 23, 619–628. [Google Scholar] [CrossRef]
- Moggia, C.; Peñaloza, O.; Torres, J.; Romero-Bravo, S.; Sepúlveda, D.; Jara, R.; Vivanco, S.; Valdés, M.; Zúñiga, M.; Beaudry, R.M.; et al. Within-plant variability in blueberry (Vaccinium corymbosum L.) II: Is a shorter harvest interval always the ideal strategy to maximize fruit firmness? Postharvest Biol. Technol. 2022, 186, 111815. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Saftner, R.; Polashock, J.; Ehlenfeldt, M.; Vinyard, B. Instrumental and sensory quality characteristics of blueberry fruit from twelve cultivars. Postharvest Biol. Technol. 2008, 49, 19–26. [Google Scholar] [CrossRef]
- Caligiani, A.; Malavasi, G.; Palla, G.; Marseglia, A.; Tognolini, M.; Bruni, R. A simple GC–MS method for the screening of betulinic, corosolic, maslinic, oleanolic and ursolic acid contents in commercial botanicals used as food supplement ingredients. Food Chem. 2013, 136, 735–741. [Google Scholar] [CrossRef]
- Belge, B.; Llovera, M.; Comabella, E.; Gatius, F.; Guillén, P.; Graell, J.; Lara, I. Characterization of cuticle composition after cold storage of ‘Celeste’ and ‘Somerset’ sweet cherry fruit. J. Agric. Food Chem. 2014, 62, 8722–8729. [Google Scholar] [CrossRef]
- Windus, N.D.; Shutak, V.G.; Gough, R.E. CO2 and C2H4 evolution by highbush blueberry fruit. HortScience 1976, 11, 515–517. [Google Scholar]
- NeSmith, D.S.; Núñez-Barrios, A.; Prussia, S.E.; Aggarwal, D. Postharvest berry quality of six rabbiteye blueberry cultivars in response to temperature. J. Am. Pomol. Soc. 2005, 59, 13–17. [Google Scholar]
- MacLean, D.D.; NeSmith, D.S. Rabbiteye blueberry postharvest fruit quality and stimulation of ethylene production by1-methylcyclopropene. HortScience 2011, 46, 1278–1281. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, L. Plant cuticles shine: Advances in wax biosynthesis and export. Curr. Opin. Plant Biol. 2009, 12, 721–727. [Google Scholar] [CrossRef]
- Lara, I. The fruit cuticle: Actively tuning postharvest quality. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Siddiqui, M.W., Ed.; Academic Press Ltd./Elsevier Science Ltd.: London, UK, 2018; pp. 93–120. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, C.M.; Lee, H.J.; Lee, S.H.; Cho, J.A.; Kim, W.S.; Park, K.H.; Moon, J.H. Caffeoyl triterpenes from pear (Pyrus pyrifolia Nakai) fruit peels and their antioxidative activities against oxidation of rat blood plasma. J. Agric. Food Chem. 2013, 61, 4563–4569. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L. A focus on the biosynthesis and composition of cuticle in fruits. J. Agric. Food Chem. 2015, 63, 4005–4019. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, L.; Riederer, M. Ecophysiology of cuticular transpiration: Comparative investigation of cuticular water permeability of plant species from different habitats. Oecologia 1996, 107, 426–432. [Google Scholar] [CrossRef]
- Leide, J.; Hildebrandt, U.; Vogg, G.; Riederer, M. The positional sterile (ps) mutation affects cuticular transpiration and wax biosynthesis of tomato fruits. J. Plant Physiol. 2011, 168, 871–877. [Google Scholar] [CrossRef]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G.T.; Lü, S.; Alkalai-Tuvia, S.; Perzelan, Y.; Paran, I.; Fallik, E.; Jenks, M.A. Fruit cuticle lipid composition and fruit post-harvest water loss in an advanced backcross generation of pepper (Capsicum sp.). Physiol. Plant. 2012, 146, 15–25. [Google Scholar] [CrossRef]
- Belge, B.; Llovera, M.; Comabella, E.; Graell, J.; Lara, I. Fruit cuticle composition of a melting and a nonmelting peach cultivar. J. Agric. Food Chem. 2014, 62, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.F.M.; Meirelles, S.T.; Salatino, A. Epicuticular waxes from caatinga and cerrado species and their efficiency against water loss. An. Acad. Bras. Ciênc. 2003, 75, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Pensec, F.; Pa̧czkowski, C.; Grabarczyk, M.; Woźniak, A.; Bénard-Gellon, M.; Bertsch, C.; Chong, J.; Szakiel, A. Changes in the triterpenoid content of cuticular waxes during fruit ripening of eight grape (Vitis vinifera) cultivars grown in the upper rhine valley. J. Agric. Food Chem. 2014, 62, 7998–8007. [Google Scholar] [CrossRef] [PubMed]
- Konarska, A. Development of fruit quality traits and comparison of the fruit structure of two Vaccinium corymbosum (L.) cultivars. Sci. Hortic. 2015, 194, 79–90. [Google Scholar] [CrossRef]
- Knoche, M.; Beyer, M.; Peschel, S.; Oparlakov, B.; Bukovac, M.J. Changes in strain and deposition of cuticle in developing sweet cherry fruit. Physiol. Plant. 2004, 120, 667–677. [Google Scholar] [CrossRef]
- Alkio, M.; Jonas, U.; Sprink, T.; van Nocker, S.; Knoche, M. Identification of putative candidate genes involved in cuticle formation in Prunus avium (sweet cherry) fruit. Ann. Bot. 2012, 110, 101–112. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).