Abstract
Fruit texture affects apples’ quality, consumer preference, and shelf life. The cell wall neutral sugar composition was reported to contribute to apples’ mechanical properties at harvest. However, the contributions of cell wall neutral sugar composition to apple texture loss during storage among different cultivars are still unclear. In this study, six cultivars of the apple fruit were stored at 25 °C for 60 days (i.e., rapid loss of texture: ‘Jiguan’, ‘Yindu’, and ‘Qinguan’; slow loss of texture: ‘Fuji’, ‘Huaguan’, and ‘Cripps pink’). The texture properties, physiological indicators, and expression of cell wall-related genes were investigated every 20 d. The results showed that apple cultivars with rapid texture loss showed a faster loss in flesh hardness and crispness, and a higher level of flesh tightness and pericarp break distance, than the cultivars with slow texture loss. Galactose content showed the closest association with the texture properties among the cell wall neutral sugar compositions. The rapid loss of galacturonic acid content and the expression of MdPG1 were higher in the cultivars with rapid texture loss than in those with slow texture loss. These results indicated that changes in cell wall neutral sugar composition contribute to apple texture loss during storage among cultivars.
1. Introduction
As one of the principal quality factors in the breeding process, apple fruit texture has been recognized as a multifactorial trait that is composed of several components, such as hardness [1,2], crispness [3,4], and mealiness [5,6]. Among them, hardness is the earliest researched element of the apple fruit [7] and has received increasing attention, such as its physiological mechanisms [8] and molecular marker-assisted breeding [9,10]. Crispness is the foremost reference indicator of perceived freshness in the apple fruit for consumers [11]. The evaluation of crispness has gradually improved, and research progress has accelerated rapidly in recent years [12,13,14]. The mealiness is also a very important indicator to assess the textural failures of fruit flesh, which is usually estimated in sensory evaluations [6]. In addition, the changes in pericarp hardness, pericarp resilience, and break distance were considered and affected the consumer’s preference [15]. To date, many efforts have focused on the physiological and biochemical alterations of the single-texture properties of apple fruit. However, there are only a few studies on the relationship between the texture properties of different cultivars during storage.
It has been demonstrated that fruit texture alterations during ripening are associated with the dissolution of pectin in the middle lamella and the modifications of the cell wall materials (CWM), which result in the loss of adhesion between adjacent cells and the disruption of the cell wall [6,16]. Pectin, which combined with ethylene affects the ripening and softening of fruits, is comprised of highly complex and heterogeneous polysaccharides [17]. The pectin backbone is composed of a linear chain of (1→4)-α-D-galacturonic acid (GalA). The linear homopolymer of α-1,4-linked D-GalA is named homogalacturonan (HG). Two structurally noteworthy pectic polysaccharide domains include rhamnogalacturonan I (RG-I) and rhamnogalacturonan II (RG-II), neutral sugar side-chains rich in rhamnose, arabinose, and galactose. They are usually polymerized to the main backbones of linear HG [18,19]. Galacturonic acid, as the backbone of pectin, is a monomer that accounts for approximately 70% of pectin molecules. Galactose, arabinose, and uronic acid contents correlated positively with the storage modulus (a measure of how much energy must be put into the sample to distort it) of fresh apple samples at harvest among different cultivars [20]. However, the relationship between texture loss and cell wall neutral sugar composition changes among the cultivars of apple fruit during storage is still not fully understood.
Many genes responsible for fruit softening and textural change during storage have been previously studied [21,22]. With the development of high-throughput sequencing technology, various functional genes related to apple texture have been identified. Cell wall-modifying genes shown to increase in expression during storage include β-galactosidase-1 (MdGAL1) [23], polyglacturonase-1 (MdPG1) [10], and agabinofuranosidase-1 and -3 (MdAFase1/3) [24,25]. Several studies have shown that MdPG1 is one of the key players that regulates fruit softening as it has been mapped to a dominant quantitative trait locus (QTL) for softening [26], and suppression of the gene results in apples that are firmer than controls [27]. Additionally, natural variations in the pectin acetylesterase gene MdPAE10 contributed to extending the shelf life of apple fruit [28]. Additionally, a QTL-based genomics-assisted prediction of the role of natural variations in MdERF3 and MdERF118 in the development and retention of apple flesh firmness/crispness was performed [13]. Although some functional cell wall degradation-related genes were identified by high-throughput technologies, many members of these gene families were not analyzed due to their large number of families and texture traits. Consequently, for different cultivars of apples during postharvest storage, this study was conducted to analyze and identify the genes related to texture changes using transcriptome data and physiological indicators.
Efforts have been focused on the physiological and biochemical alterations of a single texture characteristic of apple fruit; however, few studies have investigated the relationship between the textures of stored apples. In this study, to investigate the underlying relationship between texture properties and cell wall neutral sugar composition, six apple cultivar fruits with rapid texture loss (‘Jiguan’, ‘Yindu’, and ‘Qinguan’) and slow texture loss (‘Fuji’, ‘Huaguan’, and ‘Cripps pink’) were stored at room temperature (25 ± 1 °C) for 60 d. The apples’ texture properties, pectin content, ethylene production rate, yields of CWM, and CWM sugar composition were assayed. The correlation of each texture property, texture properties, and cell wall sugar composition were analyzed, and the expression of the key cell wall-modifying genes during storage was surveyed. The results may provide new insights for understanding apple texture loss in different apple cultivars during storage.
2. Materials and Methods
2.1. Fruit Material
The commercial maturity apple fruit (Malus × domestica Borkh.) cv. ‘Jiguan’, ‘Yindu’, ‘Qinguan’, ‘Fuji’, ‘Huaguan’, and ‘Cripps pink’ were harvested from the national apple model of Northwest A&F University in Baishui County, Weinan City, Shaanxi Province, China. Uniform ripening fruit without physical damage was selected and stored at room temperature (25 ± 1 °C). A total of 90 fruits of each cultivar were randomly divided into three groups, and they were used as three biological replicates. For each cultivar, 15 fruits (3 replicates, 5 fruits in each group) were fixed to measure the rate of ethylene production and respiration at 0, 20, 40, and 60 DAH (days after harvest). A total of 9 fruits (3 replicates, 3 fruits in each group) were used for a sensory evaluation at 40 DAH, and 6 fruits (3 replicates, 2 fruits in each group) were used for a compression observation at 0 and 60 DAH. A total of 60 fruits (3 replicates, 5 fruits in each group) at the 4 sampling points were determined for the texture properties. After the texture measurement, the samples were frozen in liquid N2 and stored at −80 °C for further experiments.
2.2. Apple Fruit Texture Puncture Test and Compression Observation
The apple fruit texture was measured with a computer-controlled texture analyzer TA-XT Plus (Stable Micro Systems Ltd., Godalming, UK). The parameters were set as follows: P/2 stigma; prepuncture speed, 10 mm·s−1; test and postpuncture speed 1.0 mm·s−1; puncture distance 10.0 mm. A multidimensional texture profile analysis was completed to determine the following phenotypic indexes for each sample [15]: F1, pericarp firmness; W1, pericarp resilience; F2, flesh fruit hardness; W3, fruit tightness; D1, pericarp break distance; F3, flesh crispness was defined as a description with slight modifications [14]. Fresh tightness is the total mechanical work during the upward retraction of the TA-XT Plus probe after the end of the puncture. The details of the program are shown in Table S1, and the image is presented in Figure 1A.
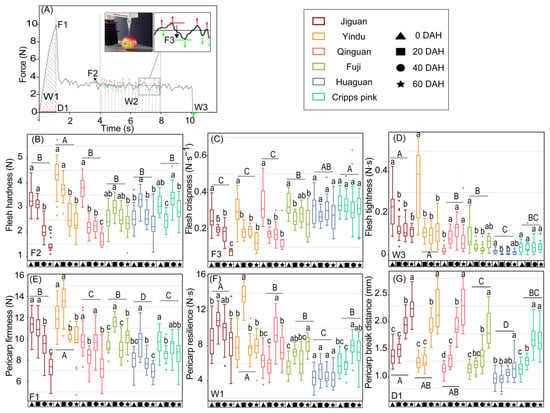
Figure 1.
Textural properties of ‘Jiguan’, ‘Yindu’, ‘Qinguan’, ‘Fuji’, ‘Huaguan’, and ‘Cripps pink’ apple fruit stored at 25 ± 1 °C for 0, 20, 40, and 60 d. (A) Apple pulp puncture force-displacement curve; (B) flesh hardness measured by F2: mean force between 4 and 8 s; (C) flesh crispness measured by F3: average drop-off of force between 4 and 8 s; (D) flesh tightness measured by W3: area after 10 s; (E) pericarp firmness measured by F1: the max force; (F) pericarp resilience measured by W1: area from 0 to max force; (G) pericarp break distance D1: the distance from 0 to max force. DHA means days after harvest. Data were presented by a boxplot which consists of the most extreme values in the data set (maximum and minimum values), the lower and upper quartiles, and the median. In each plot, the capital letters above the symbols indicate significant differences (p < 0.05) between six cultivars, and the lowercases indicate significant differences (p < 0.05) between different storage times.
The sensory evaluation was performed by an expert panel of ten judges and was consistent with Chang et al. [29]. The sensory attributes were crispness, hardness, and mealiness. They were rated on a 9-point scale from 1 (low) to 9 (very high). Longitudinal structural sections of apple flesh from the collected fruit were prepared as previously described in Hou et al. [30] with slight modifications. A stainless borer was used for the collection of 15 × 15 × 15 mm apple pulp cubes prepared for compression testing using a TA-XT plus texture analyzer (Stable Micro Systems Ltd., Godalming, UK). The probe diameter was 50 mm (P50), and the measuring seed was 0.5 mm/s. When the maximum force reached 75 N, the apple pulp compression data were recorded. The morphological features were recorded using a digital camera (Nikon D90, Nikon Corp, Tokyo, Japan) at 0 and 60 DAH.
2.3. Fruit Physiological Property Indicators
2.3.1. Determination of Ethylene Production Rate, Respiration Rate, and Pectin Content
The rates of ethylene production and respiration were measured according to our previous methods [31]. Five apple fruits were sealed in a 9 L vessel and incubated for 1 h at room temperature. To measure ethylene production, a 1 mL gas sample was collected from the vessel by a syringe, and the gas was injected into a GC-14A gas chromatograph for analysis (Shimadzu, Kyoto, Japan). The respiration rate was measured using a CO2 infrared gas analyzer (TEL7001; GE Telaire) and the results were expressed in nmol·kg−1 s−1 CO2.
Pectin was measured as described by He et al. [31]. Approximately 2 g of frozen fruit flesh was dissolved by boiling in 10 mL 95% ethanol (v/v) for 30 min with constant stirring and centrifuged at 4 °C after cooling. The supernatant was discarded, and the pellets were washed twice with hot 10 mL 95% ethanol (v/v) and centrifuged again. Then, the pellets were resuspended in distilled water at 50 °C for 30 min. The supernatant was used to measure water-soluble pectin (WSP), and the pellets were further resuspended in 10 mL 0.5 M H2SO4 and heated in a boiling water bath for 1 h. The supernatant was collected and used to measure acid-soluble pectin (ASP). One milliliter of WSP or ASP was added to 6 mL of H2SO4 for 1 h in boiling water. After the mixture was cooled, 0.2 mL of 0.15% carbazole reagent was added, and the mixture was incubated in darkness for 30 min. Finally, the pectin content was determined by measuring the absorbance at 530 nm. A standard curve was constructed using D-(+)-galacturonic acid (GalA) and was expressed as GalA equivalents per mass of fruit tissue on a fresh weight basis (g·kg−1).
2.3.2. Preparation of CWM and Determination of Neutral Sugar Composition
The CWM preparation was performed according to Renard and Ginies [32] for each of the three replicates of plant material. For each extraction, approximately 2 g of frozen fruit flesh was placed in 10 mL 70% (v/v) ethanol and stirred for 2 h. The suspension was centrifuged, and the insoluble material was collected. Then, the samples were washed repeatedly with 80% (v/v) alcohol until the absence of sugars, as shown by a negative reaction in the phenol sulfuric test. The insoluble material was resuspended in 1 mL acetone and evaporated until the solvent was dry. The dry cell wall material was called alcohol insoluble material for calculating the CWM yield and was expressed on a fresh weight basis (g·kg−1).
In each sample, 10 mg of CWM from each sample was used to determine the contents of the different cell wall components [32]. The samples were submitted to hydrolysis with 250 µL 72% (v/v) sulfuric acid for 1 h at room temperature and then diluted to 1 M sulfuric acid by the addition of water and an internal standard (1 mg/mL inositol). All the samples were incubated at 100 °C for 3 h for hydrolysis. After hydrolysis, the samples were derivatized to volatile alditol acetates [33]. They were injected into a GC-MS QP2010 Shimadzu (Kyoto, Japan). The conditions included an injector temperature of 240 °C in split mode (ratio 1:10); helium as a gas carrier at 38.3 cm·s−1; and the oven temperature programmed from 180 °C (20 min constant temperature) to 200 °C at 5 °C · min−1. Galacturonic acids were measured by the meta–hydroxyl–diphenyl assay as described by Liu et al. [34]. The absorbance was read at 520 nm using a spectrophotometer (V-530 Jasco, Tokyo, Japan), and the concentrations were calculated against a calibration curve with galacturonic acid as the external standard. All the neutral sugar composition was expressed on a fresh weight basis (g·kg−1).
2.4. Screening of Candidate Cell Wall-Related Genes in the Apple Genome
To identify cell wall-related and expressed genes in the fruit tissue, RNA-Seq data released by Busatto et al. [35] were downloaded from the NCBI. The data of the ‘Golden Delicious’ and ‘Fuji’ fruit that were stored for 14 and 21 d at room temperature were used. Based on their normalized expression values (RPKM, reads per kilobase of exon per million mapped reads), a set of cell wall-related genes (within the gene families encoding PG, PE, PL, PME, α-AF, β-GAL, and PAE) expressed in fruit tissues during postharvest storage (RPKM > 10) was selected. Their expression in ‘Jiguan’, ‘Yindu’, ‘Fiji’, and ‘Huaguan’ apples at 0, 20, 40, and 60 DHA was quantified using qRT-PCR.
2.5. RNA Extraction, cDNA Synthesis, and Real-Time PCR Quantification
The total RNA of all the samples was extracted according to a protocol described by Han et al. [36]. Three biological replicates with three independent RNA extractions and cDNA synthesis were performed for each sampling point. A total of 1 μg of RNA was used for first-stand cDNA synthesis by using a PrimeScriptTM RT Reagent Kit with gDNA Eraser (Perfect Real Time; TaKaRa, Dalian, China). The synthesized cDNA was diluted 10-fold for the following qRT-PCR analysis. qRT-PCR was conducted in a 20 μL reaction volume using SYBR Premix Ex Taq (TaKaRa, Kyoto, Japan) with the Lightcycler 480II (Roche, Basel, Switzerland) real-time PCR system. The gene encoding actin was used as an endogenous control, and the analysis was based on Li et al. [37]. The specific primers for the targeted genes were designed with Oligo 7.0, and the specific information is listed in Table S2.
2.6. Statistical Analysis
Microsoft Excel was used for the data analysis. The statistical tests were performed by SPSS Statistics (22.0, SPSS-IBM, Inc., Chicago, IL, USA) and OriginPro (Version 2022, OriginLab Corporation, Northampton, MA, USA). R software version 4.2.2 and the “ggcor” package were used to perform the Mantel test and Pearson analysis. The statistical analysis methods were a one-way ANOVA and Tukey’s multiple range test. All the experiments were repeated three times and analyzed independently.
3. Results
3.1. A Textural Evaluation System of Apple Fruit in Mechanical and Sensorial Ways
The apple fruit quality considerably decreased during room-temperature storage. In this study, the texture properties of six cultivars ‘Jiguan’, ‘Yindu’, ‘Qinguan’, ‘Fuji’, ‘Huaguan’, and ‘Cripps pink’ were investigated, and their mechanical and sensorial properties of texture showed various changes (Figure 1 and Figure 2). The results showed that the fruit flesh in the cultivars with rapid texture loss (‘Fuji’, ‘Huaguan’, and ‘Cripps pink’) maintained the original shape under identical force conditions, while that of the cultivars with slow texture loss (‘Jiguan’, ‘Yindu’, and ‘Qinguan’) was scattered and powdery at 60 DAH (Figure 2). The flesh hardness and crispness in the cultivars with rapid texture loss rapidly decreased during storage, while those of the cultivars with slow texture loss remained stable (Figure 1B,C). Moreover, the flesh crispness in the cultivars with slow texture loss was higher than that of the cultivars with rapid texture loss, which was consistent with the different appearances resulting from external pressure on the apple flesh at 60 DAH (Figure 2). The flesh tightness in the cultivars ‘Jiguan’ and ‘Fuji’ was higher than those in the cultivars ‘Huaguan’ and ‘Cripps pink’ (Figure 1D). For the textural characteristics of apple pericarp, the pericarp firmness of ‘Jiguan’ gradually decreased during storage, while there was a slow increase in ‘Yindu’, ‘Huaguan’, and ‘Fuji’ from 0 to 20 DAH (Figure 1E). The pericarp resilience of ‘Jiguan’ and ‘Yindu’ was higher than that of ‘Qinguan’, ‘Fuji’, ‘Huaguan’, and ‘Cripps pink’ (Figure 1F). Regarding the variations in the pericarp break distance, all the apple cultivars showed an increasing trend during storage; among the six cultivars, the mean value of the pericarp break distance in ‘Huaguan’ was the lowest (Figure 1G). A correlation analysis of the texture indicators showed that flesh hardness was positively correlated with flesh crispness and pericarp hardness, with correlation coefficients of 0.504 (p < 0.001) and 0.636 (p < 0.001), respectively (Figure S1). The pericarp break distance was negatively correlated with flesh hardness and flesh crispness, with correlation coefficients of −0.444 (p < 0.001) and −0.596 (p < 0.001), respectively. Flesh tightness was positively correlated with flesh hardness and pericarp hardness, with correlation coefficients of 0.279 (p < 0.05) and −0.465 (p < 0.001), respectively.
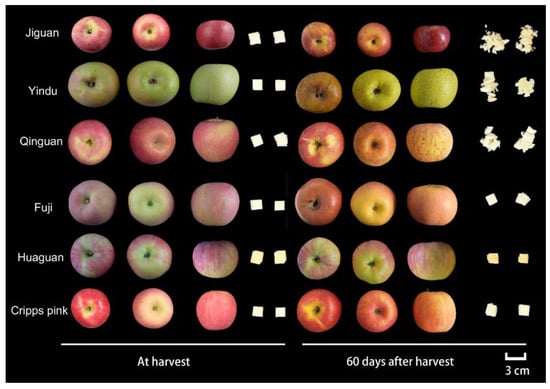
Figure 2.
Appearance of ‘Jiguan’, ‘Yindu’, ‘Qinguan’, ‘Fuji’, ‘Huaguan’, and ‘Cripps pink’ apple fruit stored at 25 ± 1 °C for 0 and 60 d. The pulp of 15 × 15 × 15 mm cube represents the shape of the apple pulp after being pressed by 75 N force using a TA-XT plus texture analyzer (Stable Micro Systems Ltd., Godalming, UK).
The sensory evaluation results showed that the crispness of the cultivars with slow texture loss (‘Fuji’, ‘Huaguan’, and ‘Cripps pink’) was higher than that of the cultivars with rapid texture loss (‘Jiguan’, ‘Yindu’, and ‘Qinguan’) at 40 DAH (Table 1); the hardness of ‘Cripps pink’ was higher than that of the other cultivars; and the mealiness of ‘Jiguan’ and ‘Yindu’ was higher than that of the other cultivars. The Pearson correlation analysis of instrument measurement and sensory evaluation showed that there was a positive correlation between flesh crispness (instrument measurement) and crispness (sensory evaluation) (p = 0.027) and a positive correlation between flesh hardness (instrument measurement) and hardness (sensory evaluation) (p = 0.025). This result indicated that the measurement of crispness could accurately reflect the natural texture of the fruit.

Table 1.
Sensory evaluation of the ‘Jiguan’, ‘Yindu’, ‘Qinguan’, ‘Fuji’, ‘Huaguan’, and ‘Cripps pink’ apple fruit stored at 25 ± 1 °C at 40 DAH. Data are presented as mean ± standard error (SE) from ten judges; in each property, the letters behind the numbers indicate significant differences (p < 0.05) between six cultivars.
3.2. Fruit Postharvest Physiological Characteristics of Six Apple Cultivars
In this study, the ethylene production rate, respiration rate, and pectin content of six apple cultivar fruits during postharvest were evaluated. The ethylene production rate of the six cultivars exhibited a typical climacteric ethylene production pattern during storage (Figure 3A), and all peaks appeared at 20 DAH. Among them, the peaks of ‘Qinguan’ were higher than those of the other cultivars. Meanwhile, the total mean of the respiration rate in ‘Jiguan’ was higher than that in the slow texture loss cultivars (‘Fuji’, ‘Huaguan’, and ‘Cripps Pink’) (Figure 3B). In addition, the ASP content of the six cultivars’ fruit gradually decreased during storage. After storage for 20 d, the ASP contents of ‘Jiguan’ and ‘Yindu’ were reduced by 5.36 and 9.21 g/kg of the fresh weight, whereas the ASP contents of ‘Fuji’, ‘Huaguan’, and ‘Cripps Pink’ were reduced by 3.90, 1.83, and 1.59 g/kg, respectively (Figure 3C). The WSP content for each cultivar gradually increased during storage, and the WSP content of ‘Jiguan’ was the highest among the six cultivars (Figure 3D).
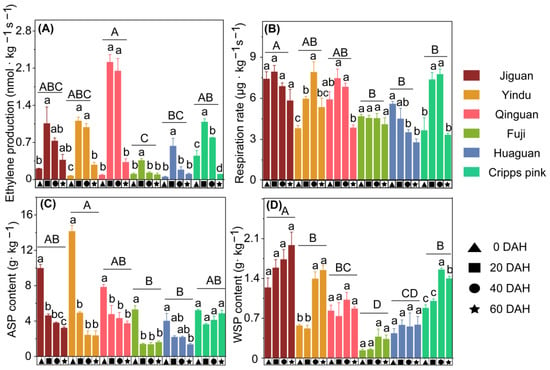
Figure 3.
Ethylene production rate (A) and respiration rate (B), acid-soluble pectin (ASP) content (C), and water-soluble pectin (WSP) content (D) of ‘Jiguan’, ‘Yindu’, ‘Qinguan’, ‘Fuji’, ‘Huaguan’, and ‘Cripps pink’ apple fruit stored at 25 ± 1 °C for 60 d. Data are presented as mean ± standard error (SE) from three biological replicate assays; in each plot, the capital letters above the symbols indicate significant differences (p < 0.05) between six cultivars, and the lowercases indicate significant differences (p < 0.05) between different storage times.
3.3. Cell Wall Material and Its Neutral Sugar Composition Postharvest Changes of Six Apple Cultivars
In this study, the CWM and neutral sugar composition of six apple cultivar fruits during postharvest were evaluated. The yields of the CWM decreased during storage in the cultivars with rapid texture loss (‘Jiguan’, ‘Yindu’, and ‘Qinguan’), while no changes were detected in the ‘Huaguan’ apple (Figure 4A). Among the six cultivars, the CWM yields of ‘Yindu’ were the highest, followed by ‘Jiguan’, ‘Cripps pink’, ‘Fuji’, and ‘Huaguan’. The glucose content was the most abundant among the seven monosaccharides in the CWM (Figure 4B), which decreased the fastest from 0 to 20 DAH (especially by 17 g·kg−1 of flesh weight in ‘Yindu’) which might be related to the rapid decrease in flesh hardness. The xylose contents were observed with no obvious pattern during storage as the main monosaccharide composed of xyloglucans (Figure 4C). Rhamnose, as the key monosaccharide of rhamnogalacturonan I (RG-I) and rhamnogalacturonan II (RG-II) is composed of pectin. The rhamnose contents of ‘Yindu’, ‘Qinguan’, and ‘Cripps pink’ were higher than those of ‘Jiguan’, ‘Fuji’, and ‘Huaguan’ (Figure 4D). Galactose and arabinose, as the main side chain monosaccharides, compose pectin. The galactose contents in the cultivars with rapid texture loss presented a downward trend over storage, especially from 0 to 20 DHA, which suggested that the rapid loss of galactose may contribute to the loss of apple texture (Figure 4E). The arabinose contents of ‘Yindu’, ‘Qinguan’, and ‘Cripps pink’ were higher than those in ‘Fuji’ and ‘Huaguan’ (Figure 4F). No obvious pattern was observed in the mannose and fucose content during storage (Figure 4G,H). The extracted galacturonic acid content of the cultivars with rapid texture loss (‘Jiguan’, ‘Yindu’, and ‘Qinguan’) generally decreased over the entire storage period, while the smallest decrease was found in the cultivars with slow texture loss (‘Fuji’, ‘Huaguan’, and ‘Cripps pink’) (Figure 4I).
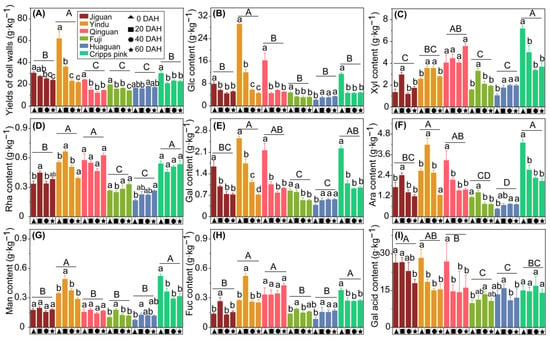
Figure 4.
Cell walls material (CWM) yields and its neutral sugar compositions content of ‘Jiguan’, ‘Yindu’, ‘Qinguan’, ‘Fuji’, ‘Huaguan’, and ‘Cripps pink’ apple fruits stored at 25 ± 1 °C for 60 d. (A) Yields of CWM; (B) glucose (Glc); (C) xylose (Xyl); (D) rhamnose (Rha); (E) galactose (Gal); (F) arabinose (Ara); (G) mannose (Man); (H) fucose (Fuc); and (I) galacturonic acid (Gal acid). Data are presented as mean ± standard error (SE) from three biological replicate assays. In each plot, the capital letters above the symbols indicate significant differences (p < 0.05) between six cultivars, and the lowercases indicate significant differences (p < 0.05) between different stored times at each apple cultivar.
3.4. Correlation of Cultivar Differences in Apple Fruit Texture during Postharvest
Mantel and Pearson correlations were sought between the texture properties and physiological indicators obtained from the six cultivars during postharvest (Figure S2). From all the parameters measured between the texture properties and physiological indicators, flesh hardness was correlated with the ASP content, yield of CWM, galacturonic acid, galactose, and glucan contents (r = 0.2–0.4, p < 0.01). Flesh crispness was only correlated with the WSP content (r = 0.2–0.4, p < 0.01), and flesh tightness was correlated with the ASP content (r = 0.2–0.4, p < 0.01). For the pericarp properties, pericarp resilience was correlated with rhamnose and fucose content (r = 0.2–0.4, p < 0.01). From all the parameters measured in the physiological indicators, a high correlation was observed between the cell wall neutral sugar compositions of the samples in six cultivars during postharvest.
To reduce the interference caused by the cultivars, the relationship between the texture properties and physiological indicators in the cultivars with rapid texture loss (‘Jiguan’ and ‘Yindu’) and slow texture loss (‘Fuji’ and ‘Huaguan’) were analyzed separately using Mantel and Pearson correlations, respectively (Figure 5). A higher correlation between the texture properties and physiological indicators was observed in ‘Jiguan’ and ‘Yindu’ than in ‘Fuji’ and ‘Huaguan’. For ‘Jiguan’ and ‘Yindu’, flesh hardness was correlated with the WSP and galactose contents (r > 0.4, p < 0.01), and flesh tightness was correlated with the ASP content, yield of CWM, galactose, and glucose contents (r > 0.4, p < 0.01). The pericarp properties were correlated with many physiological indicators in ‘Jiguan’ and ‘Yindu’. In contrast, no correlation was observed between flesh hardness and the physiological indicators in the cultivars with a slow decline in fruit hardness (‘Fuji’ and ‘Huaguan’). A correlation was observed between the pericarp break distance and rhamnose content (r > 0.4, p < 0.01) in ‘Fuji’ and ‘Huaguan’. Moreover, the results of the Pearson correlations between the physiological indicators showed that the WSP was negatively correlated with the cell wall sugar components, especially with the galactose and arabinose contents, in both the ‘Jiguan’ and ‘Yindu’ cultivars and the ‘Fuji’ and ‘Huaguan’ cultivars.
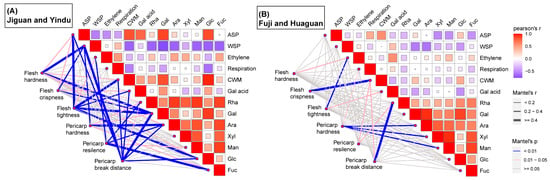
Figure 5.
Correlation analysis for texture properties, physiological indicators of ‘Jiguan’ and ‘Yindu’ (A) and ‘Fuji’ and ‘Huaguan’ (B) apple fruits during postharvest. The Mantel test was used for the six texture properties and thirteen physiological indicators and Pearson for the thirteen physiological indicators. The size and color of the square represent the correlation coefficient of indicators for Pearson. The coarseness and color of the line represent the correlation coefficient of indicators for the Mantel test.
3.5. Cell Wall Degradation-Related Genes Involved in Textural Changes of Apple Fruit during Postharvest Storage
To identify the key cell wall degradation-related genes that focus on apple texture, RNA-Seq data from the ‘Golden Delicious’ and ‘Fuji’ apple fruits stored for 21 d at room temperature (20 °C) were used [35]. The transcript abundances of the genes were estimated by reads per kilobase of exon per million reads mapped (RPKM). The selective thresholds were set using a false discovery rate (FDR) ≤ 0.01, and the highest RPKM values > 10. Thirty-four target genes (Figure S3), including three polygalacturonase-related genes, ten pectinesterase-related genes, three pectin methylesterase-related genes, four pectatelyase-related genes, nine galactosidase-related genes, one arabinofuranosidase-related gene, and four pectin acetylesterase-related genes emerged from all the RPKM values. Moreover, five genes, including one polygalacturonase-related, two pectinesterase-related, and two galactosidase-related genes were continuously upregulated in GD14d vs. GD0d and GD21d vs. GD14d of ‘Golden Delicious’. Conversely, ten genes, including two pectinesterase-related, three pectin methylesterase-related, one pectatelyase-related, three galactosidase-related, and one pectin acetylesterase-related, were continuously downregulated in GD14d vs. GD0d and GD21d vs. GD14d of ‘Golden Delicious’. These 15 genes may play a pivotal role in the crispness loss of apple fruit during storage.
The expression of the 15 genes was further investigated in the ‘Jiguan’, ‘Yindu’, ‘Fuji’, and ‘Huaguan’ apples (Figure 6). Among the 15 genes, the expression level of the MdPG1 gene was the highest and increased gradually during storage. The expression level of the MdPG1 in the cultivars with rapid texture loss (‘Jiguan’ and ‘Yindu’) was higher than that in the cultivars with slow texture loss (‘Fuji’ and ‘Huaguan’). Among the four MdPE genes, the MdPE3 (103439874) expression level was the highest and it decreased gradually during storage in the four cultivars. The expression of MdPE1 (103420337) and MdPE1 (114826808) presented an increasing trend from 0 to 60 DHA. The expression of MdPME1 (10345177), MdPME3 (103436469), and MdPL1 (103450189) increased in ‘Jiguan’, ‘Yindu’, and ‘Fuji’ but decreased in ‘Huaguan’ from 0 to 40 DHA. Among the five MdGAL genes, the expression level of MdGAL1 (103450376) was the highest. The expression of MdGAL1 (103450376) and MdGAL4 (103414756) increased in ‘Jiguan’, ‘Yindu’ and ‘Fuji’ from 0 to 40 DAH, and its expression level was higher in ‘Huaguan’ than in ‘Jiguan’ and ‘Yindu’. The MdPAE1 gene in the ‘Jiguan’ apples showed a higher expression level than those in the other three cultivars.
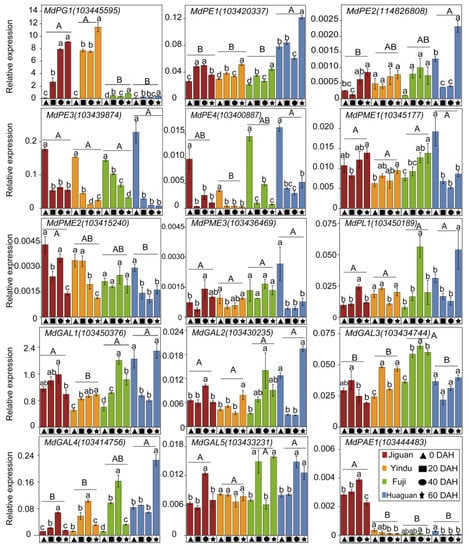
Figure 6.
Expression of the key cell wall-related genes of ‘Jiguan’, ‘Yindu’, ‘Fuji’, and ‘Huaguan’ apple fruits stored at 25 ± 1 °C for 0, 20, 40, and 60 d. Gene expression was analyzed by rRT-qPCR. Data are presented as mean ± standard error from three biological replicate assays. In each plot, the capital letters above the symbols indicate significant differences (p < 0.05) between four varieties, and the lowercases indicate significant differences (p < 0.05) between different storage times at each apple variety. PG, polygalacturonase; PE, pectinesterase; PME: pectin methylesterase; PL, pectatelyase; GAL: galactosidase; AF: arabinofuranosidase; PAE: pectin acetylesterase.
4. Discussion
4.1. The Difference in Texture Loss Rate among the Six Cultivars Was Mainly Manifested in the Flesh Hardness and Crispness of Apple
Many changes occurred in the apple flesh texture with the appearance of ethylene and respiration peaks during storage, which mainly included a decrease in hardness and crispness loss, and an increase in mealiness [38]. Apple pericarp texture on pericarp hardness and resilience also experienced a series of changes during storage [15]. In our study, rapid texture loss was mainly manifested in the apple cultivars with the rapid loss of flesh hardness and crispness (Figure 1). The flesh hardness was positively correlated with the flesh crispness, with a correlation coefficient of 0.504 (p < 0.001) (Figure S1). Similar results were reported between the crispness and hardness in five cultivars of apples [39]. These results indicated that the flesh hardness and crispness loss of the apple occurred simultaneously. Moreover, the flesh tightness and pericarp break distance were higher in cultivars with rapid texture loss than those with slow texture loss (Figure 1D,G). Zhao et al. [15] also found similar results in ‘Nagafu No. 2’. The level of flesh tightness was higher in the cultivars with rapid texture loss (‘Jiguan’, ‘Yindu’, and ‘Qinguan’) than those with slow texture loss (‘Huaguan’ and ‘Cripps Pink’), and the level of tightness at harvest was higher in the cultivars with rapid texture loss (‘Jiguan’ and ‘Yindu’) than in those with slow texture loss (‘Fuji’, ‘Huaguan’, and ‘Cripps Pink’) (Figure 1D). The high level of flesh tightness may provide the possibility for the rapid decrease in flesh hardness and crispness of the apple.
4.2. Cell Wall Neutral Sugar Composition Changes May Contribute to the Apple Texture Loss of Different Cultivars during Storage
4.2.1. The Rapid Loss of Galacturonic Acid Content May Result in the Rapid Loss of Apple Texture
Galacturonic acid, as the backbone of pectin, can be hydrolyzed by the ripening-induced enzymes endo-polygalacturonase (PG) and pectate lyase (PL) [40]. More galacturonic acid in the water-soluble pectin extracts was detected in ‘Golden Delicious’ than in ‘Fuji’ during cold storage [16]. In our study, the galacturonic acid content was higher in the cultivars with rapid texture loss (‘Jiguan’, ‘Yindu’, and ‘Qinguan’) than in those with slow texture loss (‘Fuji’ and ‘Huaguan’) during storage (Figure 4I). The results were similarly reported in ‘Gala’ and ‘Fuji’ but different from those in ‘Golden Delicious’ and ‘Fuji’ [15]. The different results should be due to the different approaches of galacturonic acid content, whereby our study compared galacturonic acid content with fresh weight, while Zhao et al. [15] compared galacturonic acid content with cell wall alcohol-insoluble material. In addition, galacturonic acid decreased from 0 to 60 DAH in the cultivars with rapid texture loss, while there was no difference in the cultivars with slow texture loss, indicating that the loss of galacturonic acid may contribute to the rapid texture loss in ‘Jiguan’, ‘Yindu’, and ‘Qinguan’ (Figure 4I). The same decreasing trend was also reported in cultivars with a rapid mealiness pattern (‘Hongjiangjun’ and ‘Gala’) [5]. No correlation was observed between the flesh hardness and galacturonic acid content in both of the cultivars ‘Jiguan’ and ‘Yindu’, and ‘Fuji’ and ‘Huaguan’, and only a correlation was observed between the flesh crispness and galacturonic acid content in the cultivars ‘Jiguan’ and ‘Yindu’ (r = 0.2–0.4, p = 0.01–0.05) (Figure 5). The low correlations between the texture properties and galacturonic acid content were because a decrease in galacturonic acid occurred from 0 to 20 DAH, while changes in the flesh hardness and crispness occurred throughout the whole storage process. Moreover, the expression of MdPG1 was higher in the cultivars with rapid texture loss than in those with slow texture loss (Figure 6). The role of MdPG1 is to hydrolyze polygalacturonic acid, resulting in a rapid loss of the galacturonic acid content in cultivars with rapid texture loss, and the role of MdPG1 in the hardness of apples has been confirmed [26,27]. In this study, the rapid increase in MdPG1 expression and the rapid loss of the galacturonic acid content occurred simultaneously in the cultivars with rapid texture loss (‘Jiguan’ and ‘Yindu’) (Figure 4I and Figure 6A). In contrast, in the varieties with slow texture loss, the decrease in galacturonic acid content was not significant due to the low expression level of MdPG1. These results further confirmed that the rapid loss of the galacturonic acid content contributed to the texture loss of the apples.
4.2.2. The Rapid Loss of Galactose Content Could Be Responsible for the Rapid Loss of Apple Texture
Galactan and arabinan are the core side chains of the polysaccharide rhamnogalacturonan-I (RG-I), and their depolymerization is believed to accelerate fruit ripening and softening [41,42]. In this study, the galactose content rapidly decreased from 0 to 20 DAH in the cultivars with rapid texture loss (‘Jiguan’, ‘Yindu’, and ‘Qinguan’) and ‘Cripps pink’, while there was no change in ‘Fuji’ and ‘Huaguan’, which was similar to the change in flesh hardness (Figure 4). The association between galactose content and flesh properties (flesh hardness, flesh crispness, and flesh tightness) was the closest among all cell wall neutral sugar compositions, which indicated that the rapid decrease in galactose was closely related to texture loss (Figure 5). Lahaye et al. [19] indicated that galactose correlated positively with the storage modulus at harvest. The expression patterns of the five MdGAL genes did not show a higher expression in the cultivars with rapid texture loss than in those with slow texture loss. In contrast, the expression levels of MdGAL3 (103434744) and MdGAL5 (103433231) were higher in ‘Fuji’ than in ‘Jiguan’ and ‘Yindu’ (Figure 6). The same results were reported in the ‘Fuji’ and ‘Qinguan’ apples, and the expression level of MdGAL genes was higher in ‘Fuji’ than in ‘Qingguan’ [43]. These results indicated that there were different expression levels in different cultivars and that the loss of galactose content may be regulated by multiple genes rather than a single gene. For the change in arabinose content, a consistency between the arabinose content and pericarp properties was observed in ‘Jiguan’ and ‘Yindu’ (Figure 5A). The significances of the arabinose content and pericarp properties were higher in the cultivars with rapid texture loss than with slow texture loss, which indicated that the rapid loss of arabinose could also be one of the reasons for the rapid loss of apple texture. A similar result was reported by Lahaye et al. [19].
4.3. A Higher Peak Ethylene Production Rate May Lead to More ASP Translated to WSP and More Cell Wall Neutral Sugar Composition Changes, Resulting in Apple Texture Loss
Ethylene plays a critical role as a ripening hormone and is implicated in controlling different facets of ripening, including texture change, acting through a range of transcriptional regulators [17]. In this study, the peak ethylene production rate was observed at 20 DAH for all six cultivars, while the peak values were higher in the cultivars with rapid texture loss than in ‘Fuji’ and ‘Huaguan’, and the same change trend was found for the respiration rate between them (Figure 3). This result was consistent between ‘McIntosh’ and ‘Honeycrisp’ [44], and those results suggested that the peak value of the ethylene production rate and respiration rate contributed to the texture loss in apples. In addition, the apples with rapid texture loss had more ASP at the beginning of storage and more WSP at the end of storage compared with the apples with slow texture loss (Figure 3C,D), which means that more ASP transformed to WSP and faster pectin solubilization coincided with texture loss. Similarly, Li et al. [5] reported a gradually increasing trend of WSP during storage, and Win et al. [45] reported that 1-MCP delayed the solubilization of pectin contents which could be reflected by changes in the cell wall components. These results implied that ethylene may affect texture loss by altering the morphology and composition of pectin. Moreover, it has been reported that one or more family members of each type of cell wall-modifying enzyme are expressed in response to ethylene during the ripening of different fruit [43]. In this study, in the cultivars with a higher ethylene production rate, the galactose content rapidly decreased and MdPG1 gene expression gradually increased. The same results reported pectin solubilization and an increase in the galactose content with increased ethylene production [46]. These results indicated that a higher peak ethylene production rate may lead to more ASP translated to WSP and more cell wall neutral sugar composition changes, resulting in apple texture loss.
5. Conclusions
The changes in the texture properties and cell wall sugar components of six different cultivars of apple fruit were compared, and the correlation between them was analyzed during storage. The apple cultivars with rapid texture loss (‘Jiguan’, ‘Yindu’, and ‘Qinguan’) showed a faster loss in flesh hardness and crispness, and a higher level of flesh tightness and pericarp break distance, than the cultivars with slow texture loss (‘Fuji’, ‘Huaguan’, and ‘Cripps pink’) (Figure 7). Among the monosaccharide components, the galactose content showed the closest association with the texture properties, which indicated that the rapid loss of galactose could be the reason for the rapid loss of apple texture. The rapid loss of galacturonic acid content and the expression level of MdPG1 were higher in the cultivars with rapid texture loss than in the cultivars with slow texture loss. In summary, the cell wall neutral sugar composition changes may be attributed to the texture loss in apple fruits during storage. These results may provide insights into understanding the textural properties of apple cultivars and developing more effective methods for the storage of high-quality apples.
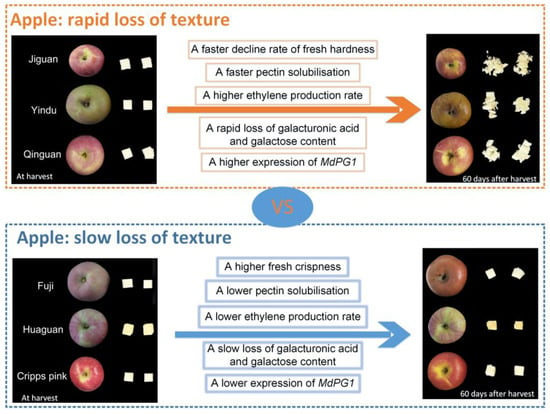
Figure 7.
The schematic illustration of key points to a different loss rate of texture among apple cultivars.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9030292/s1, Figure S1: Correlation analysis for six texture properties of ‘Jiguan’, ‘Yindu’, ‘Qinguan’, ‘Fuji’, ‘Huaguan’, and ‘Cripps pink’ apple fruits during postharvest; Figure S2: Correlation analysis for texture properties, physiological indicators of ‘Jiguan’, ‘Yindu’, ‘Qinguan’, ‘Fuji’, ‘Huaguan’, and ‘Cripps pink’ apple fruits during postharvest; and Figure S3: Expression profiles of cell wall-related gene from ‘Golden Delicious’ and ‘Fuji’ in RNA-Seq data. Table S1: Program for apple texture using TA-XT Plus (Stable Micro Systems Ltd., Godalming, UK); and Table S2: Primer sequences used for Real-Time PCR Quantification.
Author Contributions
Conceptualization, H.L. and Q.Z.; methodology, H.L.; software, S.L.; validation, S.L., M.Z. and Y.L.; formal analysis, Y.M.; investigation, H.L.; resources, J.R.; data curation, Q.Z.; writing—original draft preparation, H.L.; writing—review and editing, Q.Z. and Y.M..; visualization, H.L.; supervision, Q.Z.; project administration, J.R.; funding acquisition, Y.L. and J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Basic Research Plan in Shaanxi Province of China (project no. 2019JQ-476) for Y.L. and the National Key Research and Development Program of China (project no. 2016YFD0400102) for J.R.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
We sincerely thank Jing Zhang (Horticulture Science Research Center, Northwest A&F University, Yangling, China) and Huili Zhu (NWAFU, China) for providing professional technical assistance with GC-MS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mureșan, A.E.; Sestras, A.F.; Militaru, M.; Păucean, A.; Tanislav, A.E.; Pușcaș, A.; Mateescu, M.; Mureșan, V.; Marc, R.A.; Sestras, R.E. Chemometric comparison and classification of 22 apple genotypes based on texture analysis and physico-chemical quality attributes. Horticulturae 2022, 8, 64. [Google Scholar] [CrossRef]
- Rojas-Candelas, L.E.; Chanona-Pérez, J.J.; Méndez Méndez, J.V.; Perea-Flores, M.J.; Cervantes-Sodi, H.F.; Hernández-Hernández, H.M.; Marin-Bustamante, M.Q. Physicochemical, structural and nanomechanical study elucidating the differences in firmness among four apple cultivars. Postharvest Biol. Technol. 2021, 171, 111342. [Google Scholar] [CrossRef]
- Ni, F.; Meng, Q.; Gu, F.; Hu, Y. Building kinetic models for apple crispness to determine the optimal freshness preservation time during shelf life based on spectroscopy. J. Food Process. Preserv. 2020, 44, e14422. [Google Scholar] [CrossRef]
- Trujillo, D.I.; Mann, H.S.; Tong, C.B.S. Examination of Expansin genes as related to apple fruit crispness. Tree Genet. Genomes 2012, 8, 27–38. [Google Scholar] [CrossRef]
- Li, Q.; Xu, R.; Fang, Q.; Yuan, Y.; Cao, J.; Jiang, W. Analyses of microstructure and cell wall polysaccharides of flesh tissues provide insights into cultivar difference in mealy patterns developed in apple fruit. Food Chem. 2020, 321, 126707. [Google Scholar] [CrossRef]
- Segonne, S.M.; Bruneau, M.; Celton, J.M.; Le Gall, S.; Francin-Allami, M.; Juchaux, M.; Laurens, F.; Orsel, M.; Renou, J.P. Multiscale investigation of mealiness in apple: An atypical role for a pectin methylesterase during fruit maturation. BMC Plant Biol. 2014, 14, 375. [Google Scholar] [CrossRef]
- Brookfield, P.L.; Nicoll, S.; Gunson, F.A.; Harker, F.R.; Wohlers, M. Sensory evaluation by small postharvest teams and the relationship with instrumental measurements of apple texture. Postharvest Biol. Technol. 2011, 59, 179–186. [Google Scholar] [CrossRef]
- Johnston, J.W.; Hewett, E.W.; Hertog, M.L.A.T.M. Postharvest softening of apple (Malus domestica) fruit: A Review. N. Z. J. Crop Hortic. Sci. 2002, 30, 145–160. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Kwon, S.-I.; Kim, J.-H.; Park, M.Y.; Park, J.T.; Kim, S.A. Validation assay of Md-ACS1, Md-ACO1, and Md-PG1 molecular markers associated with storability in apples. Korean J. Breed. Sci. 2020, 52, 322–331. [Google Scholar] [CrossRef]
- Tacken, E.; Ireland, H.; Gunaseelan, K.; Karunairetnam, S.; Wang, D.; Schultz, K.; Bowen, J.; Atkinson, R.G.; Johnston, J.W.; Putterill, J.; et al. The role of ethylene and cold temperature in the regulation of the apple polygalacturonase1 gene and fruit softening. Plant Physiol. 2010, 153, 294–305. [Google Scholar] [CrossRef]
- Péneau, S.; Brockhoff, P.B.; Hoehn, E.; Escher, F.; Nuessli, J.; Plads, R.P. Relating consumer evaluation of apple freshness to sensory and physico-chemical measurements. J. Sensory Stud. 2006, 22, 313–335. [Google Scholar] [CrossRef]
- Costa, F.; Cappellin, L.; Fontanari, M.; Longhi, S.; Guerra, W.; Magnago, P.; Gasperi, F.; Biasioli, F. Texture dynamics during postharvest cold storage ripening in apple (Malus × domestica Borkh.). Postharvest Biol. Technol. 2012, 69, 54–63. [Google Scholar] [CrossRef]
- Costa, F.; Cappellin, L.; Longhi, S.; Guerra, W.; Magnago, P.; Porro, D.; Soukoulis, C.; Salvi, S.; Velasco, R.; Biasioli, F.; et al. Assessment of apple (Malus × domestica Borkh.) Fruit texture by a combined acoustic-mechanical profiling strategy. Postharvest Biol. Technol. 2011, 61, 21–28. [Google Scholar] [CrossRef]
- Yang, L.; Cong, P.; He, J.; Bu, H.; Qin, S.; Lyu, D. Differential pulp cell wall structures lead to diverse fruit textures in apple (Malus domestica). Protoplasma 2022, 259, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Quan, P.; Liu, H.; Li, L.; Qi, S.; Zhang, M.; Zhang, B.; Li, H.; Zhao, Y.; Ma, B.; et al. Transcriptomic and metabolic analyses provide new insights into the apple fruit quality decline during long-term cold storage. J. Agric. Food Chem. 2020, 68, 4699–4716. [Google Scholar] [CrossRef] [PubMed]
- Billy, L.; Mehinagic, E.; Royer, G.; Renard, C.M.G.C.; Arvisenet, G.; Prost, C.; Jourjon, F. Relationship between texture and pectin composition of two apple cultivars during storage. Postharvest Biol. Technol. 2008, 47, 315–324. [Google Scholar] [CrossRef]
- Tucker, G.; Yin, X.; Zhang, A.; Wang, M.; Zhu, Q.; Liu, X.; Xie, X.; Chen, K.; Grierson, D. Ethylene and fruit softening. Food Qual. Saf. 2017, 1, 253–267. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Lahaye, M.; Bouin, C.; Barbacci, A.; Le Gall, S.; Foucat, L. Water and cell wall contributions to apple mechanical properties. Food Chem. 2018, 268, 386–394. [Google Scholar] [CrossRef]
- Ireland, H.S.; Gunaseelan, K.; Muddumage, R.; Tacken, E.J.; Putterill, J.; Johnston, J.W.; Schaffer, R.J. Ethylene regulates apple (Malus × domestica) fruit softening through a dose × time-dependent mechanism and through differential sensitivities and dependencies of cell wall-modifying genes. Plant Cell Physiol. 2014, 55, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, B.J.; Su, G.; Zhang, M.; Grierson, D.; Chen, K.S. Transcriptional regulation of fleshy fruit texture. J. Integr. Plant Biol. 2022, 64, 1649–1672. [Google Scholar] [CrossRef]
- Ross, G.; Wegrzyn, T.; Macrae, E.; Redgwell, R. Apple beta-galactosidase activity against cell wall polysaccharides and characterization of a related cDNA clone. Plant Physiol. 1994, 106, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Goulao, L.F.; Cosgrove, D.J.; Oliveira, C.M. Cloning, Characterisation and Expression analyses of cDNA clones encoding cell wall-modifying enzymes isolated from ripe apples. Postharvest Biol. Technol. 2008, 48, 37–51. [Google Scholar] [CrossRef]
- Nobile, P.M.E.; Wattebled, F.; Quecini, V.; Girardi, C.L.; Lormeau, M.; Laurens, F. Identification of a novel α-L-arabinofuranosidase gene associated with mealiness in apple. J. Exp. Bot. 2011, 62, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Peace, C.P.; Stella, S.; Serra, S.; Musacchi, S.; Bazzani, M.; Sansavini, S.; Van de Weg, W.E. QTL dynamics for fruit firmness and softening around an ethylene-dependent polygalacturonase gene in apple (Malus × domestica Borkh.). J. Exp. Bot. 2010, 61, 3029–3039. [Google Scholar] [CrossRef]
- Atkinson, R.G.; Sutherland, P.W.; Johnston, S.L.; Gunaseelan, K.; Hallett, I.C.; Mitra, D.; Brummell, D.A.; Schröder, R.; Johnston, J.W.; Schaffer, R.J. Down-regulation of polygalacturonase1 alters firmness, tensile strength and water loss in apple (Malus × domestica) fruit. BMC Plant Biol. 2012, 12, 11–21. [Google Scholar] [CrossRef]
- Wu, B.; Shen, F.; Chen, C.J.; Liu, L.; Wang, X.; Zheng, W.Y.; Deng, Y.; Wang, T.; Huang, Z.Y.; Xiao, C.; et al. Natural variations in a pectin acetylesterase gene, MdPAE10, contribute to prolonged apple fruit shelf life. Plant Genome 2021, 14. [Google Scholar] [CrossRef]
- Chang, H.; Tong, C.B.S. The use of a combination of instrumental methods to assess change in sensory crispness during storage of a “Honeycrisp” apple breeding family. J. Texture Stud. 2018, 228–239. [Google Scholar] [CrossRef]
- Hou, J.; Sun, Y.; Chen, F.; Yu, L.; Mao, Q.; Wang, L.; Guo, X.; Liu, C. Analysis of microstructures and macrotextures for different apple cultivars based on parenchyma morphology. Microsc. Res. Tech. 2016, 79, 304–312. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Ban, Q.; Han, S.; Rao, J. Role of brassinosteroids in persimmon (Diospyros kaki L.) fruit ripening. J. Agric. Food Chem. 2018, 66, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.C.; Ginies, C. Comparison of the cell wall composition for flesh and skin from five different plums. Food Chem. 2009, 114, 1042–1049. [Google Scholar] [CrossRef]
- Englyst, H.; Wiggins, H.S.; Cummings, J.H. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1982, 107, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Busatto, N.; Tadiello, A.; Moretto, M.; Farneti, B.; Populin, F.; Vrhovsek, U.; Commisso, M.; Sartori, E.; Sonego, P.; Biasioli, F.; et al. Ethylene-auxin crosstalk regulates postharvest fruit ripening process in apple. Fruit Res. 2021, 1, 13. [Google Scholar] [CrossRef]
- Liu, X.; Renard, C.M.G.C.; Rolland-Sabaté, A.; Bureau, S.; Le Bourvellec, C. Modification of apple, beet and kiwifruit cell walls by boiling in acid conditions: Common and specific responses. Food Hydrocoll. 2021, 112. [Google Scholar] [CrossRef]
- Han, S.; Nan, Y.; Qu, W.; He, Y.; Ban, Q.; Lv, Y.; Rao, J. Exogenous γ-aminobutyric acid treatment that contributes to regulation of malate metabolism and ethylene synthesis in apple fruit during storage. J. Agric. Food Chem. 2018, 66, 13473–13482. [Google Scholar] [CrossRef]
- Li, T.; Xu, Y.; Zhang, L.; Ji, Y.; Tan, D.; Yuan, H.; Wang, A. The jasmonate-activated transcription factor mdmyc2 regulates ethylene response factor and ethylene bio-synthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell. 2017, 29, 1316–1334. [Google Scholar] [CrossRef]
- Barreiro Elorza, P.; Ortiz, C.; Ruiz-Altisent, M.; De Smedt, V.; Schotte, S.; Andani, Z.; Wakeling, I.; Beyts, P.K. Comparison between sensory and instrumental measurements for mealiness assessment in apples. A collaborative test. J. Texture Stud. 1998, 29, 509–525. [Google Scholar] [CrossRef]
- Chauvin, M.A.; Ross, C.F.; Pitts, M.; Kupferman, E.; Swanson, B. Relationship between instrumental and sensory determination of apple and pear texture. J. Food Qual. 2010, 33, 181–198. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Ng, J.K.T.; Schröder, R.; Brummell, D.A.; Sutherland, P.W.; Hallett, I.C.; Smith, B.G.; Melton, L.D.; Johnston, J.W. Lower cell wall pectin solubilisation and galactose loss during early fruit development in apple (Malus × domestica) cultivar “Scifresh” are associated with slower softening rate. J. Plant Physiol. 2015, 176, 129–137. [Google Scholar] [CrossRef]
- Schäfer, J.; Bunzel, M. Maturation-related modifications of cell wall structures of kohlrabi (Brassica oleracea var. Gongylodes). Eur. Food Res. Technol. 2018, 244, 893–902. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Dang, M.; Zhang, B.; Li, H.; Meng, R.; Qu, D.; Yang, Y.; Zhao, Z. Analysis of β-galactosidase during fruit development and ripening in two different texture types of apple cultivars. Front. Plant Sci. 2018, 9, 539. [Google Scholar] [CrossRef]
- Harb, J.; Gapper, N.E.; Giovannoni, J.J.; Watkins, C.B. Molecular analysis of softening and ethylene synthesis and signaling pathways in a non-softening apple cultivar, “Honeycrisp” and a rapidlysoftening cultivar, “McIntosh”. Postharvest Biol. Technol. 2012, 64, 94–103. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Naing, A.H.; Kwon, J.G.; Kang, I.K. 1-Methylcyclopropene (1-MCP) treatment delays modification of cell wall pectin and fruit softening in “Hwangok” and “Picnic” apples during cold storage. Postharvest Biol. Technol. 2021, 180, 111599. [Google Scholar] [CrossRef]
- Sutherland, P.W.; Fullerton, C.G.; Schröder, R.; Hallett, I.C. Cell wall changes in Actinidia arguta during softening. Sci. Hortic. 2017, 226, 173–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).