Abstract
The beneficial effects of Juniperus communis L. extracts have been known for a long time. Therefore a scientific knowledge of the chemical profile leading to this bioactivity is required. The aim of this paper is to highlight the influence of geographical origin and harvest time on compositional elements of hydroalcoholic extracts of J. communis pseudo-fruits, but also on their antibacterial properties. The samples were collected from three mountainous area of Romania, during three consecutive months. The total polyphenols were determined by the Folin-Ciocâlteu method, ranging between 12.67 and 14.77 mg GAE/g DW. High-performance liquid chromatography (HPLC) analysis was applied to identify fifteen phenolic compounds from the group of phenolic acids and flavonoids. The antibacterial effect was assessed on Gram-positive and Gram-negative bacterial strains. A good antimicrobial activity was achieved by the extracts of pseudo-fruits harvested during October and November from the Iezerele Cindrelului Botanical Nature Reserve. The obtained results denote a diverse composition of active principles in common juniper pseudo-fruits and moderate antibacterial activity dependent on the harvest time and geographical area.
1. Introduction
Scientific research on the chemical composition of herbal-derived products should be the essential evidence for the efficacy and safety that traditional therapies claim, but also for their integration in pharmacological studies and clinical practices [1]. Both plant extracts and essential oils have been shown to be effective in prevention and/or treatment of various human diseases. Due to their highly complex composition, these natural products and their derivatives are of significant importance as sources of readily accessible and affordable remedies. One of the most well-known plants about which traditional medicine provides information from ancient times is Juniperus communis L. [2]. J. communis is a dioeciously conifer in the Pinales order, Cupressaceae family, frequently found in mountainous areas, with evergreen leaves in acicular form, with edible fruit-like seeds. They are wrapped in a protective, juicy, dark-colored layer, as berry-like female cones, hence the name pseudo-fruits [3]. In J. communis, berries formation, ripening and maturation occur during three years. In the first year of reproduction cycle, the cones are small and green, in the second year the mass doubles and the color becomes blue-black matte, and in the third year ripening occur from September to October [4,5,6,7].
Both leaves and pseudo-fruits of J. communis are rich in polyphenols [8,9], volatile components [10], bitter principles, carbohydrates, and various organic acids [11,12,13,14,15,16].
Leaves of common juniper with specific geographical origin showed a significant accumulation of polyphenols that can reach up to 3.43 ± 0.17% pyrogallol equivalents, and the predominant volatile compounds are δ-3-carene, α-pinene, sabinene, β-pinene, myrcene, limonene, β-phellandrene and D-germacrene [8,10]. Using LC-ESI-QTOF/MS methods, Tang et al. [17] established the existence of 148 phenolic compounds. Significant values of polyphenols were quantified by Höferl et al. [13], Olech et al. [18], Gu et al. [19], the results being extremely useful in the subsequent research that target efficient plant utilization. Volatile organic compounds have been investigated by Antonelli et al. [20] and Meneguzzo et al. [21] in terms of favorable impact on the human body. The studies showed that terpenoids and other volatile compounds are responsible for about 50–70% of beneficial emissions from coniferous forests, with positive health effects.
J. communis contain highly bioactive compounds, especially polyphenols, with a positive impact on various conditions such as cancer, respiratory, rheumatic and circulatory disorders, diabetes, acne infections and hypertension [22,23,24,25,26,27,28,29,30,31,32,33]. The antioxidant activity of essential oils and extracts of J. communis was noted by Manvi [29], Elmastaş et al. [34], Emami et al. [35], Fernandez and Edwin [36], Fierascu et al. [37]. A seasonal fluctuation of phenolic content and antioxidant activity were reported for other herbaceous and woody plants as well [38,39]. Adams et al. [40] found significant differences in the chemical composition of essential oils obtained from leaves of Juniperus excelsa and Juniperus polycarpos depending on the plant’s origin (area and altitude).
The antimicrobial action assessed on gram-positive and gram-negative bacterial strains, but also on fungi was reported by a substantial number of studies [10,41,42,43,44,45,46,47,48,49,50,51,52]. The most important results were observed in bacterial strains such as Staphylococcus aureus, Streptococcus pyogenes and Escherichia coli, while Salmonella, Proteus, Pseudomonas and Klebsiella strains were resistant to J. communis extracts. Some authors considered that the sensitivity of Candida, Penicilium and Aspergillus species to extracts obtained from the pseudo-fruits of J. communis could be due to their richness in polyphenols [12,14,43]. Given the antioxidant and nutraceutical potential, anti-carcinogenic, anti-inflammatory and antimicrobial properties of polyphenols, there is a growing interest to accurately assess the content of these bioactive compounds in J. communis [17,18,22,32,34].
In this study, we focused on identifying the optimum harvesting time of J. communis pseudo-fruits, which would ensure an important content of target compounds, with a significant antioxidant and antimicrobial activity, for an efficient exploitation in the pharmaceutical and food industry. We conducted for the first time the comparative assessment of the chemical profile and bioactivity of J. communis pseudo-fruits extract from three mountain natural populations in Romania, harvested in three consecutive months, the results being important from economic and ecological perspectives.
2. Materials and Methods
2.1. J. communis Samples
Samples of J. communis pseudo-fruits were harvested from Iezerele Cindrelului Botanical Nature Reserve (ICR), Bâlea Lake Alpine Tundra (BLA), and Cozia National Park (CNP). The three harvesting areas were chosen as parts of the Southern Carpathians, located in south-central part of Romania, as follow: ICR is a protected area located on the northern side of the Frumoasa Plateau, in the Cindrelul Mountains, at 1999 m altitude (45°35′55″ N 23°47′51″ E); BLA is located in the Făgăraș Mountains, at 2034 m altitude (45.60335° N 24.61714° E); PNC is located in the north-east part of Vâlcea County, at 1600 m altitude (45°04″ N 24°18′02″ E). The choice of geographical areas was also made according to the dominance of J. communis species, so that in ICR, BLA and CNP juniper is the representative species of the Cupressaceae family [53,54,55].
Appreciating that in the autumn months the pseudo-fruits reach ripeness and their content in secondary metabolites is higher, ripe berries were carefully selected and hand-picked in September, October and November of 2021.
Voucher specimens of the samples were deposited at the Biotechnologies and Food Engineers Research Center within Lucian Blaga University of Sibiu and recorded with numbers 325/1–325/3.
2.2. Chemical, Reagents, Bacterial Strains and Culture Media
All chemicals and reagents used were of analytical grade. Ethanol 96% was obtained from Carl Roth GmbH, Karlsruhe, Germany). Folin-Ciocâlteu reagent, sodium carbonate 7.5%, gallic acid, caffeic acid, ellagic acid, syringic acid, coumaric acid, caftaric acid, chlorogenic acid, vanillic acid, cinnamic acid, ferulic acid, catechin, epicatechin gallate, quercetin, kaempferol p-hydroxybenzoic acid, acetic acid, acetonitrile, dimethylsulphoxide were from Sigma-Aldrich GmbH, Steinheim, Germany.
Antibacterial activity of J. communis pseudo-fruits were assessed on six standard bacterial strains: three Gram-positive (Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC29212, Bacillus subtilis ATCC23857) and three Gram-negative (Escherichia coli ATCC 25922, Salmonella enteritidis ATCC13076, Citrobacter freundii ATCC43864).
The bacterial strains are deposited and preserved in the microbial culture bank from Microbiology Department of Biotechnologies and Food Engineers Research Center (CCBIA) within the Agricultural Sciences, Food Industry and Environmental Protection Faculty/Lucian Blaga University of Sibiu, Romania. Cultures are grown on agar plates and maintained at 4 °C for short period of time, avoiding contamination. An inoculum density of 0.5 McF = 1.5 × 108 CFU/mL was used to evaluate inhibition.
Bacteria were cultured on Mueller Hinton agar and Mueller Hinton broth supplied by Sigma-Aldrich GmbH, Steinheim, Germany.
2.3. Extraction Procedure
J. communis samples consisting of 50 g of pseudo-fruits for each geographical location and each month, respectively, were dried at 50 °C in a Memmert incubator to constant mass, than grounded and stored at 12 °C. Powdered plant material was extracted with ethyl alcohol: distilled water (1:1 v/v), in a plant material: solvent ratio of 1:50 (w/v), for 24 h at a temperature of 15 °C. The crude extracts were filtered with the vacuum pump through Whatman filter paper no. 54, concentrated and dried in a rotary evaporator. The resulted dry extract was weighed and dissolved in 10 mL of distilled water, specific concentrations of the samples being obtained, depending on the harvesting period and the geographical location.
2.4. Determination of Total Phenolic Content (TPC)
Total polyphenols were determined by the spectrophotometric method using the modified Folin-Ciocâlteu method [56]. A volume of 0.20 mL extract was homogenized with 0.80 mL Folin Ciocâlteu reagent (10% v/v) and 1 mL sodium carbonate (7.5% m/v). The samples were incubated in the dark at room temperature for 60 min. The absorbance was read with a UV-1900 SHIMADZU spectrophotometer (Shimadzu Corporation, Kyoto, Japan) at a wavelength of 750 nm, the expression being in milligrams equivalent to gallic acid/gram dry weight (mg GAE/g DW). The determinations were performed in triplicate for a more accurate picture of the results.
2.5. HPLC Analysis
Phenolic compounds were identified following the HPLC method proposed by Gu et al. [19] slightly modified and using HPLC Smartline, KNAUER GmbH (Germany) equipment provided with a PDA detector, set to the following λ wavelengths: 280 nm, 320 nm, 360 nm, quaternary pump and automatic injection system. The mobile phase, eluent A consisted of a water-acetic acid solution (95/5 v/v) and eluent B from acetonitrile/water/acetic acid (100/95/5 v/v/v), using a C18 chromatographic column (Zorbax SB-Aq: 250 mm × 4.6 mm i.d., 5.0 μm p.s.). The volume of sample injected was 50 µL, the flow rate being set at 1 mL/minute. The mobile phases were degassed for 20 min at 20 °C, the gradient profile being established by mixing both phases according to the scheme: 0–25 min, 15% B; 25–35 min, 25% B; 35–45 min, 35% B; 45–70 min, 45% B; 70–75 min, 55% B; 75–80 min, 100% B; 80–85 min, 10% B. The identification and quantification of phenolic compounds was achieved by comparison with selected standards, using calibration curves for each individual compound. The experiments were performed in triplicate and the values were expressed as averaged mg/g extract. The limit of detection (LOD) and the limit of quantification (LOQ) were calculated as follows: LOD = 3 × (S/N) and LOQ = 10 × (S/N), where S/N is the signal-to -noise ratio.
2.6. Assessment of Antibacterial Activity and Minimum Inhibitory Concentration
The antimicrobial activity of J. communis pseudo-fruits hydroalcoholic extracts was determined using the modified Kirby-Bauer diffusion method. 100 µL of the test bacteria were activated in 10 mL of Mueller Hinton broth culture medium for 24 h. Prepared Petri dishes with Mueller Hinton agar culture medium were inoculated with 100 µL bacterial suspension with a concentration of 0.5 McFarland (density 0.5 McF = 1.5 × 108 CFU/mL). The extracts were tested using 6 mm diameter sterilized filter paper discs. The discs were impregnated with 10 µL of the test sample, incubated at 37 °C for 24 h and the diameters of the inhibition zone were measured with a ruler (mm). Each antimicrobial test was performed in triplicate and the mean obtained was reported. Standard disks of ampicillin (2 µg) and chloramphenicol (10 µg) were used as controls.
Disk diffusion method with successive dilutions was applied to assess the minimum inhibition concentration (MIC) of J. communis pseudo-fruits extracts. Plant extracts were diluted in distilled water over a range of 5 mg/mL to 0.1 mg/mL and 10 µL of each test dilution was pipetted onto a disk placed in Petri dishes containing solid Mueller Hinton culture medium coated with the test microorganism. After 24 h of incubation at 37 °C the areas of inhibition were measured. Depending on the concentration and the size of the inhibition zone, graphs are made, the MIC being calculated using linear regression.
2.7. Statistical Analysis
All experiments were performed in triplicate. Results are presented as the Mean ± standard deviation (SD) of more independent experiments. Analysis of variance (one-way ANOVA and two-way ANOVA) was applied to analyze the data for statistical significance and to determine the effects of categorical variables on the chemical composition of the extracts. The significant differences among means were determined using Duncan test. Significant differences were set at p ≤ 0.05. Principal component analysis (PCA) and discriminant analysis (DA) were applied to reduce the dimensionality of the data and to establish the relations between the studied variables. Pearson correlations (p < 0.05 and p < 0.01) were used to identify correlations between variables. The data were mean-centered by subtracting the mean of each variable from its values, in order not to achieve a small variance of the coefficients of the principal components This was done to remove any potential bias in the data and to ensure that the first principal component was the direction of maximum variance in the data, the data being further displayed as single point for each variable. IBM SPSS Statistics 20 and Addinsoft XLSTAT version 2014.5.03 software (Addinsoft Inc.; New York, NY, USA) were used for statistical analysis of the data.
3. Results
3.1. Dry Weight and Final Concentration of the Extracts
Dried extracts of J. communis pseudo-fruits were reconstituted in distilled water and the final concentration obtained from each sample is presented in Table 1. The extraction yield increased on average by 15–20% during the three months of harvesting. Data obtained in our study revealed that the highest extraction yield was characteristic of pseudo-fruits harvested at the end of autumn, suggesting harvest time is a critical factor in the industrial use of J. communis pseudo-fruits.

Table 1.
Extraction yield and final concentrations of J. communis pseudo-fruit extracts during the three months of harvesting (September, October and November) from the Iezerele Cindrelului Botanical Nature Reserve, Bâlea Lake Alpine Tundra and Cozia National Park.
3.2. Determination of Total Phenolic Content (TPC)
The ICR samples showed an accumulation of polyphenols between 13.69 ± 0.01 mg GAE/g DW and 24.01 ± 0.02 mg GAE/g DW, the value increasing with the maturation of the pseudo-fruits. For the BLA samples the values varied between 13.52 ± 0.01 mg GAE/g DW and 24.77 ± 0.02 mg GAE/g DW. For the CNP samples the values of polyphenols ranged between 12.67 ± 0.01 mg GAE/g DW and 19.83 ± 0.01 mg GAE/g DW registering an increased percentage of 58.2% in the last month of pseudo-fruits harvesting (Figure 1).
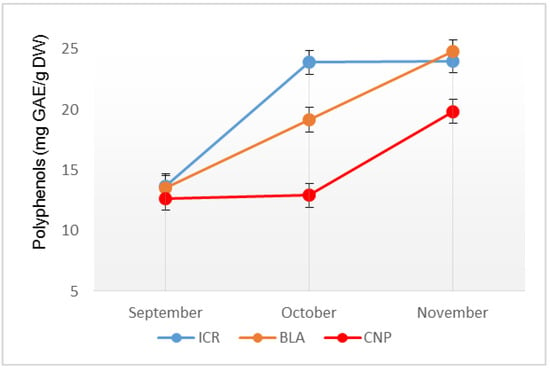
Figure 1.
Total polyphenols quantified in the samples of J. communis pseudo-fruits harvested during three months (September, October and November) from the Iezerele Cindrelului Botanical Nature Reserve, Bâlea Lake Alpine Tundra and Cozia National Park.
3.3. HPLC Profile of the Extracts
As noted in Table 2, the targeted phenolic compounds were from the group of phenolic acids and flavonoids.

Table 2.
Phenolic compounds identified and quantified in pseudo-fruits of J. communis harvested during three months (September, October and November) from the Iezerele Cindrelului Botanical Nature Reserve, Bâlea Lake Alpine Tundra and Cozia National Park.
In J. communis pseudo-fruits harvested from Southern Carpathians, caffeic acid was found between 0.092 and 0.0945 mg/g DW in the ICR samples, 0.0883 and 0.0903 mg/g DW in the BLA samples, and lower values were recorded for CNP samples where the maximum did not exceed 0.0775 mg/g DW.
The lowest content of caftaric acid reached a minimum value of 0.0013 mg/g DW in the samples collected from CNP in September and a maximum of 0.0027 mg/g DW in those collected in October from the ICR. It is important to emphasize, caftaric acid was not found in the samples collected in November from the Cindrel massif, in September from the Bâlea area and in October from CNP.
Cinnamic acid was identified at values exceeding one unit, being within the range 1.0245 and 1.0248 mg/g DW in the Cindrel area, 1.0234 and 1.0235 mg/g DW in the Bâlea area and decreased by an average of 0.7% compared to the maximum recorded, in the Cozia area. Chlorogenic acid content was detected in a range of values between a minimum of 0.0100 mg/g DW in the samples collected in November in the Cozia massif to a maximum of 0.0111 mg/g DW in the samples collected in October in the Bâlea glacier circus. Coumaric acid accumulated in more significant quantities in all samples, maximum values of 0.4677 mg/g DW being identified in November ICR samples, and of 0.4711 mg/g DW and 0.4301 mg/g DW in October BLA and CNP samples respectively.
Elagic acid, ferulic acid, and epicatechin gallate were identified in very small quantities or were not detected in the analyzed samples. Thus, elagic acid was found only in three samples, October, November in Cindrel (0.0006 mg/g DW) and November in Bâlea area (0.0023 mg/g DW).
Among the derivatives of benzoic acid, gallic acid and vanilla acid were released in extremely small amounts. The HPLC analysis revealed the average values of 0.0001 mg/g DW and 0.0002 mg/g DW in all samples collected from the Cindrel and Cozia areas. These phenolic acids were missing in the BLA samples. Syringic acid content ranged from 0.0009 mg/g DW in the BLA samples to a more significant amount of 0.0022 mg/g DW in the samples collected from the Cindrel area. This acid was not identified in the samples collected from the CNP.
High levels of P-hydroxybenzoic acid were found in all samples, the quantified values reaching up to a maximum of 5.3917 mg/g DW in the BLA, values above 5 mg/g DW being found in all samples collected both from the BLA and ICR. The minimums of this compound were around 4.1297 mg/g DW in the CNP samples.
Running the two-way Anova test revealed that for nine of the fifteen phenolic compounds, respectively caffeic acid, caftaric acid, coumaric acid, ellagic acid, ferulic acids, p-hydroxybenzoic acid, quercetin, epicatechin gallate and chlorogenic acid were highly influenced by the interaction of the two independent categorical variables, location and harvest month, for p ≤ 0.05. Over 90% of the variance of quercetin content and over 70% of the variance for the other acids content mentioned above (except epicatechin gallate and chlorogenic acid) is due to the interaction of the two variables (Table 3).

Table 3.
The effect of independent categorical variables (location*month) on the chemical composition of the extracts. Data were subjected to two-way ANOVA and Duncan test for interpretation of the significance of the differences among means, p ≤ 0.05.
Flavonoids were identified in the pseudo-fruits of J. communis in significant amounts. In our study, the samples harvested in November from CNP showed a maximum content of catechins (9.8989 mg/g DW) and the ICR-September samples revealed the minimum value of these flavonoids (7.2987 mg/g DW). Epicatechin-3-gallate was not identified and quantified in September in any sample, with very small amounts visible in October and November when the values did not exceed 0.0002 mg/g DW.
Quercetin content was quantified around 1 mg/g DW, this compound being present in all samples analyzed. Kaempferol was noted for accumulations of over 4 units in all samples taken. In the ICR, the determined values were between 4.0012 mg/g DW and 4.0111 mg/g DW in September and November respectively. In the BLA samples these values were on average 2.2% higher, and in the CNP samples ranged between 4.0082 mg/g DW in September and 5.9913 mg/g DW in October. Lower values were obtained in November when the accumulation of Kaempferol did not exceed 5.8992 mg/g DW.
The analyzed data for the PCA are represented by the 12 phenolic compounds (caffeic acid, caftaric acid, cinnamic acid, chlorogenic acid, coumaric acid, ellagic acid, ferulic acid, syringic acid, p-hydroxybenzoic acid, catechins, quercetin and kaempferol) identified and quantified in pseudo-fruits of J. communis harvested during three months (September, October and November) from the Iezerele Cindrelului Botanical Nature Reserve, Bâlea Lake Alpine Tundra and from the Cozia National Park. Three of the phenolic compounds were not included in the DA, as gallic acid, vanillic acid and epicatechin gallate were not identified in any of the samples.
The statistical approach, PCA and DA respectively, reduces the 12 measured variables to a two-dimension model without losing any information (Figure 2). The first two discriminant functions explained a 100% of the variation in the raw data, according to the preliminary findings; furthermore, their amounts were analyzed, representing phenolic compound concentration linear combinations. Both the absolute values and the signs of the coefficients (positive and negative) must be taken into account. Amounts near to zero, as in the case of cinnamic acid, indicate that this variable has limited significance in connection to the corresponding variable, while higher values, as in ellagic acid, indicate a considerable effect on the behavior of the discriminant factor. A positive or negative sign indicates that the specified discriminant variable is significant. The relative importance of the discriminant variables of the various compounds determines the positions of the points in the graphical representation. As it can be observed, samples harvest in September were clearly separated from the other two sampling periods, highlighting the fact that the concentration of phenolic compounds in that period is significantly different, the samples originating from 3 distinct regions showing a similar behavior. The other two harvest periods (October and November) had the same signs for several variables along the first discriminant function. However, an obvious separation between the latter was observed by their variable’s contribution to the second discriminant function.
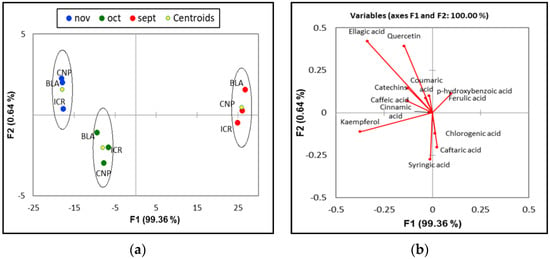
Figure 2.
(a) Discriminant analysis of the J. communis pseudo-fruits based to their harvest month; (b) Variables/Factors correlations graphical representation.
Principal component analysis (PCA) allowed the investigation of data variability and the differentiation of extract sources depending on the month of harvest, the geographical location and the compositional profile. PCA score plot showed that the first two principal components (PC1 and PC2) explained approximately 71% of the variance, with a clear grouping of samples (Figure 3). The BLA samples are located in the upper right quadrant, with a clear separation according to the harvest month. CNP samples are positioned on the left side of the score plot, while the ICR samples are positioned lower right quadrant. The loading plot presents the separation of the extracts based on their phenolic content. While ferulic acid was found in the highest concentration in BLA from September, quercetin, coumaric acid and p-hydroxybenzoic acid were detected at the highest values in BLA samples. The high content of cinnamic, caftaric, syringic and caffeic acids is specific to ICR samples, and CNP samples are distinguished by the content of kaempferol and catechins.
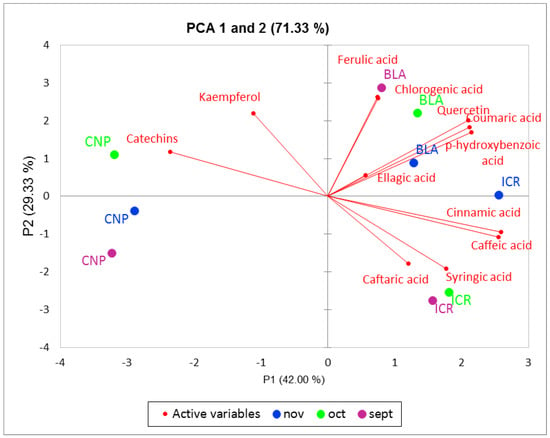
Figure 3.
Differentiation of J. communis pseudo-fruits harvested during three consecutive months (September, October and November). Colored symbols correspond to the samples of J. communis pseudo-fruits harvested from from Iezerele Cindrelului Botanical Nature Reserve, Bâlea Lake Alpine Tundra and Cozia National Park.
The results were confirmed by the Pearson correlation index performed for the extracts of J. communis pseudo-fruits. The heatmap of Pearson correlation coefficient (Figure 4) revealed a strong positive correlation between caffeic acid, cinnamic acid, coumaric acid and p-hydroxybenzoic acid and strong negative correlations with catechins and kaempferol.
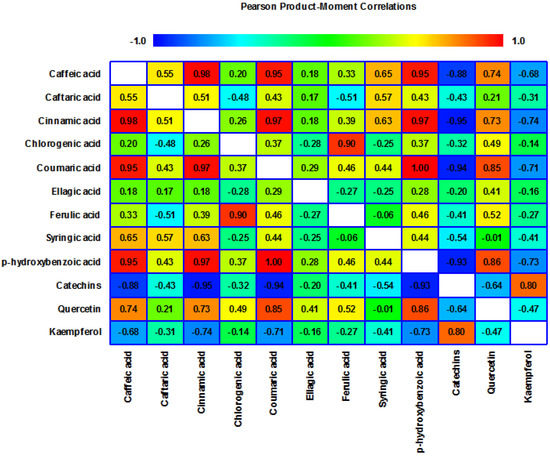
Figure 4.
Heatmap of Pearson correlation coefficient obtained from chemical compositional variables analyzed from pseudo-fruits of J. communis harvested during three months (September, October and November) from the Iezerele Cindrelului Botanical Nature Reserve, Bâlea Lake Alpine Tundra and Cozia National Park.
3.4. Antibacterial Activity and MIC of J. communis Pseudo-Fruits Extracts
In the tests performed, antibacterial activity was monitored by the diffusion method, implicitly achieving the MIC on Gram-positive and Gram-negative bacteria, and the results are presented in Table 4. Antibacterial activity is fully manifested in Gram-positive bacteria analyzed (Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC29212, Bacillus subtilis ATCC23857), regardless of the harvest month, and 66.6% in gram-negative bacteria Escherichia coli ATCC 25922, ATCC, Citrobacter freundii strain ATCC43864 not showing sensitivity to J. communis extracts. The MIC values resulting from the linear regression calculation are less than 500 µg/mL in 21.05% of the samples (susceptible microorganism), between 500 µg/mL and 1000 µg/mL in 50.87% of the samples (moderate susceptible microorganism) and over 1000 µg/mL in 28.07% of the samples (resistant microorganism).

Table 4.
Antibacterial activity established for the extracts of J. communis pseudo-fruits harvested during the three consecutive months (September, October and November) from the Iezerele Cindrelului Botanical Nature Reserve, Bâlea Lake Alpine Tundra and Cozia National Park.
4. Discussion
It is known that the ripening cycle of the pseudo-fruits of J. communis covers a period of approx. 18 months [57] and berries can be harvested throughout the growing season [58]. Although the influence of the harvest period on the chemical profile of various extracts and essential oils has been addressed by several studies [59,60,61], there is nonetheless a need to deepen this topic. To obtain the best results, it has been suggested autumn is the best season for harvesting juniper berries or from January to April for harvesting juniper branches [59,60,61,62,63]. However, the data suggest that optimization of harvesting protocols is mandatory. Falasca et al. [62] found J. communi essential oil with a similar composition in July and November. The authors stated that the change of season could be a stress factor that leads to this unusual biochemical. Based on the previous results, in our study we choose to harvest the pseudo-fruits in the autumn months and November was the most favorable month of harvest. At the same time, it is also appreciated that the geographical distribution is responsible for the extraordinary quantitative and qualitative variability of secondary metabolites in juniper-based products [4,62,63]. Total polyphenols are a relevant indicator for the antioxidant quality of a product. Following the determinations performed, it was observed the values of total polyphenols oscillate both depending on the month of harvest and the area of origin. An increase dependent on the harvest month of polyphenols was noticed for the BLA samples. Tang et al. [13] set values of 9.08 ± 0.01 mg/mL in J. communis pseudo-fruits harvested from Ozspice Store, Melbourne, Australia, while Miceli et al. [49] set an amount of 48.06 ± 0.99 mg/g DW in J. drupacea fruit from Turkey. In addition to the influence of the species, these significantly different values can be attributed to the impact of sampling season and pedoclimatic conditions factors that were previously incriminate for variation of content and composition of essential oil/extracts from various juniper species [18,60,62,63,64].
Phenolic acids varied significantly depending on their membership, but also on the geographic area and harvest time. Identified as a ubiquitous phenolic compound in all plant species, caffeic acid is a secondary metabolite with numerous and remarkable physiological properties, from antimicrobial and antiviral effects to anti-hepatocellular carcinoma activity [65]. For each mountain area, the maximum content of caffeic acid was defined by the month of November. Similarly, the highest p-hydroxybenzoic acid content was noted in November. Although a statistically significant accumulation of caffeic acid and p-hydroxybenzoic acid in November was noted only for ICR sample and BLA sample respectively, the constant increase in values during the autumn season can be attributed to cold stress. A couple of studies shows that polyphenols, including caffeic acid and p-hydroxybenzoic acid cover a wide range of biological properties, including abiotic stress response in plants and plants protection against chilling stress through various mechanisms that maintain cellular redox homeostasis [66,67,68,69].
At the same time, our data suggests the geographical location and time of ripening influence accumulation of caftaric acid. Thus, the premature pseudo-fruits from the CNP represent a better source of caftaric acid, when compared with BLA and ICR. This valuable phenolic compound is a caffeic acid derivative, with important therapeutic effects, recently reviewed by Koriem [70].
Catechins are important secondary metabolites, which are found in variable amounts in the various parts of the plants. Green tea plants (Camellia sinensis (L.) Kuntze) are well known for their high content of catechins [71]. Catechins have notable health benefits, such as strong and stable antioxidant activity [72], protective capacity against UV radiation and anti-ageing effects [73], anti-microbial activity [74], multidrug resistance in cancer [75,76] and immunomodulatory effects [77]. In our study, catechins were found at significantly higher values in CNP samples, regardless of the harvest month. However, various amounts of total polyphenols were reported in J. communis leaves and pseudo-fruits with different geographical origin [17,18,49]. In a recently published review, Gonçalves et al. [78] present a comprehensive analysis of the bioactive compounds identified in J. communis, in conjunction with their potential applications.
Antibacterial activity characterized extracts from pseudo-fruits of J. communis. The value of the zones of inhibition varies in the samples analyzed according to the month of harvest and the degree of ripening, which suggest a significant variation in accumulation of compounds with antibacterial action. Statistical analysis revealed the CNP–October and November samples were more effective against S. aureus, E. faecalis and E. coli. These results could be attributed to the significantly higher content of catechins and kaempferol that marked these samples. Significant results in the field were also obtained by Angioni et al. [10], El-Sawi et al. [12], Filipowicz et al. [44], Zhang et al. [50].
The primary aim of this research was to provide evidence of the optimal period for harvesting J. communis pseudo-fruits by analyzing their phenolic profile and identifying target compounds. Therefore, statistical analysis by DA and PCA were performed in line with the pseudo-fruits origin and the harvest period. Clear separation of the defining samples for the month of September highlights the fact that the concentration of phenolic compounds in that period is significantly different, the samples originating from 3 distinct regions showing a similar behavior. The other two harvest periods (October and November) had the same signs for several variables along the first discriminant function. However, a clearer separation between the latter was observed by their variable’s contribution to the second discriminant function. Statistical analysis revealed that the geographic location influences the phenolic content of pseudo-fruits. Therefore, the BLA samples were marked by higher content of chlorogenic, coumaric, ferulic, ellagic, p-hydroxybenzoic acids and quercetin, CNP samples were rich in kaempferol and catechins, while the ICR samples were distinguished by a higher content of caffeic, caftaric and cinnamic acids.
5. Conclusions
The variety of results obtained on extracts from the pseudo-fruits of J. communis demonstrates that they can accumulate valuable compounds in different amounts depending on the harvest period and area of origin. The total polyphenols were in a significant amount in areas with higher altitude and November was the most favorable month of harvest. The samples from Cozia National Park were dominated by kaempferol and catechins as phenolic content, while samples from Iezerele Cindrelului Botanical Nature Reserve were distinguished by the high content in cinnamic, caftaric, syringic and caffeic acids. The determined phenolic compounds are present in amounts in which they have an antibacterial effect on Gram-positive and Gram-negative bacteria, with catechin predominating, followed by p-hydroxybenzoic acid and kaempferol. The strongest antibacterial effects were noted in the case of J. communis extracts from the Iezerele Cindrelului Reserve and Bâlea Lake Alpine Tundra areas on the Staphylococcus aureus strain ATCC 29213. Further investigations into the chemical composition of these extracts under local soil and climatic conditions are recommended in order to finalize aspects that may lead to the formation of a complete picture of the relationships between environmental factors and beneficial properties of J. communis pseudo-fruits.
Author Contributions
Conceptualization, D.I.P., N.A.Ș., R.C. and O.R.B.; methodology, D.I.P., N.A.Ș. and O.R.B.; investigation, D.I.P., R.C., O.R.B. and N.A.Ș.; resources, O.R.B.; data curation, D.I.P. and O.R.B.; writing—original draft preparation, D.I.P., O.R.B. and N.A.Ș.; writing—review and editing, D.I.P., O.R.B., R.C., C.M. and N.A.Ș.; visualization, C.M. and N.A.Ș.; project administration, D.I.P.; funding acquisition, D.I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This activity was carried out with the support of the Ministry of Research, Innovation and Digitization, project no. PN 19110302 “The implementation of integrated nuclear-chemical-isotopic analytical methodologies for the authentication of traditional Romanian food products”, contract no. 20N/05.01.2023. N.A.Ş. gratefully acknowledges the support obtained through project number PN-III-P4-ID-PCE-2020-0620, within PNCDI III, a grant of the Romanian Ministry of Research, Innovation and Digitization, CNCS-UEFISCDI.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Firenzuoli, F.; Gori, L. Herbal Medicine Today: Clinical and Research Issues. Evid.-Based Complement. Altern. Med. 2007, 4, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Andersen, F.A. Final Report on the Safety Assessment of Juniperus communis Extract, Juniperus Oxycedrus Extract, Juniperus Oxycedrus Tar, Juniperus Phoenicea Extract, and Juniperus Virginiana Extract. Int. J. Toxicol. 2001, 20, 41–56. [Google Scholar] [CrossRef]
- Ciocârlan, V. Illustrated Flora of Romania—Pteridophyta et Spermatophyta; Ceres Ed.: Bucharest, Romania, 2009; ISBN 978-973-40-0817-9. [Google Scholar]
- Adams, R.P. Junipers of the World: The Genus Juniperus; Trafford Publication: Victoria, BC, Canada, 2004; ISBN 1490723250. [Google Scholar]
- Bais, S.; Gill, N.S.; Rana, N.; Shandil, S. A Phytopharmacological Review on a Medicinal Plant: Juniperus communis. Int. Sch. Res. Not. 2014, 2014, 634723. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.A.; El-Barghathi, M.; Polwart, A. Biological Flora of the British Isles: Juniperus communis L. J. Ecol. 2007, 95, 1404–1440. [Google Scholar] [CrossRef]
- Gruwez, R.; Leroux, O.; De Frenne, P.; Tack, W.; Viane, R.; Verheyen, K. Critical phases in the seed development of common juniper (Juniperus communis). Plant Biol. 2012, 15, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Modnicki, D.; Łabedzka, J. Estimation of the total phenolic compounds in juniper sprouts (Juniperus communis L.; Cupressaceae) from different places at the Kujawsko-Pomorskie province. Herba Pol. 2009, 55, 127–132. [Google Scholar]
- Miceli, N.; Trovato, A.; Marino, A.; Bellinghieri, V.; Melchini, A.; Dugo, P.; Cacciola, F.; Donato, P.; Mondello, L.; Güvenç, A.; et al. Phenolic composition and biological activities of Juniperus drupacea Labill. berries from Turkey. Food Chem. Toxicol. 2011, 49, 2600–2608. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Russo, M.T.; Coroneo, V.; Dessiä, S.; Cabras, P. Chemical Composition of the Essential Oils of Juniperus from Ripe and Unripe Berries and Leaves and Their Antimicrobial Activity. J. Agric. Food Chem. 2003, 51, 3073–3078. [Google Scholar] [CrossRef]
- Chatzopoulou, P.S.; Katsiotis, S.T. Chemical Investigation of the Leaf Oil of Juniperus communis L. J. Essent. Oil Res. 1993, 5, 603–607. [Google Scholar] [CrossRef]
- El-Sawi, S.; Motawae, H.; Ali, A. Chemical composition, cytotoxic activity and antimicrobial activity of essential oils of leaves and berries of Juniperus phoenicea L. Grown in Egypt. Afr. J. Tradit. Complement. Altern. Med. 2008, 4, 417–426. [Google Scholar] [CrossRef]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Visan, D.-C.; Oprea, E.; Radulescu, V.; Voiculescu, I.; Biris, I.-A.; Cotar, A.I.; Saviuc, C.; Chifiriuc, M.C.; Marinas, I.C. Original Contributions to the Chemical Composition, Microbicidal, Virulence-Arresting and Antibiotic-Enhancing Activity of Essential Oils from Four Coniferous Species. Pharmaceuticals 2021, 14, 1159. [Google Scholar] [CrossRef] [PubMed]
- Sela, F.; Karapandzova, M.; Stefkov, G.; Kulevanova, S. Chemical composition of berry essential oils from Juniperus communis L. (Cupressaceae) growing wild in Republic of Macedonia and assessment of the chemical composition in accordance to European Pharmacopoeia. Maced. Pharm. Bull. 2011, 57, 43–51. [Google Scholar] [CrossRef]
- Stoilova, I.S.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Stoyanova, A.S.; Krastanov, A.I. Chemical composition and antioxidant properties of juniper berry [Juniperus communis L.] essential oil. Bulg. J. Agri. Sci. 2014, 20, 227–237. [Google Scholar]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterization of Phenolic Compounds from Medicinal Plants (Hops and Juniper Berries) and Their Antioxidant Activity. Foods 2019, 9, 7. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Ivanova, D.; Tashev, A.; Boyadzhieva, S.; Kalotova, G.; Angelov, G.; Gawlik-Dziki, U. LC-ESI-MS/MS-MRM Profiling of Polyphenols and Antioxidant Activity Evaluation of Junipers of Different Origin. Appl. Sci. 2020, 10, 8921. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Bartolini, G.; Zabini, F. Temporal and Spatial Variability of Volatile Organic Compounds in the Forest Atmosphere. Int. J. Environ. Res. Public Health 2019, 16, 4915. [Google Scholar] [CrossRef]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L. as a nutraceutical in human and veterinary medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef]
- Akdogan, M.; Koyu, A.; Ciris, M.; Yildiz, K. Anti-hypercholesterolemic activity of Juniperus communis Lynn. oil in rats: A biochemical and histopathological investigation. Biomed. Res. 2012, 23, 321–328. [Google Scholar]
- Bais, S.; Gill, S.; Rana, N. Effect of Juniperus communis extract on reserpine induced catalepsy. Inventi Impact Ethnopharmacol. 2014, 2014, 1–4. [Google Scholar]
- Banerjee, S.; Mukherjee, A.T.; Chatterjee, T.K. Evaluation of analgesic activities of methanolic extract of medicinal plant Juniperus communis Linn. Int. J. Pharm. Pharm. Sci. 2012, 4, 547–550. [Google Scholar]
- Sami, B.; Jason, H.; Stephanie, J.; Annie-Pier, B.; Caitlyn, D.C.; Madison, C.; Allyson, B.; Christopher, A.G.; Gilles, A.R. Deoxypodophyllotoxin Isolated from Juniperus communis Induces Apoptosis in Breast Cancer Cells. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2014, 15, 79–88. [Google Scholar] [CrossRef]
- Kalinkevich, K.; Karandashov, V.E.; Ptitsyn, L.R. In vitro study of the anti-inflammatory activity of some medicinal and edible plants growing in Russia. Russ. J. Bioorganic Chem. 2014, 40, 752–761. [Google Scholar] [CrossRef]
- Lantto, T.A.; Laakso, I.; Dorman, H.J.D.; Mauriala, T.; Hiltunen, R.; Kõks, S.; Raasmaja, A. Cellular Stress and p53-Associated Apoptosis by Juniperus communis L. Berry Extract Treatment in the Human SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2016, 17, 1113. [Google Scholar] [CrossRef] [PubMed]
- Manvi, G.P. Screening and evaluation of pharmacognostic, phytochemical and hepatoprotective activity of J. communis L. Stems. Int. J. Pharma Bio Sci. 2010, 1, 17–23. [Google Scholar]
- Pramanik, K.C.; Biswas, R.; Bandyopadhyay, D.; Mishra, M.; Ghosh, C.; Chatterjee, T.K. Evaluation of anti-ulcer properties of the leaf extract of Juniperus communis L. in animals. J. Nat. Remedies 2007, 7, 207–213. [Google Scholar] [CrossRef]
- Rana, N.; Bais, S. Neuroprotective Effect of J. Communis in Parkinson Disease Induced Animal Models. Master’s Thesis, Pharmacology Department, Punjab Technical University, Punjab, India, 2014. [Google Scholar]
- Ved, A.; Gupta, A.; Rawat, A.K.S. Antioxidant and Hepatoprotective Potential of Phenol-Rich Fraction of Juniperus communis Linn. Leaves. Pharmacogn. Mag. 2017, 13, 108–113. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, H.; Chatterjee, T.K. Evaluation of anti-diabetic and antihyperlipidemic potential of methanolic extract of Juniperus communis [L.] in streptozotocin nicotinamide induced diabetic rats. Int. J. Pharma Bio Sci. 2013, 4, 10–17. [Google Scholar]
- Elmastaş, M.; Gülçin, I.; Beydemir, Ş.; İrfan Küfrevioğlu, Ö.; Aboul-Enein, H.Y. A Study on the In Vitro Antioxidant Activity of Juniper (Juniperus communis L.) Fruit Extracts. Anal. Lett. 2006, 39, 47–65. [Google Scholar] [CrossRef]
- Emami, S.; Javadi, B.; Hassanzadeh, M. Antioxidant Activity of the Essential Oils of Different Parts of Juniperus communis. subsp. hemisphaerica. and Juniperus oblonga. Pharm. Biol. 2007, 45, 769–776. [Google Scholar] [CrossRef]
- Fernandez, A.; Cock, I.E. The Therapeutic Properties of Juniperus communis, L.: Antioxidant Capacity, Bacterial growth Inhibition, Anticancer Activity and Toxicity. Pharmacogn. J. 2016, 8, 273–280. [Google Scholar] [CrossRef]
- Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Cimpeanu, C.; Georgescu, M.I.; Fierascu, R.C.; Ortan, A.; Sutan, A.N.; Anuta, V.; Zanfirescu, A.; et al. Genoprotective, antioxidant, antifungal and anti-inflammatory evaluation of hydroalcoholic extract of wild-growing Juniperus communis L. (Cupressaceae) native to Romanian southern sub-Carpathian hills. BMC Complement. Altern. Med. 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, C.; Landi, M.; Guidi, L.; Pardossi, A.; Incrocci, L. Seasonal Fluctuations of Crop Yield, Total Phenolic Content and Antioxidant Activity in Fresh or Cooked Borage (Borago officinalis L.), Mallow (Malva sylvestris L.) and Buck’s-Horn Plantain (Plantago coronopus L.) Leaves. Horticulturae 2022, 8, 253. [Google Scholar] [CrossRef]
- Popescu, D.I.; Lengyel, E.; Apostolescu, F.G.; Soare, L.C.; Botoran, O.R.; Șuțan, N.A. Volatile Compounds and Antioxidant and Antifungal Activity of Bud and Needle Extracts from Three Populations of Pinus mugo Turra Growing in Romania. Horticulturae 2022, 8, 952. [Google Scholar] [CrossRef]
- Adams, R.P.; Douaihy, B.; Dagher-Kharrat, M.D.; Faryaliyev, V.; Tashev, A.N.; Husnu Can Baser, K.; Christou, A.K. Geographic variation in the volatile leaf oils of Juniperus excelsa and J. polycarpos. Phytologia 2014, 96, 96–102. [Google Scholar]
- Gordien, A.Y.; Gray, A.I.; Franzblau, S.G.; Seidel, V. Antimycobacterial terpenoids from Juniperus communis L. (Cuppressaceae). J. Ethnopharmacol. 2009, 126, 500–505. [Google Scholar] [CrossRef]
- Glišić, S.B.; Milojević, S.Ž.; Dimitrijević, S.I.; Orlović, A.M.; Skala, D.U. Antimicrobial activity of the essential oil and different fractions of Juniperus communis L. and a comparison with some commercial antibiotics. J. Serb. Chem. Soc. 2007, 72, 311–320. [Google Scholar] [CrossRef]
- Pepeljnjak, S.; Kosalec, I.; Kalodera, Z.; Blǎzevíc, N. Antimicrobial activity of juniper berry essential oil Juniperus communis L., Cupressaceae. Acta Pharm. 2005, 55, 417–422. [Google Scholar]
- Filipowicz, N.; Kamiński, M.; Kurlenda, J.; Asztemborska, M.; Ochocka, J.R. Antibacterial and antifungal activity of juniper berry oil and its selected components. Phytother. Res. 2003, 17, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, S.; Rezai, M.A.; Mahmoodi, N. Analysis and antimicrobial activity of the plant Juniperus communis. Rasayan J. Chem. 2009, 2, 257–260. [Google Scholar]
- Haziri, A.; Faiku, F.; Mehmeti, A.; Govori, S.; Abazi, S.; Daci, M.; Haziri, I.; Bytyqi-Damoni, A.; Mele, A. Antimicrobial properties of the essential oil of Juniperus communis (L.) growing wild in east part of Kosovo. Am. J. Pharmacol. Toxicol. 2013, 8, 128–133. [Google Scholar] [CrossRef]
- Akkol, E.K.; Güvenç, A.; Yesilada, E. A comparative study on the antinociceptive and anti-inflammatory activities of five Juniperus taxa. J. Ethnopharmacol. 2009, 125, 330–336. [Google Scholar] [CrossRef]
- Sati, S.C.; Joshi, S. Antibacterial potential of leaf extracts of Juniperus communis L. from Kumaun Himalaya. Afr. J. Microbiol. Res. 2010, 4, 1291–1294. [Google Scholar]
- Miceli, N.; Marino, A.; Köroğlu, A.; Cacciola, F.; Dugo, P.; Mondello, L.; Taviano, M.F. Comparative study of the phenolic profile, antioxidant and antimicrobial activities of leaf extracts of five Juniperus L. (Cupressaceae) taxa growing in Turkey. Nat. Prod. Res. 2018, 34, 1636–1641. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Kuang, S.; Qing, M.; Ma, Y.; Yang, T.; Wang, T.; Li, D. Chemical composition, antioxidant, antibacterial and cholinesterase inhibitory activities of three Juniperus species. Nat. Prod. Res. 2019, 34, 3531–3535. [Google Scholar] [CrossRef]
- Kumar, P.; Bhatt, R.P.; Sati, O.P.; Dhatwalia, V.K.; Singh, L. In-vitro antifungal activity of different fraction of Juniperus communis leaves and bark against Aspergillus niger and aflatoxigenic Aspergillus flavus. Int. J. Pharma Bio Sci. 2010, 1, 1–7. [Google Scholar] [CrossRef]
- Chrapačienė, S.; Rasiukevičiūtė, N.; Valiuškaitė, A. Control of Seed-Borne Fungi by Selected Essential Oils. Horticulturae 2022, 8, 220. [Google Scholar] [CrossRef]
- Ieftime, A.; Ieftime, A. Preliminary data on the herpetofauna of the Cozia Massif (Romania). 1. Reptiles. Trav. Muséum Natl. D’histoire Nat. “Grigore Antipa” 2006, 49, 331–340. [Google Scholar]
- çobanoğlu, G.; Yavuz, M.; Costache, I.; Radu, I.; Açikgöz, B.; Baloniu, L. Epiphytic and terricolous lichens diversity in Cozia National Park (Romania). Oltenia. Stud. Comunicări. Ştiinţele Nat. 2009, 25, 17–22. [Google Scholar]
- Neblea, M.A.; Marian, M.C. Phytosociological Studies On Calcareous Screes From Meridional Carpathians (Romania). Curr. Trends Nat. Sci. 2021, 10, 209–219. [Google Scholar] [CrossRef]
- Stegăruș, D.I.; Lengyel, E.; Apostolescu, G.F.; Botoran, O.R.; Tanase, C. Phytochemical Analysis and Biological Activity of Three Stachys Species (Lamiaceae) from Romania. Plants 2021, 10, 2710. [Google Scholar] [CrossRef] [PubMed]
- Farjon, A. A Monograph of Cupressaceae and Sciadopityaceae; Royal Botanic Gardens, Kew: Richmond, UK, 2005; ISBN 9781842460689. [Google Scholar]
- Slavenas, J.; Ražinskaite, D. The dynamics of accumulation of phytoncides and essential oil in Juniperus communis L. Proc. Acad. Sci. Lith. SSR 1962, 27, 115–134. [Google Scholar]
- Lo[Zbreve]ienė, K.; Labokas, J.; Venskutonis, P.R.; Maždžierienė, R. Chromatographic Evaluation of the Composition of Essential Oil and α-Pinene Enantiomers in Juniperus communis L. Berries during Ripening. J. Essent. Oil Res. 2010, 22, 453–458. [Google Scholar] [CrossRef]
- Raal, A.; Kanut, M.; Orav, A. Annual variation of yield and composition of the essential oil of common juniper (Juniperus communis L.) branches from Estonia. Balt. For 2010, 16, 50–56. [Google Scholar]
- Falasca, A.; Caprari, C.; De Felice, V.; Fortini, P.; Saviano, G.; Zollo, F.; Iorizzi, M. GC-MS analysis of the essential oils of Juniperus communis L. berries growing wild in the Molise region: Seasonal variability and in vitro antifungal activity. Biochem. Syst. Ecol. 2016, 69, 166–175. [Google Scholar] [CrossRef]
- Fejér, J.; Gruľová, D.; Eliašová, A.; Kron, I.; De Feo, V. Influence of environmental factors on content and composition of essential oil from common juniper ripe berry cones (Juniperus communis L.). Plant Biosyst. 2018, 152, 1227–1235. [Google Scholar] [CrossRef]
- Fejér, J.; Kron, I.; Gruľová, D.; Eliašová, A. Seasonal Variability of Juniperus communis L. Berry Ethanol Extracts: 1. In Vitro Hydroxyl Radical Scavenging Activity. Molecules 2020, 25, 4114. [Google Scholar] [CrossRef]
- Shanjani, P.S.; Mirza, M.; Calagari, M.; Adams, R.P. Effects drying and harvest season on the essential oil composition from foliage and berries of Juniperus excelsa. Ind. Crops Prod. 2010, 32, 83–87. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; Da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Wan, Y.-Y.; Zhang, Y.; Zhang, L.; Zhou, Z.-Q.; Li, X.; Shi, Q.; Wang, X.-J.; Bai, J.-G. Caffeic acid protects cucumber against chilling stress by regulating antioxidant enzyme activity and proline and soluble sugar contents. Acta Physiol. Plant. 2014, 37, 1706. [Google Scholar] [CrossRef]
- Eom, S.H.; Ahn, M.-A.; Kim, E.; Lee, H.J.; Lee, J.H.; Wi, S.H.; Kim, S.K.; Bin Lim, H.; Hyun, T.K. Plant Response to Cold Stress: Cold Stress Changes Antioxidant Metabolism in Heading Type Kimchi Cabbage (Brassica rapa L. ssp. Pekinensis). Antioxidants 2022, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Koriem, K.M.M. Caftaric acid: An overview on its structure, daily consumption, bioavailability and pharmacological effects. Biointerface Res. Appl. Chem. 2020, 10, 5616–5623. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Motta, M.; Marcone, M.; Baptista, J. Variability of antioxidant properties, catechins, caffeine, L-theanine and other amino acids in different plant parts of Azorean Camellia sinensis. Curr. Res. Food Sci. 2020, 3, 227–234. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Iemma, F.; Puoci, F.; Cirillo, G.; Curcio, M.; Parisi, O.I.; Picci, N. Synthesis of Antioxidant Polymers by Grafting of Gallic Acid and Catechin on Gelatin. Biomacromolecules 2009, 10, 1923–1930. [Google Scholar] [CrossRef]
- Yoshino, S.; Mitoma, T.; Tsuruta, K.; Todo, H.; Sugibayashi, K. Effect of emulsification on the skin permeation and UV protection of catechin. Pharm. Dev. Technol. 2013, 19, 395–400. [Google Scholar] [CrossRef]
- Goyal, A.K.; Bhat, M.; Sharma, M.; Garg, M.; Khairwa, A.; Garg, R. Effect of green tea mouth rinse on Streptococcus mutans in plaque and saliva in children: An in vivo study. J. Indian Soc. Pedod. Prev. Dent. 2017, 35, 41. [Google Scholar] [CrossRef]
- Przystupski, D.; Michel, O.; Rossowska, J.; Kwiatkowski, S.; Saczko, J.; Kulbacka, J. The modulatory effect of green tea catechin on drug resistance in human ovarian cancer cells. Med. Chem. Res. 2019, 28, 657–667. [Google Scholar] [CrossRef]
- Michel, O.; Szlasa, W.; Baczyńska, D.; Saczko, J.; Tarek, M.; Kulbacka, J. The role of catechin in electroporation of pancreatic cancer cells—Effects on pore formation and multidrug resistance proteins. Bioelectrochemistry 2022, 147, 108199. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.-C.; Li, S.; Zhan, J.; Ho, C.-T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Flores-Félix, J.D.; Coutinho, P.; Alves, G.; Silva, L.R. Zimbro (Juniperus communis L.) as a Promising Source of Bioactive Compounds and Biomedical Activities: A Review on Recent Trends. Int. J. Mol. Sci. 2022, 23, 3197. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).