Evaluation of In Vitro and In Vivo Antifungal Activity of Green Synthesized Silver Nanoparticles against Early Blight in Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Green Synthesis of Silver Nanoparticles
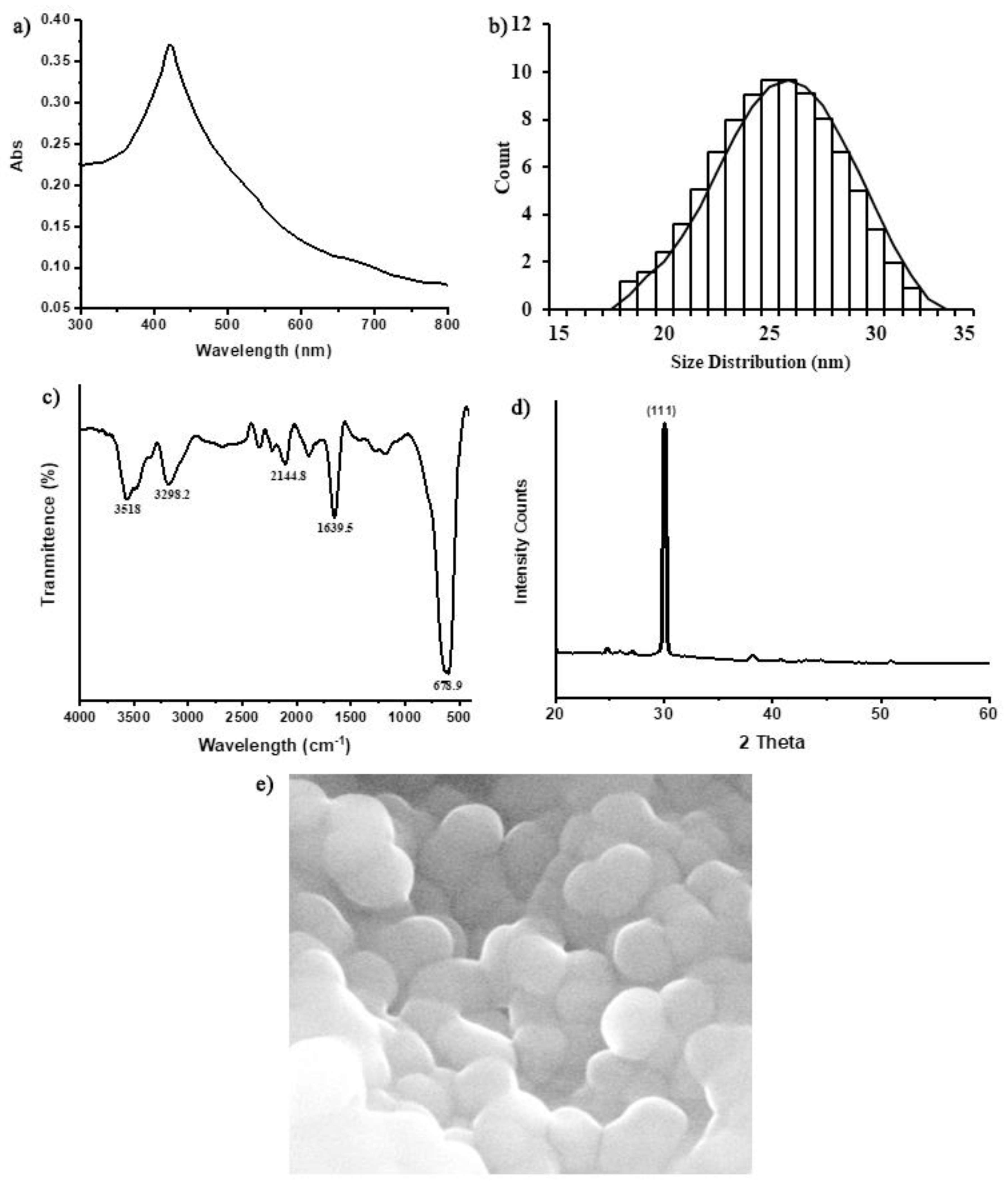
2.3. Characterization of Silver Nanoparticles
2.3.1. UV–Spectrophotometry of AgNPs
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of AgNPs
2.3.3. X-ray Diffraction (XRD) Analysis of AgNPs
2.3.4. Scanning Electron Microscopy (SEM) of AgNPs
2.4. Isolation of Pathogen
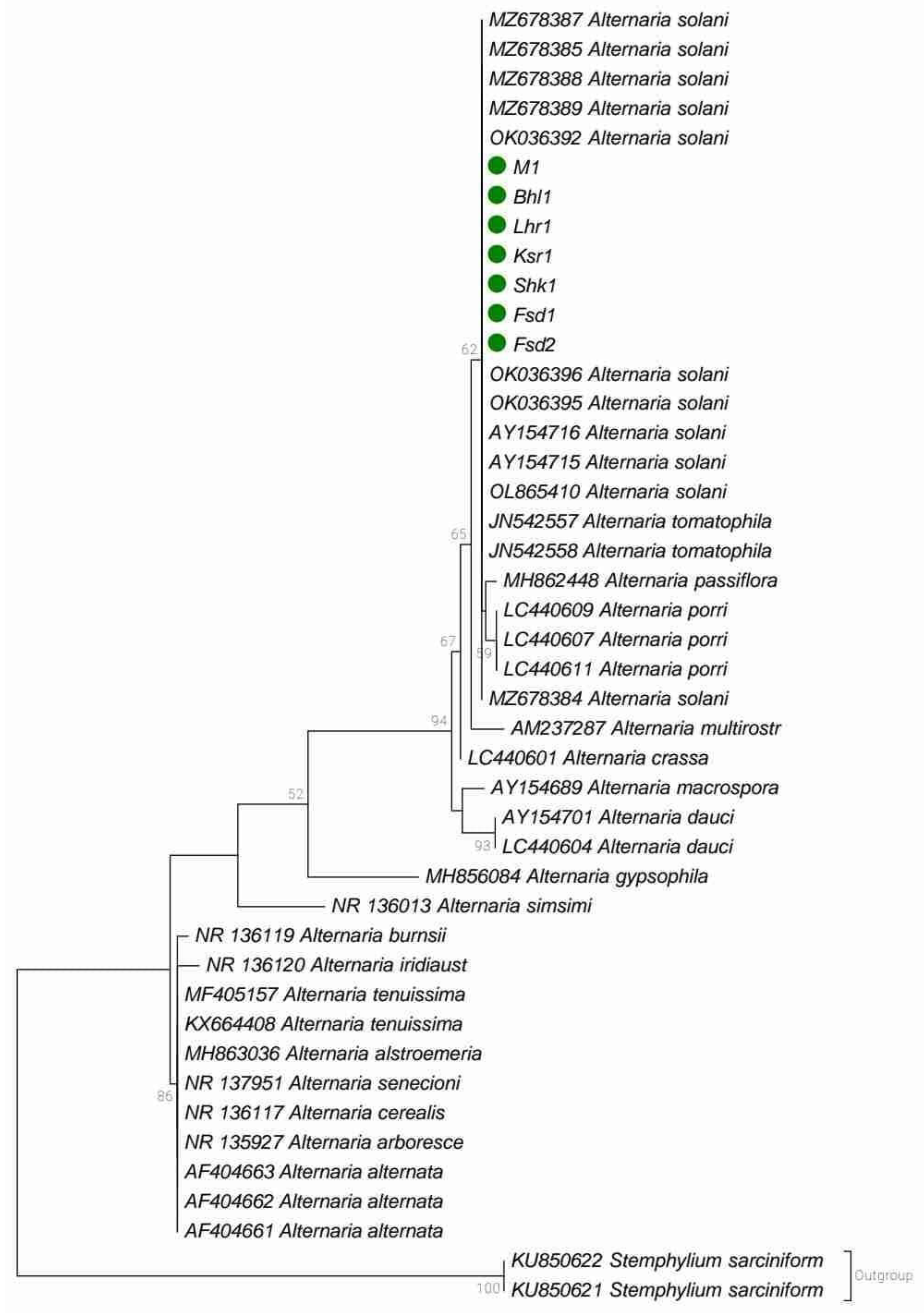
2.5. Identification of Pathogen
2.6. Pathogenicity Test of Alternaria solani
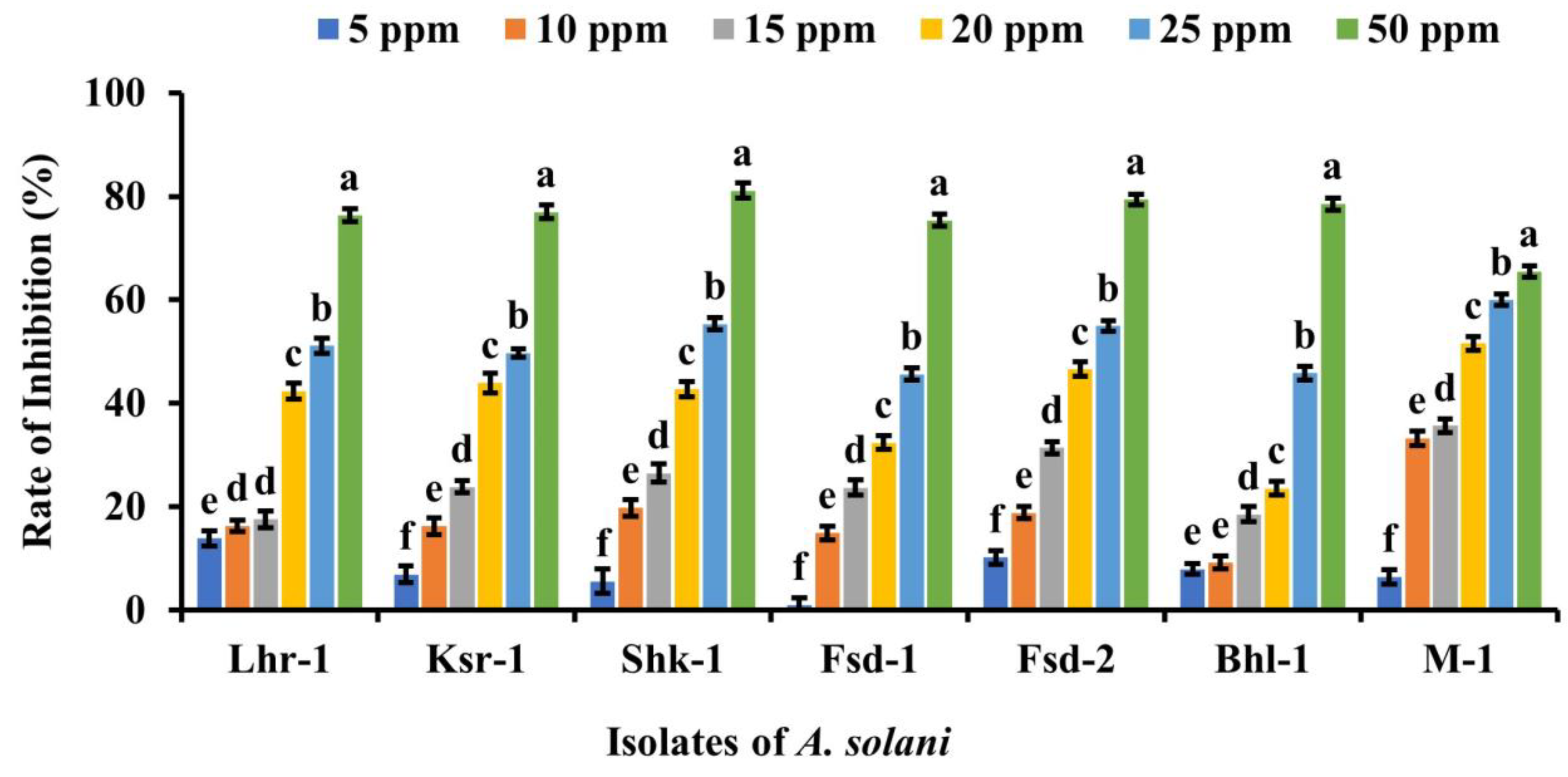
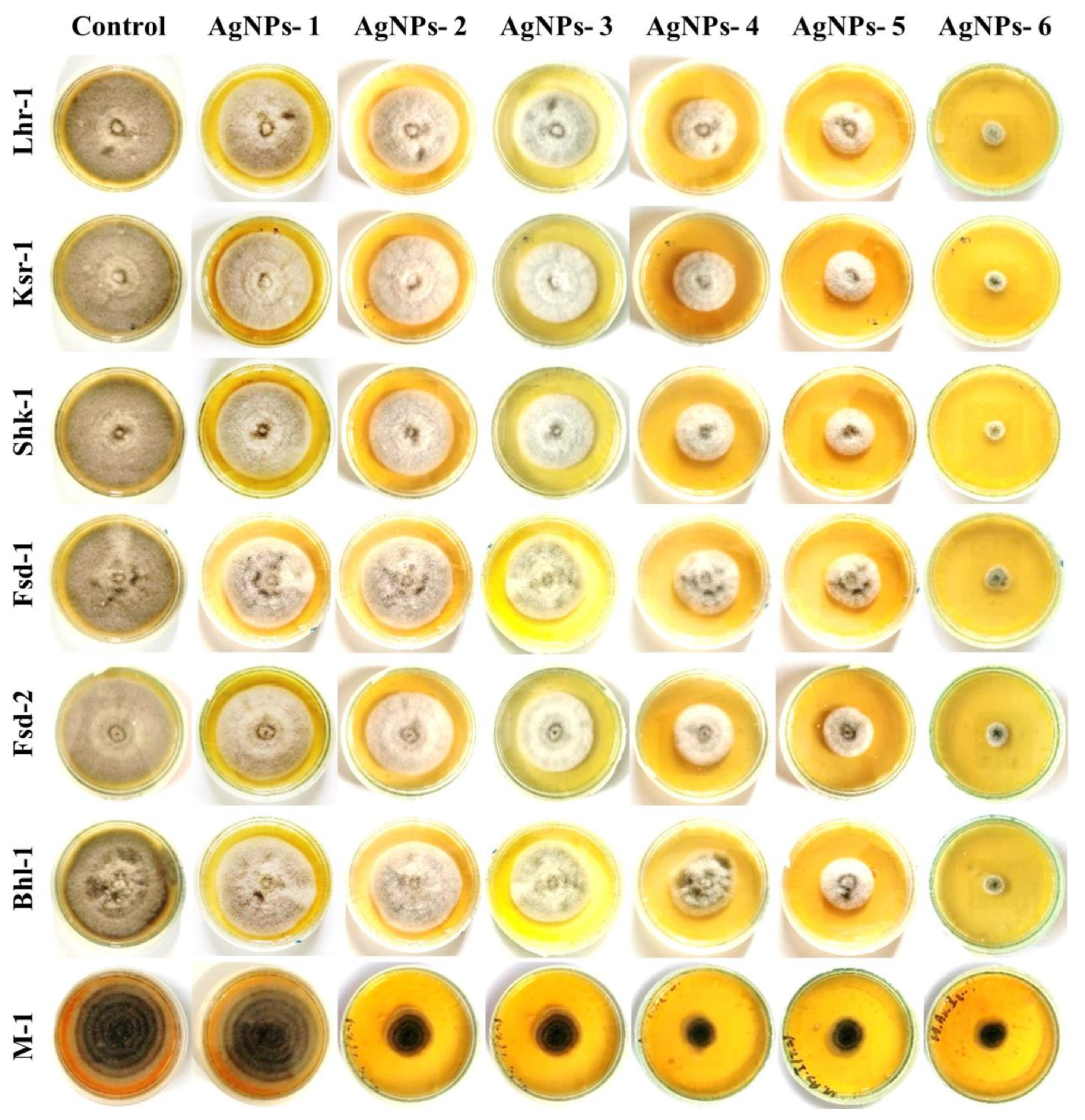
2.7. In vitro Antifungal Studies of Silver Nanoparticles
2.8. Screening of Tomato Varieties against Alternaria solani
2.9. Greenhouse Experiment for In Vivo Efficacy of Silver Nanoparticles
2.9.1. Quantification of Total Phenolics
2.9.2. Quantification of Peroxidases, Polyphenol Oxidases, and Phenylalanine Ammonia–Lyase
2.10. Data Analysis
3. Results
3.1. Characterization of Silver Nanoparticles
3.2. Morphological and Molecular Identification of Pathogen (Alternaria solani)
3.3. Pathogenicity Variability among Alternaria solani Isolates
3.4. In Vitro Antifungal Analysis of Silver Nanoparticles against Alternaria solani
3.5. Screening of Susceptible Tomato Varieties against Alternaria solani
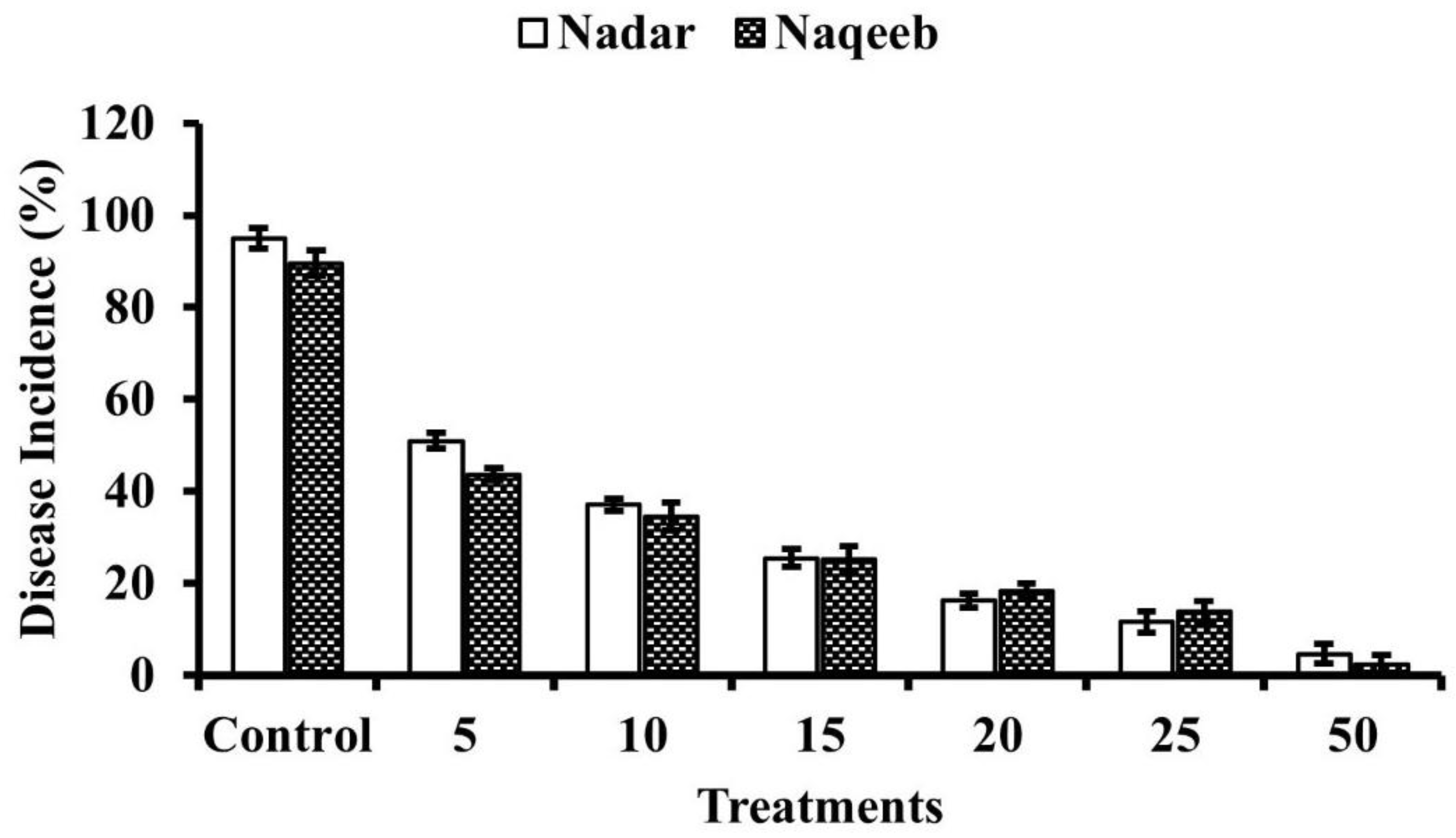
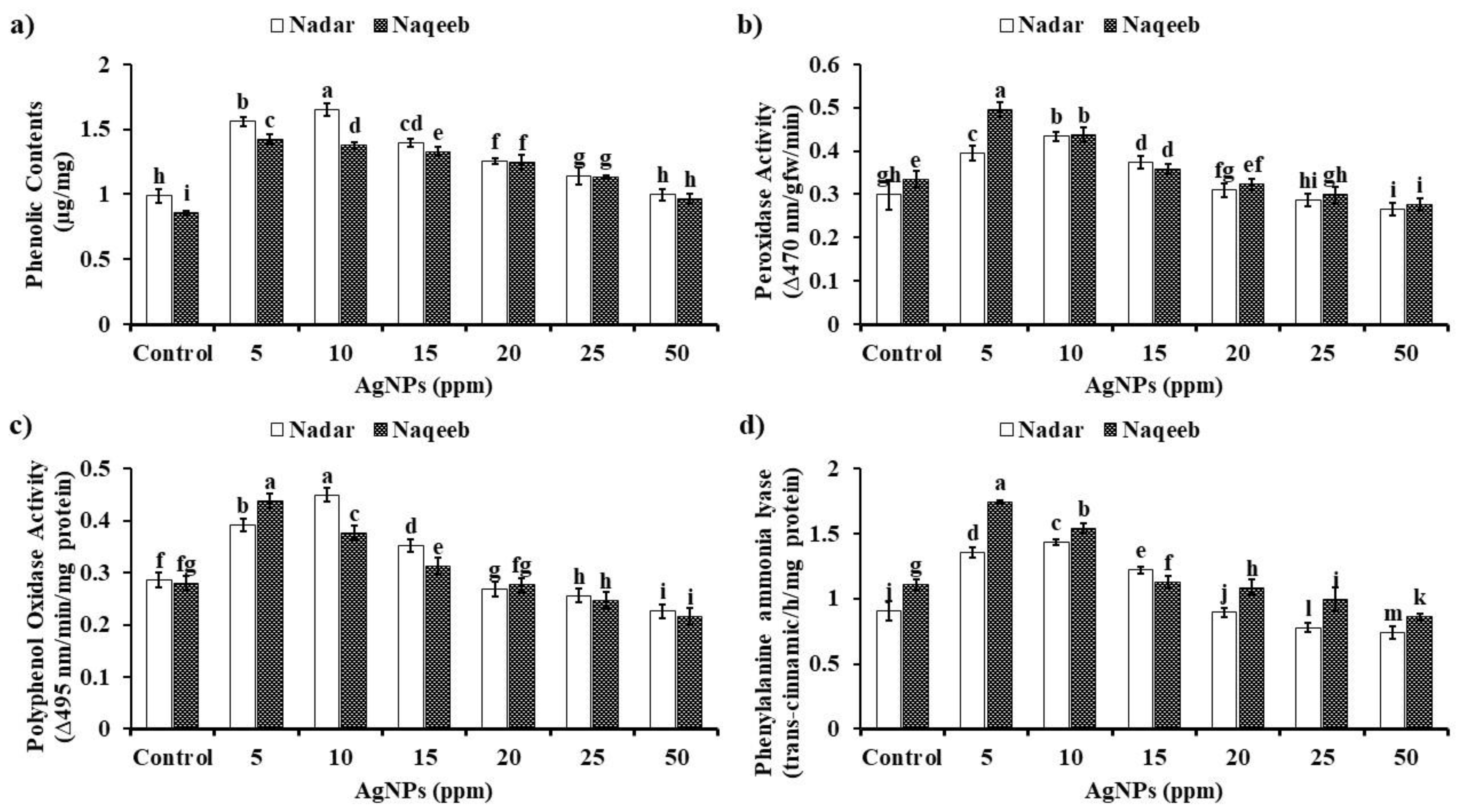
3.6. In Vivo Efficacy of Silver Nanoparticles against A. solani
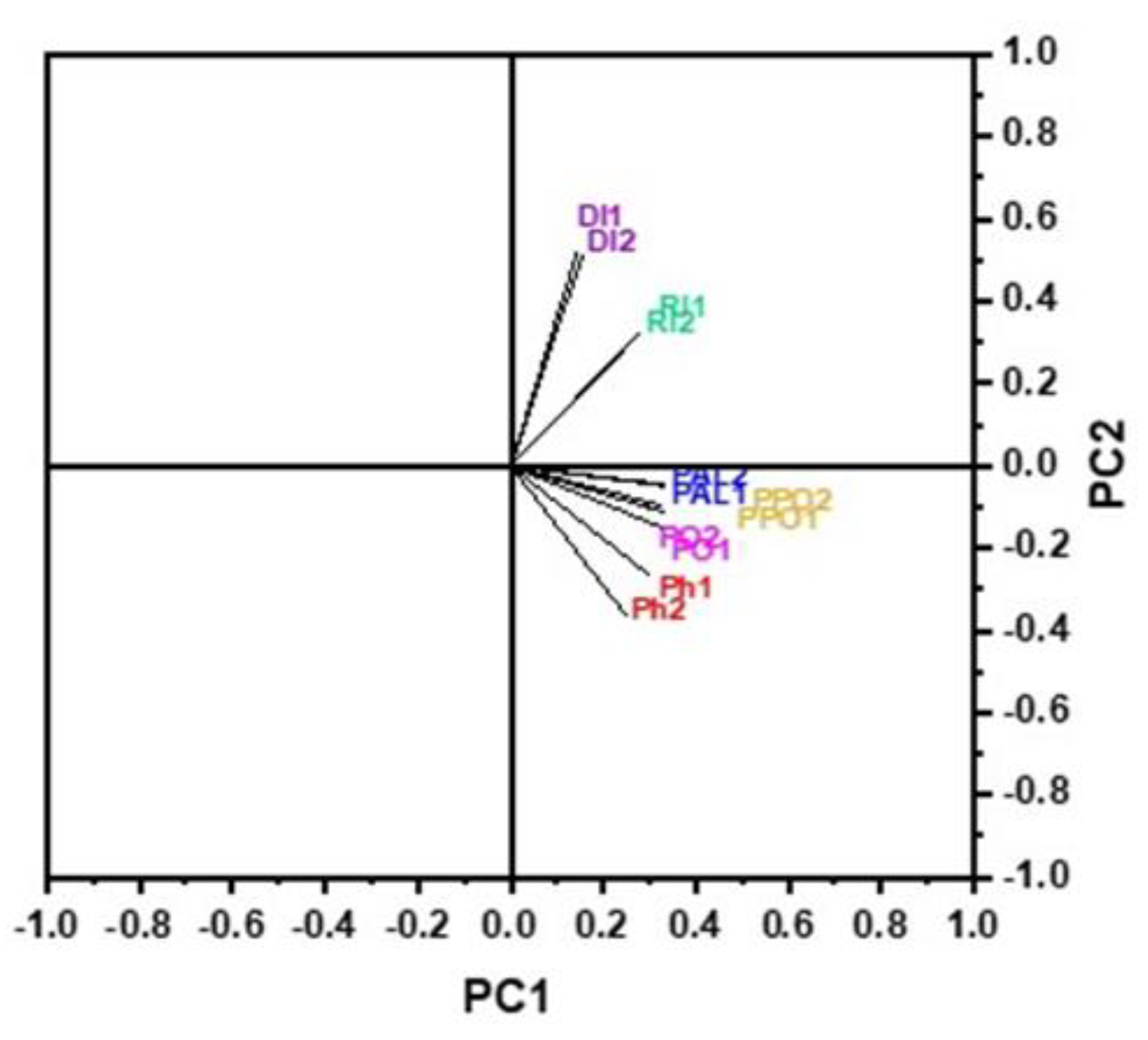
3.7. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chauhan, P.; Sharma, R.; Kumar, D. Application of Nanotechnology in Clinical Research: Present and Future Prospects. In Nanomaterials in Clinical Therapeutics: Synthesis and Applications; Wiley: Hoboken, NJ, USA, 2022; pp. 75–113. [Google Scholar]
- Findik, F. Nanomaterials and their applications. Period. Eng. Nat. Sci. 2021, 9, 62–75. [Google Scholar] [CrossRef]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef]
- Wang, H.; Wan, K.; Shi, X. Recent advances in nanozyme research. Adv. Mater. 2019, 31, 1805368. [Google Scholar] [CrossRef]
- Narayanan, M.; Devarajan, N.; He, Z.; Kandasamy, S.; Ashokkumar, V.; Raja, R.; Carvalho, I.S. Assessment of microbial diversity and enumeration of metal tolerant autochthonous bacteria from tailings of magnesite and bauxite mines. Materials 2020, 33, 4391–4401. [Google Scholar] [CrossRef]
- Haq, I.U.; Khurshid, A.; Inayat, R.; Kexin, Z.; Changzhong, L.; Ali, S.; Zuan, A.T.K.; Al-Hashimi, A.; Abbasi, A.M. Silicon-based induced resistance in maize against fall armyworm [Spodoptera frugiperda (Lepidoptera: Noctuidae)]. PLoS ONE 2021, 16, e0259749. [Google Scholar] [CrossRef]
- Subramaniam, S.; Kumarasamy, S.; Narayanan, M.; Ranganathan, M.; Rathinavel, T.; Chinnathambi, A.; Alahmadi, T.A.; Karuppusamy, I.; Pugazhendhi, A.; Whangchai, K. Spectral and structure characterization of Ferula assafoetida fabricated silver nanoparticles and evaluation of its cytotoxic, and photocatalytic competence. Environ. Res. 2022, 204, 111987. [Google Scholar] [CrossRef]
- Haq, I.U.; Zhang, K.; Ali, S.; Majid, M.; Ashraf, H.J.; Khurshid, A.; Inayat, R.; Li, C.; Gou, Y.; Al-Ghamdi, A.A. Effectiveness of silicon on immature stages of the fall armyworm [Spodoptera frugiperda (JE Smith)]. J. King Saud Univ. Sci. 2022, 34, 102152. [Google Scholar] [CrossRef]
- Thummaneni, C.; Prakash, D.S.; Golli, R.; Vangalapati, M. Green synthesis of silver nanoparticles and characterization of caffeic acid from Myristica fragrans (Nutmeg) against antibacterial activity. Mater. Today: Proc. 2022, 62, 4001–4005. [Google Scholar] [CrossRef]
- Chattha, M.U.; Amjad, T.; Khan, I.; Nawaz, M.; Ali, M.; Chattha, M.B.; Ali, H.M.; Ghareeb, R.Y.; Abdelsalam, N.R.; Azmat, S. Mulberry based zinc nano-particles mitigate salinity induced toxic effects and improve the grain yield and zinc bio-fortification of wheat by improving antioxidant activities, photosynthetic performance, and accumulation of osmolytes and hormones. Front. Plant Sci. 2022, 13, 920570. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Synthesis of silver nanoparticles using marine macroalgae Padina sp. and its antibacterial activity towards pathogenic bacteria. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Rizwan, M.; Hussain, M.; Rauf, A.; Zafar, M.N.; Mabkhot, Y.N.; Maalik, A. Green synthesis and antimicrobial evaluation of silver nanoparticles mediated by leaf extract of Syzygium cumini against poultry pathogens. Micro Nano Lett. 2020, 15, 600–605. [Google Scholar] [CrossRef]
- Akpinar, I.; Unal, M.; Sar, T. Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 2021, 3, 506. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; Shams El-Din, N.G.E.-D.; Maghraby, D.M.E.; Ibrahim, D.S.; Abdel-Megeed, A.; Abdelsalam, N.R. Nematicidal activity of seaweed-synthesized silver nanoparticles and extracts against Meloidogyne incognita on tomato plants. Sci. Rep. 2022, 12, 3841. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ali, J.; Zhang, C.; Mailhot, G.; Pan, G. Simultaneously enhanced photocatalytic and antibacterial activities of TiO2/Ag composite nanofibers for wastewater purification. J. Environ. Chem. Eng. 2020, 8, 102104. [Google Scholar] [CrossRef]
- Shah, S.S.; Shaikh, M.N.; Khan, M.Y.; Alfasane, M.A.; Rahman, M.M.; Aziz, M.A. Present status and future prospects of jute in nanotechnology: A review. Chem. Rec. 2021, 21, 1631–1665. [Google Scholar] [CrossRef] [PubMed]
- Nongbet, A.; Mishra, A.K.; Mohanta, Y.K.; Mahanta, S.; Ray, M.K.; Khan, M.; Chakrabartty, I. Nanofertilizers: A Smart and Sustainable Attribute to Modern Agriculture. Plants 2022, 11, 2587. [Google Scholar] [CrossRef]
- Sadak, M.S. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum). Bull. Natl. Res. Cent. 2019, 43, 38. [Google Scholar] [CrossRef]
- Hernández-Díaz, J.A.; Garza-García, J.J.; Zamudio-Ojeda, A.; León-Morales, J.M.; López-Velázquez, J.C.; García-Morales, S. Plant-mediated synthesis of nanoparticles and their antimicrobial activity against phytopathogens. J. Sci. Food Agric. 2021, 101, 1270–1287. [Google Scholar] [CrossRef]
- Skłodowski, K.; Chmielewska-Deptuła, S.J.; Piktel, E.; Wolak, P.; Wollny, T.; Bucki, R. Metallic Nanosystems in the Development of Antimicrobial Strategies with High Antimicrobial Activity and High Biocompatibility. Int. J. Mol. Sci. 2023, 24, 2104. [Google Scholar] [CrossRef]
- Patil, S.; Singh, N. Antibacterial silk fibroin scaffolds with green synthesized silver nanoparticles for osteoblast proliferation and human mesenchymal stem cell differentiation. Colloids Surf. B: Biointerfaces 2019, 176, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Hariram, M.; Vivekanandhan, S.; Ganesan, V.; Muthuramkumar, S.; Rodriguez-uribe, A.; Mohanty, A.; Misra, M. Tecoma stans flower extract assisted biogenic synthesis of functional Ag-Talc nanostructures for antimicrobial applications. Bioresour. Technol. 2019, 7, 100298. [Google Scholar] [CrossRef]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Takáčová, G. The Role of Silver Nanoparticles in the Diagnosis and Treatment of Cancer: Are There Any Perspectives for the Future? Life 2023, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, F.; Forghan, P.; Gutmann, J.L.; Kishen, A. Silver Nanoparticles and Their Therapeutic Applications in Endodontics: A Narrative Review. Pharmaceutics 2023, 15, 715. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; AAhmed, E.; Battah, B.; Abd Ellah, N.H.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Railean-Plugaru, V.; Pomastowski, P.; Rafińska, K.; Szultka-Mlynska, M.; Golinska, P.; Wypij, M.; Laskowski, D.; Dahm, H. Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. J. Microbiol. Immunol. Infect. 2018, 51, 45–54. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabakar, D.; Jacob, J.M.; Karuppusamy, I.; Saratale, R.G. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 2018, 114, 41–45. [Google Scholar] [CrossRef]
- Tyagi, P.K.; Mishra, R.; Khan, F.; Gupta, D.; Gola, D. Antifungal effects of silver nanoparticles against various plant pathogenic fungi and its safety evaluation on Drosophila melanogaster. Biointerface Res. Appl. Chem. 2020, 10, 6587–6596. [Google Scholar]
- El-Nagar, A.; Elzaawely, A.A.; Taha, N.A.; Nehela, Y. The antifungal activity of gallic acid and its derivatives against Alternaria solani, the causal agent of tomato early blight. Agronomy 2020, 10, 1402. [Google Scholar] [CrossRef]
- Shinde, B.A.; Dholakia, B.B.; Hussain, K.; Aharoni, A.; Giri, A.P.; Kamble, A.C. WRKY1 acts as a key component improving resistance against Alternaria solani in wild tomato, Solanum arcanum Peralta. Plant Biotechnol. J. 2018, 16, 1502–1513. [Google Scholar] [CrossRef]
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Ashraf, H.J.; Aguila, L.C.R.; Ahmed, S.; Haq, I.U.; Ali, H.; Ilyas, M.; Gu, S.; Wang, L. Comparative transcriptome analysis of Tamarixia radiata (Hymenoptera: Eulophidae) reveals differentially expressed genes upon heat shock. Comp. Biochem. Physiol.-D Genom. Proteom. 2022, 41, 100940. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; El-Abeid, S.E.; Ahmed, Y.; Iqbal, Z. Morphological and molecular characterization of large-spored I species associated with potato and tomato early blight in Egypt. Int. J. Agric. Biol. 2021, 25, 1101–1110. [Google Scholar] [CrossRef]
- Al-lami, H.F.; You, M.P.; Mohammed, A.E.; Barbetti, M.J. Virulence variability across the Alternaria spp. population determines incidence and severity of Alternaria leaf spot on rapeseed. Plant Pathol. 2020, 69, 506–517. [Google Scholar] [CrossRef]
- Aslam, M.; Habib, A.; Sahi, S.T.; Khan, R.R. Effect of Bion and Salicylic acid on peroxidase activity and total phenolics in tomato against Alternaria solani. Pak. J. Agric. Sci. 2020, 57, 53–62. [Google Scholar]
- Sarfraz, M.; Khan, S.; Moosa, A.; Farzand, A.; Ishaq, U.; Naeem, I.; Khan, W. Promising antifungal potential of selective botanical extracts, fungicides and Trichoderma isolates against Alternaria solani. Cercet. Agron. Mold. 2018, 51, 65–74. [Google Scholar] [CrossRef]
- Al-Abboud, M.A. Fungal biosynthesis of silver nanoparticles and their role in control of Fusarium wilt of sweet pepper and soil-borne fungi in vitro. Int. J. Pharmacol. 2018, 14, 773–780. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of soil fungi. Volume 1; Academic Press (London) Ltd.: London, UK, 1980. [Google Scholar]
- Ellis, M.B. Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: London, UK, 1971. [Google Scholar]
- Akhtar, K.P.; Saleem, M.Y.; Asghar, M.; Haq, M.A. New report of Alternaria alternata causing leaf blight of tomato in Pakistan. Plant Pathol. 2004, 53, 816. [Google Scholar] [CrossRef]
- Simmons, E.G. Alternaria: An Identification Manual; CBS: New York, NY, USA, 2007. [Google Scholar]
- Bruns, T.D.; Gardes, M. Molecular tools for the identification of ectomycorrhizal fungi—Taxon-specific oligonucleotide probes for suilloid fungi. Mol. Ecol. 1993, 2, 233–242. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Min, J.S.; Lee, Y.S. Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 2012, 40, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Chaerani, C.; Kardin, M.K.; Suhardi, S.; Sofiari, E.; van Ginkel, R.V.; Groenwolt, R.; Voorrips, R.E. Variation in aggressiveness and AFLP among Alternaria solani isolates from indonesia. Indones. J. Agric. Res. 2017, 18, 51–62. [Google Scholar] [CrossRef]
- Vakalounakis, D.; DJ, V. Evaluation of Tomato Cultivars for Resistance to Alternaria Blight; FAO: Rome, Italy, 1983. [Google Scholar]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Mayer, A.M.; Harel, E.; Ben-Shaul, R. Assay of catechol oxidase—A critical comparison of methods. Phytochemistry 1966, 5, 783–789. [Google Scholar] [CrossRef]
- Burrell, M.M.; Ap Rees, T. Metabolism of phenylalanine and tyrosine by rice leaves infected by Piricularia oryzae. Physiol. Plant Pathol. 1974, 4, 497–508. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Khan, S.; Almarhoon, Z.M.; Bakht, J.; Mabkhot, Y.N.; Rauf, A.; Shad, A.A. Single-Step Acer pentapomicum-Mediated Green Synthesis of Silver Nanoparticles and Their Potential Antimicrobial and Antioxidant Activities. J. Nanomater. 2022, 2022, 3783420. [Google Scholar] [CrossRef]
- Ghazali, S.Z.; Mohamed Noor, N.R.; Mustaffa, K.M.F. Anti-plasmodial activity of aqueous neem leaf extract mediated green synthesis-based silver nitrate nanoparticles. Prep. Biochem. Biotechnol. 2022, 52, 99–107. [Google Scholar] [CrossRef]
- Pawar, A.A.; Sahoo, J.; Verma, A.; Alswieleh, A.M.; Lodh, A.; Raut, R.; Lakkakula, J.; Jeon, B.-H.; Islam, M. Azadirachta indica-Derived Silver Nanoparticle Synthesis and Its Antimicrobial Applications. J. Nanomater. 2022, 2022, 4251229. [Google Scholar] [CrossRef]
- Asif, M.; Yasmin, R.; Asif, R.; Ambreen, A.; Mustafa, M.; Umbreen, S. Green Synthesis of Silver Nanoparticles (AgNPs), Structural Characterization, and their Antibacterial Potential. Dose-Response 2022, 20, 15593258221088709. [Google Scholar] [CrossRef]
- Chakravarty, A.; Ahmad, I.; Singh, P.; Sheikh, M.U.D.; Aalam, G.; Sagadevan, S.; Ikram, S. Green synthesis of silver nanoparticles using fruits extracts of Syzygium cumini and their bioactivity. Chem. Phys. Lett. 2022, 795, 139493. [Google Scholar] [CrossRef]
- AlMasoud, N.; Alhaik, H.; Almutairi, M.; Houjak, A.; Hazazi, K.; Alhayek, F.; Aljanoubi, S.; Alkhaibari, A.; Alghamdi, A.; Soliman, D.A. Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity. Green Process. Synth. 2021, 10, 518–528. [Google Scholar] [CrossRef]
- Kumar, S.; Taneja, S.; Banyal, S.; Singhal, M.; Kumar, V.; Sahare, S.; Lee, S.-L.; Choubey, R.K. Bio-synthesised silver nanoparticle-conjugated l-cysteine ceiled Mn: ZnS quantum dots for eco-friendly biosensor and antimicrobial applications. J. Electron. Mater. 2021, 50, 3986–3995. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Selvaraj, R.; Vinayagam, R. Dye degradation and antibacterial activity of green synthesized silver nanoparticles using Ipomoea digitata Linn. flower extract. Int J Environ. Sci Technol 2019, 16, 2395–2404. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Kedi, P.B.E.; Nanga, C.C.; Gbambie, A.P.; Deli, V.; Meva, F.E.; Mohamed, H.E.A.; Ntoumba, A.A.; Nko, M.H.J. Biosynthesis of silver nanoparticles from Microsorum punctatum (l.) copel fronds extract and an in-vitro anti-inflammation study. J. Nanotechnol. 2020, 2, 25–41. [Google Scholar]
- Iqbal, S.; Arifeen, S.; Akbar, A.; Zahoor, S.; Maher, S.; Khan, N.; Anwar, H.; Sajjad, A. Phytochemical screening and antibacterial assay of the crude extract and fractions of Ferula oopoda. Pure Appl. Biol. (PAB) 2019, 8, 742–749. [Google Scholar] [CrossRef]
- Abootalebi, S.N.; Mousavi, S.M.; Hashemi, S.A.; Shorafa, E.; Omidifar, N.; Gholami, A. Antibacterial effects of green-synthesized silver nanoparticles using Ferula asafoetida against Acinetobacter baumannii isolated from the hospital environment and assessment of their cytotoxicity on the human cell lines. J. Nanomater. 2021, 2021, 6676555. [Google Scholar] [CrossRef]
- Niazmand, R.; Razavizadeh, B.M. Active polyethylene films incorporated with β-cyclodextrin/ferula asafoetida extract inclusion complexes: Sustained release of bioactive agents. Polym. Test. 2021, 95, 107113. [Google Scholar] [CrossRef]
- Erenler, R.; Dag, B. Biosynthesis of silver nanoparticles using Origanum majorana L. and evaluation of their antioxidant activity. Inorg. Nano-Met. Chem. 2022, 52, 485–492. [Google Scholar]
- Asimuddin, M.; Shaik, M.R.; Adil, S.F.; Siddiqui, M.R.H.; Alwarthan, A.; Jamil, K.; Khan, M. Azadirachta indica based biosynthesis of silver nanoparticles and evaluation of their antibacterial and cytotoxic effects. J. King Saud Univ. Sci. 2020, 32, 648–656. [Google Scholar] [CrossRef]
- Aziz, S.; Hussein, G.; Brza, M.J.; Mohammed, S.T. Abdulwahid, R.; Raza Saeed, S.; Hassanzadeh, A. Fabrication of interconnected plasmonic spherical silver nanoparticles with enhanced localized surface plasmon resonance (LSPR) peaks using quince leaf extract solution. Nanomaterials 2019, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Y.M.A.; Elshahawy, I.E. Antifungal activity of photo-biosynthesized silver nanoparticles (AgNPs) from organic constituents in orange peel extract against phytopathogenic Macrophomina phaseolina. Eur. J. Plant Pathol. 2022, 162, 725–738. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Biofactories: Engineered nanoparticles via genetically engineered organisms. Green Chem. 2019, 21, 4583–4603. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Shi, Y.; Gao, B.; Wu, J.; Kirk, T.B.; Xu, J.; Xue, W. Green synthesis of lignin nanoparticle in aqueous hydrotropic solution toward broadening the window for its processing and application. J. Chem. Eng. 2018, 346, 217–225. [Google Scholar] [CrossRef]
- Riaz, H.; Chohan, S.; Abid, M. Occurrence of tomato early blight disease and associated Alternaria species in Punjab, Pakistan. J Anim Plant Sci 2021, 31, 1352–1365. [Google Scholar]
- Akhtar, K.P.; Ullah, N.; Saleem, M.Y.; Iqbal, Q.; Asghar, M.; Khan, A.R. Evaluation of tomato genotypes for early blight disease resistance caused by Alternaria solani in Pakistan. J. Plant Pathol. 2019, 101, 1159–1170. [Google Scholar] [CrossRef]
- Ayad, D.; Aribi, D.; Hamon, B.; Kedad, A.; Simoneau, P.; Bouznad, Z. Distribution of large-spored Alternaria species associated with early blight of potato and tomato in Algeria. Phytopathol. Mediterr. 2019, 58, 139–149. [Google Scholar]
- Hussain, A.; Ali, S.; Abbas, H.; Ali, H.; Hussain, A.; Khan, S.W. Spatial distribution of early blight disease on tomato, climatic factors and bio efficacy of plant extracts against Alternaria solani. Acta Sci. Pol. Hortorum Cultus 2019, 18, 29–38. [Google Scholar] [CrossRef]
- Pahnwar, S.; Khaskheli, M.; Khaskheli, A.; Wagan, K. Isolation and Identification of Different Fungal Species from Major Kharif Vegetables of Sindh Province, Pakistan. Agric. Sci. Dig. 2021, 1, 1–6. [Google Scholar] [CrossRef]
- Ding, S.; Meinholz, K.; Cleveland, K.; Jordan, S.A.; Gevens, A.J. Diversity and virulence of Alternaria spp. causing potato early blight and brown spot in Wisconsin. Phytopathology 2019, 109, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Belosokhov, A.; Belov, G.; Chudinova, E.; Kokaeva, L.Y.; Elansky, S. Alternaria spp. and Colletotrichum coccodes in potato leaves with early blight symptoms. PAGV–Spec. Rep. 2017, 18, 181–190. [Google Scholar]
- Ramezani, Y.; Taheri, P.; Mamarabadi, M. Identification of Alternaria spp. associated with tomato early blight in Iran and investigating some of their virulence factors. J. Plant Pathol. 2019, 101, 647–659. [Google Scholar] [CrossRef]
- Özer, N.; Kün, A.; İlbi, H. Detached leaf test for evaluation of resistance to powdery mildew in pepper. J. Agric. Sci. Technol. 2018, 14. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R. Antagonistic assessment of Trichoderma spp. by producing volatile and non-volatile compounds against different fungal pathogens. Arch. Phytopathol. Pflanzenschutz 2017, 50, 629–648. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Alfuzan, S.A.; Alharbi, R.I.; Al-Askar, A.A.; Al-Otaibi, R.M.; Al Subaie, H.F.; Moubayed, N.M. Comparative study of antifungal activity of two preparations of green silver nanoparticles from Portulaca oleracea extract. Saudi J. Biol. Sci. 2022, 29, 2772–2781. [Google Scholar] [CrossRef]
- Rizwana, H.; Alwhibi, M.S.; Aldarsone, H.A.; Awad, M.A.; Soliman, D.A.; Bhat, R.S. Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia. Green Process. Synth. 2021, 10, 421–429. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R.d. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Alamri, S.A.; Alrumman, S.A.; Hashem, M.; Baka, Z.A. Green Synthesis of Silver Nanoparticles Using Pomegranate and Orange Peel Extracts and Their Antifungal Activity against Alternaria solani, the Causal Agent of Early Blight Disease of Tomato. Plants 2021, 10, 2363. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Pandey, S.; Giri, V.P.; Bhattacharya, A.; Shukla, R.; Mishra, A.; Nautiyal, C. Tailoring shape and size of biogenic silver nanoparticles to enhance antimicrobial efficacy against MDR bacteria. Microb. Pathog. 2017, 105, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.; Anjum, T.; Riaz, S.; Naseem, S. Microwave-assisted green synthesis and characterization of silver nanoparticles using Melia azedarach for the management of Fusarium wilt in tomato. Front. Microbiol. 2020, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Pandey, S.; Bhattacharya, A.; Mishra, A.; Nautiyal, C.S. Protective role of biosynthesized silver nanoparticles against early blight disease in Solanum lycopersicum. Plant Physiol. Biochem. 2017, 121, 216–225. [Google Scholar] [CrossRef]
| Isolates | Colony Size (mm) | Colony Color | Mycelial Growth | Conidia Length (µm) | Conidia Width (µm) | Beak Length (µm) |
|---|---|---|---|---|---|---|
| Lhr-1 | 64.26 e ±0.188 | Whitish grey | Cottony Circular | 16.14 c ±0.866 | 9.29 bc ±1.641 | 19.56 a ±1.867 |
| Ksr-1 | 65.05 d ±0.159 | Whitish brown | Cottony Circular | 13.85 c ±2.066 | 7.66 bc ±0.613 | 23.15 a ±2.292 |
| Shk-1 | 68.59 b ±0.111 | Whitish brown | Cottony Circular | 21.20 ab ±1.170 | 10.61 ab ±0.924 | 17.63 ab ±0.918 |
| Fsd-1 | 68.56 b ±0.436 | Whitish brown | Cottony Circular | 23.33 a ±2.212 | 9.56 bc ±0.299 | 22.89 a ±0.966 |
| Fsd-2 | 66.01 c ±0.076 | Whitish brown | Cottony Circular | 18.21 bc ±1.141 | 8.51 bc ±1.164 | 11.77 bc ±0.681 |
| Bhl-1 | 69.21 a ±0.090 | Whitish grey | Cottony Circular | 23.68 a ±0.321 | 12.81 a ±0.574 | 6.53 c ±1.443 |
| M-1 | 48.21 f ±0.015 | Olive green | Not Cottony Circular | 16.27 c ±0.841 | 7.19 c ±0.565 | 13.18 b ±1.098 |
| Isolate Name | Disease Incidence (%) | No. of Lesions/Leaf | Size of Lesion (mm2) | Virulency | ||
|---|---|---|---|---|---|---|
| 5 Days | 10 Days | 15 Days | ||||
| Lhr-1 | 46.39 cd ±2.217 | 57.58 cd ±3.785 | 63.03 d ±2.389 | 5.00 a ±0.578 | 16.41 a ±2.132 | Virulent |
| Ksr-1 | 52.93 b ±1.070 | 63.54 bc ±1.470 | 76.17 bc ±1.875 | 5.67 a ±0.334 | 19.67 a ±2.502 | Virulent |
| Shk-1 | 61.69 a ±2.174 | 73.21 a ±2.566 | 88.22 a ±1.103 | 6.33 a ±0.883 | 20.77 a ±2.299 | Highly Virulent |
| Fsd-1 | 51.00 bc ±0.958 | 69.54 ab ±2.058 | 85.58 ab ±4.616 | 6.00 a ±0.578 | 21.16 a ±1.860 | Highly Virulent |
| Fsd-2 | 48.43 bcd ±1.681 | 68.37 ab ±3.278 | 85.60 ab ±3.640 | 6.67 a ±0.667 | 20.48 a ±0.168 | Highly Virulent |
| Bhl-1 | 52.52 b ±0.667 | 69.62 ab ±2.935 | 75.32 c ±3.771 | 5.33 a ±0.667 | 19.24 a ±0.688 | Virulent |
| M-1 | 44.48 d ±2.496 | 54.70 d ±0.818 | 69.69 cd ±3.059 | 5.67 a ±0.334 | 18.86 a ±1.199 | Virulent |
| Parameters | No. of Lesions per Leaf | Size of Lesion (mm2) | Infected Area of Leaf (%) | Disease Incidence (%) | Disease Index (%) | Disease Reaction | |
|---|---|---|---|---|---|---|---|
| Varieties | |||||||
| Local Varieties | Nadar | 5.07 b ±0.372 | 23.20 def ±1.656 | 38.24 bc ±0.614 | 47.12 c ±2.935 | 74.67 bc ±1.766 | Highly Susceptible |
| Naqeeb | 6.00 a ±0.116 | 30.14 bc ±1.063 | 54.98 a ±0.694 | 56.62 a ±3.636 | 80.00 a ±2.312 | Highly Susceptible | |
| Nagina | 3.80 cd ±0.347 | 25.40 cde ±1.731 | 29.16 efg ±1.223 | 28.06 fg ±0.502 | 38.67 g ±3.532 | Tolerant | |
| Roma | 3.27 d ±0.176 | 41.61 efg ±0.301 | 35.28 bcd ±2.849 | 25.10 fgh ±3.443 | 48.00 ef ±2.312 | Susceptible | |
| Rio Grande | 6.00 a ±0.2 | 41.53 a ±3.729 | 33.77 cde ±0.799 | 52.31 ab ±3.339 | 73.33 b ±1.335 | Highly Susceptible | |
| Sultan | 4.20 c ±0.116 | 34.13 b ±2.835 | 26.58 fg ±1.018 | 40.44 cd ±2.233 | 56.00 d ±2.312 | Susceptible | |
| Hybrid Varieties | Sahel | 3.67 cd ±0.133 | 26.87 cd ±3.325 | 36.81 bcd ±0.727 | 31.58 ef ±1.878 | 54.67 de ±2.669 | Susceptible |
| Cristal | 4.07 c ±0.176 | 29.36 bcd ±1.474 | 39.96 b ±4.139 | 45.71 bc ±0.371 | 66.67 c ±1.337 | Highly Susceptible | |
| Amber | 4.13 c ±0.291 | 17.82 fg ±0.367 | 31.59 def ±1.763 | 30.01 fg ±1.525 | 50.67 def ±1.335 | Susceptible | |
| Red Stone | 4.33 c ±0.241 | 16.76 g ±1.135 | 24.27 g ±2.505 | 23.74 gh ±2.305 | 45.33 fg ±2.669 | Susceptible | |
| Red Diamond | 3.37 d ±0.145 | 17.03 fg ±1.422 | 17.74 h ±0.894 | 18.98 h ±0.809 | 39.33 g ±2.909 | Tolerant | |
| Clara | 3.30 d ±0.067 | 18.27 fg ±0.754 | 31.54 def ±0.683 | 25.52 fgh ±0.656 | 40.67 g ±1.335 | Susceptible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, M.; Ahmed, S.; Khan, M.T.; Hamad, N.A.; Ali, H.M.; Abbasi, A.; Mubeen, I.; Intisar, A.; Hasan, M.E.; Jasim, I.K. Evaluation of In Vitro and In Vivo Antifungal Activity of Green Synthesized Silver Nanoparticles against Early Blight in Tomato. Horticulturae 2023, 9, 369. https://doi.org/10.3390/horticulturae9030369
Ansari M, Ahmed S, Khan MT, Hamad NA, Ali HM, Abbasi A, Mubeen I, Intisar A, Hasan ME, Jasim IK. Evaluation of In Vitro and In Vivo Antifungal Activity of Green Synthesized Silver Nanoparticles against Early Blight in Tomato. Horticulturae. 2023; 9(3):369. https://doi.org/10.3390/horticulturae9030369
Chicago/Turabian StyleAnsari, Madeeha, Shakil Ahmed, Muhammad Tajammal Khan, Najwa A. Hamad, Hayssam M. Ali, Asim Abbasi, Iqra Mubeen, Anum Intisar, Mohamed E. Hasan, and Ihsan K. Jasim. 2023. "Evaluation of In Vitro and In Vivo Antifungal Activity of Green Synthesized Silver Nanoparticles against Early Blight in Tomato" Horticulturae 9, no. 3: 369. https://doi.org/10.3390/horticulturae9030369
APA StyleAnsari, M., Ahmed, S., Khan, M. T., Hamad, N. A., Ali, H. M., Abbasi, A., Mubeen, I., Intisar, A., Hasan, M. E., & Jasim, I. K. (2023). Evaluation of In Vitro and In Vivo Antifungal Activity of Green Synthesized Silver Nanoparticles against Early Blight in Tomato. Horticulturae, 9(3), 369. https://doi.org/10.3390/horticulturae9030369








