Exploring Effects of Exogenous Selenium on the Growth and Nutritional Quality of Cabbage (Brassica oleracea var. capitata L.)
Abstract
1. Introduction
2. Materials and Method
2.1. Plant Materials and Treatment
2.2. Determination of Total Selenium and Sulfur
2.3. Detection of Se Species in Cabbage
2.4. Determination of Soluble Protein, Soluble Sugar, Free Amino Acid, and Chlorophyll Content
2.5. Detection of Ascorbic Content, Glucosinolate, Flavonoid, Anthocyanin, and Phenolic Acid
2.6. Determination of Antioxidant Enzyme Activity
2.7. Statistical Analysis
3. Results
3.1. Effects of Selenite on the Growth of Cabbage
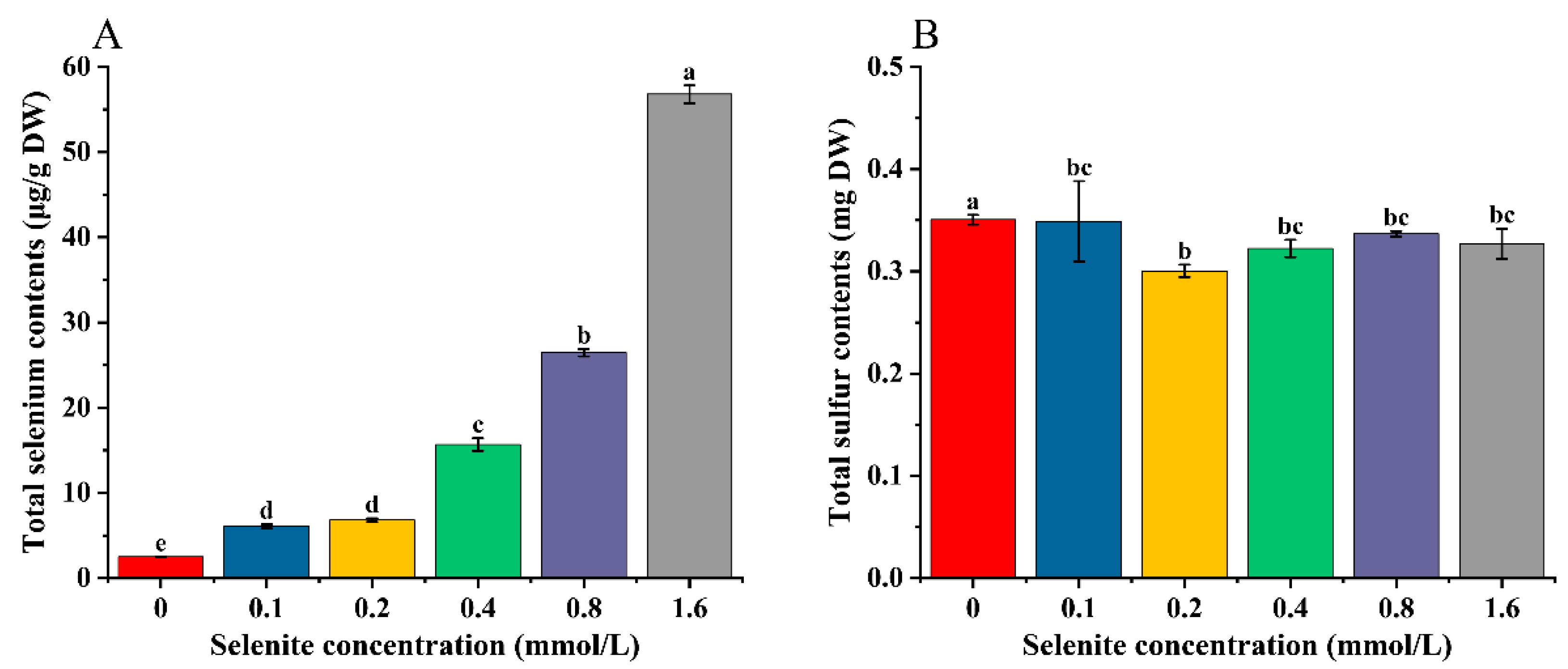
3.2. Effects of Selenite Treatments on Total Se, Sulfur, and Se Species Content of Cabbage
3.3. Effect of Different Concentration Selenite Treatments on Se Speciation of Cabbage
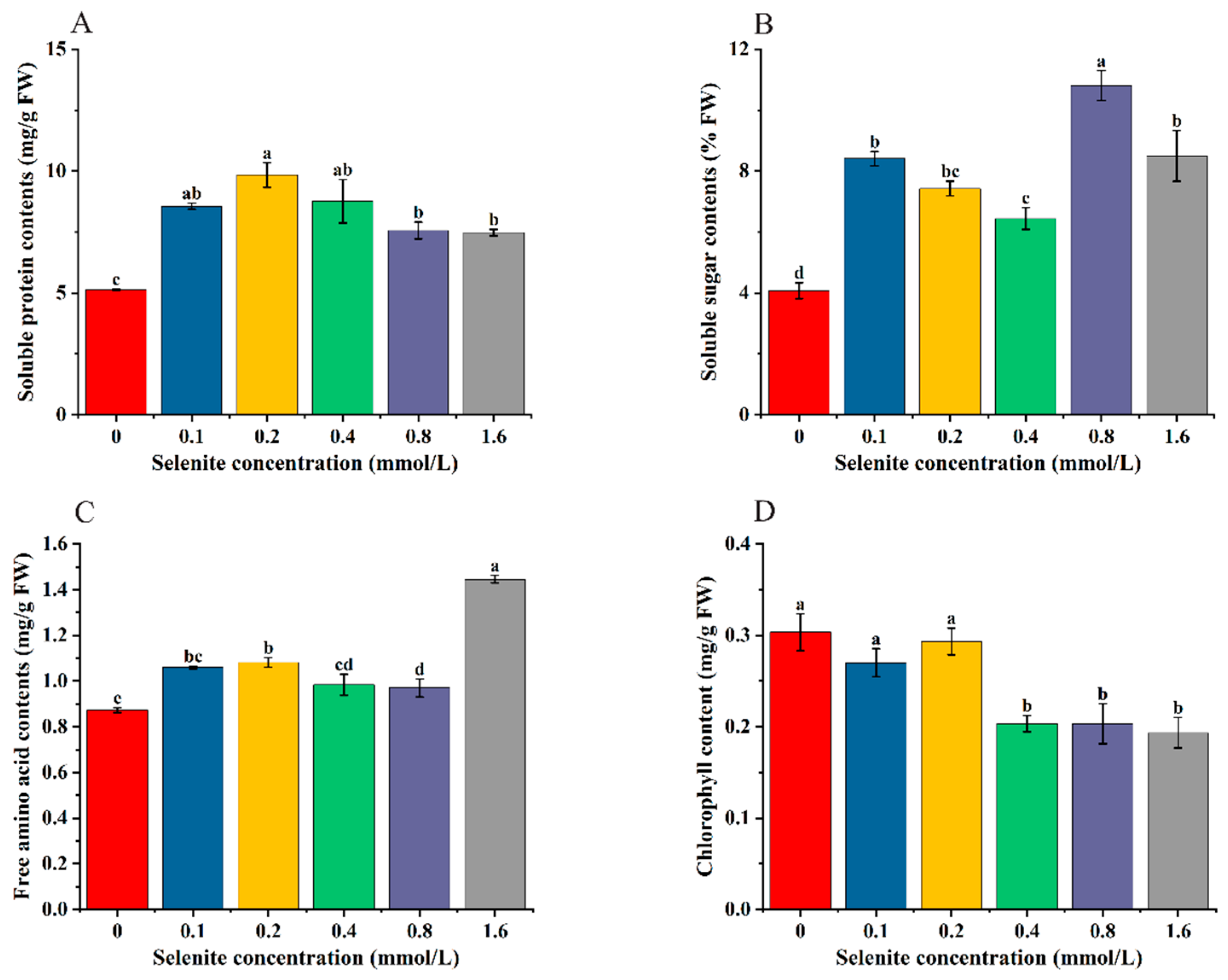
3.4. Effects of Selenite Treatment on Soluble Protein, Soluble Sugar, Free Amino Acid, and Chlorophyll Content of Cabbage
3.5. Total Ascorbic Acid, Glucosinolate, Flavonoid, Anthocyanin, and Phenolic Acid Content of Cabbage
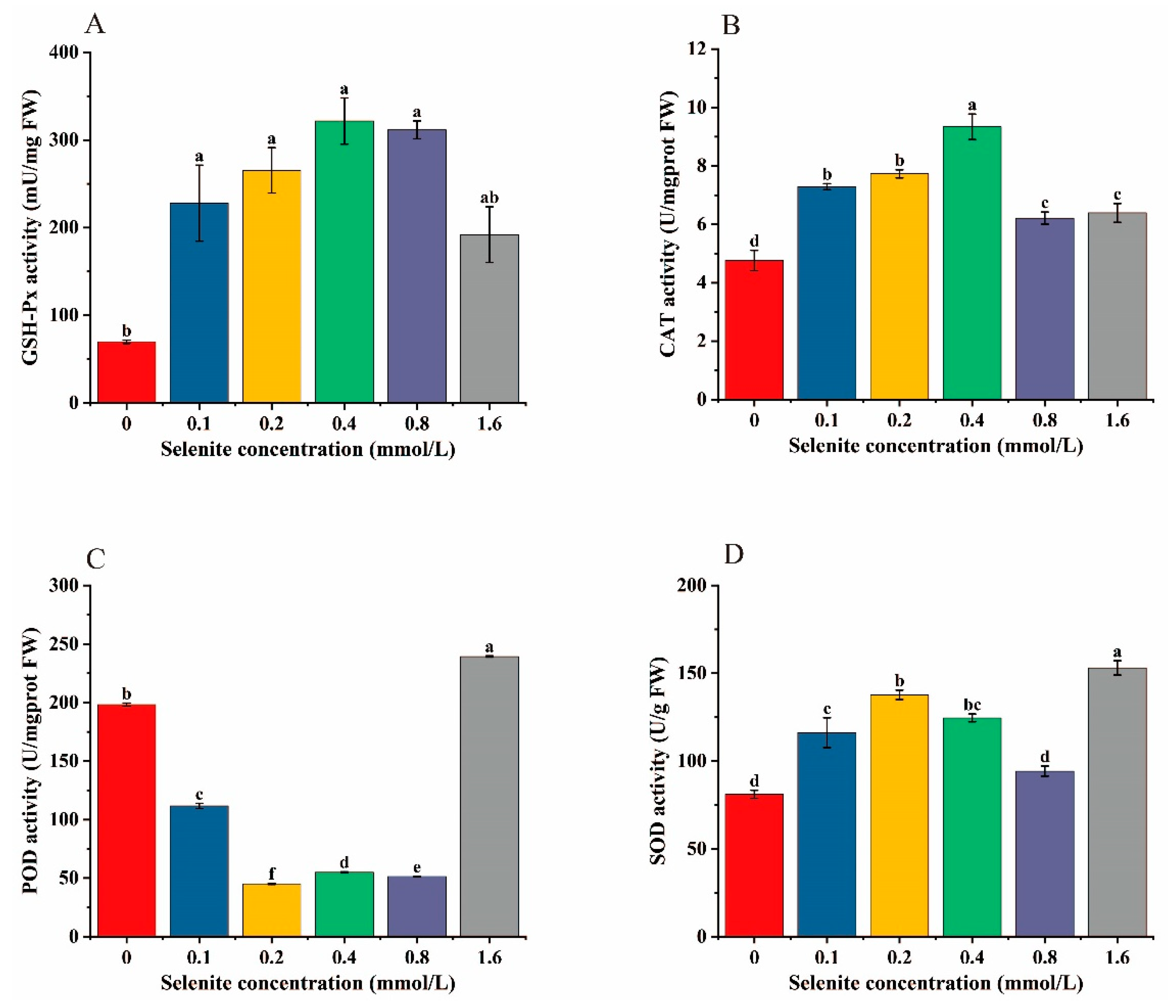
3.6. Effects of Selenite Treatment on the Antioxidant Enzyme Activity of Cabbage Heads
4. Discussion
4.1. Effect of Different Concentrations of Selenite on Total Se Content and Se Species of Cabbage
4.2. Effect of Different Concentrations of Selenite on the Primary Metabolisms of Cabbage
4.3. Effect of Different Concentration of Selenite on Ascorbic Acid Glucosinolate Flavonoid Anthocyanin and Phenolic Acid
4.4. Effect of Different Concentrations of Selenite on Antioxidant Enzymes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Rayman, M.P. The Importance of Selenium to Human Health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium in Cancer Prevention: A Review of the Evidence and Mechanism of Action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef]
- Gui, J.-Y.; Rao, S.; Gou, Y.; Xu, F.; Cheng, S. Comparative Study of the Effects of Selenium Yeast and Sodium Selenite on Selenium Content and Nutrient Quality in Broccoli Florets (Brassica oleracea L. Var. Italica). J. Sci. Food Agric. 2021, 102, 1707–1718. [Google Scholar] [CrossRef]
- Loscalzo, J. Keshan Disease, Selenium Deficiency, and the Selenoproteome. N. Engl. J. Med. 2014, 370, 1756–1760. [Google Scholar] [CrossRef]
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Irshad, S.; Abbas, Q.; Ahmad, R. A Comprehensive Review on Environmental Transformation of Selenium: Recent Advances and Research Perspectives. Environ. Geochem. Health 2019, 41, 1003–1035. [Google Scholar] [CrossRef]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium Biofortification of Agricultural Crops and Effects on Plant Nutrients and Bioactive Compounds Important for Human Health and Disease Prevention—A Review. Plant Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef]
- Wu, M.; Cong, X.; Li, M.; Rao, S.; Liu, Y.; Guo, J.; Zhu, S.; Chen, S.; Xu, F.; Cheng, S.; et al. Effects of Different Exogenous Selenium on Se Accumulation, Nutrition Quality, Elements Uptake, and Antioxidant Response in the Hyperaccumulation Plant Cardamine violifolia. Ecotoxicol. Environ. Saf. 2020, 204, 111045. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Liu, J.; Chen, Y.; Zhang, X. Exploring the Effects of Selenium Treatment on the Nutritional Quality of Tomato Fruit. Food Chem. 2018, 252, 9–15. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.; Liu, Y.; Xie, L.; Pan, S. Effect of Se Treatment on Glucosinolate Metabolism and Health-Promoting Compounds in the Broccoli Sprouts of Three Cultivars. Food Chem. 2016, 190, 374–380. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; Hwang, S.; Mel Lytle, C.; Zhu, Y.; Tai, J.C.; Bravo, R.C.; Chen, Y.; Leustek, T.; Terry, N. Overexpression of ATP Sulfurylase in Indian Mustard Leads to Increased Selenate Uptake, Reduction, and Tolerance. Plant Physiol. 1999, 119, 123–132. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Zhang, X.; Zhang, W.; Huang, L.; Zhang, Z.; Yuan, L.; Liu, X. Effects of Foliar Application of Selenate and Selenite at Different Growth Stages on Selenium Accumulation and Speciation in Potato (Solanum tuberosum L.). Food Chem. 2019, 286, 550–556. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Mitani, N.; Yamaji, N.; Shen, R.F.; Ma, J.F. Involvement of Silicon Influx Transporter OsNIP2;1 in Selenite Uptake in Rice. Plant Physiol. 2010, 153, 1871–1877. [Google Scholar] [CrossRef]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium Uptake, Translocation and Speciation in Wheat Supplied with Selenate or Selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Li, K.; Wan, Y.; Wang, Q.; Zhuang, Z.; Guo, Y.; Li, H. Uptake, Translocation and Biotransformation of Selenium Nanoparticles in Rice Seedlings (Oryza sativa L.). J. Nanobiotechnol. 2020, 18, 103. [Google Scholar] [CrossRef]
- Vasanthi, H.R.; Mukherjee, S.; Das, D.K. Retraction Notice to: Potential Health Benefits of Broccoli- A Chemico-Biological Overview. Mini-Rev. Med. Chem. 2021, 21, 1796. [Google Scholar] [CrossRef]
- Yuan, L.; Zhu, Y.; Lin, Z.; Banuelos, G.; Li, W.; Yin, X. A Novel Selenocystine-Accumulating Plant in Selenium-Mine Drainage Area in Enshi, China. PLoS ONE 2013, 8, e65615. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Z.; Shui, Y.; Liu, X.; Chen, J.; Khan, S.; Wang, J.; Gao, Z. Methods of Selenium Application Differentially Modulate Plant Growth, Selenium Accumulation and Speciation, Protein, Anthocyanins and Concentrations of Mineral Elements in Purple-Grained Wheat. Front. Plant Sci. 2020, 11, 1114. [Google Scholar] [CrossRef]
- Rao, S.; Yu, T.; Cong, X.; Xu, F.; Lai, X.; Zhang, W.; Liao, Y.; Cheng, S. Integration Analysis of PacBio SMRT- and Illumina RNA-Seq Reveals Candidate Genes and Pathway Involved in Selenium Metabolism in Hyperaccumulator Cardamine violifolia. BMC Plant Biol. 2020, 20, 492. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Bischoff, K.J. Coulometric Determination of Total Sulfur and Reduced Inorganic Sulfur Fractions in Environmental Samples. Talanta 2006, 70, 766–773. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Gill, R.A.; Ali, S.; Mwamba, T.M.; Ali, B.; Wang, J.; Huang, Q.; Aziz, R.; Zhou, W. Dual Behavior of Selenium: Insights into Physio-Biochemical, Anatomical and Molecular Analyses of Four Brassica napus Cultivars. Chemosphere 2019, 225, 329–341. [Google Scholar] [CrossRef]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The Role of 24-Epibrassinolide in the Regulation of Photosynthetic Characteristics and Nitrogen Metabolism of Tomato Seedlings under a Combined Low Temperature and Weak Light Stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, C.; Shi, L.; Liu, Z.; Zhou, D.; Meng, H.; Zhao, H.; Li, F.; Zhang, M. A Technical System for the Large-Scale Application of Metabolites from Paecilomyces Variotii SJ1 in Agriculture. Front. Bioeng. Biotechnol. 2021, 9, 671879. [Google Scholar] [CrossRef]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison between Folin-Ciocalteu and Prussian Blue Assays to Estimate the Total Phenolic Content of Juices and Teas Using 96-Well Microplates. J. Food Sci. 2015, 80, C2397–C2403. [Google Scholar] [CrossRef]
- Ratnayake, K.; Payton, J.L.; Meger, M.E.; Godage, N.H.; Gionfriddo, E.; Karunarathne, A. Blue Light-Triggered Photochemistry and Cytotoxicity of Retinal. Cell Signal. 2020, 69, 109547. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, Y.; Sun, S.; Wang, J.; Wang, W.; Han, D.; Shao, H.; Jia, H.; Fu, Y. Selenium Modulates the Level of Auxin to Alleviate the Toxicity of Cadmium in Tobacco. Int. J. Mol. Sci. 2019, 20, 3772. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, D.J.; Jiang, X.J.; Cao, Z.H. Effects of the Interactions between Selenium and Phosphorus on the Growth and Selenium Accumulation in Rice (Oryza sativa). Environ. Geochem. Health 2004, 26, 325–330. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The Dual Effects of Two Inorganic Selenium Forms on the Growth, Selected Physiological Parameters and Macronutrients Accumulation in Cucumber Plants. Acta Physiol. Plant. 2015, 37, 41. [Google Scholar] [CrossRef]
- Li, J.; Liang, D.; Qin, S.; Feng, P.; Wu, X. Effects of Selenite and Selenate Application on Growth and Shoot Selenium Accumulation of Pak Choi (Brassica chinensis L.) during Successive Planting Conditions. Environ. Sci. Pollut. Res. Int. 2015, 22, 11076–11086. [Google Scholar] [CrossRef]
- Yang, X.; Liao, X.; Yu, L.; Rao, S.; Chen, Q.; Zhu, Z.; Cong, X.; Zhang, W.; Ye, J.; Cheng, S.; et al. Combined Metabolome and Transcriptome Analysis Reveal the Mechanism of Selenate Influence on the Growth and Quality of Cabbage (Brassica oleracea Var. capitata L.). Food Res. Int. 2022, 111135. [Google Scholar] [CrossRef]
- Hartikainen, H.; Xue, T.; Piironen, V. Selenium as an Anti-Oxidant and pro-Oxidant in Ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Lin, Z.Q.; Wu, L.; Terry, N. Phytoremediation of Selenium-Contaminated Soils and Waters: Fundamentals and Future Prospects. Re.v Environ. Health 2002, 17, 291–306. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Wang, M.; Ali, F.; Wang, M.; Dinh, Q.T.; Zhou, F.; Bañuelos, G.S.; Liang, D. Understanding Boosting Selenium Accumulation in Wheat (Triticum aestivum L.) Following Foliar Selenium Application at Different Stages, Forms, and Doses. Environ. Sci. Pollut. Res. Int. 2020, 27, 717–728. [Google Scholar] [CrossRef]
- Slekovec, M.; Goessler, W. Accumulation of Selenium in Natural Plants and Selenium Supplemented Vegetable and Selenium Speciation by HPLC-ICPMS. Chem. Speciat. Bioavailab. 2015, 17, 63–73. [Google Scholar] [CrossRef]
- Yu, Q.; Boyanov, M.I.; Liu, J.; Kemner, K.M.; Fein, J.B. Adsorption of Selenite onto Bacillus subtilis: The Overlooked Role of Cell Envelope Sulfhydryl Sites in the Microbial Conversion of Se(IV). Environ. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Dai, Z.; Imtiaz, M.; Rizwan, M.; Yuan, Y.; Huang, H.; Tu, S. Dynamics of Selenium Uptake, Speciation, and Antioxidant Response in Rice at Different Panicle Initiation Stages. Sci. Total Environ. 2019, 691, 827–834. [Google Scholar] [CrossRef]
- Sors, T.G.; Martin, C.P.; Salt, D.E. Characterization of Selenocysteine Methyltransferases from Astragalus Species with Contrasting Selenium Accumulation Capacity. Plant J. 2009, 59, 110–122. [Google Scholar] [CrossRef]
- Ávila, F.W.; Yang, Y.; Faquin, V.; Ramos, S.J.; Guilherme, L.R.G.; Thannhauser, T.W.; Li, L. Impact of Selenium Supply on Se-Methylselenocysteine and Glucosinolate Accumulation in Selenium-Biofortified Brassica Sprouts. Food Chem. 2014, 165, 578–586. [Google Scholar] [CrossRef]
- Hu, L.; Yang, C.; Zhang, L.; Feng, J.; Xi, W. Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck). Molecules 2019, 24, 3440. [Google Scholar] [CrossRef]
- Khatkar, D.; Kuhad, M.S. Short-Term Salinity Induced Changes in Two Wheat Cultivars at Different Growth Stages. Biol. Plant. 2000, 43, 629–632. [Google Scholar] [CrossRef]
- Guo, X.; Ji, Q.; Rizwan, M.; Li, H.; Li, D.; Chen, G. Effects of Biochar and Foliar Application of Selenium on the Uptake and Subcellular Distribution of Chromium in Ipomoea Aquatica in Chromium-Polluted Soils. Ecotoxicol. Environ. Saf. 2020, 206, 111184. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, Y.; An, X.; Kong, F.; Gao, D.; Yin, H. Changes in Sugar Content and Related Enzyme Activities in Table Grape (Vitis vinifera L.) in Response to Foliar Selenium Fertilizer. J. Sci. Food Agric. 2017, 97, 4094–4102. [Google Scholar] [CrossRef]
- Van Hoewyk, D. A Tale of Two Toxicities: Malformed Selenoproteins and Oxidative Stress Both Contribute to Selenium Stress in Plants. Ann. Bot. 2013, 112, 965–972. [Google Scholar] [CrossRef]
- Ren, G.; Ran, X.; Zeng, R.; Chen, J.; Wang, Y.; Mao, C.; Wang, X.; Feng, Y.; Yang, G. Effects of Sodium Selenite Spray on Apple Production, Quality, and Sucrose Metabolism-Related Enzyme Activity. Food Chem. 2021, 339, 127883–127889. [Google Scholar] [CrossRef]
- Babaei, A.; Ranglová, K.; Malapascua, J.R.; Masojídek, J. The Synergistic Effect of Selenium (Selenite, −SeO32−) Dose and Irradiance Intensity in Chlorella cultures. AMB Express 2017, 7, 56. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The Roles of Selenium in Protecting Plants against Abiotic Stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Hu, Q.; Pan, G.; Zhu, J. Effect of Selenium on Green Tea Preservation Quality and Amino Acid Composition of Tea Protein. J. Hortic. Sci. Biotechnol. 2001, 76, 344–346. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium Enrichment Enhances the Quality and Shelf Life of Basil Leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, C.; Fan, Y.; Chen, C.; He, H.; Wang, X.; Zhang, Z.; Zuo, Z.; Peng, G.; Hu, Y.; et al. Toxicity of DON on GPx1-Overexpressed or Knockdown Porcine Splenic Lymphocytes in Vitro and Protective Effects of Sodium Selenite. Oxid. Med. Cell. Longev. 2019, 2019, 5769752. [Google Scholar] [CrossRef]
- Chen, Z.; Young, T.E.; Ling, J.; Chang, S.-C.; Gallie, D.R. Increasing Vitamin C Content of Plants through Enhanced Ascorbate Recycling. Proc. Natl. Acad. Sci. USA 2003, 100, 3525–3530. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Selenium Influences Glucosinolate and Isothiocyanates and Increases Sulfur Uptake in Arabidopsis Thaliana and Rapid-Cycling Brassica oleracea. J. Agric. Food Chem. 2013, 61, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Halkier, B.A.; Gershenzon, J. Biology and Biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Yang, Y.; Ávila, F.W.; Fish, T.; Yuan, H.; Hui, M.; Pan, S.; Thannhauser, T.W.; Li, L. Effects of Selenium Supplementation on Glucosinolate Biosynthesis in Broccoli. J. Agric. Food Chem. 2018, 66, 8036–8044. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.-M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid Oxidation in Plants: From Biochemical Properties to Physiological Functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Riaz, R.S.; Elsherif, M.; Moreddu, R.; Rashid, I.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Anthocyanin-Functionalized Contact Lens Sensors for Ocular PH Monitoring. ACS Omega 2019, 4, 21792–21798. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.-J.; Kang, H.-M.; Lee, Y.-T. Influence of Selenium Biofortification on the Bioactive Compounds and Antioxidant Activity of Wheat Microgreen Extract. Food Chem. 2020, 309–328, 125763. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, Q.; Huang, Y.; Fulton, A.N.; Hannah-Bick, C.; Adeleye, A.S.; Keller, A.A. Activation of Antioxidant and Detoxification Gene Expression in Cucumber Plants Exposed to a Cu(OH)2 Nanopesticide. Environ. Sci. Nano 2017, 4, 1750–1760. [Google Scholar] [CrossRef]
- Lüthje, S.; Meisrimler, C.-N.; Hopff, D.; Möller, B. Phylogeny, Topology, Structure and Functions of Membrane-Bound Class III Peroxidases in Vascular Plants. Phytochemistry 2011, 72, 1124–1135. [Google Scholar] [CrossRef]
- Azpilicueta, C.E.; Pena, L.B.; Tomaro, M.L.; Gallego, S.M. Modifications in Catalase Activity and Expression in Developing Sunflower Seedlings under Cadmium Stress. Redox Rep. 2008, 13, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, H.; Tong, Y.; Wang, Y. Insights into the Superoxide Dismutase Gene Family and Its Roles in Dendrobium Catenatum under Abiotic Stresses. Plants 2020, 9, 1452. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, S.; Pérez-Labrada, F.; García-Enciso, E.L.; Leija-Martínez, P.; Medrano-Macías, J.; Dávila-Rangel, I.E.; Juárez-Maldonado, A.; Rivas-Martínez, E.N.; Benavides-Mendoza, A. Selenium and Sulfur to Produce Allium Functional Crops. Molecules 2017, 22, 558. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.G.D.B.; Silva, V.M.; Montanha, G.S.; Lavres, J.; Pereira de Carvalho, H.W.; Reis, A.R.D. Assessment of Selenium Spatial Distribution Using μ-XFR in Cowpea (Vigna unguiculata (L.) Walp.) Plants: Integration of Physiological and Biochemical Responses. Ecotoxicol. Environ. Saf. 2021, 207, 111216. [Google Scholar] [CrossRef]
- Janská, A.; Marsík, P.; Zelenková, S.; Ovesná, J. Cold Stress and Acclimation—What Is Important for Metabolic Adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef]
- Song, Y.; Li, J.; Liu, M.; Meng, Z.; Liu, K.; Sui, N. Nitrogen Increases Drought Tolerance in Maize Seedlings. Funct. Plant Biol. 2019, 46, 350–359. [Google Scholar] [CrossRef]


| Sodium Selenite Concentration (mmol/L) | Head Size (cm) | Head Weight (g) | Total Plant Weight (g) |
|---|---|---|---|
| 0 | 10.4 ± 0.40 b | 303.33 ± 60.82 bc | 539.46 ± 68.47 ab |
| 0.1 | 13 ± 0.607 ab | 375.9 ± 6.95 ab | 555.46 ± 31.35 ab |
| 0.2 | 12.57 ± 0.80 a | 367.37 ± 20.21 ab | 573.1 ± 27.70 a |
| 0.4 | 13.03 ± 0.63 a | 424.03 ± 6.38 a | 603.7 ± 20.70 a |
| 0.8 | 11.33 ± 0.60 a | 352.13 ± 9.81 abc | 533.27 ± 11.10 ab |
| 1.6 | 10.63 ± 0.41 b | 275.57 ± 6.70 c | 452.07 ± 21.25 b |
| Treatment (mmol/L) | SeCys2 (μg/g DW) | SeMeCys (μg/g DW) | SeMet (μg/g DW) | SeO32− (μg/g DW) | SeO42− (μg/g DW) |
|---|---|---|---|---|---|
| 0 | ND | ND | ND | ND | ND |
| 0.1 | ND | ND | ND | ND | ND |
| 0.2 | ND | ND | ND | ND | ND |
| 0.4 | ND | ND | 3.37 ± 0.111 b | ND | 0.87 ± 0.019 c |
| 0.8 | 1.76 ± 0.009 a | 0.024 ± 0.002 b | 2.36 ± 0.102 c | 0.24 ± 0.009 a | 1.04 ± 0.027 b |
| 1.6 | 1.17 ± 0.068 b | 0.116 ± 0.005 a | 6.24 ± 0.163 a | 0.09 ± 0.004 b | 1.76 ± 0.012 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Chen, Q.; Liao, X.; Yang, X.; Chao, W.; Cong, X.; Zhang, W.; Liao, Y.; Ye, J.; Qian, H.; et al. Exploring Effects of Exogenous Selenium on the Growth and Nutritional Quality of Cabbage (Brassica oleracea var. capitata L.). Horticulturae 2023, 9, 330. https://doi.org/10.3390/horticulturae9030330
Yu L, Chen Q, Liao X, Yang X, Chao W, Cong X, Zhang W, Liao Y, Ye J, Qian H, et al. Exploring Effects of Exogenous Selenium on the Growth and Nutritional Quality of Cabbage (Brassica oleracea var. capitata L.). Horticulturae. 2023; 9(3):330. https://doi.org/10.3390/horticulturae9030330
Chicago/Turabian StyleYu, Li, Qiangwen Chen, Xiaoli Liao, Xiaoyan Yang, Wei Chao, Xin Cong, Weiwei Zhang, Yongling Liao, Jiabao Ye, Hua Qian, and et al. 2023. "Exploring Effects of Exogenous Selenium on the Growth and Nutritional Quality of Cabbage (Brassica oleracea var. capitata L.)" Horticulturae 9, no. 3: 330. https://doi.org/10.3390/horticulturae9030330
APA StyleYu, L., Chen, Q., Liao, X., Yang, X., Chao, W., Cong, X., Zhang, W., Liao, Y., Ye, J., Qian, H., Zhao, Y., Cheng, S., & Xu, F. (2023). Exploring Effects of Exogenous Selenium on the Growth and Nutritional Quality of Cabbage (Brassica oleracea var. capitata L.). Horticulturae, 9(3), 330. https://doi.org/10.3390/horticulturae9030330






