Polyploid Induction and Karyotype Analysis of Dendrobium officinale
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of D. officinale Protocorms
2.3. Colchicine Configuration
2.4. Induction of Protocorms of D. officinale
2.5. Morphological Observations
2.6. Stomatal Observation
2.7. Chromosomal Observation
2.8. Chromosome Karyotype Analysis
3. Results
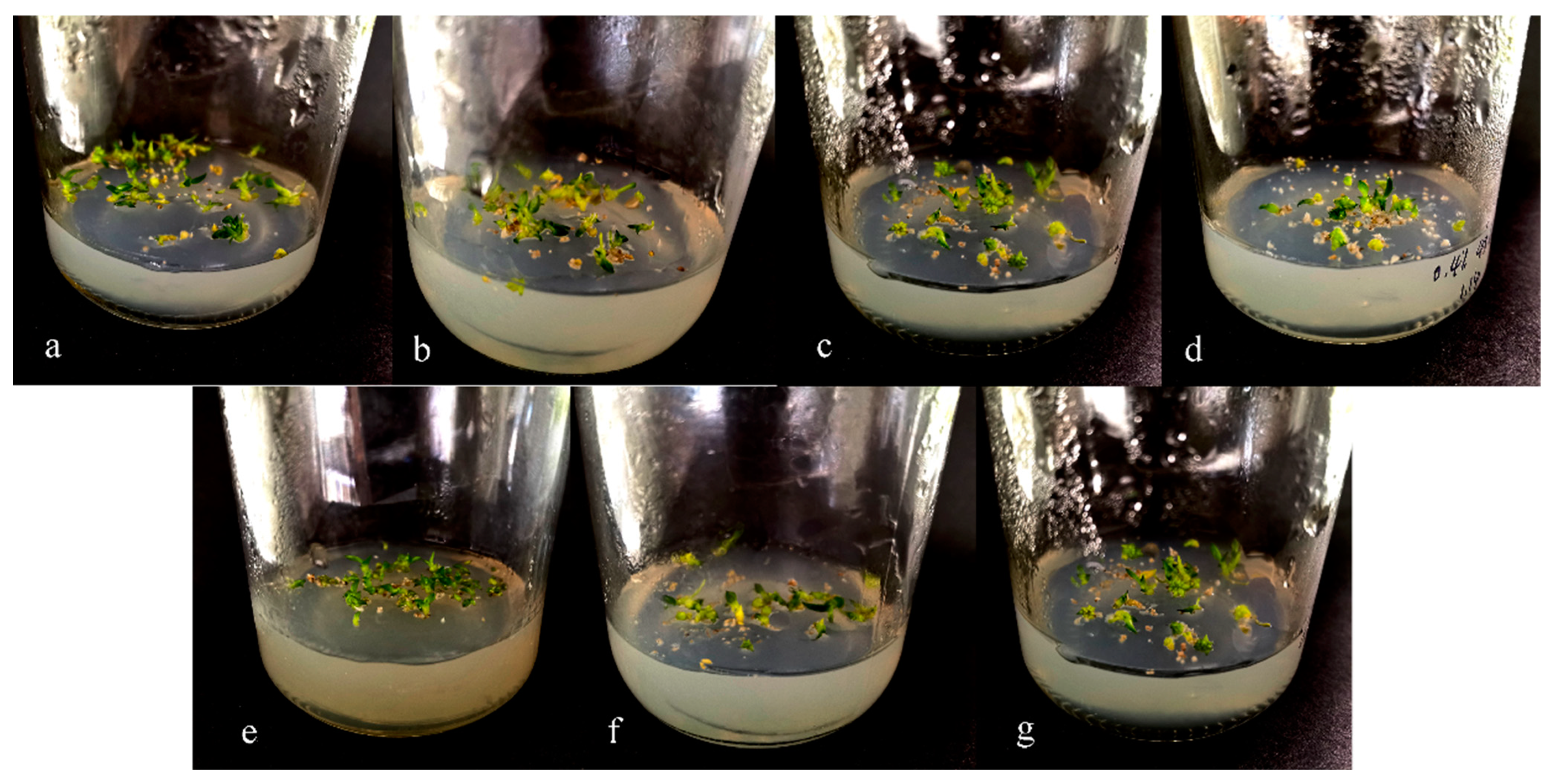
3.1. Induction of Protocorms of D. officinale
3.2. Morphological Observations
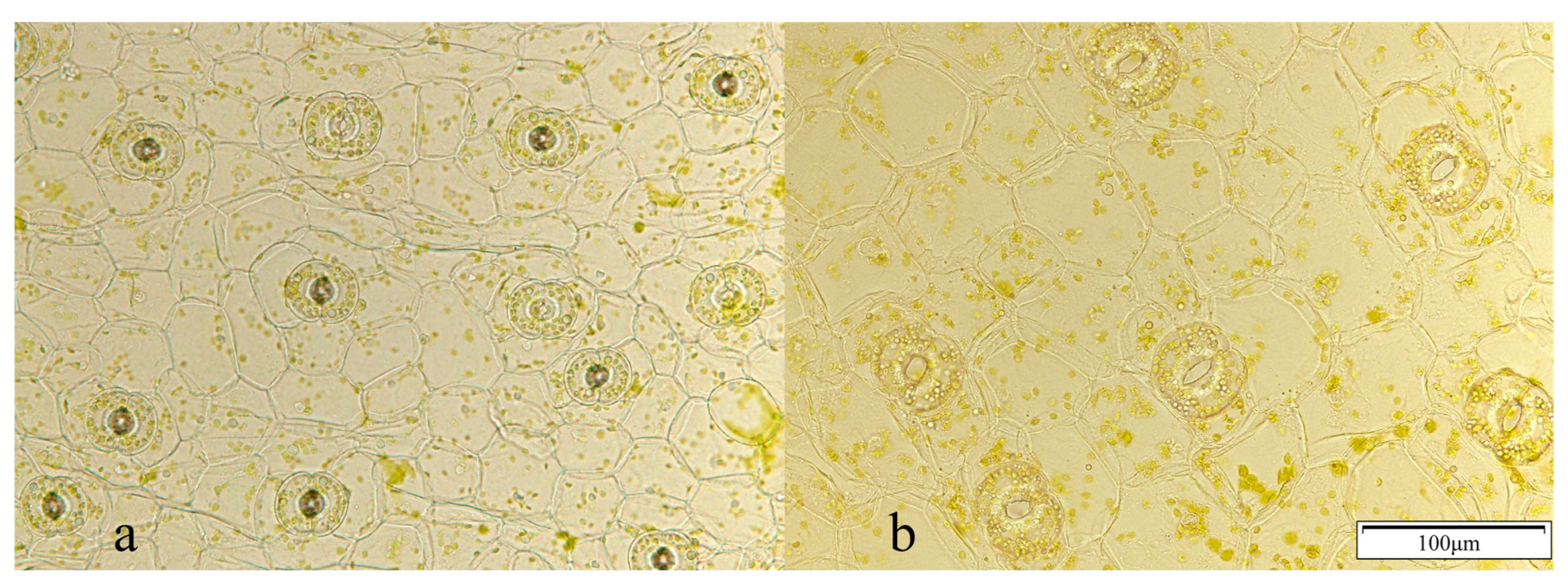
3.3. Stomatometric Observations
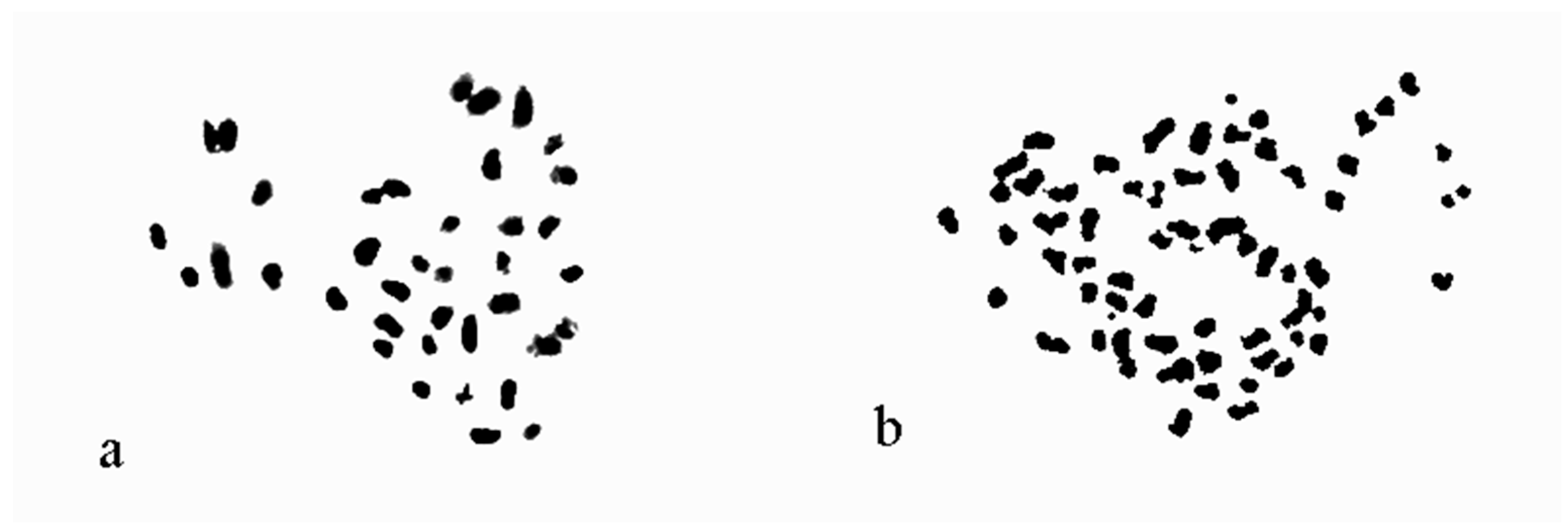
3.4. Chromosome Observations
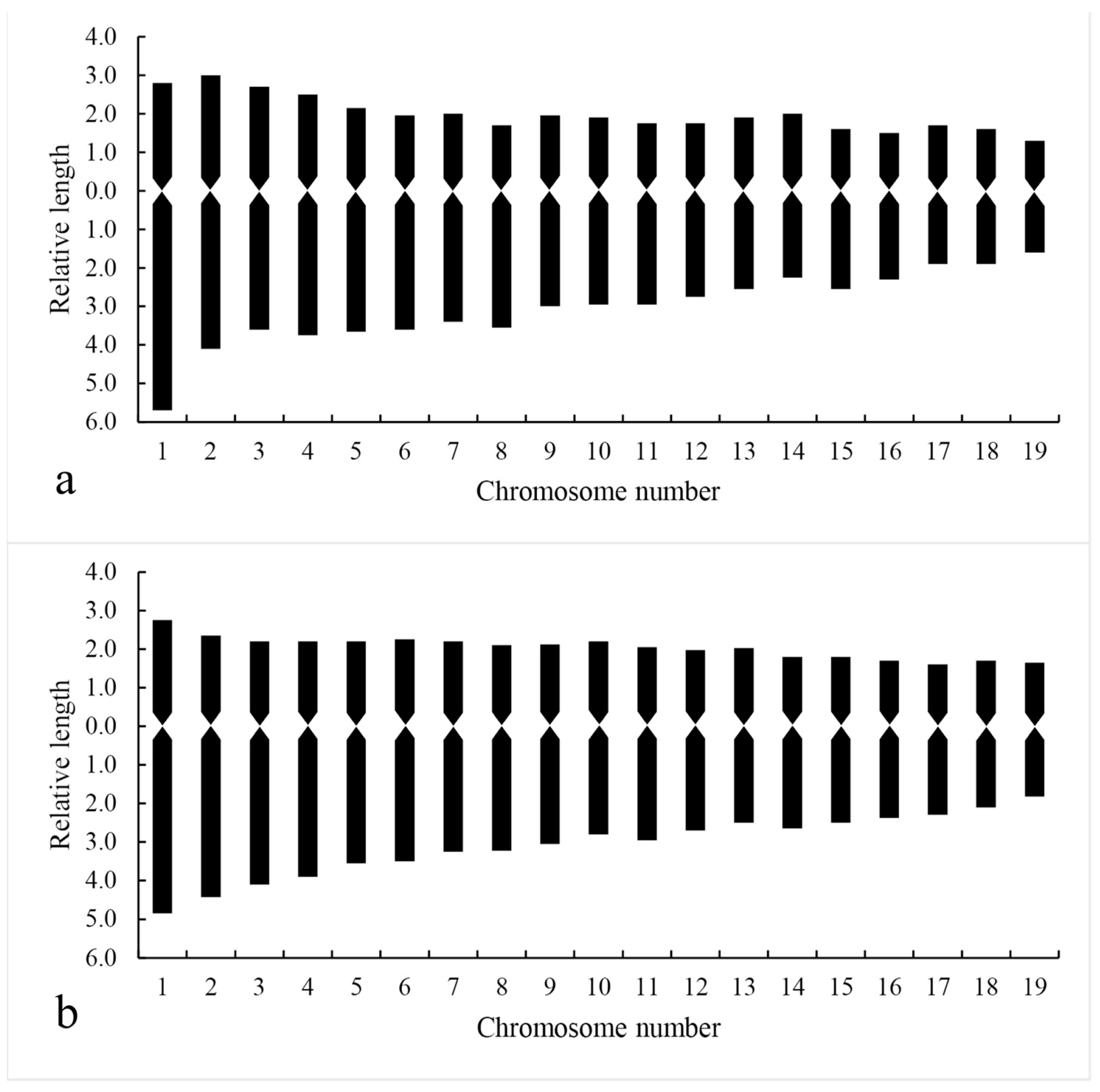
3.5. Chromosome Karyotype Analysis
4. Discussion
4.1. Polyploid Protocorm Induction of D. officinale
4.2. Morphological Observations
4.3. Stomatometric Observations
4.4. Chromosome Observations
4.5. Karyotype Analysis of Chromosome Polyploidy
4.6. Polyploidy Effects on Gene Expression
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, H.; Zhao, T.; Sheng, Y.; Zheng, T.; Fu, L.; Zhang, Y. Dendrobium officinale Kimura et Migo: A Review on Its Ethnopharmacology, Phytochemistry, Pharmacology, and Industrialization. Evid. Based Complement. Altern. Med. 2017, 2017, 7436259. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, C.; Wang, N.; Xu, Y.; Tang, G.; Xu, L.; Feng, Y. Bioactivities and Mechanism of Actions of Dendrobium officinale: A Comprehensive Review. Oxidative Med. Cell. Longev. 2022, 2022, 6293355. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yuan, Y.; Zhang, J. How Climate Change Will Alter the Distribution of Suitable Dendrobium Habitats. Front. Ecol. Evol. 2020, 8, 536339. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, X.; Jia, Z.; Li, C.; Ma, J.; Zhang, J. The Effects of Ecological Factors on the Main Medicinal Components of Dendrobium officinale under Different Cultivation Modes. Forests 2020, 11, 94. [Google Scholar] [CrossRef]
- Hassanzadeh, F.; Zakaria, R.A.; Azad, N.H. Polyploidy Induction in Salvia officinalis L. and its Effects on Some Morphological and Physiological Characteristics. Cytologia 2020, 85, 157–162. [Google Scholar] [CrossRef]
- Chen, J.T.; Coate, J.E.; Meru, G. Artificial Polyploidy in Plants. Front. Plant Sci. 2020, 11, 621849. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, H.; Jiang, Y.; Wang, L.; Kong, X.; Huang, Y.; Jia, G. Effects of Polyploidization on Morphology, Photosynthetic Parameters and Sucrose Metabolism in Lily. Plants 2022, 11, 2112. [Google Scholar] [CrossRef]
- Mo, L.; Chen, J.; Lou, X.; Xu, Q.; Dong, R.; Tong, Z.; Huang, H.; Lin, E. Colchicine-Induced Polyploidy in Rhododendron fortunei Lindl. Plants 2020, 9, 424. [Google Scholar] [CrossRef]
- Trojak-Goluch, A.; Kawka-Lipińska, M.; Wielgusz, K.; Praczyk, M. Polyploidy in Industrial Crops: Applications and Perspectives in Plant Breeding. Agronomy 2021, 11, 2574. [Google Scholar] [CrossRef]
- Niazian, M.; Nalousi, A.M. Artificial Polyploidy Induction for Improvement of Ornamental and Medicinal Plants. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 142, 447–469. [Google Scholar] [CrossRef]
- Iannicelli, J.; Elechosa, M.A.; Juarez, M.A. Effect of Polyploidization in the Production of Essential Oils in Lippia integrifolia. Ind. Crops Prod. 2016, 81, 20–29. [Google Scholar] [CrossRef]
- Madani, H.; Escrich, A.; Hosseini, B. Effect of Polyploidy Induction on Natural Metabolite Production in Medicinal Plants. Biomolecules 2021, 11, 899. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Chen, P.Y.; To, K.Y. Induction of Polyploidy and Metabolic Profiling in the Medicinal Herb Wedelia chinensis. Plants 2021, 10, 1232. [Google Scholar] [CrossRef]
- Leitch, A.R.; Leitch, I.J. Genomic Plasticity and The Diversity of Polyploid Plants. Science 2008, 320, 481–483. [Google Scholar] [CrossRef]
- Lan, M.O.; Chen, J.; Fei, C. Induction and Characterization of Polyploids from Seeds of Rhododendron fortunei Lindl. J. Integr. Agric. 2020, 19, 2016–2026. [Google Scholar] [CrossRef]
- Bolaños-Villegas, P.; Chen, F.C. Advances and Perspectives for Polyploidy Breeding in Orchids. Plants 2022, 11, 1421. [Google Scholar] [CrossRef]
- Zhang, J.J. Polyploid Induction and Identification of Dendrobium officinale. Master’s Thesis, Zhejiang Agriculture and Forestry University, Hangzhou, China, 2013. [Google Scholar]
- Zhang, Q.H.; Li, Z.L.; Tang, M. Study on the Use of Colchicine to Induce Polyploidy of Dendrobium candidum Wall. ex Lindl. J. Yunnan Agric. Univ. 2011, 26, 678–682. [Google Scholar] [CrossRef]
- Wu, R. Analysis of the Polyploid Induction, Differential Gene Expression of Dendrobium officinale and the Development of Its EST-SSR Markers. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2016. [Google Scholar]
- Vilcherrez-Atoche, J.A.; Iiyama, C.M.; Cardoso, J.C. Polyploidization in Orchids: From Cellular Changes to Breeding Applications. Plants 2022, 11, 469. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Martinelli, A.P.; da Silva, J.A.T. A Novel Approach for the Selection of Cattleya Hybrids for Precocious and Season-independent Flowering. Euphytica 2016, 210, 143–150. [Google Scholar] [CrossRef]
- Vilcherrez-Atoche, J.A.; Silva, J.C.; Clarindo, W.R.; Mondin, M.; Cardoso, J.C. In Vitro Polyploidization of Brassolaeliocattleya Hybrid Orchid. Plants 2023, 12, 281. [Google Scholar] [CrossRef]
- Luo, Z.; Iaffaldano, B.J.; Cornish, K. Colchicine-induced Polyploidy has the Potential to Improve Rubber Yield in Taraxacum kok-saghyz. Ind. Crops Prod. 2018, 112, 75–81. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, F.; Fu, J. In Vitro Polyploid Induction Using Colchicine for Zingiber officinale Roscoe cv. ‘Fengtou’ginger. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 142, 87–94. [Google Scholar] [CrossRef]
- Sadat Noori, S.A.; Norouzi, M.; Karimzadeh, G. Effect of Colchicine-induced Polyploidy on Morphological Characteristics and Essential Oil Composition of Ajowan (Trachyspermum Ammi L.). Plant Cell Tissue Organ Cult. (PCTOC) 2017, 130, 543–551. [Google Scholar] [CrossRef]
- Fang, C.P.; Shan, C.G.; Wang, W.T. Preliminary Study on Induction and Identification of Polyploidy of Shandong Salvia miltiorrhiza Buds Treated by Colchicine. Agric. Sci. Technol. Hunan 2011, 12, 1338–1341. [Google Scholar] [CrossRef]
- Li, M.X.; Chen, R.Y. A Suggestion on the Standardization of Karyotype Analysis in Plants. Plant Sci. J. 1985, 3, 297–302. [Google Scholar]
- Stebbins, G.L. Chromosomal Evolution in Higher Plants; Edward Arnold: London, UK, 1971; pp. 87–89. [Google Scholar]
- Arano, H. Cytological Studies in Subfamily Carduoideae (Compositae) of Japan IX. Bot. Soc. Jpn. 1963, 76, 32–39. [Google Scholar]
- Vichiato, M.R.M.; Vichiato, M.; Pasqual, M. Tetraploidy Induction and Identification in Dendrobium nobile Lindl (Orchidaceae). Rev. Cienc. Agron. 2007, 38, 385. [Google Scholar]
- Lv, Z.; Zhu, F.; Jin, D.; Wu, Y.; Wang, S. Seed Germination and Seedling Growth of Dendrocalumus brandisii in Vitro, and the Inhibitory Mechanism of Colchicine. Front. Plant Sci. 2021, 12, 784581. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Chen, J.J.; Cao, Y.M. Induction of Tetraploids in ‘Red Flash’caladium Using Colchicine and Oryzalin: Morphological, Cytological, Photosynthetic and Chilling Tolerance Analysis. Sci. Hortic. 2020, 272, 109524. [Google Scholar] [CrossRef]
- Ren, Y.; Jing, Y.; Kang, X. In Vitro Induction of Tetraploid and Resulting Trait Variation in Populus alba× Populus glandulosa Clone 84 K. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 146, 285–296. [Google Scholar] [CrossRef]
- Liao, S.M. Polyploid Mutation and Level Determination of Dendrobium candidum. Master’s Thesis, Zhejiang University, Hangzhou, China, 2002. [Google Scholar]
- Zhan, Z.G.; Xu, C. Study on Polyploid Induction of Dendrobium officinale by Colchicine. J. Zhejiang Univ. Sci. Ed. 2011, 38, 321–325. [Google Scholar] [CrossRef]
- Wei, K.H.; Xu, J.P.; Li, L.X. In Vitro Induction and Generation of Tetraploid Plants of Sophora tonkinensis Gapnep. Pharmacogn. Mag. 2018, 14, 149. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Wu, H. In Vitro Segregation of Tetraploid and Octoploid Plantlets from Colchicine-induced Ploidy Chimeras in Echinacea purpurea L. HortScience 2016, 51, 549–557. [Google Scholar] [CrossRef]
- Tokumoto, Y.; Kajiura, H.; Takeno, S. Induction of Tetraploid Hardy Rubber Tree, Eucommia ulmoides, and Phenotypic Differences from Diploid. Plant Biotechnol. 2016, 15, 1219. [Google Scholar] [CrossRef]
- Gao, F.H.; He, L.J.; Chen, H.J.; Liu, J.W.; Dan, Y.M.; Wang, W.J.; Yu, L.X. Chromosome Doubling and Polyploid Karyotype Analysis of Wild Lycium ruthenicum. Mol. Plant Breed. 2020, 18, 7522–7529. [Google Scholar] [CrossRef]
- Xu, Y.L. Preliminary Study on the Relationship Between Flower Color Type and Intracellular Environment of Camellia yunnanensis. J. Yunnan Agric. Univ. (Nat. Sci.) 2015, 30, 9. [Google Scholar] [CrossRef]
- Samatadze, T.E.; Yurkevich, O.Y.; Khazieva, F.M.; Basalaeva, I.V.; Konyaeva, E.A.; Burova, A.E.; Zoshchuk, S.A.; Morozov, A.I.; Amosova, A.V.; Muravenko, O.V. Agro-Morphological and Cytogenetic Characterization of Colchicine-Induced Tetraploid Plants of Polemonium caeruleum L. (Polemoniaceae). Plants 2022, 11, 2585. [Google Scholar] [CrossRef]
- Zeng, J.; Wang, D.; Wu, Y. Karyotype Analysis of Gazania rigens Varieties. Hortic. Plant J. 2016, 2, 279–283. [Google Scholar] [CrossRef]
- Noedoost, F.; Sheidai, M.; Riahi, H. Karyotype Analysis in Some Chara Species (Charales, Charophyceae) in Iran. Caryologia 2016, 69, 162–169. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, D.; Kang, L. Ploidy Variation and Karyotype Analysis in Hemerocallis spp. (Xanthorrhoeaceae) and Implications on Daylily Breeding. N. Z. J. Crop Hortic. Sci. 2014, 42, 183–193. [Google Scholar] [CrossRef]
- Li, T.J.; Qu, Y.; Wang, B. Karyotype Analysis of Different Ploidy Camellia yunnanensis. J. Cent. South Univ. For. Technol. 2023, 2, 1–8. [Google Scholar] [CrossRef]
- Yu, C.; Luo, L.; Pan, H. Karyotype Analysis of Wild Rosa Species in Xinjiang, Northwestern China. J. Am. Soc. Hortic. Sci. 2014, 139, 39–47. [Google Scholar] [CrossRef]
- Soltis, D.E.; Albert, V.A.; Leebens-Mack, J. Polyploidy and Angiosperm Diversification. Am. J. Bot. 2009, 96, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Wendel, J.F. Novel patterns of gene expression in polyploid plants. Trends Genet. 2005, 21, 539–543. [Google Scholar] [CrossRef]
- Mishra, B.K.; Pathak, S.; Sharma, A. Modulated gene expression in newly synthesized auto-tetraploid of Papaver somniferum L. S. Afr. J. Bot. 2010, 76, 447–452. [Google Scholar] [CrossRef]
- Tarkesh, E.S.; Karimzadeh, G.; Naghavi, M.R.; Vrieling, K. Altered gene expression and root thebaine production in polyploidized and methyl jasmonate-elicited Papaver bracteatum Lindl. Plant Physiol. Biochem. 2021, 158, 334–341. [Google Scholar] [CrossRef]



| Processing Time/h | Treatment Concentration/% | Number of Samples | Number of Deaths | Survival Rate/% |
|---|---|---|---|---|
| 0 (CK) | 0 (CK) | 300 | 1.00 ± 0.58 h* | 99.00 |
| 24 | 0.1 | 300 | 8.33 ± 0.67 g | 91.67 |
| 24 | 0.2 | 300 | 16.67 ± 0.33 ef | 83.33 |
| 24 | 0.3 | 300 | 17.33 ± 1.76 ef | 82.67 |
| 24 | 0.4 | 300 | 21.00 ± 0.58 def | 79.00 |
| 36 | 0.1 | 300 | 14.67 ± 1.33 f | 85.33 |
| 36 | 0.2 | 300 | 22.67 ± 1.20 de | 77.33 |
| 36 | 0.3 | 300 | 26.33 ± 0.89 cd | 74.67 |
| 36 | 0.4 | 300 | 29.67 ± 1.45 bc | 70.33 |
| 48 | 0.1 | 300 | 18.33 ± 1.20 ef | 81.67 |
| 48 | 0.2 | 300 | 24.00 ± 0.58 cde | 76.00 |
| 48 | 0.3 | 300 | 34.33 ± 2.19 b | 65.66 |
| 48 | 0.4 | 300 | 42.00 ± 4.93 a | 58.00 |
| Processing Time/h | Treatment Concentration/% | Plant Height/mm | Internodes Length/mm | Stem Diameter/mm | Leaf Shape Index/mm |
|---|---|---|---|---|---|
| 0 (CK) | 0 (CK) | 22.40 ± 0.77 a* | 3.86 ± 0.09 a | 2.72 ± 0.09 d | 1.97 ± 0.08 a |
| 24 | 0.1 | 18.97 ± 0.91 bcd | 3.98 ± 0.19 a | 3.45 ± 0.9 a | 1.77 ± 0.04 bc |
| 24 | 0.2 | 21.27 ± 0.99 abc | 3.89 ± 0.11 a | 3.37 ± 0.10 b | 1.55 ± 0.05 d |
| 24 | 0.3 | 21.59 ± 1.08 ab | 3.99 ± 0.13 a | 3.32 ± 0.12 bc | 1.61 ± 0.05 cd |
| 24 | 0.4 | 18.27 ± 0.70 cd | 3.82 ± 0.10 ab | 2.97 ± 0.08 bcd | 1.77 ± 0.05 bcd |
| 36 | 0.1 | 18.79 ± 0.81 bcd | 3.60 ± 0.07 bcd | 3.24 ± 0.33 bcd | 1.66 ± 0.04 bcd |
| 36 | 0.2 | 20.46 ± 0.95 abcd | 3.50 ± 0.09 bcd | 2.91 ± 0.09 cd | 1.78 ± 0.07 bc |
| 36 | 0.3 | 18.88 ± 0.66 bcd | 3.45 ± 0.07 bcd | 2.88 ± 0.09 cd | 1.77 ± 0.08 bc |
| 36 | 0.4 | 18.17 ± 0.87 d | 3.35 ± 0.09 cd | 2.85 ± 0.21 cd | 1.77 ± 0.05 bc |
| 48 | 0.1 | 19.07 ± 0.73 bcd | 3.67 ± 0.09 abc | 2.95 ± 0.09 bcd | 1.73 ± 0.04 bc |
| 48 | 0.2 | 20.62 ± 1.17 abcd | 3.91 ± 0.10 ab | 3.06 ± 0.12 bcd | 1.79 ± 0.07 b |
| 48 | 0.3 | 18.80 ± 0.88 bcd | 3.14 ± 0.05 d | 2.98 ± 0.09 d | 1.62 ± 0.04 cd |
| 48 | 0.4 | 20.29 ± 0.95 abcd | 3.28 ± 0.08 cd | 2.98 ± 0.08 bcd | 1.64 ± 0.06 bcd |
| Morphological Characteristics | Diploid Plants | Tetraploid Plants |
|---|---|---|
| Plant height/mm | 22.40 ± 0.77 b* | 21.47 ± 1.67 b |
| Internodes length/mm | 3.86 ± 0.09 a | 3.36 ± 0.17 b |
| Stem diameter/mm | 2.72 ± 0.09 b | 3.27 ± 0.18 a |
| Leaf shape index | 1.97 ± 0.08 a | 1.79 ± 0.75 b |
| Processing Time/h | Treatment Concentration/% | Number of Samples | Stomatal Variation Rate/% | Number of Chromosome Doubles |
|---|---|---|---|---|
| 0 (CK) | 0 (CK) | 30 | 0.00 | 0 |
| 24 | 0.1 | 30 | 23.33 | 0 |
| 24 | 0.2 | 30 | 26.76 | 1 |
| 24 | 0.3 | 30 | 30.00 | 1 |
| 24 | 0.4 | 30 | 20.00 | 0 |
| 36 | 0.1 | 30 | 10.00 | 0 |
| 36 | 0.2 | 30 | 20.00 | 1 |
| 36 | 0.3 | 30 | 13.33 | 1 |
| 36 | 0.4 | 30 | 6.67 | 0 |
| 48 | 0.1 | 30 | 26.67 | 0 |
| 48 | 0.2 | 30 | 13.33 | 3 |
| 48 | 0.3 | 30 | 13.33 | 2 |
| 48 | 0.4 | 30 | 16.67 | 1 |
| Stomatal Indicators | Diploid Plants | Tetraploid Plants |
|---|---|---|
| Stomatal length diameter/μm | 38.09 ± 0.94 b* | 53.50 ± 2.86 a |
| Stomatal short diameter/μm | 34.84 ± 0.79 b | 48.13 ± 2.33 a |
| Stomatal density/mm2 | 9.56 ± 0.38 a | 6.00 ± 0.41 b |
| Ploidy | Relative Length | Arm Ratio | Length Ratio of Longest Chromosome to Shortest Chromosome | Chromosomes with Arm Ratio > 2/% | Karyotype Formula | Karyotype | Asymmetry Coefficient/% |
|---|---|---|---|---|---|---|---|
| Diploid plants | 2.9~8.5 | 1.10~2.15 | 2.93 | 36.84 | 2n = 2x = 38 = 24m + 14sm | 2B | 60.59 |
| Tetraploid plants | 3.2~8.1 | 1.06~2.2 | 2.53 | 23.68 | 2n = 4x = 76 = 58m + 18sm | 2B | 60.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Duan, S.-D.; Jia, Y.; Hao, L.-H.; Xiang, D.-Y.; Chen, D.-F.; Niu, S.-C. Polyploid Induction and Karyotype Analysis of Dendrobium officinale. Horticulturae 2023, 9, 329. https://doi.org/10.3390/horticulturae9030329
Liu Y, Duan S-D, Jia Y, Hao L-H, Xiang D-Y, Chen D-F, Niu S-C. Polyploid Induction and Karyotype Analysis of Dendrobium officinale. Horticulturae. 2023; 9(3):329. https://doi.org/10.3390/horticulturae9030329
Chicago/Turabian StyleLiu, Yang, Shan-De Duan, Yin Jia, Li-Hong Hao, Di-Ying Xiang, Duan-Fen Chen, and Shan-Ce Niu. 2023. "Polyploid Induction and Karyotype Analysis of Dendrobium officinale" Horticulturae 9, no. 3: 329. https://doi.org/10.3390/horticulturae9030329
APA StyleLiu, Y., Duan, S.-D., Jia, Y., Hao, L.-H., Xiang, D.-Y., Chen, D.-F., & Niu, S.-C. (2023). Polyploid Induction and Karyotype Analysis of Dendrobium officinale. Horticulturae, 9(3), 329. https://doi.org/10.3390/horticulturae9030329





