GIS-Facilitated Seed Germination, Fertilization Effects on Growth, Nutrient and Phenol Contents and Antioxidant Potential in Three Local Endemic Plants of Crete (Greece) with Economic Interest: Implications for Conservation and Sustainable Exploitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Local Cretan Endemic Plants
2.2. Seed Collections
2.3. Ecological Profiles
2.4. Germination Tests and Seed Appearance
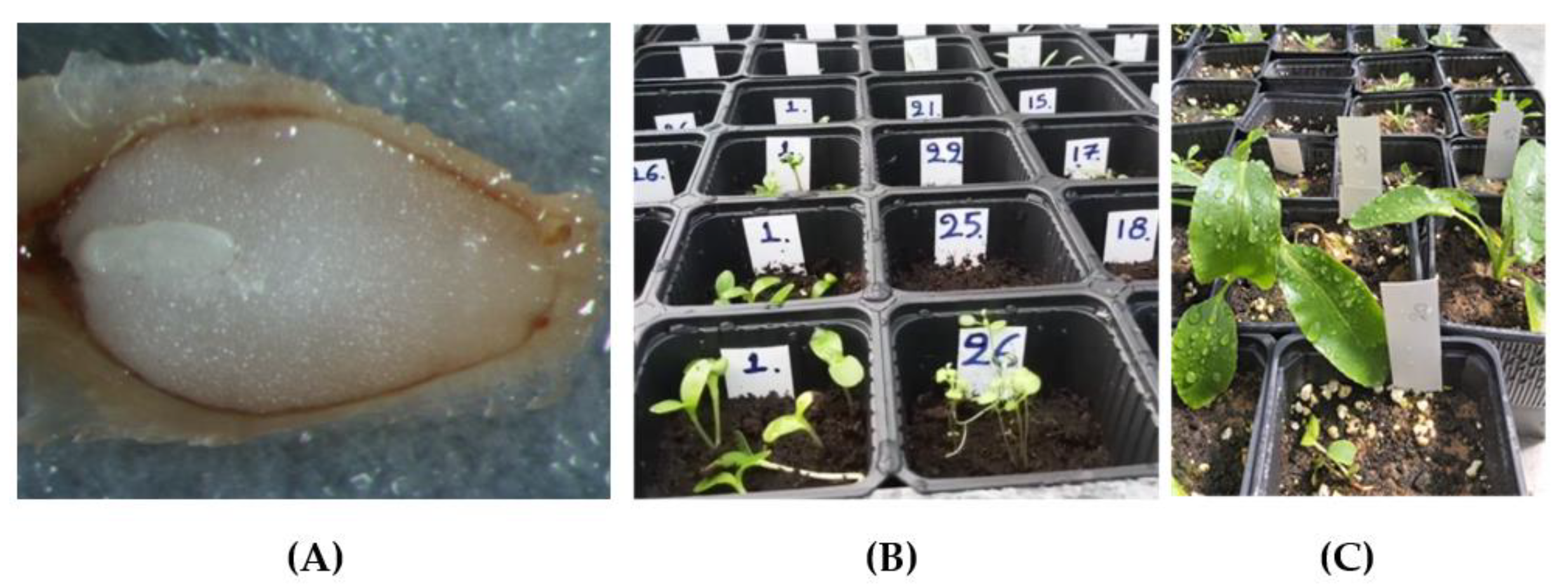
2.5. Plant Production
2.6. Morphological and Physiological Measurements of Seedlings
2.7. Plant Tissue Analyses of Seedlings
2.8. Determination of Total Phenol Content and Antioxidant Capacity
2.9. Statistical Analysis
3. Results
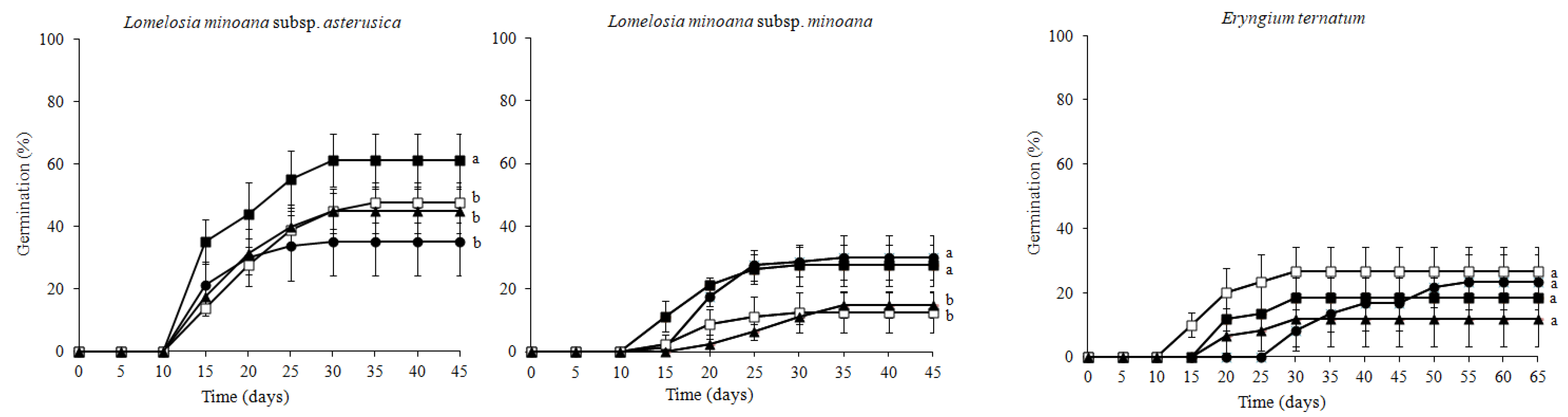
3.1. Seed Germination Success
3.2. Seedlings Growth
3.3. Leaf Nutrient Concentration
3.4. Total Phenol Content and Antioxidant Capacity
4. Discussion
4.1. Seed Germination Protocols
4.2. Fertilization Protocols
4.3. Leaf Nutrient Content
4.4. Re-Evaluation of Feasibility and Readiness Timescale for Sustainable Exploitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A.; et al. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Padulosi, S.; Thompson, J.; Rudebjer, P. Fighting Poverty, Hunger and Malnutrition with Neglected and Underutilized Species (NUS): Needs, Challenges and the Way Forward; Biodiversity International: Rome, Italy, 2013; Available online: https://hdl.handle.net/10568/68927 (accessed on 13 February 2023).
- Rivera, D.; Obón, C.; Heinrich, M.; Inocencio, C.; Verde, A.; Fajardo, J. Gathered Mediterranean food plants—Ethnobotanical investigations and historical development. In Local Mediterranean Food Plants and Nutraceuticals; Heinrich, M., Müller, W.E., Galli, C., Eds.; Karger Publishing: Basel, Switzerland, 2006; Volume 59, pp. 18–74. [Google Scholar] [CrossRef]
- Libiad, M.; Khabbach, A.; El Haissoufi, M.; Anestis, I.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Greveniotis, V.; Tsiripidis, I.; et al. Agro-alimentary potential of the neglected and underutilized local endemic plants of Crete (Greece), Rif-Mediterranean coast of Morocco and Tunisia: Perspectives and challenges. Plants 2021, 10, 1770. [Google Scholar] [CrossRef]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef]
- Scariot, V.; Seglie, L.; Gaino, W.; Devecchi, M. Evaluation of European native bluebells for sustainable floriculture. Acta Hortic. 2012, 937, 273–280. [Google Scholar] [CrossRef]
- Gkika, P.I.; Krigas, N.; Menexes, G.; Eleftherohorinos, I.G.; Maloupa, E. Conservation of the rare Erysimum naxense Snogerup and the threatened Erysimum krendlii Polatschek: Effect of temperature and light on seed germination. Open Life Sci. 2013, 8, 1194–1203. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, Ν.; Maloupa, E. GIS-facilitated in vitro propagation and ex situ conservation of Achillea occulta. Plant Cell Tiss. Organ Cult. 2011, 107, 531–540. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In vitro propagation of medicinal and aromatic plants: The case of selected Greek species with conservation priority. In Vitro Cell. Dev. Biol. Plant 2019, 55, 635–646. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. GIS-facilitated effective propagation protocols of the Endangered local endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving ex-situ conservation needs and its future sustainable exploitation as an ornamental. Plants 2020, 9, 1465. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Maloupa, E.; Tsoktouridis, G. Propagation and ex-situ conservation of Lomelosia minoana subsp. minoana and Scutellaria hirta-two ornamental and medicinal Cretan endemics (Greece). Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12168. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; El Haissoufi, M.; Pipinis, E.; Kostas, S.; Libiad, M.; Khabbach, A.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; et al. GIS-Facilitated seed germination and multifaceted evaluation of the Endangered Abies marocana Trab. (Pinaceae) enabling conservation and sustainable exploitation. Plants 2021, 10, 2606. [Google Scholar] [CrossRef]
- Fanourakis, D.; Paschalidis, K.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Liapaki, E.; Jouini, M.; Ipsilantis, I.; Maloupa, E.; et al. Pilot cultivation of the local endemic Cretan marjoram Origanum microphyllum (Benth.) Vogel (Lamiaceae): Effect of fertilizers on growth and herbal quality features. Agronomy 2021, 12, 94. [Google Scholar] [CrossRef]
- Kostas, S.; Hatzilazarou, S.; Pipinis, E.; Bourgou, S.; Ben Haj Jilani, I.; Ben Othman, W.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; et al. DNA Barcoding, GIS-Facilitated seed germination and pilot cultivation of Teucrium luteum subsp. gabesianum (Lamiaceae), a Tunisian local endemic with potential medicinal and ornamental value. Biology 2022, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Khabbach, A.; Libiad, M.; El Haissoufi, M.; Bourgou, S.; Megdiche-Ksouri, W.; Lamchouri, F.; Ghrabi-Gammar, Z.; Menteli, V.; Vokou, D.; Tsoktouridis, G.; et al. Electronic commerce of the endemic plants of Northern Morocco (Mediterranean Coast-Rif) and Tunisia over the Internet. Bot. Sci. 2021, 100, 139–152. [Google Scholar] [CrossRef]
- Krigas, N.; Mouflis, G.; Grigoriadou, K. Conservation of important plants from the Ionian Islands at the Balkan Botanic Garden of Kroussia, N Greece: Using GIS to link the in situ collection data with plant propagation and ex situ cultivation. Biodivers. Conserv. 2010, 19, 3583–3603. [Google Scholar] [CrossRef]
- Krigas, N.; Lykas, C.; Ipsilantis, I.; Matsi, T.; Weststrand, S.; Havström, M.; Tsoktouridis, G. Greek tulips: Worldwide electronic trade over the internet, global ex situ conservation and current sustainable exploitation challenges. Plants 2021, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Krigas, N.; Karapatzak, E.; Panagiotidou, M.; Sarropoulou, V.; Samartza, I.; Karydas, A.; Damianidis, C.K.; Najdovski, B.; Teofilovski, A.; Mandzukovski, D.; et al. Prioritizing plants around the cross-border area of Greece and the Republic of North Macedonia: Integrated conservation actions and sustainable exploitation potential. Diversity 2022, 14, 570. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot cultivation of the Vulnerable Cretan endemic Verbascum arcturus L. (Scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13, 14030. [Google Scholar] [CrossRef]
- Pipinis, E.; Hatzilazarou, S.; Kostas, S.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; et al. Facilitating conservation and bridging gaps for the sustainable exploitation of the Tunisian local endemic plant Marrubium aschersonii (Lamiaceae). Sustainability 2022, 14, 1637. [Google Scholar] [CrossRef]
- Sefi, O.; Bourgou, S.; Megdiche-Ksouri, W.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Krigas, N.; Ghrabi-Gammar, Z. Bioactivities and phenolic composition of Limonium boitardii Maire and L. cercinense Brullo & Erben (Plumbaginaceae): Two Tunisian strict endemic plants. Inter. J. Environ. Health Res. 2022, 32, 2496–2511. [Google Scholar] [CrossRef]
- Kloukina, C.; Tomou, E.M.; Krigas, N.; Sarropoulou, V.; Madesis, P.; Maloupa, E.; Skaltsa, E. Non-polar secondary metabolites and essential oil of ex situ propagated and cultivated Sideritis syriaca L. subsp. syriaca (Lamiaceae) with consolidated identity (DNA Barcoding): Towards a potential new industrial crop. Ind. Crops Prod. 2020, 158, 112957. [Google Scholar] [CrossRef]
- Beisheim, K.; Otte, A. Evaluating the ornamental value of the Caucasian flora in Georgia. Tuexenia 2017, 37, 33–354. [Google Scholar] [CrossRef]
- Krigas, N.; Panagiotidou, M.; Maloupa, E. Incorporating biogeographical principles in horticulture: Design and creation of the Ionian islands unique rock garden in Thessaloniki, Greece. Sibbaldia 2017, 15, 129–146. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kanellou, E.; Economou, G. Integrated design and management of vegetation at archaeological sites to protect monuments and enhance the historical landscape. Acta Hortic. 2017, 1189, 1–10. [Google Scholar] [CrossRef]
- Cristaudo, A.; Catara, S.; Impelluso, C. Conservation of native germplasm of the Mediterranean Region: Ecological characterization of Sicilian flora species for sustainable use. In Conservation of Threatened Species: Activities and Collaborations within the Network; RIBES Series 1; Mariotti, M., Magrini, S., Eds.; RIBES: Tuscia, Italy, 2015; pp. 53–56. [Google Scholar]
- Küpeli, E.; Kartal, M.; Aslan, S.; Yesilada, E. Comparative evaluation of the anti-inflammatory and antinociceptive activity of Turkish Eryngium species. J. Ethnopharmacol. 2006, 107, 32–37. [Google Scholar] [CrossRef]
- Marčetić, M.; Petrović, S.; Milenković, M.; Niketić, M. Composition, antimicrobial and antioxidant activity of the extracts of Eryngium palmatum Pančić and Vis. (Apiaceae). Open Life Sci. 2014, 9, 149–155. [Google Scholar] [CrossRef]
- Macdonald, B. Practical Woody Plant Propagation for Nursery Growers; Timber Press Inc.: Portland, OR, USA, 2006; p. 669. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Kostopoulou, P.; Radoglou, K.; Dini-Papanastasi, O.; Spyroglou, G. Enhancing planting stock quality of Italian cypress (Cupressus sempervirens L.) by pre-cultivation in mini-plugs. Ecol. Eng. 2010, 36, 912–919. [Google Scholar] [CrossRef]
- O’Reill, C.; Arnott, J.T.; Owens, J.N. Effects of photoperiod and moisture availability on shoot growth, seedling morphology, and cuticle and epicuticular wax features of container-grown western hemlock seedlings. Can. J. Forest Res. 1989, 19, 122–131. [Google Scholar] [CrossRef]
- Buendia-Velazquez, M.V.; Lopez-Lopez, M.A.; Cetina-Alcala, V.M.; Diakite, L. Substrates and nutrient addition rates affect morphology and physiology of Pinus leiophylla seedlings in the nursery stage. iForest 2016, 10, 115–120. [Google Scholar] [CrossRef]
- Oliet, J.; Planelles, R.; Artero, F.; Valverde, R.; Jacobs, D.F.; Segura, M. Field performance of Pinus halepensis planted in Mediterranean arid conditions: Relative influence of seedling morphology and mineral nutrition. New Forests 2009, 37, 313–331. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Puertolas, J.; Penuelas, J.L.; Planelles, R. Effect of nitrozen fertilization in the nursery on the drought and frost resistance of Mediterranean forest species. Forest Syst. 2005, 14, 408–418. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction risk assessment of the Greek endemic flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Fournaraki, C. Conservation of Threatened Plants of Crete—Seed Ecology, Operation and Management of a Gene Bank. Ph.D. Thesis, Department of Botany, Faculty of Biology, National and Kapodistrian University of Athens, Athens, Greece, 2010. (In Greek). [Google Scholar]
- Strid, A. Atlas of the Aegean Flora Part 1 (Text & Plates) & Part 2 (Maps), 1st ed.; Englera 33 (1 & 2); Botanic Garden and Botanical Museum: Berlin, Germany; Freie Universität: Berlin, Germany, 2016. [Google Scholar]
- Krigas, N.; Papadimitriou, K.; Mazaris, A.D. GIS and Ex-Situ Plant Conservation. In Application of Geographic Information Systems; Alam, B.M., Ed.; InTechopen.com: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Cessna, S.; Demmig-Adams, B.; Adams, W.W., III. Exploring photosynthesis and plant stress using inexpensive chlorophyll fluorometers. J. Nat. Resourc. Life Sci. Educ. 2010, 39, 22–30. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Rowland, A.P. Chemical analysis. In Methods in Plant Ecology; Moore, P.D., Chapman, S.B., Eds.; Blackwell Scientific Publication: Oxford, UK, 1986; pp. 285–344. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Bremmer, J.M.; Mulvaney, C.S. Nitrogen-Total, 2nd ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic compounds with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wissen. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Klockars, A.; Sax, G. Multiple Comparisons; Sage Publications: Newbury Park, CA, USA, 1986; p. 87. [Google Scholar]
- Snedecor, G.W.; Cochran, W.C. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1980; p. 507. [Google Scholar]
- Lionello, P.; Malanotte-Rizzoli, P.; Boscolo, R.; Alpert, P.; Artale, V.; Li, L.; Luterbacher, J.; May, W.; Trigo, R.; Tsimplis, M.; et al. The Mediterranean climate: An overview of the main characteristics and issues. Develop. Earth Environ. Sci. 2006, 4, 1–26. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005; p. 250. [Google Scholar]
- Perez-Garcıa, F.; Iriondo, J.M.; Gonzalez-Benito, M.E.; Carnes, L.F.; Tapia, J.; Prieto, C.; Plaza, R.; Perez, C. Germination studies in endemic plant species of the Iberian Peninsula. Israel J. Plant Sci. 1995, 43, 239–247. [Google Scholar] [CrossRef]
- Doussi, M.A.; Thanos, C.A. Ecophysiology of seed germination in Mediterranean geophytes. 1. Muscari spp. Seed Sci. Res. 2002, 12, 193–201. [Google Scholar] [CrossRef]
- Kadis, C.; Kounnamas, C.; Georghiou, K. Seed germination and conservation of endemic, rare and threatened aromatic plants of Cyprus. Israel J. Plant Sci. 2010, 58, 251–261. [Google Scholar] [CrossRef]
- Kupferschmid, A.D.; Stampfli, A.; Newbery, D.M. Dispersal and microsite limitation in an abandoned calcareous grassland of the southern Prealps. Folia Geobot. 2000, 35, 125–141. [Google Scholar] [CrossRef]
- Ayuso, M.; Ramil-Rego, P.; Landin, M.; Gallego, P.P.; Barreal, M.E. Computer-assisted recovery of threatened plants: Keys for breaking seed dormancy of Eryngium viviparum. Front. Plant Sci. 2017, 8, 2092. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Schutte, B.; Knee, M. The effects of rudimentary embryos and elevated oxygen on seed dormancy of Eryngium yuccifolium Michx. (Apiaceae). Seed Sci. Technol. 2005, 33, 53–62. [Google Scholar] [CrossRef]
- Necajeva, J.; Ievinsh, G. Seed dormancy and germination of an endangered coastal plant Eryngium maritimum (Apiaceae). Estonia J. Ecol. 2013, 62, 150. [Google Scholar] [CrossRef]
- Wolkis, D.; Blackwell, S.; Villanueva, S.K. Conservation seed physiology of the ciénega endemic, Eryngium sparganophyllum (Apiaceae). Conserv. Physiol. 2020, 8, coaa017. [Google Scholar] [CrossRef]
- Hammouda, M.A.; Bark, Z.Y. Some aspects of germination of desert seeds. Phyton 1969, 13, 183–201. [Google Scholar]
- Li, J.; Zhu, Z.; Gerendás, J. Effects of nitrogen and sulfur on total phenolics and antioxidant activity in two genotypes of leaf mustard. J. Plant Nutr. 2008, 31, 1642–1655. [Google Scholar] [CrossRef]
- Arena, M.E.; Postemsky, P.D.; Curvetto, N.R. Changes in the phenolic compounds and antioxidant capacity of Berberis microphylla G. Forst. berries in relation to light intensity and fertilization. Sci. Hortic. 2017, 218, 63–71. [Google Scholar] [CrossRef]
- Amarowicz, R.; Cwalina-Ambroziak, B.; Janiak, M.A.; Bogucka, B. Effect of N fertilization on the content of phenolic compounds in Jerusalem artichoke (Helianthus tuberosus L.) tubers and their antioxidant capacity. Agronomy 2020, 10, 1215. [Google Scholar] [CrossRef]
- Xiao, W.X.; Xie, F.T.; Zhang, H.J.; Wang, H.Y.; Wang, H. Effect of fertilizer and planting density on photosynthetic characteristics and yield of super-high- yielding soybean cultivar. Chin. J. Oil Crop Sci. 2009, 31, 190–195. [Google Scholar]
- Siedliska, A.; Baranowski, P.; Pastuszka-Woźniak, J.; Zubik, M.; Krzyszczak, J. Identification of plant leaf phosphorus content at different growth stages based on hyperspectral reflectance. BMC Plant Biol. 2021, 21, 28. [Google Scholar] [CrossRef]
- Sági-Kazár, M.; Solymosi, K.; Solti, A. Iron in leaves: Chemical forms, signalling, and in-cell distribution. J. Exp. Bot. 2022, 73, 1717–1734. [Google Scholar] [CrossRef]
- Miller, G.W.; Huang, I.J.; Welkie, G.W.; Pushnik, J.C. Function of Iron in Plants with Special Emphasis on Chloroplasts and Photosynthetic Activity; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar] [CrossRef]
- Nam, H.I.; Shahzad, Z.; Dorone, Y.; Clowez, S.; Zhao, K.; Bouain, N.; Katerina, S.L.P.; Huikyong, C.; Seung, Y.R.; Rouached, H. Interdependent iron and phosphorus availability controls photosynthesis through retrograde signaling. Nat. Commun. 2021, 12, 7211. [Google Scholar] [CrossRef]
- Burns, I.G. Influence of plant nutrient concentration on growth rate: Use of a nutrient interruption technique to determine critical concentrations of N, P and K in young plants. Plant Soil 1992, 142, 221–233. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Yao, X.; Li, J.; Deng, Q. Co-evaluation of plant leaf nutrient concentrations and resorption in response to fertilization under different nutrient-limited conditions. Diversity 2022, 14, 385. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Luo, F.; Wu, L.; Zhang, Y.; Lan, T. Determination and analysis of leaf P and K concentrations of several plant species in Jinan city. E3S Web Conf. 2018, 53, 03055. [Google Scholar] [CrossRef]
- See, C.R.; Yanai, R.D.; Fisk, M.C.; Vadeboncoeur, M.A.; Quintero, B.A.; Fahey, T.J. Soil nitrogen affects phosphorus recycling: Foliar resorption and plant-soil feedbacks in a northern hardwood forest. Ecology 2015, 96, 2488–2498. [Google Scholar] [CrossRef]
- Tripathi, D.; Singh, V.; Chauhan, D.; Prasad, S.; Dubey, N. Role of macronutrients in plant growth and acclimation: Recent advances and future prospective. In Improvement of Crops in the Era of Climatic Changes; Ahmad, P., Wani, M., Azooz, M., Phan Tran, L.S., Eds.; Springer: New York, NY, USA, 2014; pp. 197–216. [Google Scholar] [CrossRef]
- Chiaregato, C.G.; França, D.; Messa, L.L.; Santos Pereira, T.; Faez, R. A review of advances over 20 years on polysaccharide-based polymers applied as enhanced efficiency fertilizers. Carboh. Polym. 2022, 279, 119014. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Marschner, H. 8-Functions of Mineral Nutrients: Macronutrients, 2nd ed.; Academic Press: Cambridge, MA, USA, 1995; pp. 229–312. [Google Scholar]
- Yruela, I. Transition metals in plant photosynthesis. Metallomics 2013, 5, 1090–1109. [Google Scholar] [CrossRef]
- Dalcorso, G.; Manara, A.; Piasentin, S.; Furini, A. Nutrient metal elements in plants. Metallomics 2014, 10, 1770–1788. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, M.; Ma, Q. Copper-based foliar fertilizer and controlled release urea improved soil chemical properties, plant growth and yield of tomato. Sci. Hortic. 2012, 143, 109–114. [Google Scholar] [CrossRef]
- Gemin, L.G.; Bocchetti de Lara, G.; Mógor, Á.F.; Mazaro, S.M.; Sant‘Anna-Santos, B.F.; Mógor, G.; Oliveira Amatussi, J.; Negrelli Cordeiro, E.C.; Costa Marques, H.M. Polysaccharides combined to copper and magnesium improve tomato growth, yield, anti-oxidant and plant defense enzymes. Sci. Hortic. 2023, 310, 111758. [Google Scholar] [CrossRef]

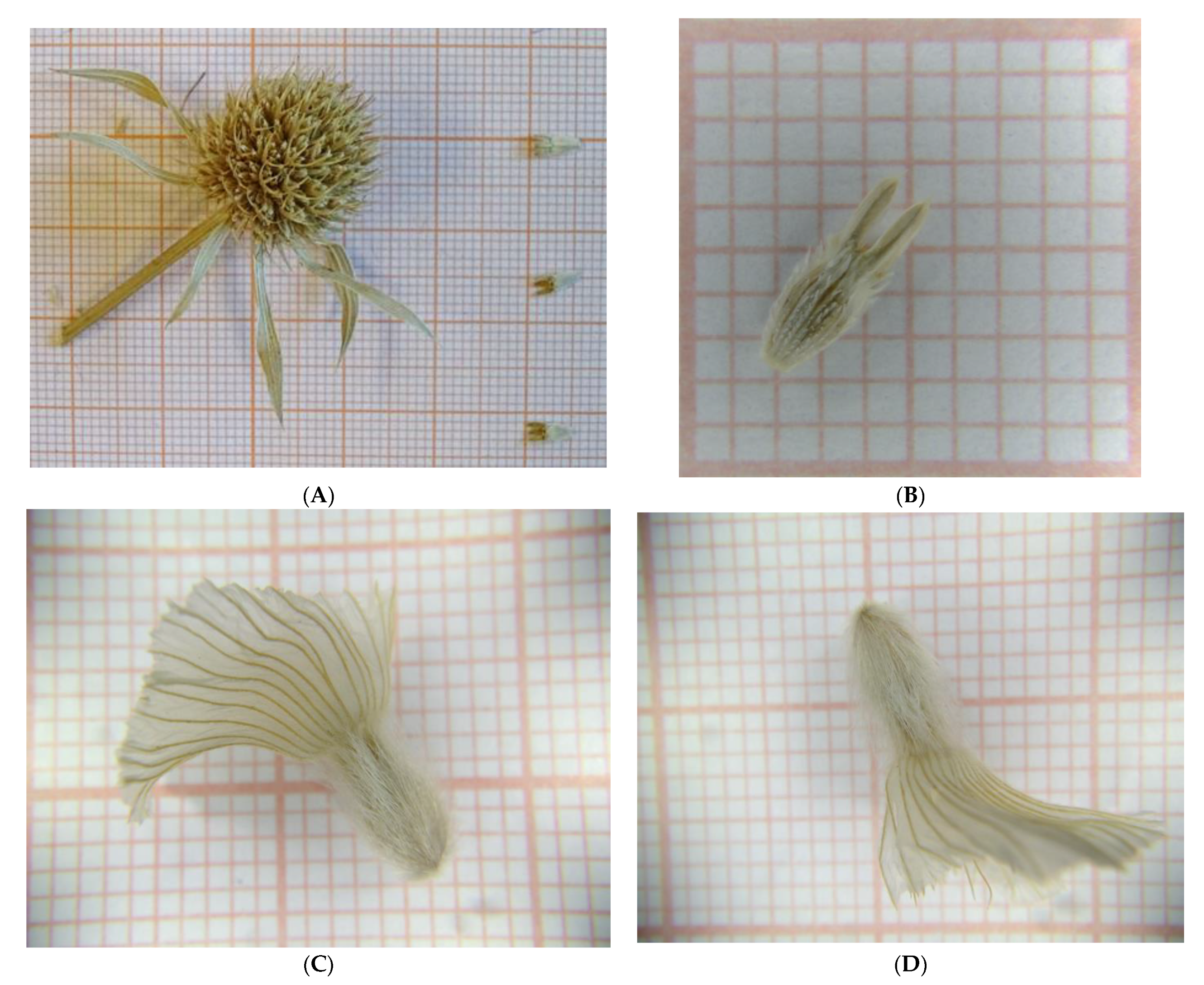


| Scientific Name /Family | IPEN Accession | Collection Date | Collection Site and Area | Latitude (North) | Longitude (East) |
|---|---|---|---|---|---|
| * Eryngium ternatum Poir./Apiaceae | GR-BBGK-1- 19,1140 | 21 August 2019 | Samaria Gorge, Chania | 35.2724 | 23.9616 |
| * Lomelosia minoana (P.H. Davis) Greuter and Burdet s subsp. asterusica (Greuter) Greuter & Burdet/Dipsacaceae | GR-BBGK-1- 20,364 | 17 August 2020 | Kofinas, Asterousia, Heraklion | 35.1833 | 24.2427 |
| Lomelosia minoana (P.H. Davis) Greuter & Burdet subsp. minoana/Dipsacaceae | GR-BBGK-1- 19,16 | 10 July 2018 | Kalami, Ano Viannos, Heraklion | 35.0301 | 25.5073 |
| pH | Organic Matter (%) | Soluble Salts (mS/cm) | CaCO3 (%) | Mechanical Analysis | ||
|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | ||||
| 8.12 | 0.36 | 0.35 | 5.50 | 56.00 | 28.00 | 16.00 |
| Macronutrient Concentrations (ppm) | ||||||
| N-NO3 | Phosphorus (P) | Potassium (K) | Magnesium (Mg) | Calcium (Ca) | ||
| 8.00 | 8.00 | 104.00 | 842.00 | >2000 | ||
| Micronutrient Concentrations (ppm) | ||||||
| Fe | Zinc (Zn) | Manganese (Mn) | Copper (Cu) | |||
| 4.7 | 2.00 | 7.06 | 0.77 | |||
| Attribute (Unit) | Control | Integrated Nutrient Management (INM) | Chemical Fertilization (ChF) |
|---|---|---|---|
| Shoot height (cm) | 10.83 ± 1.29 a * | 11.43 ± 1.21 a | 11.57 ± 1.44 a |
| Root collar diameter (mm) | 3.30 ± 0.45 b | 3.94 ± 0.48 a | 3.93 ± 0.50 a |
| Number of leaves | 15.93 ± 1.62 a | 17.60 ± 2.80 a | 16.53 ± 3.11 a |
| Root dry biomass (g) | 0.52 ± 0.12 a | 0.31 ± 0.06 b | 0.53 ± 0.09 a |
| Above-ground dry biomass (g) | 1.93 ± 0.39 ab | 1.52 ± 0.29 b | 2.10 ± 0.14 a |
| Chlorophyll content index (CCI) | 46.16 ± 7.99 c | 85.58 ± 9.56 b | 103.34 ± 13.97 a |
| Chlorophyll fluorescence (Fv/Fm) | 0.741 ± 0.011 b | 0.762 ± 0.015 a | 0.760 ± 0.010 a |
| Attribute (Unit) | Control | Integrated Nutrient Management (INM) | Chemical Fertilization (ChF) |
|---|---|---|---|
| Shoot height (cm) | 13.80 ± 1.48 b * | 16.90 ± 1.19 a | 13.30 ± 1.09 b |
| Root collar diameter (mm) | 3.36 ± 0.25 a | 3.24 ± 0.17 a | 2.63 ± 0.33 b |
| Number of leaves | 17.00 ± 2.45 a | 16.20 ± 2.77 ab | 13.40 ± 0.89 b |
| Root dry biomass (g) | 0.55 ± 0.04 a | 0.22 ± 0.04 b | 0.19 ± 0.03 b |
| Above-ground dry biomass (g) | 2.66 ± 0.05 a | 1.32 ± 0.07 b | 1.51 ± 0.20 b |
| Chlorophyll content index (CCI) | 50.81 ± 9.16 c | 74.50 ± 11.22 a | 65.67 ± 9.09 b |
| Chlorophyll fluorescence (Fv/Fm) | 0.750 ± 0.010 b | 0.765 ± 0.011 a | 0.747 ± 0.011 b |
| Attribute (Unit) | Control | Integrated Nutrient Management (INM) | Chemical Fertilization (ChF) |
|---|---|---|---|
| Shoot height (cm) | 20.80 ± 3.11 ab * | 20.00 ± 2.74 b | 24.10 ± 2.35 a |
| Root collar diameter (mm) | 4.09 ± 0.89 a | 3.97 ± 0.66 a | 4.92 ± 0.31 a |
| Number of leaves | 6.80 ± 1.30 a | 7.40 ± 1.14 a | 8.00 ± 0.71 a |
| Root dry biomass (g) | 0.69 ± 0.22 c | 2.16 ± 0.03 b | 2.79 ± 0.43 a |
| Above-ground dry biomass (g) | 0.35 ± 0.07 c | 0.85 ± 0.06 b | 1.08 ± 0.06 a |
| Chlorophyll content index (CCI) | 12.01 ± 1.17 b | 18.03 ± 2.02 a | 18.94 ± 3.90 a |
| Chlorophyll fluorescence (Fv/Fm) | 0.696 ± 0.018 b | 0.750 ± 0.009 a | 0.748 ± 0.012 a |
| Treatment | Nitrogen (N) (%) | Phosphorus (P) (mg/g) | Potassium (K) (mg/g) | Calcium (Ca) (mg/g) | Magnesium (Mg) (mg/g) | Sodium (Na) (mg/g) |
|---|---|---|---|---|---|---|
| Lomelosia minoana subsp. asterusica | ||||||
| Control | 1.91 a * | 2.77 b | 21.79 a | 10.84 a | 3.53 a | 4.80 a |
| INM | 1.96 a | 2.92 a | 21.11 b | 10.41 b | 3.35 b | 3.63 c |
| ChF | 1.55 b | 2.58 c | 16.55 c | 9.19 c | 3.16 c | 3.95 b |
| Lomelosia minoana subsp. minoana | ||||||
| Control | 1.54 c | 1.88 c | 13.98 b | 10.11 c | 3.39 c | 5.07 b |
| INM | 2.19 a | 2.90 a | 17.70 a | 12.57 a | 3.77 a | 4.12 c |
| ChF | 1.98 b | 2.51 b | 17.18 a | 10.96 b | 3.64 b | 5.70 a |
| Eryngium ternatum | ||||||
| Control | 1.69 b | 3.77 a | 15.42 c | 14.55 a | 3.59 a | 7.90 c |
| INM | 2.30 a | 2.88 b | 22.87 a | 11.92 b | 3.80 a | 14.93 b |
| ChF | 1.70 b | 3.07 b | 20.95 b | 11.34 c | 3.61 a | 15.86 a |
| Treatment | Iron (Fe) (ppm) | Manganese (Mn) (ppm) | Zind (Zn) (ppm) | Copper (Cu) (ppm) |
|---|---|---|---|---|
| Lomelosia minoana subsp. asterusica | ||||
| Control | 213.41 b * | 16.06 a | 15.48 a | 7.32 b |
| INM | 385.61 a | 16.53 a | 15.53 a | 102.74 a |
| ChF | 227.15 b | 11.76 a | 10.00 b | 1.54 b |
| Lomelosia minoana subsp. minoana | ||||
| Control | 181.83 b | 15.54 a | 26.50 a | 6.73 b |
| INM | 205.53 b | 15.43 a | 14.18 b | 76.02 a |
| ChF | 263.84 a | 13.00 a | 20.32 ab | 7.54 b |
| Eryngium ternatum | ||||
| Control | 377.08 a | 48.58 a | 13.17 a | 10.83 b |
| INM | 161.23 b | 44.82 a | 14.07 a | 131.89 a |
| ChF | 170.15 b | 35.36 b | 12.20 a | 2.18 c |
| Treatment | Total Phenol Content (mg GAE/g FW) | Antioxidant Capacity (mg AAE/g FW) |
|---|---|---|
| Lomelosia minoana subsp. asterusica | ||
| Control | 3.084 ± 0.065 a * | 2.279 ± 0.047 a |
| INM | 1.572 ± 0.090 c | 2.037 ± 0.035 b |
| ChF | 2.694 ± 0.044 b | 2.197 ± 0.044 a |
| Lomelosia minoana subsp. minoana | ||
| Control | 2.096 ± 0.152 b | 2.312 ± 0.025 b |
| INM | 2.167 ± 0.080 b | 2.085 ± 0.041 c |
| ChF | 2.658 ± 0.071 a | 2.419 ± 0.024 a |
| Eryngium ternatum | ||
| Control | 0.748 ± 0.004 c | 0.101 ± 0.0005 c |
| INM | 0.897 ± 0.017 b | 0.109 ± 0.0013 b |
| ChF | 1.210 ± 0.007 a | 0.122 ± 0.0008 a |
| Cretan Endemic Plant | General Ornamental Interest [1] | * Special Ornamental Interest [1] | Agro-Alimentary Interest [4] | Medicinal Interest [5] | Feasibility and Readiness Timescale for Sustainable Exploitation (Level II/III Assessments **) | Increase in the Sum of Individual Scores (+) and Upgraded Percentage (%) Compared with Previous Level II/III Assessments |
|---|---|---|---|---|---|---|
| Eryngium ternatum | Low | Low-to-below average | Very low | Below average | 54.17%/ Achievable in medium-term | +13.89% (68.06%)/ Achievable in short-term |
| 33.33% | 34.37%/38.70%/47.31%/49.41% | 23.81 | 44.44% | |||
| Lomelosia minoana subsp. asterusica | Average | Very high | No | Very low | 50%/ Achievable in medium-term | +13.89% (63.89%)/ Achievable in short-term |
| 56.67% | 65.62%/63.64%/69.89%/71.54% | 0 | 20.37% | |||
| Lomelosia minoana subsp. minoana | Average | Very high | No | Very low | 40.28%/ Achievable in long-term | +30.55% (70.83%)/ Already achieved |
| 51.67% | 62.50%/62.08%/68.28%/70.75% | 0 | 20.37% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatzilazarou, S.; Kostas, S.; Pipinis, E.; Anestis, I.; Papaioannou, E.; Aslanidou, V.; Tsoulpha, P.; Avramakis, M.; Krigas, N.; Tsoktouridis, G. GIS-Facilitated Seed Germination, Fertilization Effects on Growth, Nutrient and Phenol Contents and Antioxidant Potential in Three Local Endemic Plants of Crete (Greece) with Economic Interest: Implications for Conservation and Sustainable Exploitation. Horticulturae 2023, 9, 335. https://doi.org/10.3390/horticulturae9030335
Hatzilazarou S, Kostas S, Pipinis E, Anestis I, Papaioannou E, Aslanidou V, Tsoulpha P, Avramakis M, Krigas N, Tsoktouridis G. GIS-Facilitated Seed Germination, Fertilization Effects on Growth, Nutrient and Phenol Contents and Antioxidant Potential in Three Local Endemic Plants of Crete (Greece) with Economic Interest: Implications for Conservation and Sustainable Exploitation. Horticulturae. 2023; 9(3):335. https://doi.org/10.3390/horticulturae9030335
Chicago/Turabian StyleHatzilazarou, Stefanos, Stefanos Kostas, Elias Pipinis, Ioannis Anestis, Evgenia Papaioannou, Vasiliki Aslanidou, Parthena Tsoulpha, Manolis Avramakis, Nikos Krigas, and George Tsoktouridis. 2023. "GIS-Facilitated Seed Germination, Fertilization Effects on Growth, Nutrient and Phenol Contents and Antioxidant Potential in Three Local Endemic Plants of Crete (Greece) with Economic Interest: Implications for Conservation and Sustainable Exploitation" Horticulturae 9, no. 3: 335. https://doi.org/10.3390/horticulturae9030335
APA StyleHatzilazarou, S., Kostas, S., Pipinis, E., Anestis, I., Papaioannou, E., Aslanidou, V., Tsoulpha, P., Avramakis, M., Krigas, N., & Tsoktouridis, G. (2023). GIS-Facilitated Seed Germination, Fertilization Effects on Growth, Nutrient and Phenol Contents and Antioxidant Potential in Three Local Endemic Plants of Crete (Greece) with Economic Interest: Implications for Conservation and Sustainable Exploitation. Horticulturae, 9(3), 335. https://doi.org/10.3390/horticulturae9030335








