Abstract
India is one of the leading citrus−producing countries, next to China and Brazil. Despite the sizeable production, especially of lemons and limes, India contributes meagerly to the world’s citrus market. Of the various factors responsible for the restricted quantum of citrus export, citrus canker (Xanthomonas citri pv. citri) is one of the leading serious causes and directly impacts the appearance of the fruits. Considering the extent of damage, the present study aimed to understand the impact of Xanthomonas citri pv. citri (Xcc) on the physio−biochemical responses in contrasting Citrus spp. Two genotypes, each of three citrus species, namely Citrus aurantifolia (Pusa Udit and ALC−35), C. limon (Kagzi Kalan and Konkan Seedless), and C. paradisi (Redblush and Marsh Seedless), were artificially inoculated with Xcc (108 to 109 cfu/ mL) by the pinprick method. The physio−biochemical changes in the host were evaluated after 48 h post inoculation (hpi). The chlorophyll content (total, a, and b) degradation and reduction in leaf gas exchange parameters, such as photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs), and intrinsic water−use efficiency (iWUE), were measured to a greater extent in susceptible than resistant genotypes. The microscopic observations also evidenced higher stomatal density with larger stomatal areas in susceptible genotypes, favoring the easier penetration of Xcc in host tissues than resistant species or genotypes. The higher activities of various antioxidant enzymes, viz., superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and glutathione reductase (GR), the contents of soluble protein, and phenolics were measured in resistant genotypes in contrast to susceptible genotypes. The activities of phenyl ammonia lyase (PAL) and polyphenol oxidase (PPO) were also higher in resistant genotypes, whilst the levels of ROS (superoxide radical and hydrogen peroxide) production were enhanced in susceptible genotypes. Based on the host–pathogen interaction, the order of resistance in a descending manner was found as Kagzi Kalan, Marsh Seedless, Redblush, Konkan Seedless, Pusa Udit, and ALC−35. Further, the results will enhance the understanding of the pathogen mechanism during pathogenesis in resistant and susceptible Citrus species.
1. Introduction
Citrus, belonging to the Rutaceae family, is the third most important fruiting plant in the world. Globally, citrus fruits are grown on 200,400 ha, with an annual production of 158 million tons. As of 2020, China bestowed 28.21% of the world’s citrus fruit production, followed by Brazil, India, Mexico, and the US (accounting for 59.45% of citrus fruit production). India is one of the largest citrus producers, ranking third next to China and Brazil [1]. Citrus spp. (including lemon, lime, sweet orange, mandarin, grapefruit, citron, pummelo, tangelo, tangerine, etc.) is the third most important fruit crop in India after mango (Mangifera indica L.) and banana (Musa paradisiaca L.), contributing 12.5% of production, and occupies a 1091 thousand ha area with an annual production of 14,150 thousand tonnes [2].
India largely produced limes and lemons to generate revenue of about 9 million USD by export and contributed 17.42% of world production [3,4]. Several diseases such as foot rot, fruit drop, anthracnose, citrus canker, citrus scab, sooty mold, ringspot, citrus tristeza, citrus greening, and citrus exocortis threaten citrus cultivation [5]. Despite having favorable soil and climate conditions, citrus trees have low productivity due to biotic stresses. Among them, citrus canker is the most destructive disease, affecting eclectic commercial varieties of Citrus spp. and accounting for 5 to 30% yield losses [6]. The disease is still spreading even after spending an estimated 12 million USD annually on its management [7,8]. Citrus canker caused by Xanthomonas citri pv. citri (Xcc) affects limes, lemons, trifoliate oranges, grapefruit, sweet oranges, and tangelo. Tangerines and mandarins are less susceptible to infection, whereas lemons and tangelo are moderately susceptible. Citrus fruits, including calamondin, citron, and oval kumquat, have been reported resistant to canker [9,10,11,12]. All above−ground plant parts of citrus plants are susceptible to canker. The disease symptoms are characterized by the appearance of conspicuous raised necrotic lesions on the leaves, twigs, and fruits of citrus. Severe infections can cause a range of symptoms, from defoliation, blemished fruit, premature fruit drop, and twig dieback to general tree decline [13,14]. These symptoms result from the hypertrophy of parenchymal cells, which is followed by a significant amount of bacterial exudation and multiplication [15,16]. In particular during rain splash, Xcc spreads through active lesions on canker−infected leaves or fruits. This process may be hastened by wind or by the movement of infected material to other places [6,17,18].
The occurrence of disease in citrus plants reduces their quality by causing premature fruit drop and causes the most economically significant damage to the plants. In addition to direct yield loss, Xcc has an impact on fruit trade due to its appearance. Younger leaf tissues are more susceptible to canker than older tissues, and lesions develop after inoculations when bacteria come into direct contact with mesophyll cells. Furthermore, “symptom” expression depends upon the weather conditions. Additionally, citrus leaf miner (Phyllocnistis citrella Stainton) larvae damage caused during the flush periods increased X. citri pv. citri infection [14].
The pathogenesis of Xcc starts when bacteria land on the leaf’s surface and make contact with the cuticle. Many foliar pathogenic bacteria have evolved the ability to adhere and form biofilm on the host surface before entering the intercellular spaces of the mesophyll tissue through stomata [19,20]. Moreover, stomatal density and structure could also play a crucial role as physical barriers to bacterial infection [20,21]. The mechanisms underlying plant−preformed defense and its relevance for limiting bacterial pathogen entry to the apoplast remain poorly understood. According to studies, the resistant kumquat genotype “Meiwa” and the susceptible navel orange genotype “Newhall” respond differently to Xcc infection due to differences in stomatal density, size, aperture, and epicuticular wax content [22,23]. Additionally, in the “Pera” IAC orange, a smaller stomatal aperture was associated with lower susceptibility to Xcc. However, there were no differences observed in terms of stomatal density between this genotype and the more susceptible cultivar “Washington navel” [24].
Plants have evolved a delicate network of strategies to protect themselves against pathogenic invasion during their life cycle, including pre−existing physical (structural), pre−formed bio−chemical obstacles, and the activation of physiological and metabolic responses. These barriers are claimed to be the first line of plant defense that plays a crucial role in combating the ingression of pathogens [25,26,27,28]. It is reasonable to speculate that the resistant and susceptible genotypes of a given plant species may have different structures and show divergent responses when the same pathogen attacks them [29]. Therefore, a comparative investigation of these different citrus genotypes with variable disease resistance will help to unravel some structural and physio−biochemical elements that may account for the contrasting resistance, based on the development of new preventive methods or the creation of novel germplasm via state−of−the−art techniques.
Plants have a complex array of cell−based defense systems that they use to maintain their resistance to potentially harmful pathogens [30,31]. Reactive oxygen species (ROS), including superoxide (O2−) and H2O2, are formed as a first line of defense by oxidative bursts produced in apoplasts in response to the early pathogen recognition response [27]. In the current models of plant responses, ROS and other radicals work as regulators of resistance−related genes [32], catalysts for covalent cell−wall modifications, and signals for cell−death reactions [30,31]. High ROS levels in plants act to strengthen the cell wall and prevent pathogen growth, which enhances host resistance to pathogens through hypersensitive responses and modifies gene expression via signaling molecules [32,33]. However, a high accumulation of ROS can be toxic to plant cells by preventing plant growth and development [31]. As a result, antioxidant compounds and enzymes are required to maintain ROS homeostasis [34,35,36,37]. ROS are produced in plant cells by Class III peroxidases (CIII Prxs, or POD) and their associated pathways, which include photosynthesis, photorespiration, and respiration. NADPH oxidase, which is resident at the cell surface, is also involved in the production of ROS [38,39,40,41,42]. In addition, the ROS scavenger’s superoxide dismutase (SOD), catalase (CAT), and glutathione s-transferase (GST) cooperate with ROS producers to maintain ROS homeostasis [43].
The hypersensitive response induces apoptosis and is suggested to play a role in host–pathogen interactions. It is characterized by rapid cell death in response to pathogen invasion and causes the accumulation of reactive oxygen species [44]. The enzymatic activities of antioxidants tightly regulate the metabolism of reactive oxygen species (ROS) [11]. The roles of ROS and antioxidants’ enzymatic activities in host resistance and pathogen invasion are highly dependent on the type of plant–microbe interactions [45]. Cellular resistance to Xcc in various citrus genotypes is a complex process. The recognition of bacterial cells and the subsequent transduction of signaling molecules may lead to very different physiological and structural modifications between resistant and susceptible genotypes.
Superoxide dismutases (SODs) cause the dismutation of the superoxide radical (O2−) into molecular oxygen and hydrogen peroxide (H2O2). They generally act in concert with catalases that further dismutate H2O2 into innocuous oxygen and water [46]. Although many aspects of metabolism have been characterized in Xcc-citrus interactions, antioxidant metabolism, which plays a crucial role in plants’ ability to respond to biotic stress, is poorly understood [47]. Reactive oxygen species (ROS), such as superoxide radicals (O2−) and hydrogen peroxide (H2O2), are produced by altered oxidative metabolism in plants in response to pathogens [48]. H2O2 evolution is essential and integral to pathogen-induced programmed cell death (PCD) [49]. Superoxide dismutase activity, which converts superoxide radicals into H2O2, is one of the crucial components of ROS metabolism. A major source of H2O2 is the dismutation of O2– via the activity of SOD [50]. SODs are regarded as a first step in reducing oxidative stress by converting O2– to H2O2 during normal metabolism. Peroxidases catabolize H2O2, a byproduct of normal metabolism, into non-toxic forms. H2O2 concentrations increased when the activities of POD, CAT, and APOD were reduced [50], which in turn promoted systemic acquired resistance [9]. PODs play a variety of roles in disease resistance, including lignification, suberization, and protein cross-linking of cell-wall protein [51]. PODs of healthy plants are involved in cell elongation.
Scientists worldwide have paid significant attention to canker disease because of its extensive damage to the citrus industry. They have done a lot of work to develop potential strategies to control or prevent it. So far, significant efforts have been directed toward evaluating and screening the susceptibility of a large spectrum of commercial citrus species or relatives widely used in producing regions [10,52,53,54]. In addition, physiological or biochemical analysis has been conducted, such as investigating changes in enzymatic activity [55] and ROS accumulation [56]. However, it has to be mentioned that, currently, little information is available on a comprehensive comparative analysis of the structure and biochemical response to Xcc infection between different citrus genotypes with contrasting Xcc resistance, except for work that has conducted a comparative analysis between “Mexican” lime and “Yuzu” for Xcc infection in spray-inoculated young leaves. Despite the fact that some work on host response to Xcc has been done, a pairwise comparison of stomatal characteristics and physio-biochemical reactions to the Xcc challenge between resistant and susceptible genotypes is largely lacking. To address this issue, in the present study, we attempted to understand the anatomical interrelationship with disease intensity in different citrus genotypes with contrasting reactions. Meanwhile, a physiological and biochemical assay was performed to analyze the activities of several biochemical constituents following the Xcc attack.
2. Materials and Methods
2.1. The Site Conditions and Plant Material
The present investigation was carried out in the citrus experimental block of the Division of Fruits and Horticultural Technology, Indian Council of Agriculture Research, Indian Agricultural Research Institute, New Delhi, in 2021. The experimental area is situated at 77°12′ E longitude, 28°40′ N latitude, and an altitude of 228.6 m above mean sea level, having typical subtropical climatic conditions defined by a hot and dry summer followed by a cold winter. May and June are the hottest months, with the maximum temperature varying between 38 and 44.0 °C, and December and January are the coolest months, with temperatures ranging between 6.1 and 16.9 °C. In the present experiment, six different citrus genotypes (two from each group),viz., acid lime (Pusa Udit and Acid lime collection (ALC-35)), lemon (Kagzi Kalan and Konkan Seedless), and grapefruit (Marsh seedless and Redblush), showing contrasting reactions (most tolerant or susceptible) to Xcc infection were selected. The plants of acid lime were raised through seeds, while the plants of lemon were propagated through air layering. The grapefruit varieties were propagated through T-budding on Citrus Jambhiri rootstock. The propagated materials (one year old) were transplanted into plastic pots (12″) containing about 8 kg sterilized mixture of soil (3 parts) and farm yard manure (1 part). The soil type was a virgin Inceptisol with a pH of 7.4 and an electrical conductivity (EC; 1:2 (w/v) soil/water) of 0.75 dS m−1, a cation exchange capacity (CEC) of 10.72 c mol kg−1, an organic carbon content of 0.48% (w/w), a soil N concentration of 240.23 kg ha−1, a soil P2O5 concentration of 58.65 kg ha−1, and a soil K2O concentration of 555.92 kg ha−1.The plants were inoculated with X. citri pv. citri using the pinprick method in the first week of August 2021.After inoculation, the plants were covered with polythene sheet overnight to maintain the proper humidity for disease development. The plants inoculated with sterilized distilled water were used as control. The data were recorded from pathogen and mock-inoculated plants at 48 h post inoculation.
2.2. Leaf Gas Exchange Parameters
The leaf gas exchange parameters, including net photosynthesis (A), transpiration rate (E), and stomatal conductance (gs), were measured on three mature leaves on each replication between 10 a.m. and 12 a.m. using the LCi-SD Ultra Compact Photosynthesis System (ADC Bio Scientific Ltd., Global House, Hoddesdon, UK). The intrinsic water-use efficiency (iWUE) is defined as the ratio of the fluxes of net photosynthetic rate and conductance for water vapor, which indicates the cost of assimilation per unit of water, expressed in units of µmol mol−1 [57]. iWUE was calculated by dividing the net photosynthetic rate (A) by stomatal conductance (gs). The studies on the gas exchange were recorded under the following conditions: day temperature of 34.6 °C; night temperature of 27 °C; relative humidity (RH) of 78.9%; and a photoperiod of 12 h.
2.3. Stomatal Observations
Stomatal distribution was examined using epidermal imprints of the abaxial surfaces of mature, fully expanded leaves [58]. Feviquick adhesive was smeared uniformly to the middle portion of the underside of a leaf, away from the leaf margins, to form epidermal imprints. This leaf position has stomatal dimensions and density that represent the average of the entire leaf [25]. After 5 min, the dried membrane was cautiously skinned off and mounted on microscopic slides. A digital fluorescence microscope with an inbuilt camera (Leadz camera-GSS (United Kingdom)) was used to measure size and count. Stomata size (pore length and width) and stomata complex (pore + guard cell (length and width) were measured at 40× magnification to the nearest 16 µm.
The stomatal density was determined by counting the number of stomata per field (200×200 μm2) of view at 40x magnification. These values were then converted into the number of stomata per μm2. The sum of the pore length and width was used to calculate the stomatal area. Similarly, the stomatal area complex (pore + guard cell) was measured by adding the stomatal complex length and width. The stomatal index (I) was calculated using the number of stomatal cells per square millimeter of the leaf’s epidermis. It is the ratio of stomatal cells to epidermal cells. The stomatal conductance index (SCI) was computed by multiplying the pore length with stomatal density. To estimate stomatal density, the average minimal sample size was three leaves per genotype. The length and width of the stomata cells were also measured three times.
2.4. Chlorophyll Contents
Chlorophyll contents (“a”, “b”, and total) were quantified by a non-maceration method using dimethyl sulfoxide (DMSO) [59]. A total of 100 mg of the finely chopped leaves were placed in test tubes with 10 mL of DMSO. The test tubes were covered with aluminum foil and kept in an oven at 65 °C for 4 h. After that, the Spectro UV-VIS Double Beam PC Ver. 3.3 Labomed, Inc., was used to measure the absorbance of the chlorophyll solution at 645 and 663 nm using dimethyl sulfoxide as a blank. The amount of chlorophyll is given as mg g−1 FW. Chlorophyll “a”, “b”, and total were calculated by using formulae:
A = Absorbance, W = Weight of the sample, V = Volume of DMSO (dimethyl sulfoxide) in mL.
2.5. Antioxidant Enzyme Assay
Preparation of Enzyme Extract
Fresh leaves from each replication (plant) were taken 48 h after inoculation and stored in an ice box before being washed in tap water followed by double-distilled water. One gram of the leaf sample was weighed and homogenized with liquid nitrogen in a pre-chilled mortar and pestle, and then 10 mL of phosphate buffer (100 mM; pH 7.5, containing 0.5 mM ethylene diamine tetra acetic acid) was added. The homogenate was collected in oak-ridge tubes and centrifuged at 15,000× g for 20 min at 4 °C. The supernatant was strained through two layers of muslin cloth and kept in a refrigerator (−20 °C). Using this supernatant’s extract, the following antioxidant enzymes were measured:
Superoxide Dismutase (SOD) (EC 1.15.1.1)
SOD activity in the leaf was measured using the method outlined in [60]. The assay was predicated on SOD’s ability to inhibit the photochemical reduction of nitro blue tetrazolium (NBT). The reaction mixture (3.0 mL) was prepared in tubes consisting of 0.2 mL of 200 mM methionine, 0.2 mL of 3 mM ethylene diamine tetra acetic acid (EDTA), 0.1 mL of 2.25 mM NBT, 0.1 mL of 60 M riboflavin, 100 µL of enzyme extract, and phosphate buffer. Riboflavin was added as the last component, and the tubes were stirred well. The reaction was initiated for 15 min by keeping the tubes 30 cm below a light bank consisting of two 15-watt fluorescent lamps. After 15 min, the lights were switched off, and the tubes were immediately covered with a black cloth to stop the reaction. Afterward, the absorbance of the mixture was read on UV-VIS double beam PC 8 scanning Auto cell spectrophotometer (Specord 200, Analytik Jena, India) at 560 nm wavelength. A complete reaction mixture containing 0 µL of enzyme extract, developing maximum color, served as a control. The amount of enzyme extract in the reaction mixture was used to plot the absorbance (log A 560). The resultant graph was used to read the volume of enzyme extract, resulting in a 50% reduction in absorbance compared to the control. One unit of SOD activity in the sample was taken as the amount of enzyme, which resulted in a 50% reduction in absorbance compared with the control tube, lacking enzyme extract. Finally, SOD was quantified using the sample’s soluble protein content and expressed as mg−1 TSP minutes−1.
Catalase (EC 1.11.1.6)
The catalase assay was based on hydrogen peroxide (H2O2) absorption in the UV range at 240 nm. Over time, a reduction in absorbance was observed [61]. The reaction mixture (3.0 mL), which consisted of 1.5 mL of 100 mM potassium phosphate buffer (pH 7.0), 0.5 mL of 75 mM H2O2, and 200 µL of enzyme extract, was prepared in test tubes, and the rest was water to make up the volume. In addition to H2O2, the reaction started instantly, and a decrease in absorbance was observed for 1 min at 240 nm. Enzyme activity was calculated as the concentration of H2O2 reduced (initial reading − final reading = quantity of H2O2 reduced) and expressed as μmoles H2O2 hydrolyzed mg−1 TSP minutes–1.
Guaiacol Peroxidase (EC 1.11.1.7)
Guaiacol peroxidase activity in leaf samples was measured using the method suggested by [62]. In the assay, guaiacol was utilized as the enzyme substrate. One gram of leaf sample was homogenized to prepare the enzyme extract. The reaction mixture was made in tubes by adding 3 mL of phosphate buffer (0.1 M; pH 6.1), 500 µL of H2O2 (12 mM), 500 L of guaiacol (96 mM) as an enzyme substrate, and 200 µL of enzyme extract. The absorbance was measured using a UV-VIS double beam PC 8 scanning Auto cell spectrophotometer, Specord 200, Analytik Jena, India at a wavelength of 470 nm. The absorbance increased at 30 s intervals until it reached a constant reading. The guaiacol peroxidase activity was measured in units of µmol tetra-guiacol mg−1min−1 total soluble protein (TSP).
Glutathione Reductase (GR) (EC 1.8.1.7)
The glutathione reductase enzyme’s activity was measured according to the method of [63]. The 3.0 mL reaction mixture contained 1.0 mL of potassium phosphate buffer (0.2 M, pH 7.5) containing 1.0 mM ethylene diamine tetra acetic acid, 0.5 mL of 3.0 mM DTNB (5, 5-dithiobis 2-nitrobenzoic acid) in potassium phosphate buffer (10 mM, pH 7.5), 0.1 mL NADPH (2.0 mM), 0.1 mL of enzyme extract, and the remaining distilled water. The reaction started by adding 0.1 mL of 2.00 GSSG (oxidized glutathione). The absorbance increase at 412 nm was recorded at 25 °C for 10 min on a UV-VIS double beam PC 8 scanning Auto cell spectrophotometer (Specord 200, Analytik Jena, India). The activity of glutathione reductase was measured in mmoles DNTB reduced mg−1 total soluble proteins (TSP) min−1.
2.6. Reactive Oxygen Species (Oxidative Stress)
2.6.1. Superoxide Radical (O2−)
The total superoxide radical content was measured according to the method of [64]. It was based on the principle of the formation of blue-colored formazone by nitroblue tetrazolium chloride with superoxide radical (O2−) in the absence of total superoxide dismutase (SOD) activity. Plant tissue was homogenized in pre-cooled phosphate buffer (0.2 M, pH 7.2) containing 1 mM diethyl di-thio carbamate (to inhibit SOD). In a Sigma refrigerated centrifuge (Model 3K−30 Osterode, Germany), the homogenate was centrifuged at 10,000 rpm for 10 min. The supernatant was used immediately for the estimation of superoxide radicals. A 3 mL reaction mixture was prepared in tubes consisting of 0.2 mL of 200 mM methionine, 0.1 mL of 3 mM EDTA, 0.1 mL of 2.25 mM NBT, 50 µL of 1.5 M sodium carbonate, 250 µL of enzyme extract, and 2.30 mL water. It was incubated at 30 °C for 10–15 min, and absorbance was recorded at 540 nm. The superoxide content was expressed as Δ540 Mm−1 g−1 FW.
2.6.2. Hydrogen Peroxide (H2O2)
Hydrogen peroxide was estimated by forming a titanium-hydroperoxide complex [65]. Hydrogen peroxide forms a light-yellow-colored titanium-hydroperoxide complex with the titanium reagent. This yellow complex absorbs at 415 nm.
Reagents: acetone: analytical-grade reagent; ammonium hydroxide/ liquid ammonia: analytical-grade reagent; and titanium reagent.
Titanium reagent: One gram of titanium dioxide and 10 g of potassium sulfate were digested in 150 mL of concentrated sulfuric acid over a hot plate for 4 h. The digested mixture was diluted to 500–600 mL and stirred with a magnetic stirrer cum heater at 70–80 °C until a clear, transparent solution was obtained. It was diluted to 1.5 L and stored in a dark brown bottle [66].
Estimation of H2O2
One gram of leaf tissue was ground to a fine powder with the help of liquid nitrogen, followed by the addition of 10 mL of cooled acetone in a cold room (10 °C). The mixture was filtered through Whatman No. 1 filter paper, and then 4 mL of titanium reagent and 5 mL of ammonium solution were added to precipitate the titanium-hydroperoxide complex. The reaction mixture was centrifuged at 10,000× g for 10 min in a refrigerated centrifuge. The precipitate was dissolved in 10 mL of 2 M H2SO4 and then recentrifuged. The supernatant was read at 415 nm against a reagent blank in a UV-visible spectrophotometer (Specord 200, Analytik Jena, India). A standard curve of hydrogen peroxide was prepared by taking a range of concentrations of H2O2, to which 10 mL of cold acetone was added. Various steps followed this, as in the case of the sample, and a curve was drawn by plotting concentrations against respective absorbance. The hydrogen peroxide activity was given in mmoles of H2O2 mg−1 FW.
2.7. Phenyl Ammonia Lyase (PAL) Activity
PAL activity was determined using the method described in [67]. The homogenization buffer contained 25 mM Tris buffer (pH 8.8). The reaction mixture was prepared by adding 0.1 mL of enzyme extract to 0.4 mL of 0.05 M Tris buffer (pH 8.8) containing 0.2 mM L-phenylalanine and then incubated in a water bath at 37 °C for 60 min. The reaction was stopped by adding 0.1 mL of 0.5 N HCL. The trans-cinnamic acid was extracted by adding 2 mL of toluene. The absorbance was measured at 412 nm, and the enzyme activity was calculated. A standard curve of phenylalanine-lyase activity was prepared for the calculation, and the results were expressed in µg cinnamic acid min−1g−1 fresh weight.
2.8. Soluble Protein Content
The Bradford protein assay [68] was used to calculate the content of soluble protein in the enzyme extract. It was based on the principle that Coomassie Brilliant blue G250′s maximum absorbance for an acidic solution shifts from 465 to 595 nm after binding with protein. A standard curve was created using a bovine serum albumin (BSA) stock solution of 100 g/mL, after which dilutions of this stock solution ranging from 0 to 100 g/mL were made. Standard BSA was added to the Bradford reagent (2 mL). It was incubated at room temperature in the dark for 10 min. In a UV-Visible spectrophotometer (Specord 200, Analytik Jena, India), absorbance was measured at 595 nm. The protein content in the sample was measured in mg g−1 FW.
2.9. Total Phenolic Content (TPC) Content
The leaves were used for the estimation of total phenolic content. The extraction of phenolic compounds was performed according to [69] with slight modifications. In brief, 1 g of leaf was mixed with 20 mL of 100% aqueous methanol. Then, it was kept at 37 °C overnight with a 300 rpm incubator shaker, followed by centrifugation at 10,000 rpm for 5 min, before being filtered through Whatman No. 1 paper. Total phenolic content was estimated by the Folin–Ciocalteu method [70]. Here, phenolic samples (250 µL) and distilled water (1 mL) were mixed in a test tube, followed by the addition of Folin–Ciocalteu reagent (250 µL). After 6 min of reaction, 2.5 mL of 7% Na2CO3 was added. The reaction mixture was then incubated at room temperature for 90 min, and absorbance was recorded at 760 nm. A standard curve of gallic acid was prepared to calculate total phenolic content, and results were expressed as micrograms of gallic acid equivalent (GAE) per 100 g of dry weight (µg GAE/100 g DW).
2.10. Polyphenol Oxidase (PPO) Activity
Polyphenol oxidase activity was measured using the method described in [71]. Potassium phosphate buffer (2 mL) and pyrogallol (1 mL) were mixed together in the reaction mixture. Then, 0.1 mL of the enzyme extract was added, and the mixture was kept at room temperature for 3 min to “incubate”. The reaction was stopped by adding sulfuric acid at 0.5 mL, and an OD at 480 nm was taken immediately using a UV-VIS spectrophotometer (Hitachi, U-2900, Japan) against the control, which was a mixture of potassium phosphate buffer (2 mL) and pyrogallol (1 mL). Sulfuric acid (0.5 mL) was added after 3 min of incubation before recording the OD value. The reaction mixture without the enzyme served as the blank. The change in absorbance of 0.1 per minute was used to describe one unit of enzyme activity. The following formula was used to calculate specific activity:
where A is the difference in absorbance of the sample and the control (OD S–OD C).
(A × 3/0.1)/3 = specific activity/minutes/mg protein
2.11. Statistical Analysis
The experiment was laid out in a factorial, completely randomized block design and replicated thrice with five plants per replication. The data were analyzed using the SAS package (9.3 SAS Institute, Cary, Inc., Addison, IL, USA) to calculate F values followed by Tukey’s honest significant test. p values ≤ 0.05 were considered significant.
3. Results
3.1. Physiological Response of Different Citrus Genotypes to X. citri pv. citri Infection
The leaf gas exchange parameters, namely photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs), intrinsic water-use efficiency (iWUE), and chlorophyll (total, “a”, and “b”) content, were significantly influenced by Xcc inoculation in the six citrus genotypes (Table 1 and Figure 1). Of the tested genotypes, Kagzi Kalan lemon proved superior, having the highest A and E. The highest gs was statistically registered in Marsh Seedless grapefruit. Among the six citrus genotypes, Konkan Seedless lemon had significantly higher total, “a”, and “b” chlorophyll contents than other genotypes. In addition, the intrinsic water-use efficiency (iWUE) was recorded in the leaves of Redblush grapefruit; however, it was statistically similar to Kagzi Kalan and Konkan Seedless lemons and ALC-35 lime. Xcc inoculation tended to reduce the A, E, gs, and leaf chlorophyll (total, “a”, and “b”) content in all the genotypes statistically; however, iWUE expressed the reverse trend in response to bacterial inoculation. Following the bacterial inoculation, the highest reduction in A was registered in ALC-35 acid lime (41.17%), followed by Pusa Udit (22.77%), while the lowest reduction in A was noticed in Kagzi Kalan lemon (6.31%). Similarly, the highest and lowest reduction in E was observed in ALC-35 lime (41.27%) and Kagzi Kalan lemon (10.78%), respectively. The decrease in gs in the tested genotypes following Xcc inoculation over control ranged from 15.38 to 55.26%, being lowest in Kagzi Kalan lemon and highest in ALC-35 lime. The iWUE followed an upward trend following Xcc inoculation in all the Citrus spp., which was highest in ALC-35 lime (34.09%) and lowest in Kagzi Kalan lemon (10.53%). The content of total chlorophyll (leaf) also decreased significantly after the inoculation with Xcc, ranging from 9.91% to 42.16%. However, the decrease was rather lower in grapefruit and Kagzi Kalan lemon (9.91–22.93%) than in limes (25.74–42.16%). The highest and lowest decrease in chl“a” content due to citrus canker inoculation wasfound in ALC-35 (47.82%) and Marsh Seedless (10.52%), respectively. Canker-inoculated plants showed lower chl“b” than untreated plants, ranging from −8.51% to −30.30% over non-inoculated plants. The highest decrease in chl“b” content was registered in ALC-35, while it was lowest in Marsh Seedless.

Table 1.
Effect of X. citri pv. citri inoculation on leaf gas exchange and chlorophyll (total, “a”, and “b”) content of citrus genotypes.

Figure 1.
Citrus canker disease symptoms developed on the leaves of susceptible and resistant genotypes of citrus incited by X. citri pv. citri via artificial inoculation (40 days after inoculation). Highly susceptible genotype: ALC-35 lime (A) healthy leaves and (B) infected leaves; immune: Kagzi Kalan lemon (C) healthy leaves and (D) infected leaves; moderately susceptible: Konkan Seedless lemon (E) healthy leaves and (F) infected leaves; susceptible genotype: Pusa Udit lime (G) healthy leaves and (H) infected leaves; moderately resistant genotype: Redblush grapefruit (I) healthy leaves and (J) infected leaves; highly resistant genotype: Marsh Seedless (K) healthy leaves and (L) infected leaves.
3.2. Stomatal Density and Dimensions and Their Correlation with Disease Intensity
The microscopic examination of leaf surface imprints in Citrus spp. is presented in Table 2 and Figure 2. The stomatal density per leaf surface exhibited a significant variation among the tested genotypes, which was highest in ALC-35 (687.68 mm−2) and lowest in Kagzi Kalan (408.31 mm−2) lemon with no significant difference with Pusa Udit (429.80 mm−2) lime. A similar trend was observed in the case of stomatal pore length, which was the highest in ALC-35 (11.50 µm), followed by Konkan Seedless (10.35 µm), without showing a significant difference. Likewise, the highest stomatal pore width was found in ALC-35 (8.27 µm), having similarity statistically with other genotypes except Kagzi Kalan. The stomatal complex length was higher in Konkan Seedless (20.28 µm), which was statistically similar to ALC-35 (20.1 µm) and Pusa Udit (19.39 µm), while it was lowest in Redblush (16.90 µm). All the genotypes were found statistically similar with regards to complex stomatal width; the stomatal area was highest in ALC-35 (95.12 µm2) without any significant difference with Konkan Seedless. Similarly, a trend was found in the case of the complex stomatal area, being highest in Konkan Seedless (332.79 µm2), with no significant differences with Pusa Udit, Kagzi Kalan, and Redblush, while it was lowest in Marsh Seedless grapefruit (246.58 µm2). A higher percentage of the stomatal index at (40x) was found in Konkan Seedless (11.01 %), which was statistically similar to ALC-35 and Redblush without showing a significant difference. The lowest stomatal index was recorded in Pusa Udit (7.01 %). In the case of the stomatal conductance index, no significant difference was found among the genotypes tested in the present study. ALC-35 (7.89) proved superior statistically in respect of the stomatal conductance index, which was lowest in Kagzi Kalan (3.65).

Table 2.
Stomatal density and dimension of Citrus spp.
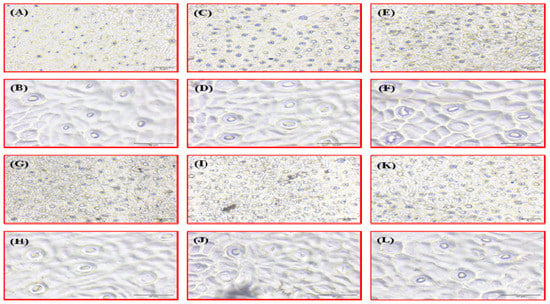
Figure 2.
Comparison of stomatal distribution and aperture size between resistant and susceptible genotypes of Citrus spp. Immune: Kagzi Kalan lemon, (A) 10× and (B) 40×; highly susceptible: ALC-35, (C) 10× and (D) 40×; moderately susceptible: Konkan Seedless lemon, (E) 10× and (F) 40×; susceptible genotype: Pusa Udit lime, (G) 10× and (H) 40×; moderately resistant genotype: Redblush grapefruit, (I) 10× and (J) 40×; highly resistant genotype: Marsh seedless grapefruit, (K) 10× and (L) 40×.
The percentage disease incidence (PDI) was found to be correlated with various stomatal parameters (Figure 3). The correlation coefficient ranged between 0.647 and 0.981 and showed a significant to highly significant positive correlation. The PDI showed significantly positive correlations with stomatal density (0.707), stomata pore length (0.961), stomata pore width (0.949), stomata area (0.981), stomata complex length (0.674), stomata complex width (0.647), stomata complex area (0.688), and stomatal conductance index (0.951). However, PDI showed a strong positive correlation with all the stomatal parameters except the stomatal index.
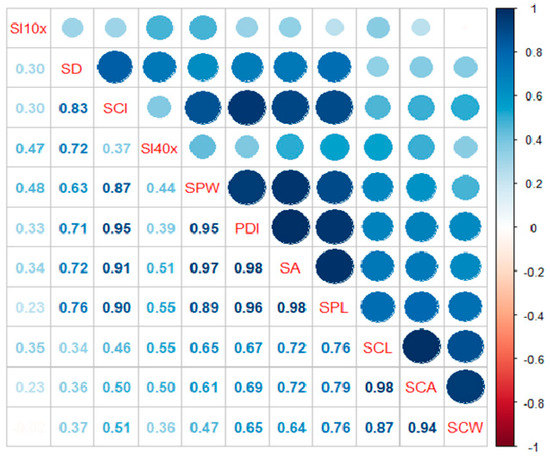
Figure 3.
Correlation of percentage disease index (PDI) with stomatal parameters in different Citrus spp. where PDI: Percentage of disease index, SPL: Stomata pore length, SPW: Stomata pore width, SCL: Stomata complex length, SCW: Stomata complex width, SA: Stomata area, SCA: Stomata complex area, SD: Stomatal density, SI: Stomatal index, SCI: Stomatal conductance index.
3.3. Protein and Total Phenol Content (TPC)
The effect of Xcc inoculation on the contents of soluble protein and phenol and the activities of antioxidant enzymes in the leaves of six citrus genotypes, as influenced significantly, are presented in Table 3. Of the various citrus genotypes, Kagzi Kalan lemon was found to have the highest contents of soluble protein and phenol statistically over other genotypes. The soluble protein content increased in the leaves of the tested citrus genotypes following Xcc inoculation; however, the increase was relatively lower in lemon and grapefruit cultivars (13.92–15.50%) than in limes (34.02–71.63%). The level of phenolic content tended to increase after the artificial inoculation of Xcc with no definite trend. However, Kagzi Kalan lemon, Pusa Udit acid lime, and grapefruit cultivars showed a higher increase (43.47–50.91%) in phenol content than Konkan Seedless lemon and ALC-35 lime (34.28–38.45%).

Table 3.
Effect of X. citri pv. citri inoculation on the contents of soluble protein, phenol, and the activity of antioxidant enzymes of citrus genotypes.
3.4. Antioxidant Enzyme (SOD, CAT, and GR) Activity
The leaves of Marsh Seedless grapefruit had the highest activity of the superoxide dismutase (SOD) enzyme, while the highest activity of guaiacol peroxidase (POD) was recorded in Kagzi Kalan lemon statistically (Table 3). The limes (Pusa Udit and ALC-35) were statistically superior concerning catalase (CAT) activity than other genotypes. Further, Redblush grapefruit statistically proved its superiority in respect of glutathione reductase (GR) activity over the rest of the genotypes studied. Similar to protein and phenol content, all antioxidant enzymes, namely superoxide dismutase (SOD), guaiacol peroxidase (POD), catalase (CAT), and glutathione reductase (GR), were also upregulated by Xcc inoculation in all the Citrus spp. tested in the present study. The highest upregulation of GR was registered in ALC-35 (37.68%) followed by Pusa Udit (31.17%) limes over non-inoculated seedlings. Contrary to this, ALC-35 lime showed the lowest upregulation of CAT (7.69%) than other genotypes, wherein it increased from 23.22 to 41.94%. The highest upregulation of POD (41.48%) and GR (37.68%) was observed in ALC-35 lime, whereas the lowest increase in GR (9.80%) and POD (21.58%) was recorded in Marsh Seedless grapefruit and Kagzi Kalan lemon, respectively.
3.5. Reactive Oxygen Species
The levels of reactive oxygen species (ROS), namely hydrogen peroxide and superoxide, were significantly altered by the inoculation of Xcc in all Citrus species (Table 4). The levels of ROS tended to increase in the leaves of tested citrus genotypes following the inoculation of Xcc. The highest increase in the level of hydrogen peroxide was observed in ALC-35 lime, followed by Pusa Udit (43.47%), while it was lowest in Kagzi Kalan lemon (4.84%) over the non-inoculated state. ALC-35 lime also showed the highest-percent upregulation of the superoxide radical (35.71%), ranging from 9.74 to 18.38% in the rest of the genotypes.

Table 4.
Effect of X. citri pv. citri inoculation on reactive oxygen species (H2O2 and superoxide radical) in citrus genotypes.
3.6. Phenyl Ammonia Lyase (PAL) Activity
Various Citrus species studied in the present study significantly influenced the activity of phenyl ammonia lyase (PAL) due to the inoculation of Xcc (Figure 4), which was further enhanced over non-inoculation. Kagzi Kalan lemon proved to have the highest activity of PAL over the rest of the genotypes. However, the highest increase in its activity following Xcc inoculation was recorded in Redblush grapefruit (78.89%) followed by Konkan Seedless lemon (65.51%) and ALC-35 lime (62.53%), while the lowest upregulation of PAL activity was noticed in Marsh Seedless grapefruit (9.74%).
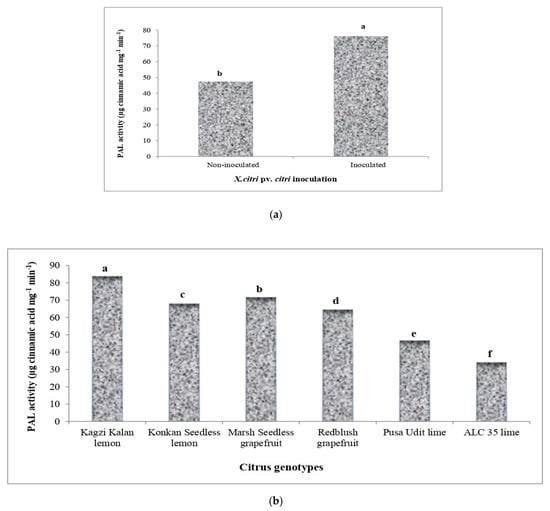
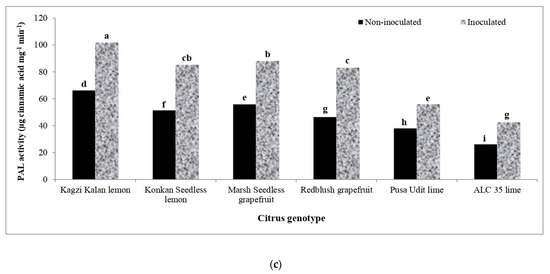
Figure 4.
Effect of X. citri pv. citri (Xcc) on the activity of phenyl ammonia lyase (PAL) enzyme in Citrus spp. (a) Activity of PAL in Xcc-inoculated and mock-inoculated plants. (b) Mean activity of PAL in different citrus genotypes. (c) The interaction effect of Xcc on PAL activity in inoculated and non-inoculated plants. Means for inoculation, genotypes and their interaction followed by different lowercase letters are significantly different at p ≤ 0.05 by Tukey’s HSD test.
3.7. Polyphenol Oxidase Activity (PPO)
The data relating to the activity of polyphenol oxidase (PPO), which was significantly upregulated in the leaves of various Citrus species due to Xcc inoculation, are presented in Figure 5. In non-inoculated genotypes, the highest activity of PPO was registered in Kagzi Kalan lemon, which was further increased to the highest extent in this genotype (73.58%), followed by Pusa Udit lime (62.50%). The genotypes Konkan Seedless lemon and ALC-35 lime showed a lower increase in the activity of PPO (26.79–34.29%) than the rest of the genotypes studied.
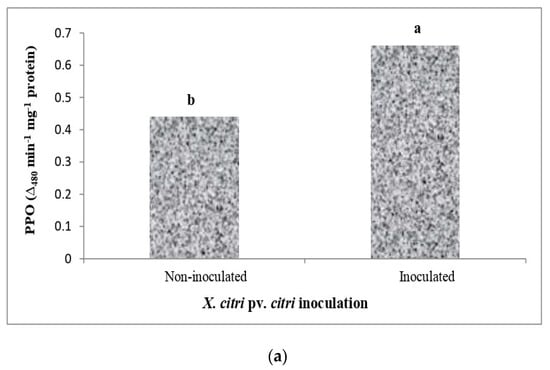
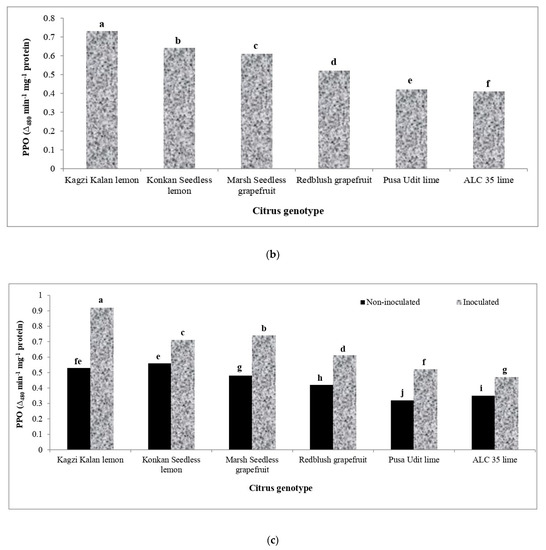
Figure 5.
Effect of X. citri pv. citri (Xcc) on the polyphenol oxidase (PPO) enzyme activity in Citrus spp. (a) The activity of PPO in Xcc inoculated and mock-inoculated plants. (b) Mean activity of PPO in different citrus genotypes. (c) The interaction effect of Xcc on PPO activity in inoculated and non-inoculated plants. Means for inoculation, genotypes and their interaction followed by different lowercase letters are significantly different at p ≤ 0.05 by Tukey’s HSD test.
4. Discussion
4.1. Physiological Response of Different Citrus Genotypes to X. citri pv. citri Infection
When a pathogen attacks, plants prioritize the biosynthesis of defense-related substances while reducing other (e.g., growth-related) cellular activities. This permits a reduction in physiological parameters until pathogenic growth has been halted [72]. This could help the plant by depriving biotrophic pathogens of nutrition. Various physiological parameters, such as chlorophyll contents (a, b, and total), photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs), and intrinsic water-use efficiency (iWUE), were studied in six different citrus genotypes after artificial inoculation. The tested genotypes showed a reduction in their activities due to X. citri pv. citri inoculation. However, the level of reduction was genotype-specific. ALC-35 acid lime was found to be worse affected due to Xcc inoculation, as the highest reduction in total chlorophyll, chl a, and chl b was registered in ALC-35. The reductions in A, E, gs, and iWUE were recorded in ALC-35; these reductions were lowest in Marsh Seedless (total chlorophyll, chl a, and chl b). Kagzi Kalan expressed the lowest reduction in A, E, gs, and iWUE. The results of the current study agree with the findings of [73], who found a significant reduction in chlorophyll content in infected plants with the turnip mosaic virus. Probably, declining chlorophyll content per unit area of leaf, stomatal closure, and reduced stomatal closure upon infection are the reasons for a reduced photosynthetic rate [74,75,76,77].
Similar to the present study, reduced chlorophyll content in infected plants was found by [78,79]. They suggested that the detoxification of pathogenic compounds may lead to the declining chlorophyll content. The defense response involves active processes that consume energy to retaliate against the pathogens; therefore, the induction of carbohydrate metabolisms was observed in plants [80].
4.2. Comparison of Stomatal Distribution and Aperture Size between Resistant and Susceptible Genotypes of Citrus spp.
The internal tissues involving natural openings and wounds are the entry sites that assist the pathogens’ entry into the host cell [81]. In the present study, the stomatal parameters, viz., area, density, pore length and width, and the conductance index, were highest in genotype ALC 35, while the stomata complex length and stomatal index were highest in genotype Konkan Seedless. Our findings were parallel to an earlier study reported by [82], where they found that stomatal characteristics (density and size) and stomatal response (apertures) affected the bacterial population by preventing them from entering the host tissues. We observed that, compared to susceptible genotypes, the resistant genotypes had significantly lower stomatal density and smaller stomatal openings.
Bacteria cannot get through the plant’s epidermis like fungi can, so they get into the host’s internal tissues through natural openings or accidental wounds. Stomata are essential microscopic surface openings that act as the main ports of bacterial entry during an infection [82]. In the case of stomatal parameters, ALC-35 had the highest stomatal density, stomata pore length, stomata pore width, stomata area, stomata complex area, and stomatal conductance index, while Konkan Seedless had the highest stomata complex length and stomatal index. The results of the current study were consistent with the findings of [24], who found that the stomatal characteristics (density and size) and stomatal response (apertures) have an effect on the population of bacteria entering the internal tissues, which are thus a factor influencing the magnitude of the resistance of the host plant to the invading bacteria. The resistant citrus genotypes had significantly lower stomatal density and smaller stomatal openings than the susceptible genotype. There may be a greater chance of more attacking bacteria entering the internal tissues if more and larger stomatal pores are on the surface.
4.3. Biochemical Change in Different Citrus Genotypes during X. citri pv. citri Infection
In the present study, the canker inoculation significantly interacted with different citrus genotypes in respect of reactive oxygen species (ROS), antioxidant enzymes, total phenolic compounds, phenyl ammonia lyase, polyphenol oxidase, and protein contents. The contents of hydrogen peroxide, superoxide radicals, and protein and the activities of catalase, guaiacol peroxidase, glutathione reductase, superoxide dismutase, total phenolic content, phenyl ammonia lyase, and polyphenol oxidase were low in non-inoculated plants. At the same time, canker inoculation significantly increased/upregulated their levels in all the genotypes tested, although the percent change following Xcc inoculation was specific to the biochemical constituent and genotype.
4.3.1. Soluble Protein Content
In order to prevent the growth and spread of diseases in healthy tissues, the synthesis of host and pathogenesis-related proteins takes place. When a plant goes under stress, it produces proteins that have a defensive role against the pathogen [83]. Plants inoculated with Xcc had a higher absolute protein content than the control. Among all the tested genotypes, Kagzi Kalan had the highest absolute protein content over other genotypes and was low in acid lime collections (ALC-35 and Pusa Udit) but proved worse in respect of absolute protein content after Xcc inoculation. However, the percentage increase in absolute soluble protein content in ALC-35 and Pusa Udit over control was relatively high. The lowest percent increase in soluble protein content was registered in Kagzi Kalan, followed by Marsh Seedless and Red Blush. The findings of our investigation confirmed that proteins play a vital role in defense mechanisms, both directly and indirectly, by triggering defense systems in plants, which have also been reported by [80]. Most plants synthesize protein rapidly when attacked by any pathogen. Plants cannot become immune against pathogens, but they can develop resistance against these pathogens through a complex defense system [84,85,86,87]. It was also found that resistant Kirumakki nucellar has a higher amount of protein content with a lower disease index as compared to susceptible rough lemon. Similarly, during the present experimentation, Kagzi Kalan had a higher soluble protein content than susceptible genotypes.
4.3.2. Total Phenolic Content
Secondary metabolites such as phenolics and chemically heterogeneous chemicals, mainly flavonoids, lignin, and tannins, play a significant role in disease resistance in Citrus spp. [88]. Xcc-inoculated plants showed higher total phenolic content (TPC) than non−inoculated plants. The highest absolute TPC was found in Kagzi Kalan lemon, followed by Marsh Seedless grapefruit, and the lowest in Pusa Udit. The highest percent increase in absolute total phenol content was observed in Redblush, followed by Marsh Seedless and Kagzi Kalan, while it was significantly lower in both genotypes Konkan Seedless and ALC-35. The present study’s findings are consistent with those of [86], which observed the significant role of specific phenolic compounds in plant defense mechanisms. Inoculated plants have a higher TPC content than non-inoculated, healthy plants. It is also observed that the TPC content was lower in susceptible genotype leaves than in resistant ones. These results are also in agreement with the findings of [89]. Higher levels of total phenolic content were observed in resistant genotypes compared to susceptible ones because it restricts pathogen invasion [90]. TPC has a significant role in controlling citrus canker disease [91,92]. Total phenolic content (TPC) helps in plant resistance against the mechanical penetration of pathogens by decreasing their susceptibility to enzymes, which degrade cell walls, inhibit the entrance of pathogen-secreted toxins and enzymes, and stop the flow of nutrients toward the pathogens [93].
4.3.3. Phenyl Ammonia Lyase Activity
Phenyl ammonia lyase, the first enzyme in the metabolic pathway for phenylpropanoids, is responsible for the biosynthesis of phytoalexin, lignin, and p-coumaric acid derivatives that contribute to the plant’s defense mechanism. PAL also participates in the biosynthesis of salicylic acid, a plant defense hormone that is required for both systemic and local resistance [94]. In this study, Xcc inoculation significantly increased PAL activity compared to the control. Kagzi Kalan lemon exhibited the highest absolute PAL activity, which was lowest in ALC-35 after Xcc inoculation. The highest-percent upregulation of PAL activity was observed in Redblush, Konkan Seedless, and Marsh Seedless, while it was lowest in Pusa Udit. This result is consistent with the previous finding that showed the involvement of PAL in increasing the resistance in response to the stimulation of various resistance elicitors in Citrus spp. [24,95,96]. The resistant citrus genotypes had higher PAL activity than susceptible ones upon exposure to Xcc infection, indicating that the former may synthesize more abundant secondary metabolites that are all associated with the localized resistance [97].
4.3.4. Polyphenol Oxidase Activity
Enzyme polyphenol oxidase (PPO) converts phenolics into highly toxic quinones and is also involved in the terminal oxidation of diseased plant tissues, which is attributed to its role in disease resistance [98]. Few polyphenol molecules have been found in the RNA of pathogens [99], and when these molecules are exposed to PPO, they react to produce quinones that inactivate the pathogen’s RNA. According to this, PPO may itself inhibit pathogens. In this study, the leaves of Kagzi Kalan, Konkan Seedless, and Marsh Seedless, which are resistant species, had more PPO activity than susceptible genotypes. The highest−percent upregulation in PPO activity due to canker inoculation was exhibited by Kagzi Kalan lemon, followed by Pusa Udit. The upregulation in the activity of PAL was lower in Konkan Seedless, ALC-35, and Redblush than in the rest of the genotypes studied. Our results are consistent with the findings of [90,91]. The PPO content of the infected tissues was slightly higher than that of the healthy ones. The accumulation of phenolics was ten times higher in apple leaves with a slight infection of Erwinia amylovora than in leaves with pronounced disease symptoms [100].
4.3.5. Reactive Oxygen Species
The bacterial pathogens have a potential source of oxidative stress during their interactions with plants [101]. The absolute contents of hydrogen peroxide and superoxide radicals were higher in ALC-35 and Marsh Seedless after Xcc inoculation. The ALC-35 genotype exhibited the highest-percent increase in H2O2 and superoxide radicals over non-inoculated plants, while the lowest increase in H2O2 and superoxide radicals was registered in Kagzi Kalan lemon. In the present study, there was a remarkable difference in the concentration of H2O2 and superoxide radicals between inoculated and non-inoculated leaves of different citrus genotypes. During the pathogen attack, ROS such as superoxide radicals (O2−), H2O2, and hydroxyl radicals are produced [102]. H2O2 is associated with the host plant’s resistance to pathogen attack and plays a crucial role during programmed cell death by regulating one of the early events of a hypersensitivity reaction [103]. The impairment of endogenous H2O2 accumulation in bacterial pathogens causes the accumulation of H2O2 in plants induced during the interaction. Similarly, [86] observed an increase in H2O2 activity when inoculated with X. citri pv. citri as compared to healthy control plants. The production of ROS at the site of infection, including O2−, H2O2, and the hydroxyl radical, is the plant’s first defense mechanism against a pathogen attack [104]. Accumulation of H2O2 in the infected zone may be toxic and suppress the bacterial population [105]. H2O2 is produced when the superoxide radical reacts with multiple antioxidant enzymes, including superoxide dismutase. Catalase and peroxidase scavenge hydrogen peroxide. Peroxidases are known for their role in H2O2 detoxification in plants [45]. H2O2 levels in plant tissues are regulated by catalase [40,44,72,106].
4.3.6. Antioxidant Enzyme Activity
Plants can defend themselves against pathogen attacks by triggering defense mechanisms through antimicrobial actions such as strengthening cell walls and the hypersensitive response (HR) that results in programmed cell death (PCD) in infected tissues [107,108]. During the host–pathogen interaction, antioxidants play a crucial role in the defense mechanism at the site of the pathogen attack. Antioxidant enzymes such as CAT, POD, and SOD play a significant role in pathogen inhibition [109].
Peroxidase Activity
Peroxidase performs a variety of biological processes, such as auxin metabolism, lignin biosynthesis, and the stiffening of cell walls to protect against pathogens [72]. Additionally, it helps in the lignification process, wound curing, and defense against biotic and abiotic stresses. In the present study, Kagzi Kalan lemon maintained the highest absolute guaiacol peroxidase activity (POD) after Xcc inoculation. However, a higher-percent upregulation over control was registered in ALC-35, Marsh Seedless, and Redblush than in other genotypes. It was least upregulated in Kagzi Kalan. In comparison to healthy plants, inoculated plants had higher absolute POD activity. The increase in catalase and peroxidase activity following Xcc inoculation has also been reported [110,111].
Catalase, SOD, and GR Activity
Catalase (CAT), the most critical enzyme involved in scavenging H2O2, has been seen to increase in response to Xcc attack, and CAT showed qualitative and temporal changes in Xcc-inoculated plants [112]. The H2O2 level is mediated by lower CAT and higher SOD activity in resistant genotypes, which induces the resistance and metabolic activity of hydrogen peroxide in Citrus spp., regulated by the production and destruction of these antioxidant enzymes [113]. Our findings showed that resistant genotypes of different Citrus spp. had higher SOD and lower CAT absolute activities over susceptible ones. Compared to the control, canker inoculation exhibited a higher upregulation of CAT activity in Konkan Seedless, Kagzi Kalan, and Redblush than the rest of the genotypes; however, it was poorly upregulated in the ALC-35 genotype.
In this study, Xcc-inoculated plants had higher SOD activity than non−inoculated plants. The highest−percent upregulation of SOD activity was observed in Pusa Udit, and it was lowest in ALC-35. Similarly, [114] also observed that susceptible genotype leaves inoculated with Xcc had higher absolute catalase activity than healthy and non−inoculated plants. Moreover, POD, CAT, and H2O2 levels increased following Xac inoculation on citrus leaves [79]. SOD activity involved in the production of H2O2 was responsible for maximizing the H2O2 concentration in resistant genotypes. However, a recent study shows that the pathogen suppressed the increased level of H2O2 due to increased levels of CATs and lower SODs. SODs produce H2O2 by converting superoxide radicals into hydrogen peroxide, while catalases are responsible for their removal [46]. The dismutation of H2O2 is achieved by catalase activity, which helps retain it at a relatively low concentration by changing millions of H2O2 molecules into water and oxygen due to photorespiration [115]. In the present study, low activity of SOD was observed in susceptible genotypes compared to resistant ones, and these results were also witnessed by [45]. Xcc also affects the activity of enzymes related to cellular redox regulation and SOD, CAT, GR, and glutathione peroxidase activities. Susceptible Mexican lime leaves exhibited higher GR activity over resistant Kumaquat and Calamondin [116].
5. Conclusions
In conclusion, we demonstrated that the Kagzi Kalan lemon is more resistant to Xcc than other citrus genotypes we tested in the present study using the pinprick inoculation method. The physical barriers, such as the smaller density and size of stomata and the unique genetically controlled structure of the former, may be involved in the resistance. It is possible that the resistance is due to the first plant’s unique structure, which is controlled by genes, and its physical barriers, such as its smaller number and size of stomata. Furthermore, Kagzi Kalan lemon possibly deploys powerful physio−biochemical defense machinery against pathogens via more drastic activation of the related enzymes involved in the stress response, leading to the phenotypic difference as we observed herein. Photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs), and total, “a”, and “b” chlorophyll content were reduced more in the susceptible genotypes than the resistant ones after inoculation with Xcc. The highest stomatal density, stomatal pore (length and width), stomata area, complex stomatal area, and stomatal conductance index were found in susceptible genotypes compared to resistant ones. The activities of POD, TPC, PAL, PPO, CAT, GR, and soluble protein content were higher in resistant genotypes than in susceptible genotypes following Xcc inoculation. Based on the physio−biochemical analysis, the order of resistance of different citrus genotypes was found to be Kagzi Kalan > Marsh Seedless >Redblush> Konkan Seedless > Pusa Udit > ALC-35.
Author Contributions
Conceptualization and writing—original draft preparation and investigation, A.K.M. and R.M.S.; Trail acquisition, A.K.M., R.M.S. and A.K.D.; writing—review and editing, A.K.M., R.M.S., A.S.K., A.K.K. and A.K. (Aditiya Kulshreshtha); Methodology, A.K.M., R.M.S., A.K.D., D.S., O.P.A., A.D., A.K.G., N.S. and A.M.S.; Software—Formal analysis, A.K. (Amrender Kumar), R.M.S., A.K.M., J.Y., R.P.S. and A.M..; funding acquisition, A.K.M., R.M.S., J.Y., A.K.K., A.S.K., A.K, R.P.S., A.M. and A.K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ICAR-IARI, New Delhi; IARI−Ashok_11039.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors thank the Head, Division of Fruits and Horticultural Technology, ICAR-IARI, for providing the necessary facilities to conduct the research. We also thank the Head, Division of Plant Pathology, ICAR-IARI, for providing all technical support in conducting experiments. This research work is part of the Ph.D. program of PG School, IARI.
Conflicts of Interest
On behalf of all authors, the corresponding author confirms that there is no conflict of interest.
References
- FAOSTAT, 2019–2020, Statistics Division, Food and Agriculture Organization of the United Nations. Agriculture Statistics; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- MOAFW, 2021–2022, Final Estimates of 2020-21 and First Advance Estimates of 2021-22 of Area and Production of Horticultural Crops. Available online: https://agricoop.nic.in/en/StatHortEst#gsc.tab=0 (accessed on 31 December 2022).
- APEDA. 2021. Available online: https://agriexchange.apeda.gov.in/InternationalProductions/International_Prodtion.aspx?ProductCode=0497 (accessed on 31 December 2022).
- Workman, D. Lemons and Limes Exporters by Country. 2021. Available online: https://www.worldstopexports.com/lemons-exporters-by-country (accessed on 31 December 2022).
- Rattanpal, H.S.; Singh, G.; Singh, S.; Arora, A. Citrus Cultivation in Punjab; Punjab Agricultural University: Ludhiana, India, 2017. [Google Scholar]
- Schubert, T.S.; Rizvi, S.A.; Sun, X.; Gottwald, T.R.; Graham, J.H.; Dixon, W.N. Meeting the challenge of eradicating citruscanker in Florida—Again. Plant Dis. 2001, 85, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Zhang, Y.; Wang, N. Diffusible signal factor (DSF)-mediated quorum sensing modulates expression of diverse traits in Xanthomonas citri and responses of citrus plants to promote disease. BMC Genom. 2019, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A. Further data on the susceptibility of rutaceous plants to citrus-canker. J. Agric. Res. 1918, 15, 661–665. [Google Scholar]
- Gottwald, T.R.; Graham, J.H.; Civerolo, E.L.; Barrett, H.C.; Hearn, C.J. Differential host range reaction of citrus and citrus relatives to citrus canker and citrus bacterial spot determined by leaf mesophyll susceptibility. Plant Dis. 1993, 77, 1004–1009. [Google Scholar] [CrossRef]
- Viloria, Z.; Drouillard, D.L.; Graham, J.H.; Grosser, J.W. Screening triploid hybrids of Lakeland’ limequat for resistance to citrus canker. Plant Dis. 2004, 88, 1056–1060. [Google Scholar] [CrossRef]
- Khalaf, A.; Moore, G.A.; Jones, J.B.; Gmitter, F.G., Jr. New insights into the resistance of Nagami kumquat to canker disease. Physiol. Mol. Plant Pathol. 2007, 71, 240–250. [Google Scholar] [CrossRef]
- Francis, M.I.; Peña, A.; Graham, J.H. Detached leaf inoculation of germplasm for rapid screening of resistance to citrus canker and citrus bacterial spot. Eur. J. Plant Pathol. 2010, 127, 571–578. [Google Scholar] [CrossRef]
- Dewdney, M.; Graham, J. Florida Citrus Pest Management Guide: Citrus Canker (EDIS); The Institute of Food and Agricultural Sciences (IFAS) University of Florida: Gainesville, FL, USA, 2014; p. 182. [Google Scholar]
- Moreira, R.R.; Machado, F.J.; Lanza, F.E.; Trombin, V.G.; Bassanezi, R.B.; Miranda, M.P.; Barbosa, J.C.; Junior, G.J.S.; Behlau, F. Impact of diseases and pests on premature fruit drop in sweet orange orchards in São Paulo state citrus belt, Brazil. Pest Manag. Sci. 2022, 78, 2643–2656. [Google Scholar] [CrossRef]
- Gottwald, T.R.; Graham, J.H.; Schubert, T.S. Citrus canker: The pathogen and its impact. Plant Health Prog. 2002, 3, 15. [Google Scholar] [CrossRef]
- Kobayashi, A.K.; Vieira, L.G.E.; Bespalhok Filho, J.C.; Leite, R.P.; Pereira, L.F.P.; Molinari, H.B.C.; Marques, V.V. Enhanced resistance to citrus canker in transgenic sweet orange expressing the sarcotoxin IA gene. Eur. J. Plant Pathol. 2017, 149, 865–873. [Google Scholar] [CrossRef]
- Bock, C.H.; Graham, J.H.; Gottwald, T.R.; Cook, A.Z.; Parker, P.E. Windspeed andwind-associated leaf injury affect severity of citrus canker on Swingle citrumelo. Eur. J. Plant Pathol. 2010, 128, 21–38. [Google Scholar] [CrossRef]
- Pruvost, O.; Boher, B.; Brocherieux, C.; Nicole, M.; Chiroleu, F. Survival of Xanthomonas axonopodis pv. citri in leaf lesions under tropical environmental conditions and simulated splash dispersal of inoculum. Phytopathology 2002, 92, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Melotto, M.; Underwood, W.; He, S.Y. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008, 46, 101. [Google Scholar] [CrossRef] [PubMed]
- Rigano, L.A.; Siciliano, F.; Enrique, R.; Sendín, L.; Filippone, P.; Torres, P.S.; Qüesta, J.; Dow, J.M.; Castagnaro, A.P.; Vojnov, A.A.; et al. Biofilm formation, epiphytic fitness, and canker development in Xanthomonas axonopodis. pv. citri. Mol. Plant Microbe. Interact. 2007, 20, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Vojnov, A.A.; Marano, M.R. Biofilm formation and virulence in bacterial plant pathogens. In Virulence Mechanisms of Plant Pathogenic Bacteria; The American Phytopathological Society: St. Paul, MN, USA, 2015; pp. 1–492. [Google Scholar]
- Gonçalves-Zuliani, A.M.O.; Cardoso, K.A.K.; Belasque Junior, J.; Zanutto, C.A.; Hashiguti, H.T.; Bock, C.H.; Nakamura, C.V.; Nunes, W.M.D.C. Reaction of detached leaves from different varieties of sweet orange to inoculation with Xanthomonas citri pv.citri. Summa Phytopathol. 2016, 42, 125–133. [Google Scholar] [CrossRef]
- Teper, D.; Xu, J.; Li, J.; Wang, N. The immunity of Meiwa kumquat against Xanthomonas citri is associated with a known susceptibility gene induced by a transcription activator-like effector. PLoS Pathog. 2020, 16, e1008886. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.Z.; Liu, J.H.; Hong, N. Differential structure and physiological response to canker challenge between‘ Meiwa’ kumquat and ‘Newhall’ navel orange with contrasting resistance. Sci. Hortic. 2011, 128, 115–123. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Leitch, I.J.; Patel, S.; Pendharkar, A.; Knight, C.A. Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol. 2008, 179, 975–986. [Google Scholar] [CrossRef]
- Duan, S.; Long, Y.; Cheng, S.; Li, J.; Ouyang, Z.; Wang, N. Rapid evaluation of the resistance of citrus germplasms against Xanthomonas citri subsp. citri. Phytopathology 2022, 112, 765–774. [Google Scholar] [CrossRef]
- Licciardello, G.; Caruso, P.; Bella, P.; Boyer, C.; Smith, M.W.; Pruvost, O.; Robene, I.; Cubero, J.; Catara, V. Pathotyping citrus ornamental relatives with Xanthomonas citri pv. citri and X. citri pv. aurantifolii refines our understanding of their susceptibility to these pathogens. Microorganisms 2022, 10, 986. [Google Scholar] [CrossRef]
- Dutton, C.; Horak, H.; Hepworth, C.; Mitchell, A.; Ton, J.; Hunt, L.; Gray, J.E. Bacterial infection systemically suppresses stomatal density. Plant Cell Environ. 2019, 42, 2411–2421. [Google Scholar] [CrossRef]
- Thordal-Christensen, H. Fresh insights into processes of non-host resistance. Curr. Opin. Plant Biol. 2003, 6, 351–357. [Google Scholar] [CrossRef]
- Molina, L.; Kahmann, R. An ustilago may disgene involved in H2O2 detoxification is required for virulence. Plant Cell 2007, 19, 2293–2309. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.P.; Carvalho, G.; Vilhena, M.B.; Creste, S.; Azevedo, R.A.; Monteiro-Vitorello, C.B. Functional analysis of oxidative burstin sugarcane smut-resistant and-susceptible genotypes. Planta 2017, 245, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Pitino, M.; Armstrong, C.M.; Duan, Y. Rapid screening for citrus canker resistance employing pathogen-associated molecular pattern- triggered immunity responses. Hortic. Res. 2015, 2, 15042. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sheng, X.; Greenshields, D.L.; Ogieglo, A.; Kaminskyj, S.; Selvaraj, G.; Wei, Y. Profiling of wheat class III peroxidase genes derived from powdery mildew-attacked epidermis reveals distinct sequence- associated expression patterns. Mol. Plant Microbe. Interact. 2005, 18, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Han, F.P.; Fedak, G.; Ouellet, T.; Dan, H.; Somers, D.J. Mapping of genes expressed in Fusarium graminearum-infected heads of wheat cultivar‘ Frontana’. Genome 2005, 48, 88–96. [Google Scholar] [CrossRef]
- Passardi, F.; Penel, C.; Dun, C. Performing the paradoxical: How plant peroxidases modify the cell wall. Trends Plant Sci. 2004, 9, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, P. Tissue-specific expression of a defence-related peroxidase in transgenic wheat potentiates cell death in pathogen-attacked leaf epidermis. Mol. Plant Pathol. 2008, 9, 45–57. [Google Scholar] [CrossRef]
- Mittler, R.; Herr, E.H.; Orvar, B.L.; VanCamp, W.; Willekens, H.; Inzé, D.; Ellis, B.E. Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyper responsive to pathogen infection. Proc. Natl. Acad. Sci. USA 1999, 96, 14165–14170. [Google Scholar] [CrossRef]
- Ma, W.; Pang, Z.; Huang, X.; Xu, J.; Pandey, S.S.; Li, J.; Achor, D.S.; Vasconcelos, F.N.C.; Hendrich, C.; Huang, Y.; et al. Citrus Huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin. Nat. Commun. 2022, 13, 529. [Google Scholar] [CrossRef]
- Barna, B.; Fodor, J.; Harrach, B.D.; Pogány, M.; Király, Z. The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiol. Biochem. 2012, 59, 37–43. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Torres, M.A. ROS in biotic interactions. Physiol. Plant 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Aviello, G.; Knaus, U.G. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal. Immunol. 2018, 11, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.P.; Carvalho, G.; Martins, P.F.; Dourado, M.N.; Vilhena, M.B.; Pileggi, M.; Azevedo, R.A. Differential responses of the antioxidant system of ametryn and clomazone tolerant bacteria. PLoS ONE 2014, 9, e112271. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszek, P. Oxidative burst: A nearly plant response to pathogen infection. Biochem. J. 1997, 322, 681–692. [Google Scholar] [CrossRef]
- Kumar, N.; Ebel, R.C.; Roberts, P.D. H2O2 degradation is suppressed in kumquat leaves infected with Xanthomonas axonopodis. pv. citri. Sci. Hortic. 2011, 130, 241–247. [Google Scholar] [CrossRef]
- Voloudakis, A.E.; Marmey, P.; Delannoy, E.; Jalloul, A.; Martinez, C.; Nicole, M. Molecular cloning and characterizationof Gossypium hirsutum superoxide dismutase genes during cotton–Xanthomonas campestris pv. malvacearum interaction. Physiol. Mol. Plant Pathol. 2006, 68, 119–127. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Babita, M.; Maheswari, M.; Rao, L.M.; Shanker, A.K.; Rao, D.G. Osmotic adjustment, drought tolerance and yield in castor (Ricinus communis L.) hybrids. Environ. Exp. Bot. 2010, 69, 243–249. [Google Scholar] [CrossRef]
- Cao, H.; Glazebrook, J.; Clarke, J.D.; Volko, S.; Dong, X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 1997, 88, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, M.; Guerrero, C.; Botella, M.A.; Barceló, A.; Amaya, I.; Medina, M.I.; Alonso, F.J.; de Forchetti, S.M.; Tigier, H.; Valpuesta, V. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol. 2000, 122, 1119–1128. [Google Scholar] [CrossRef]
- Myung, I.S.; Hyun, J.W.; Kim, K.S.; Lee, S.C.; Lim, H.C. Evaluation of Shiranuhi, a hybrid of Kiyomi tangor andNakano No. 3 Ponkan, for resistance to citrus canker in growth chamber. Plant Pathol. J. 2003, 19, 253–256. [Google Scholar] [CrossRef]
- Shiotani, H.; Yoshioka, T.; Yamamoto, M.; Matsumoto, R. Susceptibility to citrus canker caused by Xanthomonas axonopodis. pv. citri depends on the nuclear genome of the host plant. J. Gen. Plant Pathol. 2008, 74, 133–137. [Google Scholar] [CrossRef]
- Ishihara, H.; Uchida, S.; Masuda, Y.; Tamura, K.; Tsuyumu, S. Increase in telomerase activity in citrus inoculated with Xanthomonas axonopodis. pv. citri. J. Gen. Plant Pathol. 2004, 70, 218–220. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Gmitter, F.G.; Conesa, A.; Dopazo, J.; Moore, G.A. Fortunella margarita transcriptional reprogramming triggered by Xanthomonas citri pv. citri. BMC Plant Biol. 2011, 11, 159. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Q.; Selimi, D.; Wang, Q.; Charkowski, A.O.; Chen, X.; Yang, C.H. The plant phenolic compound p-coumaric acid represses gene expression in the Dickeya dadantii type III secretion system. Appl. Environ. Microbiol. 2009, 75, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Nayem, S.A.; Chowdhury, M.S.M.; Sultana, N.; Masum, G.Z.H.; Rahman, M.S.; Jamal, M.A.H.M. Combined effect of salt stress and Xanthomonas axonopodis. pv.citri on citrus (Citrus aurantifolia). Heliyon 2020, 6, e03403. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Cerling, T.E. Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree. Physiol. 1995, 15, 105–111. [Google Scholar] [CrossRef]
- Sampson, J. A method of replicating dry or moist surfaces for examination by light microscopy. Nature 1961, 191, 932–933. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Canad J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 19, pp. 121–126. [Google Scholar]
- Thomas, R.L.; Jen, J.J.; Morr, C.V. Changes in soluble and bound peroxidase-IAA oxidase during tomato fruit development. J. Food Sci. 1982, 47, 158–161. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis (2-nitrobenzoicacid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, K.K.; Naithani, S.C. Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability in seeds of Shorear obusta Gaertn. f. New Phytol. 1994, 126, 623–627. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet -B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef]
- Teranishi, Y.; Tanaka, A.; Osumi, M.; Fukui, S. Catalase activities of hydrocarbon-utilizing Candida yeasts. Agric. Biol. Chem. 1974, 38, 1213–1220. [Google Scholar] [CrossRef]
- Edward, E.A.; Kessmann, H. Isoflavonoids phytoalexins and their biosynthesis enzymes. In Moecular Plant Pathology: A Practical Approach; Gurr, S.J., McPherson, M.J., Bowles, D.J., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 45–62. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Emmons, C.L.; Peterson, D.M.; Paul, G.L. Antioxidant capacity of oat (Avena sativa L.) extracts. 2. In vitro antioxidant activity and contents of phenolic and tocol antioxidants. J. Agric. Food Chem. 1999, 47, 4894–4898. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenol oxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Bolwell, G.P.; Wojtaszek, P. Mechanisms for the generation of reactive oxygen species in plant defence–abroad perspective. Physiol. Mol. Plant Pathol. 1997, 51, 347–366. [Google Scholar] [CrossRef]
- Guo, D.P.; Guo, Y.P.; Zhao, J.P.; Liu, H.; Peng, Y.; Wang, Q.M.; Chen, J.S.; Rao, G.Z. Photosynthetic rate and chlorophyll fluorescence in leaves of stem mustard (Brassica juncea var. tsatsai) after turnip mosaic virus in fection. Plant Sci. 2005, 168, 57–63. [Google Scholar] [CrossRef]
- Funayama, S.; Hikosaka, K.; Yahara, T. Effects of virus infection and growth irradiance on fitness components and photosynthetic properties of Eupatorium makinoi (Compositae). Am. J. Bot. 1997, 84, 823–829. [Google Scholar] [CrossRef]
- Sayed, O.H. Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica 2003, 41, 321–330. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Berova, M.; Stoeva, N.; Zlatev, Z.; Stoilova, T.; Chavdarov, P. Physiological changes in bean (Phaseolus vulgaris L.) Leaves, infected by the most important bean disease. J. Cent. Eur. Agric. 2007, 8, 57–62. [Google Scholar]
- Garavaglia, B.S.; Thomas, L.; Gottig, N.; Dunger, G.; Garofalo, C.G.; Daurelio, L.D.; Ndimba, B.; Orellano, E.G.; Gehring, C.; Ottado, J. Aeukaryotic-acquired gene by abiotrophic phytopathogen allows prolonged survival on the host by counteracting the shut-down of plant photosynthesis. PLoS ONE 2010, 5, 8950. [Google Scholar] [CrossRef]
- Hameed, A.; Atiq, M.; Sahi, S.T.; Rajput, N.A.; Ahmed, Z.; Alam, M.W.; Alsamadany, H.; Alzahrani, Y.; Sarfraz, S.; Altaf, J.; et al. Biochemical base of resistance in citrus against canker disease. Pak. J. Agri. Sci. 2021, 58, 1850–1858. [Google Scholar]
- Rasoulnia, A.; Alavi, S.M.; Askari, H.; Farrokhi, N.; Soltani Najafabadi, M. Effects of Xanthomonas citri pv.citri infection on chlorophyll pigment content, chlorophyll fluorescence and proteins change in Citrus aurantifolia. J. Agric. Sci. Technol. 2018, 20, 571–582. [Google Scholar]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant–pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata functioning innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Niedz, R.P.; Doostdar, H.; McCollum, T.G.; McDonald, R.E.; Mayer, R.T. Plant defensive proteins and disease resistance in citrus. Proc. Fla. State Hortic. Soc. 1994, 107, 79–82. [Google Scholar]
- Dangl, J.L.; Jones, J.D. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Sharma, R.R. Citrus canker approaching century: A review. Tree Sci. Biotech. 2009, 2, 54–56. [Google Scholar]
- Li, X.; Zhao, C.; Li, H.; Zhu, W.; Ma, H.; Feng, H. Bacterial impact on H2O2 accumulation during the interaction between Xanthomonas and rice. Plant Prod. Sci. 2009, 12, 133–138. [Google Scholar] [CrossRef]
- Khan, K.; Ikram, S.; Ashfaq, M.; Jaskani, M.J.; Shafqat, W. Citrus rootstock characterization against citrus canker and evaluation of antibiotics effect against Xanthomonas axonopodis. pv. citri. J. Innov. Agric. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Taizand, Z. Photosynthesis: Physiological and ecological considerations. Plant Physiol. 2006, 9, 171–192. [Google Scholar]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic compounds and the role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Abid, M.; Khan, M.A.; Wahid, A. Screening and determinationofphenolicsinrelationtoresistanceagainstcitruscanker. Pak. J. Phytopathol. 2008, 20, 109–116. [Google Scholar]
- Raza, M.K.; Khan, M.M.; Naqvi, S.A.; Jaskani, M.J.; Mahmood, K.; Husnain, S.K. Efficacy of citrus rootstocks against citrus canker (Xanthomonas axonopod Dis. pv. citri) infestation. Pak. J. Phytopathol. 2016, 28, 241–247. [Google Scholar]
- Łaźniewska, J.; Macioszek, V.K.; Kononowicz, A.K. Plant-fungus interface: The role of surface structures in plant resistanceand susceptibility to pathogenic fungi. Physiol. Mol. Plant Pathol. 2012, 78, 24–30. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest. Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Ballester, A.R.; Izquierdo, A.; Lafuente, M.T.; González-Candelas, L. Biochemical and molecular characterization of induced resistance against Penicillium digitatum in citrus fruit. Postharvest. Biol. Technol. 2010, 56, 31–38. [Google Scholar] [CrossRef]
- Ryals, J.A.; Neuenschwander, U.H.; Willits, M.G.; Molina, A.; Steiner, H.Y.; Hunt, M.D. Systemic acquired resistance. Plant Cell 1996, 8, 1809–1819. [Google Scholar] [CrossRef]
- Vidhyasekaran, P. Physiology of Disease Resistance in Plants; CRC Press, Inc.: Boca Raton, FL, USA, 1998; Volume 2. [Google Scholar]
- Kosuge, T. The role of phenolics in host response to infection. Annu. Rev. Phytopathol. 1969, 7, 195–222. [Google Scholar] [CrossRef]
- Woods, T.L.; Agrios, G.N. Inhibitory effects of a polyphenol-polyphenol oxidase system on the infectivity of cowpea. Phytopathology 1974, 64, 35–37. [Google Scholar] [CrossRef]
- Jiao, H.J.; Wang, S.Y.; Civerolo, E.L. Enzymatic activities of citrus leaves from plants reresistant and susceptible to citrus bacterial canker disease. Env. Exp. Bot. 1992, 32, 465–470. [Google Scholar] [CrossRef]
- Bakhtawar, F.; Wang, X.; Manan, A.; Iftikhar, Y.; Atta, S.; Bashir, M.A.; Mubeen, M.; Sajid, A.; Hannan, A.; Hashem, M.; et al. Biochemical characterization of citrus Bentleaf viroid infecting citrus cultivars. J. King Saud. Univ. Sci. 2022, 34, 101733. [Google Scholar] [CrossRef]
- Roemmelt, S.; Plagge, J.; Treutter, D.; Gutmann, M.; Feucht, W.; Zeller, W. October. Defence reaction of apple against fireblight: Histological and biochemical studies. VIII Int. Workshop Fire Blight 1998, 489, 335–336. [Google Scholar]
- Chen, X.R.; Wang, X.L.; Zhang, Z.G.; Wang, Y.C.; Zheng, X.B. Differences in the induction of the oxidative burst in compatible and incompatible interactions of soybean and Phytophthora sojae. Physiol. Mol. Plant Pathol. 2008, 73, 16–24. [Google Scholar] [CrossRef]
- Majid, M.U.; Awan, M.F.; Fatima, K.; Tahir, M.S.; Ali, Q.; Rashid, B.; Rao, A.Q.; Nasir, I.A.; Husnain, T. Genetic resources of chili pepper (Capsicum annuum L.) against Phytophthora capsica and their induction through various biotic and abiotic factors. Cytol. Genet. 2017, 51, 296–304. [Google Scholar] [CrossRef]
- Peng, M.; Kuc, J. Peroxidase-generate dhydrogen peroxide as a source of anti-fungal activity in vitro and on tobacco leafdisks. Phytopathology 1992, 82, 696–699. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Chitosan-induced anti virala ctivity and innate immunity in plants. Environ. Sci. Pollut. Res. 2015, 22, 2935–2944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, H.; Wang, G.; Xu, L.; Shen, Z. Cadmium-induced accumulation of hydrogen peroxide in the leaf apoplastof Phaseolus aureus and Vicia sativa and the roles of differentanti oxidant enzymes. J. Hazard. Mater. 2009, 168, 76–84. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Handa, N.; Sharma, R.; Kaur, H.; Kohli, S.; Kumar, V.; Kaur, P. Lignins and abiotic stress: An overview. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Ahmad, P., Wani, M., Eds.; Springer: New York, NY, USA, 2014; pp. 267–296. [Google Scholar] [CrossRef]
- Kumar, N.; Ebel, R.C.; Roberts, P.D. Superoxide dismutase activity in kumquat leaves in fected with Xanthomonas axonopodis. pv. citri. J. Hortic. Sci. Biotechnol. 2011, 86, 62–68. [Google Scholar] [CrossRef]
- Rasoulnia, A.; Alavi, S.M.; Askari, H.; Farrokhi, N.; Najafabadi, M.S. Antioxidant activity and lipid peroxidation in response to citrus canker bacterial infection. Intl. J. Farm. Alli. Sci. 2013, 2, 1179–1184. [Google Scholar]
- Hu, Y.Q.; Liu, S.; Yuan, H.M.; Li, J.; Yan, D.W.; Zhang, J.F.; Lu, Y.T. Functional comparison of catalase genes in the elimination of photorespiratory H2O2 using promoter-and3′-untranslated region exchange experiments in the Arabidopsis CAT2 photo respiratory mutant. Plant Cell Environ. 2010, 33, 1656–1670. [Google Scholar] [CrossRef]
- Imlay, J.A. Cellular defences against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Ebel, R.C.; Roberts, P.D. Antioxidant metabolism of grape fruit infected with Xanthomonas axonopodis. pv. citri. Environ. Exp. Bot. 2011, 71, 41–49. [Google Scholar] [CrossRef]
- Treus, M.; Salas, C.O.; Gonazález, M.A.; Estévez, J.C.; Tapia, R.A.; Estévez, R.J. (Z)-Ethy l2-phenyl-1- (2-vinylphenyl) vinyl carbamates. Part 1: Synthesis and preliminary studies on the irdivergent transformation into benzo [c] phenanthridines and 2-phenyl-12010, 4-naphthoquinones. Tetrahedron 2010, 66, 9986–9995. [Google Scholar] [CrossRef]
- Chen, P.S.; Wang, L.Y.; Chen, Y.J.; Tzeng, K.C.; Chang, S.C.; Chung, K.R.; Lee, M.H. Understanding cellular defence inkumquat and calamondin to citrus canker caused by Xanthomonas citri pv. citri. Physiol. Mol. Plant Pathol. 2012, 79, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).