UV-A Radiation Stimulates Tolerance against Fusarium oxysporum f. sp. lycopersici in Tomato Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Tomato Crop Development
2.2. UV-A and F. oxysporum f. sp. lycopersici Treatments of Tomato Plants
2.3. Supplementation with UV-A Radiation on Tomato Plants
2.4. Inoculation of F. oxysporum f. sp. lycopersici on Tomato Plants
2.5. F. oxysporum f. sp. lycopersici Incidence and Severity in Tomato Plants
2.6. Tomato Plants Agronomic Parameters
2.7. Biochemical Parameters Measurement
2.8. Stress Biomarkers Test
2.9. Secondary Metabolites Measurement
2.10. Photosynthetic Pigments Measurement
2.11. Statistical Analysis
3. Results
3.1. Impact of UV-A Radiation on F. oxysporum f. sp. lycopersici Incidence and Severity
3.2. Impacts of F. oxysporum f. sp. lycopersici and UV-A Treatments on Agronomic Parameters
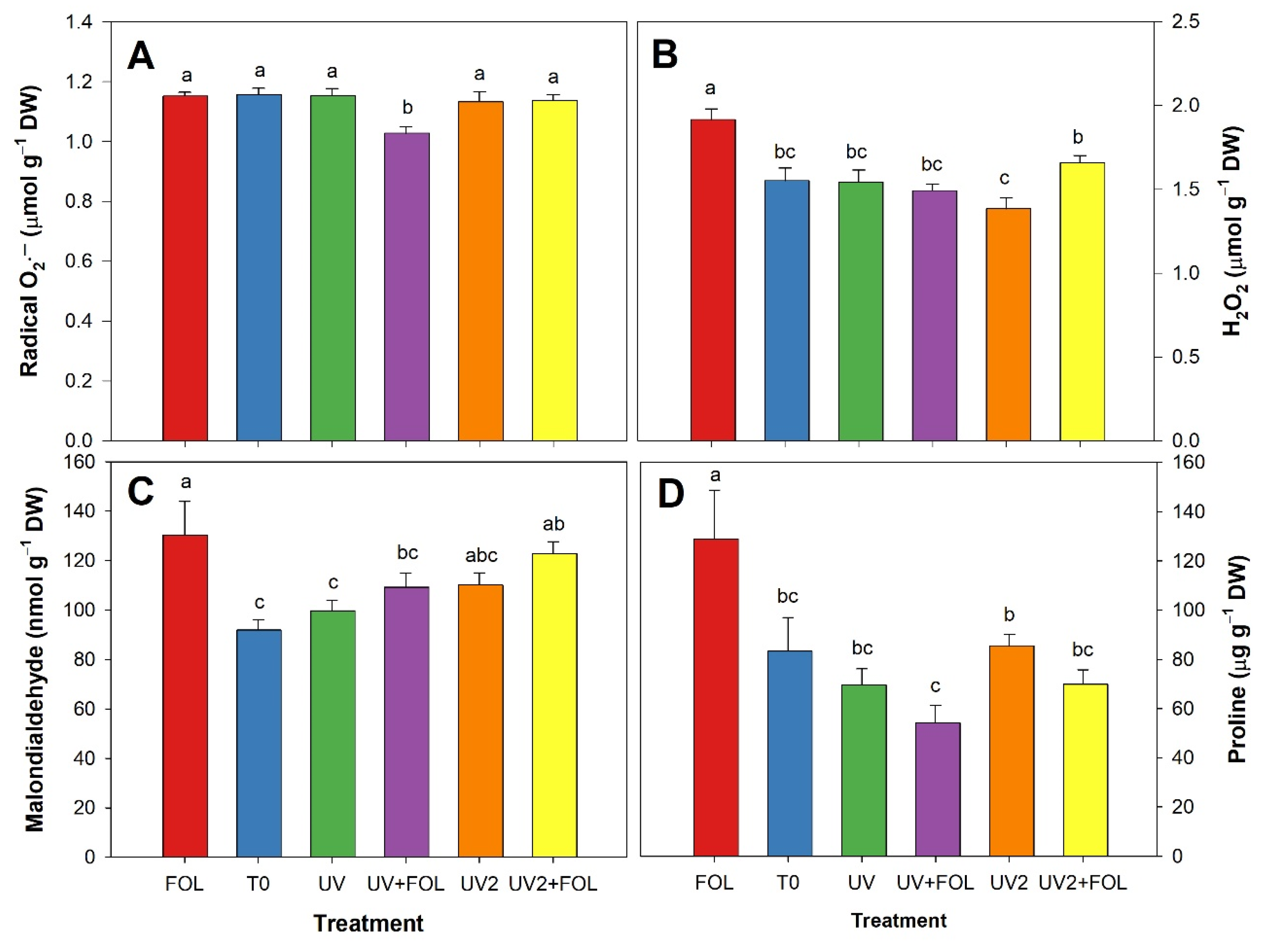
3.3. Impacts of F. oxysporum f. sp. lycopersici and UV-A Treatments on Stress Biomarkers
3.4. Impacts of F. oxysporum f. sp. lycopersici and UV-A Treatments on Secondary Metabolites
3.5. Impacts of F. oxysporum f. sp. lycopersici and UV-A Treatments on Photosynthetic Pigments
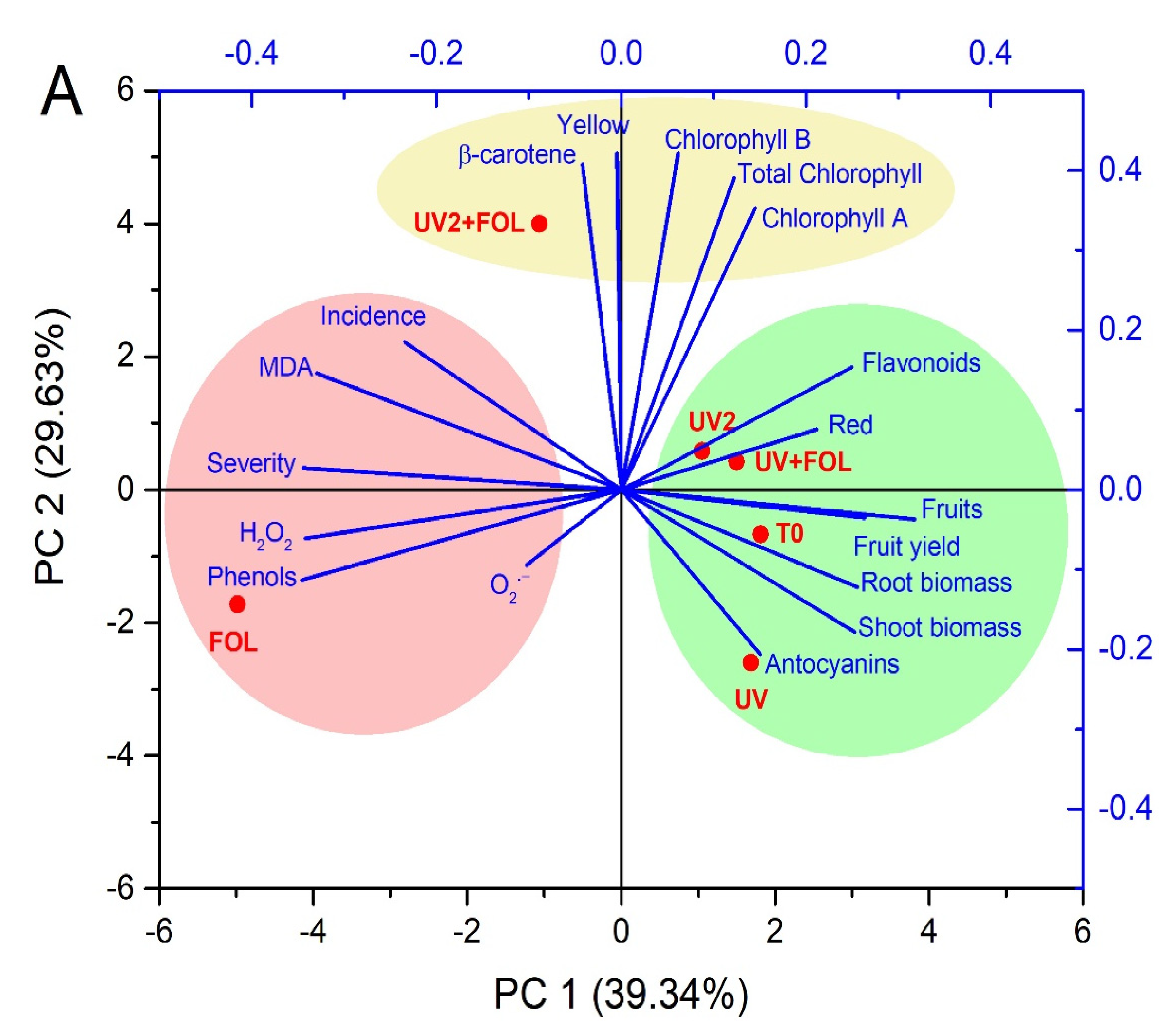
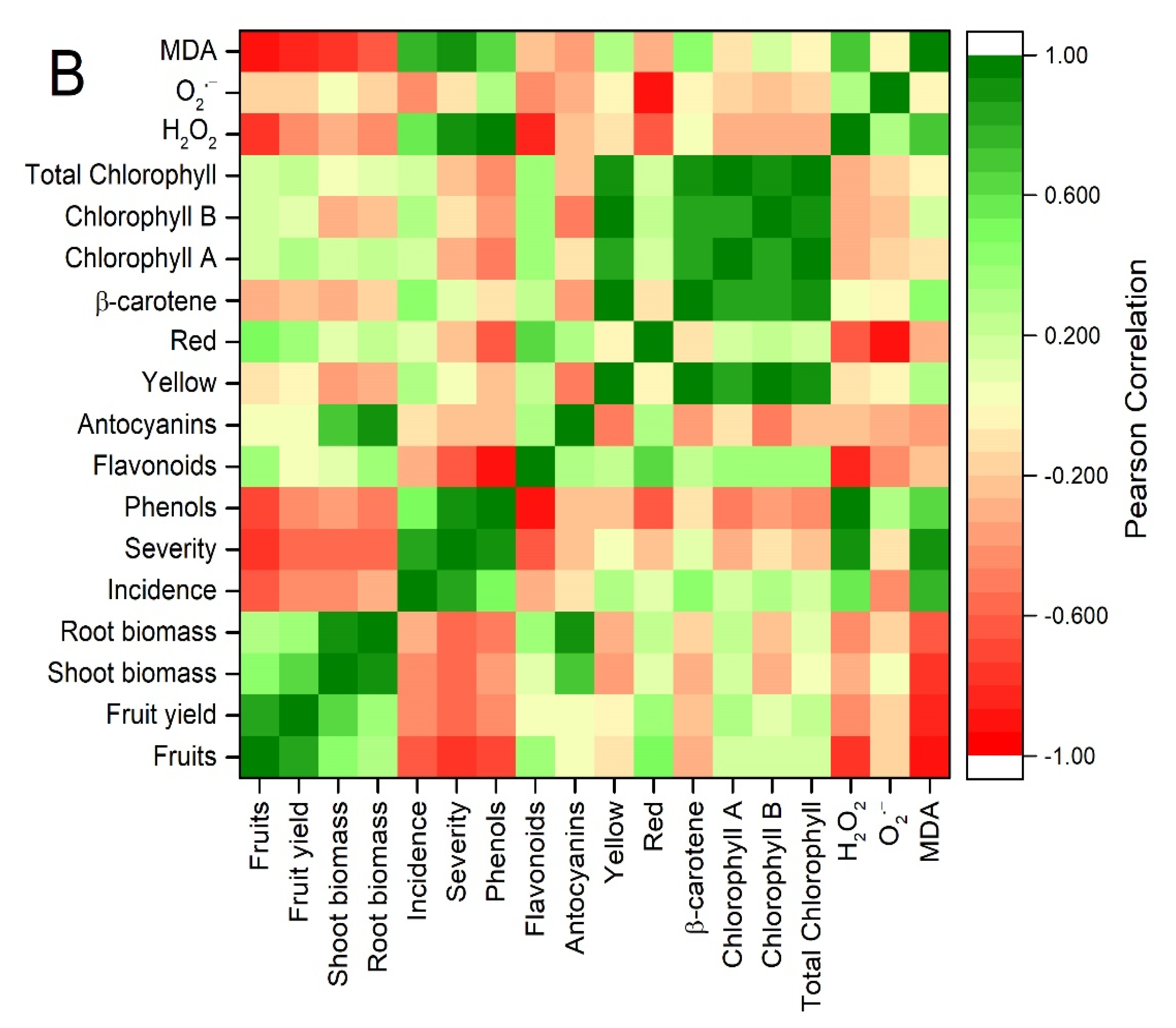
3.6. Principal Component Analysis and Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, M.H.P.; Furlong, E.B. Fungal Diseases and Natural Defense Mechanisms of Tomatoes (Solanum lycopersicum): A Review. Physiol. Mol. Plant Pathol. 2022, 122, 101906. [Google Scholar] [CrossRef]
- Raza, M.M.; Bebber, D.P. Climate Change and Plant Pathogens. Curr. Opin. Microbiol. 2022, 70, 102233. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifah, D.H.M.; Damra, E.; Melhem, M.B.; Hozzein, W.N. Fungus under a Changing Climate : Modeling the Current and Future Global Distribution of Fusarium oxysporum Using Geographical Information System Data. Microorganisms 2023, 2, 468. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, Toxicity, Production and Detection of Fusarium Mycotoxin: A Review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A Review of the Most Common and Economically Important Diseases That Undermine the Cultivation of Tomato Crop in the Mediterranean Basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J. Biological Control Agents against Fusarium Wilt of Banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bilal, R.M.; Gewida, A.G.A.; Dhama, K.; Abdel-Latif, H.M.R.; Amer, M.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Binnaser, Y.S.; et al. An Overview on the Potential Hazards of Pyrethroid Insecticides in Fish, with Special Emphasis on Cypermethrin Toxicity. Animals 2021, 11, 1880. [Google Scholar] [CrossRef]
- Onorati, F.; Tornambé, A.; Paina, A.; Maggi, C.; Sesta, G.; Berducci, M.T.; Bellucci, M.; Rivella, E.; D’Antoni, S. Ecotoxicological and Chemical Approach to Assessing Environmental Effects from Pesticide Use in Organic and Conventional Rice Paddies. Water 2022, 14, 4136. [Google Scholar] [CrossRef]
- Olea, A.; Bravo, A.; Martínez, R.; Thomas, M.; Sedan, C.; Espinoza, L.; Zambrano, E.; Carvajal, D.; Silva-Moreno, E.; Carrasco, H. Antifungal Activity of Eugenol Derivatives against Botrytis Cinerea. Molecules 2019, 24, 1239. [Google Scholar] [CrossRef]
- Booth, J.; Schenk, P.M.; Mirzaee, H. Microbial Biopesticides against Bacterial, Fungal and Oomycete Pathogens of Tomato, Cabbage and Chickpea. Appl. Microbiol. 2022, 2, 288–301. [Google Scholar] [CrossRef]
- Malik, M.S.; Haider, S.; Rehman, A.; Rehman, S.U.; Jamil, M.; Naz, I.; Anees, M. Biological Control of Fungal Pathogens of Tomato (Lycopersicon esculentum) by Chitinolytic Bacterial Strains. J. Basic Microbiol. 2022, 62, 48–62. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Li, H.; Wang, X.; Zhang, R.; Yang, X.; Jiang, Q.; Shi, Q. Revealing the Mechanisms for Linalool Antifungal Activity against Fusarium xysporum and Its Efficient Control of Fusarium Wilt in Tomato Plants. Int. J. Mol. Sci. 2022, 24, 458. [Google Scholar] [CrossRef]
- Cárdenas-Laverde, D.; Barbosa-Cornelio, R.; Coy-Barrera, E. Antifungal Activity against Fusarium oxysporum of Botanical End-Products: An Integration of Chemical Composition and Antifungal Activity Datasets to Identify Antifungal Bioactives. Plants 2021, 10, 2563. [Google Scholar] [CrossRef]
- Cao, M.; Cheng, Q.; Cai, B.; Chen, Y.; Wei, Y.; Qi, D.; Li, Y.; Yan, L.; Li, X.; Long, W.; et al. Antifungal Mechanism of Metabolites from Newly Isolated Streptomyces sp. Y1-14 against Banana Fusarium Wilt Disease Using Metabolomics. J. Fungi 2022, 8, 1291. [Google Scholar] [CrossRef]
- Ma, M.; Taylor, P.W.J.; Chen, D.; Vaghefi, N.; He, J.-Z. Major Soilborne Pathogens of Field Processing Tomatoes and Management Strategies. Microorganisms 2023, 11, 263. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Li, M.; Shakoor, N.; Adeel, M.; Zhou, P.; Guo, M.; Jiang, Y.; Zhao, W.; Lou, B.; et al. Application and Mechanisms of Metal-based Nanoparticles in the Control of Bacterial and Fungal Crop Diseases. Pest Manag. Sci. 2023, 79, 21–36. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.E.; Zhen, S.; Kim, J. Mild-Intensity UV-A Radiation Applied Over a Long Duration Can Improve the Growth and Phenolic Contents of Sweet Basil. Front. Plant Sci. 2022, 13, 858433. [Google Scholar] [CrossRef]
- Akhila, P.P.; Sunooj, K.V.; Aaliya, B.; Navaf, M.; Sudheesh, C.; Sabu, S.; Sasidharan, A.; Mir, S.A.; George, J.; Mousavi Khaneghah, A. Application of Electromagnetic Radiations for Decontamination of Fungi and Mycotoxins in Food Products: A Comprehensive Review. Trends Food Sci. Technol. 2021, 114, 399–409. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Martins, S.; Gonçalves, A.; Correia, C.M.; Ribeiro, C.; Celeste, M.; Santos, C. The Potential Use of the UV-A and UV-B to Improve Tomato Quality and Preference for Consumers. Sci. Hortic. 2019, 246, 777–784. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light Signaling and Plant Responses to Blue and UV Radiations-Perspectives for Applications in Horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.K.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A Radiation Effects on Higher Plants: Exploring the Known Unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Miao, N.; Yun, C.; Shi, Y.; Gao, Y.; Wu, S.; Zhang, Z.; Han, S.; Wang, H.; Wang, W. Enhancement of Flavonoid Synthesis and Antioxidant Activity in Scutellaria baicalensis Aerial Parts by UV-A Radiation. Ind. Crops Prod. 2022, 187, 115532. [Google Scholar] [CrossRef]
- Miao, N.; Yun, C.; Han, S.; Shi, Y.; Gao, Y.; Wu, S.; Zhao, Z.; Wang, H.; Wang, W. Postharvest UV-A Radiation Affects Flavonoid Content, Composition, and Bioactivity of Scutellaria baicalensis Root. Postharvest Biol. Technol. 2022, 189, 111933. [Google Scholar] [CrossRef]
- Steiner, A.A. A Universal Method for Preparing Nutrient Solutions of a Certain Desired Composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Yang, P.M.; Huang, Q.C.; Qin, G.Y.; Zhao, S.P.; Zhou, J.G. Different Drought-Stress Responses in Photosynthesis and Reactive Oxygen Metabolism between Autotetraploid and Diploid Rice. Photosynthetica 2014, 52, 193–202. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Yu, Z.; Dahlgren, R.A. Evaluation of Methods for Measuring Polyphenols in Conifer Foliage. J. Chem. Ecol. 2000, 26, 2119–2140. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of a Propolis Extract and Identification of the Main Constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Minguez-Mosquera, M.I. Rapid Spectrophotometric Determination of Red and Yellow Isochromic Carotenoid Fractions in Paprika and Red Pepper Oleoresins. J. Agric. Food Chem. 2001, 49, 3584–3588. [Google Scholar] [CrossRef]
- Samuoliene, G.; Virsile, A.; Miliauskienė, J.; Haimi, P.; Laužikė, K.; Jankauskienė, J.; Novičkovas, A.; Kupčinskienė, A.; Brazaitytė, A. The Photosynthetic Performance of Red Leaf Lettuce under UV-A Irradiation. Agronomy 2020, 10, 761. [Google Scholar] [CrossRef]
- Yu, L.; Xu, S.-Y.; Luo, X.-C.; Ying, S.-H.; Feng, M.-G. Rad1 and Rad10 Tied to Photolyase Regulators Protect Insecticidal Fungal Cells from Solar UV Damage by Photoreactivation. J. Fungi 2022, 8, 1124. [Google Scholar] [CrossRef]
- Cohrs, K.C.; Schumacher, J. The Two Cryptochrome/Photolyase Family Proteins Fulfill Distinct Roles in DNA Photorepair and Regulation of Conidiation in the Gray Mold Fungus Botrytis Cinerea. Appl. Environ. Microbiol. 2017, 83, e00812–17. [Google Scholar] [CrossRef]
- Yu, Z.; Fischer, R. Light Sensing and Responses in Fungi. Nat. Rev. Microbiol. 2019, 17, 25–36. [Google Scholar] [CrossRef]
- Kvam, E.; Benner, K. Mechanistic Insights into UV-A Mediated Bacterial Disinfection via Endogenous Photosensitizers. J. Photochem. Photobiol. B Biol. 2020, 209, 111899. [Google Scholar] [CrossRef]
- Kvam, E.; Davis, B.; Benner, K. Comparative Assessment of Pulsed and Continuous LED UV-A Lighting for Disinfection of Contaminated Surfaces. Life 2022, 12, 1747. [Google Scholar] [CrossRef]
- Peng, H.; Pang, Y.; Liao, Q.; Wang, F.; Qian, C. The Effect of Preharvest UV Light Irradiation on Berries Quality: A Review. Horticulturae 2022, 8, 1171. [Google Scholar] [CrossRef]
- Badmus, U.O.; Crestani, G.; Cunningham, N.; Havaux, M.; Urban, O.; Jansen, M.A.K. UV Radiation Induces Specific Changes in the Carotenoid Profile of Arabidopsis Thaliana. Biomolecules 2022, 12, 1879. [Google Scholar] [CrossRef]
- Jin-Hui, L.; Myung-Min, O.; Ki-Ho, S. Short-Term Ultraviolet (UV)-A Light-Emitting Diode (LED) Radiation Improves Biomass and Bioactive Compounds of Kale. Front. Plant Sci. 2019, 10, 01042. [Google Scholar] [CrossRef]
- Vodnik, D.; Vogrin, Ž.; Šircelj, H.; Grohar, M.C.; Medič, A.; Carović-Stanko, K.; Safner, T.; Lazarević, B. Phenotyping of Basil (Ocimum basilicum L.) Illuminated with UV-A Light of Different Wavelengths and Intensities. Sci. Hortic. 2023, 309, 111638. [Google Scholar] [CrossRef]
- Lee, M.; Kim, J.; Oh, M.-M.; Lee, J.-H.; Rajashekar, C.B. Effects of Supplemental UV-A LEDs on the Nutritional Quality of Lettuce: Accumulation of Protein and Other Essential Nutrients. Horticulturae 2022, 8, 680. [Google Scholar] [CrossRef]
- Hayes, M.M.; Dewberry, R.J.; Babujee, L.; Moritz, R.; Allen, C. Validating Methods To Eradicate Plant-Pathogenic Ralstonia Strains Reveals That Growth In Planta Increases Bacterial Stress Tolerance. Microbiol. Spectr. 2022, 10, 1–15. [Google Scholar] [CrossRef]
- Suthaparan, A.; Stensvand, A.; Solhaug, K.A.; Torre, S.; Telfer, K.H.; Ruud, A.K.; Mortensen, L.M.; Gadoury, D.M.; Seem, R.C.; Gislerød, H.R. Suppression of Cucumber Powdery Mildew by Supplemental UV-B Radiation in Greenhouses Can Be Augmented or Reduced by Background Radiation Quality. Plant Dis. 2014, 98, 1349–1357. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; Correia, C.V.; Correia, C.M.; Moutinho-Pereira, J.; Melo, P.; Dias, M.C.; Santos, C. Physiological, Biochemical and Molecular Assessment of UV-A and UV-B Supplementation in Solanum lycopersicum. Plants 2021, 10, 918. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; Melo, P.; Santos, C. Moderate UV-A Supplementation Benefits Tomato Seed and Seedling Invigoration: A Contribution to the Use of UV in Seed Technology. Sci. Hortic. 2018, 235, 357–366. [Google Scholar] [CrossRef]
- Choi, D.-S.; Nguyen, T.K.L.; Oh, M.-M. Growth and Biochemical Responses of Kale to Supplementary Irradiation with Different Peak Wavelengths of UV-A Light-Emitting Diodes. Hortic. Environ. Biotechnol. 2022, 63, 65–76. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Li, D.; Liu, X.; Pan, Y.; He, J.; Zhang, J. ROS Regulate NCF2, Key Metabolic Enzymes and MDA Levels to Affect the Growth of Fusarium Solani. Agriculture 2022, 12, 1840. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and Oxidative Burst: Roots in Plant Development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- Abreu, A.L.; Gratão, P.L.; Rodriguez, C.A.V.; Sousa Junior, G.S. A Novel Mineral Composition Increases Soybean Crop Yield by Mitigating Stress Induced by Ultraviolet-A and -B Radiation. Agronomy 2022, 13, 138. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Yadav, V.; Zhao, W.; He, Y.; Zhang, X.; Wei, C. Drought-Induced Proline Is Mainly Synthesized in Leaves and Transported to Roots in Watermelon under Water Deficit. Hortic. Plant J. 2022, 8, 615–626. [Google Scholar] [CrossRef]
- Salama, H.M.H.; Al Watban, A.A.; Al-Fughom, A.T. Effect of Ultraviolet Radiation on Chlorophyll, Carotenoid, Protein and Proline Contents of Some Annual Desert Plants. Saudi J. Biol. Sci. 2011, 18, 79–86. [Google Scholar] [CrossRef]
- Lee, J.-W.; Park, S.-Y.; Oh, M.-M. Supplemental Radiation of Ultraviolet-A Light-Emitting Diode Improves Growth, Antioxidant Phenolics, and Sugar Alcohols of Ice Plant. Hortic. Environ. Biotechnol. 2021, 62, 559–570. [Google Scholar] [CrossRef]
- Loconsole, D.; Santamaria, P. UV Lighting in Horticulture: A Sustainable Tool for Improving Production Quality and Food Safety. Horticulturae 2021, 7, 9. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA Radiation Is Beneficial for Yield and Quality of Indoor Cultivated Lettuce. Front. Plant Sci. 2019, 10, 1563. [Google Scholar] [CrossRef]
- Chen, L.; Mao, H.; Lin, S.; Mohi, A.; Din, U.; Yin, X.; Yuan, M.; Zhang, Z.; Yuan, S.; Zhang, H.; et al. Different Photosynthetic Response to High Light in Four Triticeae Crops. Int. J. Mol. Sci. 2023, 24, 1569. [Google Scholar] [CrossRef]
- Huihui, Z.; Yue, W.; Xin, L.; Guoqiang, H.; Yanhui, C.; Zhiyuan, T.; Jieyu, S.; Nan, X.; Guangyu, S. Chlorophyll Synthesis and the Photoprotective Mechanism in Leaves of Mulberry (Morus alba L.) Seedlings under NaCl and NaHCO3 Stress Revealed by TMT-Based Proteomics Analyses. Ecotoxicol. Environ. Saf. 2020, 190, 110164. [Google Scholar] [CrossRef]
- Maina, J.N.; Wang, Q. Seasonal Response of Chlorophyll a/b Ratio to Stress in a Typical Desert Species: Haloxylon ammodendron. Arid Land Res. Manag. 2015, 29, 321–334. [Google Scholar] [CrossRef]
- Biswas, D.K.; Ma, B.-L.; Xu, H.; Li, Y.; Jiang, G. Lutein-Mediated Photoprotection of Photosynthetic Machinery in Arabidopsis thaliana Exposed to Chronic Low Ultraviolet-B Radiation. J. Plant Physiol. 2020, 248, 153160. [Google Scholar] [CrossRef]
- Badmus, U.O.; Ač, A.; Klem, K.; Urban, O.; Jansen, M.A.K. A Meta-Analysis of the Effects of UV Radiation on the Plant Carotenoid Pool. Plant Physiol. Biochem. 2022, 183, 36–45. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-García, Y.; Escobar-Hernández, D.I.; Benavides-Mendoza, A.; Morales-Díaz, A.B.; Olivares-Sáenz, E.; Juárez-Maldonado, A. UV-A Radiation Stimulates Tolerance against Fusarium oxysporum f. sp. lycopersici in Tomato Plants. Horticulturae 2023, 9, 499. https://doi.org/10.3390/horticulturae9040499
González-García Y, Escobar-Hernández DI, Benavides-Mendoza A, Morales-Díaz AB, Olivares-Sáenz E, Juárez-Maldonado A. UV-A Radiation Stimulates Tolerance against Fusarium oxysporum f. sp. lycopersici in Tomato Plants. Horticulturae. 2023; 9(4):499. https://doi.org/10.3390/horticulturae9040499
Chicago/Turabian StyleGonzález-García, Yolanda, Diego Iván Escobar-Hernández, Adalberto Benavides-Mendoza, América Berenice Morales-Díaz, Emilio Olivares-Sáenz, and Antonio Juárez-Maldonado. 2023. "UV-A Radiation Stimulates Tolerance against Fusarium oxysporum f. sp. lycopersici in Tomato Plants" Horticulturae 9, no. 4: 499. https://doi.org/10.3390/horticulturae9040499
APA StyleGonzález-García, Y., Escobar-Hernández, D. I., Benavides-Mendoza, A., Morales-Díaz, A. B., Olivares-Sáenz, E., & Juárez-Maldonado, A. (2023). UV-A Radiation Stimulates Tolerance against Fusarium oxysporum f. sp. lycopersici in Tomato Plants. Horticulturae, 9(4), 499. https://doi.org/10.3390/horticulturae9040499







