Abstract
Selection of sunflower varieties with greater cadmium (Cd) tolerance and detecting physiological variation under different Cd concentrations are important to study the potential of sunflower (Helianthus annuus L.) in the phytoremediation of Cd. The aim of this study was to investigate the variation in the Cd tolerance among four sunflower varieties (79−79, 363, 8361, ADT). Photosynthesis was determined using a Li−6400 XT portable photosynthesis system. Inductively coupled plasma mass spectrometry was used to detect the accumulation of Cd in different plant parts (leaf, stem and root). Subsequently, the Cd amount per plant, bio−concentration factor (BCF), and translocation factor (TF) were calculated. Cd exposure caused a decline in photosynthesis in four sunflower varieties. The 79−79 species displayed the highest Cd concentrations in tissues and 363 displayed a higher BCF in aerial parts under Cd exposure among the four species. Under Cd stress, the total soluble sugars in roots remained unaffected in 363. Based on the results of this experiment, the cultivar of 79−79 and 363 were more tolerant to Cd when compared to the other sunflower cultivars ADT and 8361. The present investigation results indicate that 79−79 and 363 can be further applied in the field trials of phytoremediation practices in contaminated soil.
1. Introduction
Agricultural and industrialization activities including the cement industry, mining, and the ashes of human beings lead to the contamination of heavy metals Cd [1]. More than 80% of the contaminated soil is contaminated with excess metals, 7% of which is Cd [2,3]. Cd can give rise to harmful health risks to microorganisms, plants, animals, and humans due to their toxicity and cumulative behavior [4,5].
A number of ways have been developed to mitigate cadmium pollution in soil. One of these technologies is phytoremediation, which is the application of hyperaccumulation plants to absorb Cd from the soil and to accumulate this toxic metal in the aboveground parts of plants [6]. The most critical element of phytoremediation in Cd polluted soils is mainly due to the proper plants we selected, which can grow in Cd-polluted soils and accumulate Cd in the aerial parts. Attention has been paid to finding Cd hyper-accumulation plants such as maize (Zea mays L.) [7,8], rice (Oryza sativa L.) [9,10], and fava bean (Vicia faba L.) [11,12]. However, only a few plant species such as Sedum alfredii have been verified, which can tolerate high Cd concentrations [13,14,15]. Since these Cd hyper-accumulators have little biomass production, the application of these plants in Cd polluted land is limited [15]. The usage of sunflower varieties has been proposed for phytoremediation because these species are characterized by a rapid growth rate, well−developed root system, and the ability to produce high biomass, therefore, it is regarded as one of the best plants for phytoremediation [16,17,18,19].
Previous studies have shown that sunflower varieties display different accumulation of Cd [20]. The sunflower root can accumulate 5 mg·kg−1 of Cd when the plants grow in metal-polluted soil (6 mg·kg−1) [21]. Nehnevajova reported that sunflower leaves and stems grown in Cd polluted soil (levels of 0.81 mg kg−1) accumulated 1.58 and 0.67 mg Cd, respectively [22].
The responses of sunflower cultivars to Cd exposure have been investigated at the physiological level [23,24,25,26]. The toxicity symptoms of Cd in plants can be related to changes such as sub-cellular division, enzyme activity, photosynthesis, carbohydrate metabolism, and respiration [27]. Cd exposure caused a decline in the plant roots, decreased the root activity, and directly affected the absorption of nutrients and water [28]. Furthermore, Cd stress can also destroy photosynthesis organs and reduce stomatal conductance, which seriously affects photosynthesis, reduces the photosynthetic rate and transpiration rate, alters the synthesis of sugar and sugar alcohols, and ultimately inhibits the growth. Meanwhile, Cd entry into the cells inevitably leads to the production of reaction oxygen species (ROS) such as H2O2 in plant cells [29,30]. However, there have been few reports on cadmium among different sunflower varieties.
Here, sunflower varieties (79−79, ADT, 363, 8361) grown in hydroponic cultivation were exposed to either 0, 50, and 100 μM CdCl2 for 15 days and to investigate Cd accumulation, translocation, and the biochemical stress and defense reactions (H2O2 and O2−) as the basis of Cd tolerance. Variation in Cd tolerance and the physiological mechanisms (photosynthesis, starch, total soluble sugar) involved in these variations in the four sunflower varieties were studied. We then hypothesis that there are Cd differences in the four sunflower cultivars and the Cd differences are related in the change in physiological and transport activity to the plant tissue. The implications for Cd tolerance and the underlying physiological mechanisms are discussed in the whole context of the evaluation and selection of sunflowers for phytoremediation practices in contaminated soil.
2. Materials and Methods
2.1. Sample Collection
Four different sunflower cultivars (Helianthus annuus L.) (79−79 and ADT, which belong to the oil sunflower, and 363 and 8361, which belong to the sunflower for eating, widely cultivated in China) were continuously exposed to three concentrations of Cd: 0 μM, 50 μM, and 100 μM in hydroponics for 15 days when the sunflowers grew in the fifth week. Photosynthesis, net photosynthetic rate, stomatal conductance, and chlorophyll were determined. Meanwhile, plant height and stem diameter were recorded and photographed. The plants were sampled to determine the dry matter production and cadmium content in different plant tissues. Three biological replicates were performed per cultivar and per Cd treatment.
2.2. Plant Culture
Seeds of the sunflower cultivars 79−79, ADT, 363, and 8361 purchased from seed markets were germinated in a bowl of sand in a greenhouse. These plants were transferred to a prepared hydroponic device filled with nutrient solution after three weeks to adapt (the sunflower was left to acclimate to water for a while). Four-leaf uniform size seedlings were selected and submitted to different concentrations of Cd: 0 μM, 50 μM, and 100 μM (Figure 1). The nutrient solution (full strength Hoagland solution) consisted of Ca(NO3)2, KNO3, MgSO4, KH2PO4, H3BO3, MnCl2, ZnSO4, CuSO4, Na2MoO4, and FeNaEDTA. The nutrient solution was changed every three days. At the same time, we changed the Cd concentration. All of the plants were cultivated under 24/20 °C (day/night temperature), 60−70% relative humidity, and a 14/8 h (light/night) photoperiod.
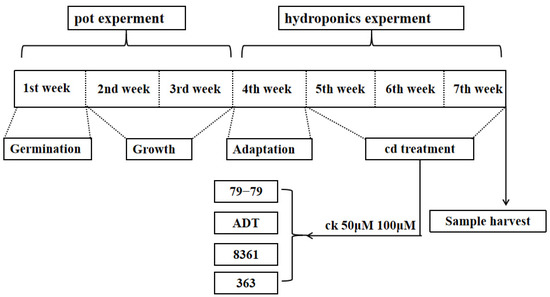
Figure 1.
Scheme of the used treatments to study the effect of cadmium (Cd) toxicity in the sunflower varieties.
2.3. Plant Growth Parameters
Plant height, thick stems, and soil and plant analyzer development (SPAD) dynamics were measured every two days on sunflower cultivar until the plants were harvested. Similarly, the root length, TotVolume, ProjArea, SurfArea, and volume were determined. Three independent replicates were used for the morphological and biochemical analyses. The DIGITAL CALIPER WITHIN 300 mm OPERATING INSTRUCTION and EPSON TWAIN PRO root scanner (32 bit, Canada Regent Instrument Inc, Quebec City, QC, Canada) were used to determine the thick stems and root parameters, respectively.
2.4. Gas Exchange Measurement
Leaves of each plant from three mature sunflower varieties were selected for gas exchange measurement before they were harvested. Net photosynthetic rate (Pn), stomatal conductance (Cond), transpiration rate (Trm), and intercellular CO2 concentration (Ci) were determined on fully expanded leaves, using a portable photosynthesis system Li−6400 XT (LiCor−6400; LiCor Inc., Lincoln, NE, USA), as described previously by [31]. The developing plants were harvested at maturity after 15 days. The root system of each plant was carefully washed using tap water and then rinsed in 20 mM EDTA for 5 min to remove the Cd2+ at the root surface. Subsequently, the sunflower cultivars we harvested were separated into the root, stem, and leaves. Further physiological and biochemical analyses were performed on different tissues of the sunflower species, as stated in the following sections.
2.5. Determination of Cd Concentrations
The Cd concentrations in different tissues were determined by inductively coupled plasma mass spectrometry (ICP−MS, aurora M90). Before the determination, the residual Cd adsorbed on the surface of sunflower was removed with 20 mM EDTA solution (w/v) for 5 min and then blotted on filter paper. In order to measure the total cadmium concentration, sunflower powder (about 0.1 g) from the roots, stem, and leaves was digested in a mixture of nitric–acid perchloric acid (HNO3:HClO4; 4:1 v/v). Cd accumulation in different roots, shoots, and leaves was calculated according to [32]. The translocation factor (TF) among each part of the plant was calculated according to [33]. The formula of TF and BCF is as follows:
TF = aerial tissues of a plant/the Cd concentration in roots × 100%;
BCF = metal concentration in plant roots or aerial tissues/metal concentration in the soil or solution.
2.6. NBT, DAB Analysis, Starch, Total Soluble Sugar, and Conductivity Determination
The accumulation of H2O2 and O2− was visually detected in plant leaves with the 3,3−diaminobenzidine (DAB) and nitro−blue tetrazolium (NBT) methods described by Liu with slight modification [34]. In brief, the leaves were submerged in a 1 mg/mL water solution of DAB and NBT for 8–10 h and 1 h, respectively, in the dark, then decolorized by the immersion of the leaves in 90% ethanol. The content of total soluble sugars and starch was determined by the anthrone method, as described by [6,35]. Fresh leaves were divided into strips of appropriate length and soaked in sterile water at 4 °C for 2 h to measure the electrolyte leakage. The conductivity meter DDS−307 (Leici Corporation, Shanghai, China) was used to detect the first conductivity value, named L1. The homogenate was boiled at 100 °C in a water bath for 20 min and cooled down to room temperature. Then, the second conductivity value was recorded and named L2 [36]. The relative electrical conductivity (REC) was calculated by using the following formula: The relative electrical conductivity = L1/L2 × 100%
2.7. Statistical Analysis
The SPSS statistics 17.0 package and one−way ANOVA method were used for all results we possessed. All data were mean ± SD of the three independent replicas, and the Duncan’s multiple range test was applied with the level of significance fixed at p < 0.05. Graphs were performed using the routines available in GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. Phenotype Changes under Different Cd Treatments
The phenotype aspect of different sunflower cultivars cultivated under different Cd regimes are displayed in Figure 2 and Table 1. Cadmium treatment significantly inhibited the growth of sunflowers such as the plant height and root morphology. Compared to the control, the phenotype of sunflower plants changed significantly under cadmium treatment. After six days of cadmium treatment, only 363 showed certain resistance under a high concentration of cadmium stress (Figure 2b), and the resistance of 79−79, 8361, and ADT was weaker, showing growth inhibition. There was more significant inhibition in 8361 and ADT when exposed to a high concentration of Cd than those grown under the control.

Figure 2.
Morphological aspects of the four different sunflower varieties cultivated under Cd stress for six days. Plant culture was conducted using a hydroponic system. (a) 79−79 and ADT; (b) 363 and 8361.

Table 1.
TotVolume, length, ProjArea, SurfArea, and volume of sunflower root under different concentrations of cadmium. Different letters indicate significant differences between treatments (p < 0.05, Duncan’s test).
The root scanner was used to analyze the root system (Table 1). 79−79 had the strongest resistance to cadmium, followed by the 363 variety. While 8361 and ADT were weaker, the roots were destroyed. The root length, projection area, and volume of 79−79 decreased by 34.99%, 40.86%, and 65.79%, respectively, under high concentration treatment. Root length, projection area, and volume of ADT decreased by 72.04%, 72.28%, 84.44%, respectively. The root length, projection area, and volume of 363 and 8361 decreased by 60.24%, 62.71%, 88.04%, 69.7%, 68.46%, and 78.2%, respectively. The roots of 79−79 were unaffected except for the projection area. Similarly, the roots of 363 were unaffected during the exposure, except for the TotVolume, which was significantly inhibited.
When exposed to a high concentration of cadmium, the plant heights of the various varieties decreased gradually (p < 0.05) (Figure 3). ADT and 79−79 sunflower varieties grow slowly after cadmium stress. Although the cultivars 363 and 8361 were inhibited by cadmium stress, their increments were higher than ADT and 79−79. Among them, cultivar 363 had the highest increment.
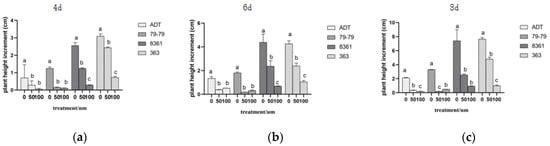
Figure 3.
Plant height increments after 4, 6, and 8 days of cadmium treatment. Within each sunflower variety, the different letters indicate a significant difference at the p < 0.05 level, according to Duncan’s multiple range test. (a) is 4 d of plant height increment, (b) is 6 d of plant height increment, (c) is 8 d of plant height increment.
3.2. Effects on the Photosynthesis of Sunflowers
Cadmium stress has a significant impact on the photosynthesis of sunflower. As shown in Table 2, the net photosynthetic rate (Pn), stomatal conductance (Cond), and transpiration rate (Trm) of CK were the highest, and decreased significantly under high cadmium treatment (p < 0.05). The photosynthetic rates (Pn) of the mature leaves of the four sunflower varieties 79−79, ADT, 363, and 8361 declined by 22.11%, 17.31%, 26.5%, 22.79%, respectively, under 50 μM Cd treatment while in the 100 μM Cd treatment, the photosynthetic rates declined by 40.96%, 53.51%, 50.46%, and 54.74% in 79−79, ADT, 363, and 8361, respectively. Stomatal conductance (Cond) in 79−79, ADT, and 8361 decreased markedly under cadmium stress, while in sunflower variety 363, the stomatal conductance (Cond) was not inhibited by cadmium exposure.

Table 2.
Net CO2 assimilation rate (Pn), stomatal conductance (Cond), transpiration rate (Trm), and intercellular CO2 concentration (Ci) of sunflower root under different concentrations of cadmium. Different letters indicate significant differences between treatments (p < 0.05, Duncan’s test).
The SPAD of different sunflower varieties were significantly reduced after cadmium treatment, especially exposed to the 100 μm treatment. The SPAD of 8361 and ADT was significantly inhibited under different Cd treatment. The leaves showed a strong growth reduction under a high concentration of Cd treatment (Figure 4), which may lead to the decline in chlorophyll content.
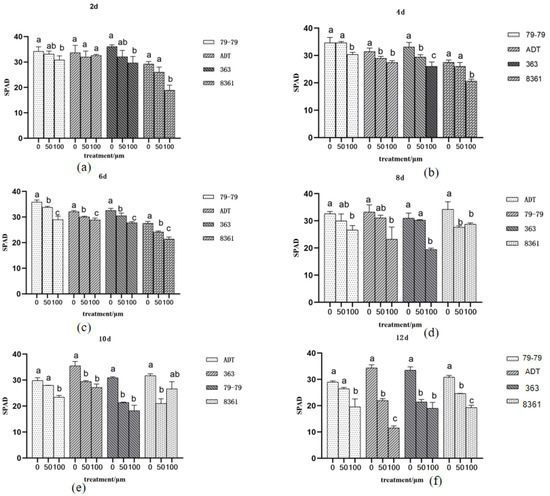
Figure 4.
Changes in the SPAD after 2, 4, 6, 8, 10, and 12 days of cadmium treatment. Within each sunflower varieties, the different letters indicate a significant difference at the p < 0.05 level, according to Duncan’s multiple range test. (a) is 2 d, (b) is 4 d, (c) is 6 d, (d) is 8 d, (e) is 10 d, (f) is 12 d.
3.3. Relative Electrical Conductivity and Water Content Changed under Different Cd Concentration
The results of the relative electrical conductivity of the roots, stems, and leaves of the sunflower varieties were obviously different (Figure 5). The electrical conductivity of cultivars 363, 8361, and 79−79 in the roots increased after cadmium treatment, which increased by 23.49%, 22.92%, and 62.44% under low concentration and increased by 41.2%, 44.64%, and 57.75% under high concentration, respectively, while the relative electrical conductivity of cultivar ADT decreased after cadmium treatment, which decreased to 80.08%. The electrical conductivity of cultivars 363 and ADT were not significantly inhibited in the stem after cadmium treatment, while in cultivars 79−79 and 8361, it was obviously decreased after cadmium treatment (Figure 5b). In leaves, the electrical conductivity of variety ADT in the Cd treatment group and control group was higher than that of the other three cultivars. There was significant change in the other three sunflower species except variety 8361 (Figure 5c).
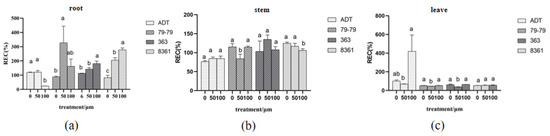
Figure 5.
Relative conductivity of the roots, stems, and leaves treated with cadmium. Within each sunflower variety, the different letters indicate a significant difference at the p < 0.05 level, according to Duncan’s multiple range test. (a) is root, (b) is stem, (c) is leave.
The water content in sunflower decreased after 10 and 15 days of cadmium treatment, and the water content in the roots and stems decreased more obviously than that of the leaves at 10 days (Figure 6c). Sunflower cultivar 79−79 treated with cadmium for 15 days showed better resistance in the roots, stems, and leaves than the other three cultivars (Figure 6d–f).
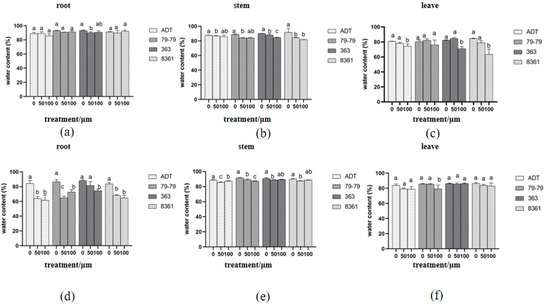
Figure 6.
Changes in the water content in the roots, stems, and leaves treated with cadmium for 10 days (a–c), 15 days (d–f). Within each sunflower variety, the different letters indicate a significant difference at the p < 0.05 level, according to Duncan’s multiple range test.
3.4. Changes of Starch and Total Soluble Sugar Content
Soluble sugars and starch are important metabolites that are derived from photosynthesis and may be involved in membrane lipid biosynthesis and oxidant detoxification under Cd exposure. The starch content in the roots and leaves were affected differently under Cd treatment (Figure 7a,b). The starch content in the roots of the sunflower varieties of 363 and 79−79 were not affected, while the starch in the two varieties 8361 and ADT were significantly inhibited in the treatment group (Figure 7a). The 8361 variety was reduced by 7–23.3% after cadmium treatment, and ADT variety was reduced by 11.5–19.3%. Only 8361 showed a significant change in the leaves. The content of starch in the leaves increased by 3.4–6.1% when exposed to cadmium (Figure 7b).
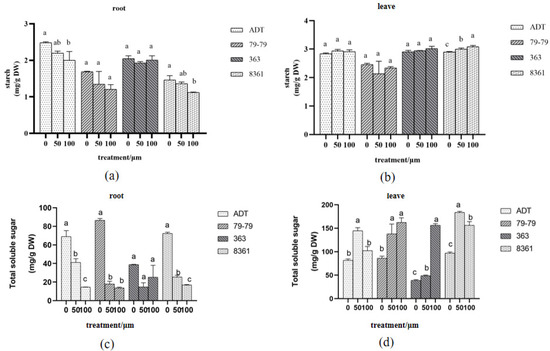
Figure 7.
Changes in the starch (a,b) and total soluble sugar (c,d) content in the roots and leaves of plants treated with cadmium. Within each sunflower species, the different letters indicate a significant difference at the p < 0.05 level, according to Duncan’s multiple range test.
The content of total soluble sugars in the roots and leaves of the sunflowers is shown in Figure 7c,d. The total soluble sugar content in the roots was reduced in sunflower varieties 79−79, ADT, and 8361. The ADT variety was reduced by 40.4–78.9% after cadmium treatment; the 79−79 variety was reduced by 79.2–84.1% (Figure 7c); and the 8361 variety was reduced by 25.4–77.1%. No significant changes were observed in the 363 plants. The total soluble sugar in the leaves treated with cadmium was significantly increased in the other three varieties.
3.5. Histochemical Detection of H2O2 and O2−
The first and second true leaves of different sunflower varieties were used to detect the H2O2 and O2− by 3′,3′−diaininobenzidine (DAB) staining and nitroblue tetrazolium (NBT) staining (Figure 8) after ten days. The concentration of H2O2 and O2− in the leaves of the control plants was unchanged during the NBT and DAB staining, but the color of the plants treated with different cadmium levels was significantly different. The staining of sunflower variety ADT was more obvious than that of 79−79 after treatment with different concentrations of cadmium (Figure 8a). Similarly, the staining of sunflower variety 363 was more obvious than that of sunflower variety 8361 (Figure 8b).
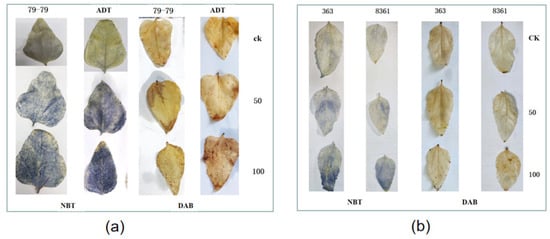
Figure 8.
Histochemical detection of H2O2 and O2− using DAB and NBT staining in the leaves of sunflower varieties exposed to Cd after ten days. The intense brown color indicates the presence and accumulation of H2O2 and O2−. (a) 79−79 and ADT, (b) 363 and 8361.
3.6. Cadmium Content in Sunflowers
The Cd concentrations in the roots, stems, and leaves of the different sunflower varieties were analyzed to evaluate the plant’s ability to accumulate Cd from the soil after 15 days. The results showed that, in general, the content of Cd in the different tissues was significantly different (root > stem > leaf) (Figure 9). In the root, after different concentrations of cadmium treatment, the cadmium content of different varieties of sunflower were significantly different (Figure 9a). The cadmium content in the sunflower root was ADT > 79−79 > 8361 > 363 under the low cadmium concentration treatment. The cadmium content in the sunflower root was 8361 > 363 > ADT > 79−79 under the high cadmium concentration treatment. There was no significant change in the cadmium content in the stems (Figure 9b). In the leaves, regardless of whether there were high or low concentrations of cadmium, sunflower variety 363 had the highest cadmium content (Figure 9c).
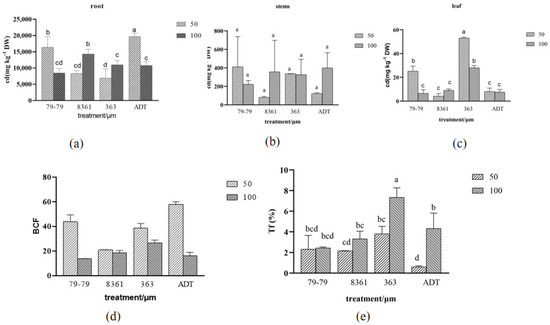
Figure 9.
Changes in the cadmium content in the roots, stems, and leaves treated with cadmium (a–c) after 15 days. The different enrichment and transport coefficients in the four sunflower cultivars exposed to Cd (d,e). Within each sunflower species, the different letters indicate a significant difference at the p < 0.05 level, according to Duncan’s multiple range test.
The four sunflower cultivars of the bio−concentration factor (BCF) were determined (Figure 9d) in order to evaluate the ability of different sunflower species to accumulate Cd. BCF in the aerial parts of the four species displayed a gradual decrease in the order ADT > 79−79 > 8361 > 363 under a low concentration of cadmium; while under the high concentration of cadmium, BCF in the aerial parts of the four sunflower varieties displayed a gradual decrease in the order 79−79 > 8361 > 363 > ADT. TF were significantly higher in 363 and 79−79 than in the other two species (Figure 9e).
3.7. Correlation Analysis
As shown in Figure 10, the positive correlation between the content of cadmium and root parameter was much higher in TotVolume than in the root length and SurfArea, and the correlation between the content of cadmium and root parameter were obviously different in the four sunflower varieties (79−79 (0.8711) > 8361 (0.6959) > ADT (0.514) > 363 (0.2599)). The correlation coefficients between the physiological and photosynthetic indices by the Pearson correlation analysis indicated that Pn was positively related to TSS, WC, Cond, while Ci was significantly related to Cond and Trm (p < 0.01). Meanwhile, WC was related to PH and TSS, and RC was positively related to SPAD and S (Figure 10f).
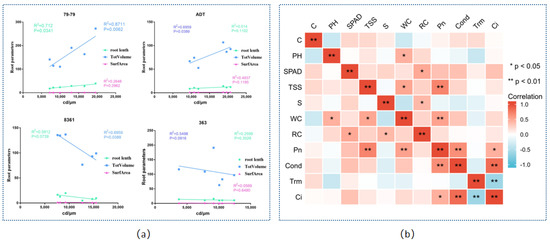
Figure 10.
The correlation analysis between the cadmium content and root parameters (root length, TotVolume, SurfArea) in four different sunflower cultivars. Blue is TotVolume, green is root length, pink is SurfArea (a). Correlation heat−map of the Cd content, plant height, SPAD, total soluble sugar, starch, water content, relative conductivity under Cd treatment. C: content, PH: plant height, TSS: total soluble sugar, S: starch, WC: water content, RC: Relative conductivity (b). ** p < 0.01, * p < 0.05.
4. Discussion
4.1. Cd Effect on Growth
It is generally accepted that heavy metal stress plays a significant role in various kinds of physiological processes. Although numerous studies with regard to the Cd responses of plants have been carried out, there are no straightforward critical factors available to assess the Cd tolerance in different sunflower varieties. In this study, integrated plant photosynthesis, relative electrical conductivity, starch and total soluble sugar, and Cd accumulation capacity were documented to evaluate the Cd tolerance in four sunflower varieties. The plants interfering with excessive cadmium concentration are usually characterized by a decline in plant growth including necrotic on leaves and decreased leaf area, but the toxic effects of Cd differ among plant species [37,38]. In this study, Cd stress, especially under high concentration treatment, significantly reduced the root parameters of TotVolume (ADT, 363, 8361), length, ProjArea, SurfArea, and volume in 8361 and ADT, but not in 79−79 and 363 (Figure 2 and Figure 3 and Table 1), which is consistent with previous studies [39,40]. The results indicate that 79−79 and 363 can grow better in the Cd polluted soil than ADT and 8361. Plant inhibition is caused by many reasons such as Cd stress, which can significantly affect the level of endogenous hormones in plants as well as damage the hormonal balance, hence affecting the normal growth and development of plants. The leaves of the plant can carry out photosynthesis, and the root system can transport the nutrients the plant needs. 79−79 and 363 grow better than 8361, and ADT may suggest that they can adapt to Cd after a long time exposure to minimize the negative effect on photosynthesis and growth.
4.2. Cd Tolerance Is Associated with Physiological Changes
The availability of carbohydrates may play an important role in defense and repair the harm caused by Cd. Data showed that starch and total soluble sugar increased in the leaves under Cd treatment, in contrast, Cd treatment significantly decreased the starch and total soluble sugar (p < 0.05) in ADT and 8361 but not in 363 in the plant roots. Our results showed that increases in the total soluble sugars and starch in the leaves of sunflower varieties may have contributed to oxidant detoxification under Cd exposure. There were similar results in the study of heavy metal cadmium in poplar, where they found that the increase in total soluble sugars in the roots of P. cathayana may be conducive to the improvement in osmotic regulation and antioxidant capacity. Cd is reported to disturb carbohydrate metabolism through the deprived effects to photosynthesis [41]. The results indicate that net CO2 assimilation rate (Pn), stomatal conductance (Cond), transpiration rate (Trm), and intercellular CO2 concentration (Ci) were significantly suppressed under different cadmium treatment, especially at high concentration (p < 0.05) (Table 2). Similarly, SPAD was obviously affected after being treated with cadmium except for 79−79 and 363 at day 12 (p < 0.05) (Figure 4). Cd from the surroundings is a strong inhibitor to photosynthesis because of its negative effect on leaves, particularly in mesophyll cells. Many studies have proven that Cd reduces the synthesis of chlorophyll and affects the chlorophyll content in many different plant species leaves [42,43]. The availability of carbohydrates for defense and repair may play an important role. Cd treatment had no significant effect on the content of starch and total soluble sugar in plant varieties 79−79 and 363 (Figure 7a–c).
The level of ROS is the well−known standard for detecting the degree of oxidative stress and is believed to be a major cause of growth inhibition [44]. It has often been noticed that ROS production is induced in plants under Cd exposure. As in previous studies with other species, Cd−exposure−induced ROS (O2− and H2O2) are produced in the roots and leaves. Our data indicate that Cd treatment promoted the accumulation of O2− and H2O2 in four sunflower cultivars, which affected the cell membrane functionality and integrity. These severely damaged the biosynthesis of photosynthetic machinery and delayed the subsequent growth [45]. An outstanding increase in H2O2 was proven to suppress organ elongation though the limitation extensibility of the plant cell walls [46]. This may explain the growth suppression observed in sunflower leaves at 50 and 100 µM of Cd in ADT and 8361.
4.3. Accumulation and Distribution of Cd in Sunflowers
The higher accumulation of Cd in the roots of ADT and 79−79 under low concentrations indicate that ADT and 79−79 have the ability to accumulate relatively higher Cd in roots than the other species. Under high concentrations, there was a higher accumulated Cd in the roots of 8361, 363, and ADT (Figure 9), which indicates that 8361, 363, and ADT have the ability to accumulate relatively higher Cd in the roots than the other sunflower variety. The cadmium content of sunflower varieties 79−79 and 363 was higher than that of ADT and 8361 in the aboveground part, indicating that 79−79 and 363 are superior to the other sunflower varieties for Cd phytoremediation because Cd accumulation in overground parts is ideal for easy harvest. The transfer factor (TF) of heavy metals, which is defined as transported metals from the soil to overground parts of plants, is regarded as an important factor for phytoremediation potential. Plenty of datasets have been used regarding the TF of Cd for different plant species and there is a criterion that a TF value >1 has been regarded as a key feature for metal (hyper) accumulators [47,48]. Our results confirmed that sunflower cultivars 363 and 79−79 have the potential for the remediation of Cd−contaminated soils.
The highest accumulation of Cd in the roots of ADT, 79−79, and 363 under low concentration indicate that these sunflower varieties have the ability to accumulate relatively higher Cd in the roots. The highest accumulated Cd concentrations in roots were ADT and 8361 under high concentration (Figure 9), indicating that this sunflower variety has the ability to accumulate relatively higher Cd in the roots. However, the growth of varieties ADT and 8361 was seriously inhibited under a high concentration treatment. This may be related to the absorption of more cadmium at high concentrations. Furthermore, the largest Cd amount in the stem and leaf parts of 79−79 and 363 indicating that these sunflower cultivars are superior to the other four sunflower varieties for Cd phytoremediation because aboveground Cd accumulation is ideal for easy harvest [49]. Additionally, the highest BCF in 79−79 also illustrates that the 79−79 species has a stronger capacity to accumulate and translocate Cd to the aboveground parts of sunflower varieties. (Supplementary Figure S1). Furthermore, the highest TF in the 363 aerial parts also show that 363 is the preferential plant for phytoremediation.
5. Conclusions
In this study, four sunflower cultivars (79−79, ADT, 363, 8361) grown in hydroponics were selected as potential candidates for phytoremediation. These findings show that 79−79 and 363 have the ability for phytoremediation due to its better resistance as well as high accumulation of Cd. Photosynthetic rates (Pn) of the mature leaves of the four sunflower varieties 79−79, ADT, 363, and 8361 declined by 40.96%, 53.51%, 50.46%, and 54.74%, respectively. Cd treatment significantly decreased the starch and total soluble sugar (p < 0.05) in ADT and 8361 but not in 363 and 79−79 in the plant roots. The cadmium content of sunflower varieties 79−79 and 363 was higher than that of ADT and 8361 in the aboveground part. TF were significantly higher in 363 and 79−79 than in the other two species. These data show that well−coordinated physiological changes in 79−79 and 363 confer the greater Cd tolerance. Further studies should focus on advanced and optimal practices to evaluate the field evaluation of high Cd−accumulating sunflower cultivars. The metal tolerance as well as absorption mechanisms in high Cd−accumulating sunflower cultivars 79−79 and 363 also need to be investigated to better understand the accumulation pathways.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9030320/s1, Figure S1: Schematic diagram of the Cd absorption and enrichment efficiency of different sunflower varieties.
Author Contributions
Conceptualization, D.T., B.C., L.Z. and S.Z.; Methodology, D.T., B.C., L.Z. and S.Z.; Software, D.T.; Validation, D.T., B.C., L.Z. and S.Z.; Formal analysis, D.T. and S.Z.; Investigation, D.T., B.C., L.Z. and S.Z.; Resources, S.Z.; Data curation, D.T. and S.Z.; Writing—original draft preparation, D.T., B.C. and S.Z.; Writing—review and editing, D.T., B.C. and S.Z.; Visualization, D.T., L.Z., B.C. and S.Z.; Supervision, B.C. and S.Z.; Project administration, S.Z.; Funding acquisition, S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Tianchi Hundred Talents Program for High–level Talents of Xinjiang Uygur Autonomous Region, the Natural Science Foundation of Xinjiang Uygur Autonomous Region (Grant No. 2019D01C017).
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
This work was supported by the Tianchi Hundred Talents Program for High−level Talents of Xinjiang Uygur Autonomous Region, the Natural Science Foundation of Xinjiang Uygur Autonomous Region (Grant No. 2019D01C017).
Conflicts of Interest
The authors declare no competing interests.
References
- Benavides, M.P.; Groppa, M.D.; Recalde, L.; Verstraeten, S.V. Effects of polyamines on cadmium−and copper−mediated alterations in wheat (Triticum aestivum. L.) and sunflower (Helianthus annuus. L.) seedling membrane fluidity. Arch. Biochem. Biophys. 2018, 654, 27–39. [Google Scholar] [CrossRef]
- Luo, Q.; Bai, B.; Xie, Y.; Yao, D.; Zhang, D.; Chen, Z.; Zhuang, W.; Deng, Q.; Xiao, Y.; Wu, J. Effects of Cd uptake, translocation and redistribution in different hybrid rice varieties on grain Cd concentration. Ecotoxicol. Environ. Saf. 2022, 240, 113683. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Ma, H.; Li, L.L.; Gao, Y.F.; Li, Y.Z.; Xu, H. Subcellular distribution, chemical forms and physiological responses involved in cadmium tolerance and detoxification in Agrocybe Aegerita. Ecotoxicol. Environ. Saf. 2019, 171, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Li, L.; Duan, Q.; Liu, X.; Chen, M. Progress in our understanding of plant responses to the stress of heavy metal cadmium. Plant Signal. Behav. 2021, 16, 1836884. [Google Scholar] [CrossRef]
- Cornu, J.Y.; Bussière, S.; Coriou, C.; Robert, T.; Maucourt, M.; Deborde, C.; Moing, A.; Nguyen, C. Changes in plant growth, Cd partitioning and xylem sap composition in two sunflower cultivars exposed to low Cd concentrations in hydroponics. Ecotoxicol. Environ. Saf. 2020, 205, 111145. [Google Scholar] [CrossRef] [PubMed]
- He, J.L.; Ma, C.F.; Ma, Y.L.; Li, H.; Kang, J.Q.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Cadmium tolerance in six poplar species. Environ. Sci. Pol. 2013, 20, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Nan, Z.R.; Zhao, Z.J. Bioaccumulation and translocation of cadmium in wheat (Triticumaestivum L.) and maize (Zea mays L.) from the polluted oasis soil of Northwestern China. Chem. Speciat. Bioavailab. 2014, 26, 43–51. [Google Scholar] [CrossRef]
- Xu, X.H.; Liu, C.Y.; Zhao, X.Y.; Li, R.Y.; Deng, W.J. Involvement of an antioxidant defense system in the adaptive response to cadmium in maize seedlings (Zea mays L.). Bull. Environ. Contam. Toxicol. 2014, 93, 618–624. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.C.; Tam, N.F.C.; Ye, Z.H. Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J. Environ. Sci. 2019, 75, 296–306. [Google Scholar] [CrossRef]
- Ashraf, U.; Mahmood, M.H.; Hussain, S.; Abbas, F.; Anjum, S.A.; Tang, X. Lead (Pb) distribution and accumulation in different plant parts and its associations with grain Pb contents in fragrant rice. Chemosphere 2020, 248, 126003. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, P.; Zhang, S.; Dai, J.; Chen, H.P.; Lombi, E.; Howard, D.L.; van der Ent, A.; Zhao, F.J.; Kopittke, P.M. Chemical speciation and distribution of cdmium in rice grain and implications for bioavailability to humans. Environ. Sci. Technol. 2020, 54, 12072–12080. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Hamid, Y.; Zehra, A.; Sahito, Z.A.; He, Z.; Hussain, B.; Yang, X. Characterization of fava bean (Viciafaba L.) genotypes for phytoremediation of cadmium and lead co−contaminated soils coupled with agro−production. Ecotoxicol. Environ. Saf. 2019, 171, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, J.L.; Ji, X.H.; Luo, X.G. Unraveling response mechanism of photosynthetic metabolism and respiratory metabolism to uranium−exposure in Vicia faba. J. Hazard. Mater. 2020, 398, 122997. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Tian, S.K.; Yang, X.E.; Wang, X.C.; Brown, P.; Li, T.Q.; He, Z.L. Enhanced root−to−shoot translocation of cadmium in the hyperaccumulating ecotype of Sedum alfredii. J. Exp. Bot. 2008, 59, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.K.; Lu, L.L.; Yang, X.E.; Webb, S.M.; Du, Y.H.; Brown, P.H. Spatial imaging and speciation of lead in the accumulator plant Sedum alfredii by microscopically focused synchrotron X-ray investigation. Environ. Sci. Technol. 2010, 44, 5920–5926. [Google Scholar] [CrossRef]
- Sarwar, N.; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.C.; Zhang, Y.J.; He, X.F.; Li, X.F.; Zhang, X.X. Analysis of the report on the national general survey of soil contamination. J. Agro−Environ. Sci. 2017, 36, 1689–1692. [Google Scholar]
- Zehra, A.; Sahito, Z.A.; Tong, W.; Tang, L.; Hamid, Y.; Wang, Q.; Cao, X.; Khan, M.B.; Hussain, B.; Jatoi, S.A.; et al. Identification of high cadmium−accumulating oilseed sunflower (Helianthus annuus) cultivars for phytoremediation of an Oxisol and an Inceptisol. Ecotoxicol. Environ. Saf. 2020, 187, 109857. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.J.; Najeeb, U.; Li, X.; Pan, J.M.; Huang, Q.; Zhou, W.J.; Liang, Z.S. Synergistic effects of EDDS and ALA on phytoextraction of cadmium as revealed by biochemical and ultrastructural changes in sunflower (Helianthus annuus L.) tissues. J. Hazard. Mater. 2021, 407, 124764. [Google Scholar] [CrossRef]
- Marques, A.P.; Moreira, H.; Franco, A.R.; Rangel, A.O.; Castro, P.M. Inoculating sunflower (Helianthus annuus) grown in zinc and cadmium contaminated soils with plant growth promoting bacteria−effects on phytoremediation strategies. Chemosphere 2013, 92, 74–83. [Google Scholar] [CrossRef]
- Wang, A.; Wang, M.; Liao, Q.; He, X. Characterization of Cd translocation and accumulation in 19 maize cultivars grown on Cd−contaminated soil: Implication of maize cultivar selection for minimal risk to human health and for phytoremediation. Environ. Sci. Pollut. Res. 2016, 23, 5410–5419. [Google Scholar] [CrossRef]
- Nehnevajova, E.; Herzig, R.; Federer, G.; Erismann, K.H.; Schwitzguébel, J.P. Screening of sunflower cultivars for metal phytoextraction in a contaminated field prior to mutagenesis. Int. J. Phytoremediation 2005, 7, 337–349. [Google Scholar] [CrossRef]
- Adesodun, J.K.; Atayese, M.O.; Agbaje, T.A.; Osadiaye, B.A.; Mafe, O.F.; Soretire, A.A. Phytoremediation potentials of sunflowers (Tithonia diversifolia and Helianthus annuus) for metals in soils contaminated with zinc and lead nitrates. Water Air Soil Pollut. 2010, 207, 195–201. [Google Scholar] [CrossRef]
- Seth, C.S.; Misra, V.; Singh, R.R.; Zolla, L. EDTA−enhanced lead phytoremediation in sunflflower (Helianthus annuus L.) hydroponic culture. Plant Soil 2011, 347, 231. [Google Scholar] [CrossRef]
- Doncheva, S.; Moustakas, M.; Ananieva, K.; Chavdarova, M.; Gesheva, E.; Vassilevska, R.; Mateev, P. Plant response to lead in the presence or absence EDTA in two sunflower genotypes (cultivated H. annuus cv. 1114 and interspecific line H. annuus×H. argophyllus). Environ. Sci. Pol. 2013, 20, 823–833. [Google Scholar] [CrossRef]
- Motesharezadeh, B.; Navabzadeh, M.; Liyaghat, A.M. Modeling phytoremediation of cadmium contaminated soil with sunflower (Helianthus annus) under salinity stress. Int. J. Environ. Res. 2016, 10, 109–118. [Google Scholar]
- Hu, Z.Y.; Zhang, Y.F.; He, Y.; Cao, Q.Q.; Zhang, T.; Lou, L.Q.; Cai, Q.S. Full length transcriptome assembly of italian ryegrass root integrated with RNA−seq to identify genes in response to plant cadmium stress. Int. J. Mol. Sci. 2020, 21, 1067. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Monteiro, C.; Moutinho−Pereira, J.; Correia, C.; Goncalves, B.; Santos, C. Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiol. Plant. 2013, 35, 1281–1289. [Google Scholar] [CrossRef]
- Rasafi, T.E.; Oukarroum, A.; Haddioui, A.; Song, H.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2020, 5, 675–726. [Google Scholar] [CrossRef]
- Guo, Z.H.; Zeng, P.; Xiao, X.Y.; Peng, C. Physiological, anatomical, and transcriptional responses of mulberry (Morus alba L.) to Cd stress in contaminated soil. Environ. Pollut. 2021, 284, 117387. [Google Scholar] [CrossRef]
- Manaa, A.; Goussi, R.; Derbali, W.; Cantamessa, S.; Essemine, J.; Barbato, R. Photosynthetic performance of quinoa (Chenopodium quinoa.) after exposure to a gradual drought stress followed by a recovery period. Bba−Bioenerg. 2021, 1862, 148383. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, F.; Gao, S.; Wang, X. Heavy metal accumulation in different rice cultivars as influenced by foliar application of nano−silicon. Water Air Soil Pollut. 2016, 227, 1–13. [Google Scholar] [CrossRef]
- Huang, S.; Rao, G.; Ashraf, U.; Deng, Q.; Dong, H.; Zhang, H. Ultrasonic seed treatment improved morpho−physiological and yield traits and reduced grain Cd concentrations in rice. Ecotoxicol. Environ. Saf. 2021, 214, 112119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Lei, X.J.; Wang, P.L.; Wang, Y.Y.; Lv, J.X.; Li, X.P.; Gao, C.Q. Overexpression of ThSAP30BP from Tamarix hispida improves salt tolerance. Plant Physiol. Bioch. 2020, 146, 124–132. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Ismayilov, A.I.; Mamedov, A.I.; Fujimaki, H.; Tsunekawa, A.; Levy, G.J. Soil salinity type effects on the relationship between the electrical conductivity and salt content for 1:5 soil−to−water extract. Sustainability 2021, 13, 3395. [Google Scholar] [CrossRef]
- Yan, B.F.; Nguyen, C.; Pokrovsky, O.S.; Candaudap, F.; Coriou, C.; Bussiere, S.; Robert, T.; Cornu, J.Y. Cadmium allocation to grains in durum wheat exposed to low Cd concentrations in hydroponics. Ecotoxicol. Environ. Saf. 2019, 184, 109592. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Yang, C.Y.; Zhang, L.B.; Li, L.Z.; Liu, S.J.; Yu, J.B.; You, L.P.; Zhou, D.; Xia, C.H.; Zhao, J.M.; et al. Metabolic profiling of cadmium−induced effects in one pioneer intertidal halophyte Suaeda salsa by NMR−based metabolomics. Ecotoxicology 2011, 20, 1422–1431. [Google Scholar] [CrossRef]
- Zhao, D.; Li, T.; Shen, M.; Wang, J.L.; Zhao, Z.W. Diverse strategies conferring extreme cadmium (Cd) tolerance in the dark septate endophyte (DSE), Exophiala pisciphila: Evidence from RNA−seq data. Microbiol. Res. 2015, 170, 27–35. [Google Scholar] [CrossRef]
- He, J.; Ren, Y.; Chen, X.; Chen, H. Protective roles of nitric oxide on seed germination and seedling growth of rice (Oryza sativa L.) under cadmium stress. Ecotoxicol. Environ. Saf. 2014, 108, 114–119. [Google Scholar] [CrossRef]
- Peco, J.D.; Campos, J.A.; Romero−Puertas, M.C.; Olmedilla, A.; Higueras, P.; Sandalio, L.M. Characterization of mechanisms involved in tolerance and accumulation of Cd in Biscutella auriculata L. Ecotoxicol. Environ. Saf. 2020, 201, 110784. [Google Scholar] [CrossRef] [PubMed]
- Shahabivand, S.; Maivan, H.Z.; Goltapeh, E.M.; Sharifi, M.; Aliloo, A.A. The effects of root endophyte and arbuscular mycorrhizal fungi on growth and cadmium accumulation in wheat under cadmium toxicity. Plant Physiol. Bioch. 2012, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Mangal, M.; Agarwal, M.; Bhargava, D. Effect of cadmium and zinc on growth and biochemical parameters of selected vegetables. J. Pharmacogn. Phytochem. 2013, 2, 106–114. [Google Scholar]
- Ren, L.; Wang, M.R.; Wang, Q.C. ROS−induced oxidative stress in plant cryopreservation: Occurrence and alleviation. Planta 2021, 254, 124. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Back, K. 2−Hydroxymelatonin Promotes Seed Germination by Increasing Reactive Oxygen Species Production and Gibberellin Synthesis in Arabidopsis thaliana. Antioxidants 2022, 11, 737. [Google Scholar] [CrossRef]
- Li, W.Y.; Chen, B.X.; Chen, Z.J.; Gao, Y.T.; Chen, Z.; Liu, J. Reactive Oxygen Species Generated by NADPH Oxidases Promote Radicle Protrusion and Root Elongation during Rice Seed Germination. Int. J. Mol. Sci. 2017, 18, 110. [Google Scholar] [CrossRef]
- Kacalkova, L.; Tlustos, P.; Szakova, J. Phytoextraction of risk elements by willow and poplar trees. Int. J. Phytoremediation 2015, 17, 414–421. [Google Scholar] [CrossRef]
- Balabanova, B.; Stafifilov, T.; Liping, F.; Meicong, W. Bioavailability and bioaccumulation characterization of essential and heavy metals contents in various plant food from polluted and referent areas. J. Environ. Health Sci. Eng. 2016, 13, 2. [Google Scholar]
- Tang, X.; Pang, Y.; Ji, P.; Gao, P.; Nguyen, T.H.; Tong, Y. Cadmium uptake in above−ground parts of lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2016, 125, 102–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).